Abstract
Introduction
Postpartum depression (PPD) is a significant and common public health problem for women.
Aims
To examine the efficacy of an intervention based on the principles of interpersonal therapy (IPT) in reducing the risk of PPD in pregnant women.
Methods
Randomized controlled trial of 205 pregnant women who were 18 years old or older, on public assistance, and at risk for PPD. Participants (mean age=23; 38% Hispanic and 23% Black) were randomized to either the IPT group intervention (n=104) or the treatment as usual control (TAU) program (n=101).
Results
At 6 months, the overall depression rate in the intervention group (16%) was lower than the control group (31%) and the effect of the intervention was statistically significant at p<0.05.
Limitations
It is unknown if findings will generalize to a more heterogeneous sample of women than the current study, such as women from a range of socio-economic and cultural backgrounds, or marital status. There was a differential amount of contact between TAU and intervention conditions.
Conclusions
An IPT based intervention during the prenatal period has the potential to reduce cases of PPD within 6 months postpartum in at risk mothers on public assistance.
Keywords: Postpartum, Pregnancy, Depression, Intervention, Interpersonal therapy
1. Introduction
Postpartum depression (PPD) is a significant public health problem for women occurring in 10–15% of recently delivered mothers, (Horowitz et al., 2011; O’Hara and Swain, 1996) and among financially disadvantaged women the prevalence rates of PPD are even higher (Hobfoll et al., 1995; Scholle et al., 2003). Timely and effective interventions to reduce the risk of PPD are critical because the postpartum period can be a period of increased risk for depression (Dave et al., 2010; Vesga-Lopez et al., 2008) and for negative infant and later child outcomes (Grace et al., 2003; Murray and Cooper, 1997).
A proportion of women who suffer from PPD do not receive treatment, especially low-income women (Campbell et al., 1995; Abrams et al., 2009), which is due in part to the stigma associated with the disorder as well as limited access to effective treatments (Abrams et al., 2009; Callister et al., 2011; Sword et al., 2008). Further, when there is routine screening and regular follow-up of women with PPD, overall rates of treatment use are low (O’Mahen and Flynn, 2008; Yonkers et al., 2009). Moreover, certain treatments (e.g., pharmacological) are often met with hesitancy or resistance from breastfeeding mothers (Ferreira et al., 2007; Djulus et al., 2006). The burden of PPD is especially high in low-income women and their offspring (Murray et al., 1996). Although the literature remains equivocal regarding the causes of PPD, a number of risk factors have been shown to independently predict it, such as prenatal depression, child-care and life stress, and social support (see meta-analyses by Beck, 2001; Robertson et al., 2004). Risk factors for PPD, including inadequate social support and interpersonal conflict (Muzik et al., 2010), are modifiable and can be the focus of a preventative intervention.
A recent Cochrane review of 28 preventative interventions for PPD (Dennis and Doswell, 2013a) suggest, overall, women who received a psychosocial or psychological intervention were significantly less likely to experience PPD or depressive symptoms compared to those who received standard care. The authors concluded that the most promising interventions in the prevention of PPD were professionally-based home visits, (e.g., intensive home-nurse visits) postpartum lay (peer)-based telephone support, and those based in interpersonal psychotherapy. Others in their review (O’Hara and McCabe, 2013) have noted that more current preventive intervention trials for PPD provide only observed effects that have been small with modest evidence for these intervention studies and they have relied entirely on self-report of depressive symptoms. Additionally, another recent review reported that no definite conclusions can be made regarding which interventions are most likely to prevent PPD because few preventive interventions have demonstrated efficacy, replicated findings, used validated diagnostic measures for PPD, or included heterogeneous samples of women (Tzilos et al., 2015).
Interpersonal therapy (IPT) is an empirically-based treatment for a major depressive episode (MDE) (Elkin et al., 1989) and IPT-based trials and trials that target an at-risk population appear to hold the most promise for further study (Werner et al., 2015). IPT-based interventions target those factors that appear to play a role in PPD (e.g., social support, role transitions, life stressors). Previously, we conducted two randomized control pilot studies which used a selective IPT-based intervention to reduce the likelihood of PPD in pregnant women on public assistance and at risk for PPD. The first study (Zlotnick et al., 2001) was a pilot study (n=37) that randomized women on public assistance who were between 20 and 35 weeks’ gestation and at high risk for developing PPD to the IPT-based intervention and usual care condition or to only the usual care condition. The intervention significantly decreased depressive symptoms and decreased rates of PPD within the 3-month postpartum period, such that none of the women in the intervention condition developed PPD as compared to 33% of the women in the usual care condition. In a larger trial, a selective intervention (Zlotnick et al., 2006), 99 pregnant women who were at risk for PPD were randomized to the IPT-based intervention or to usual care. Compared to usual care, participation in the IPT-based preventive intervention significantly decreased PPD cases within 3 months postpartum.
Since it is unknown if the findings from these prior pilot studies will generalize to a larger sample of adult women whose onset of PPD was strictly after delivery and to a time period beyond three months postpartum, the objective of the present study was to examine the efficacy of this IPT-based intervention on reducing the risk of PPD in a sample of 205 pregnant women at risk for PPD and on public assistance who were followed up to 12 months after delivery.
2. Materials and methods
2.1. Trial design
Recruitment was conducted at three prenatal clinics in the Northeast, including a University-affiliated hospital, and two primary care sites. We conducted a blinded, randomized controlled trial to evaluate the efficacy of the intervention to reduce the risk of PPD in our sample. The intervention took place during pregnancy, and the booster took place within two weeks after delivery. Episodes that began during pregnancy were not considered a PPD case, and any new episodes of major depression after delivery were assessed up to 12-months postpartum.
2.2. Participants
Inclusion criteria for the study were: 1) pregnant status, 2) 18 years or older, 3) between 20 and 35 weeks gestation, 4) received public assistance, 5) English-speaking, 6) attended an urban, pre-natal medical clinic in the Northeast, and 7) a score of 27 or more on the Cooper Survey Questionnaire (First et al., 2002) (CSQ; see Procedures), which is the empirically derived threshold for high-risk status. Exclusion criteria prior to randomization included: 1) currently receiving mental health services, 2) did not understand English (Spanish-speaking only), or 3) met criteria for a current mood disorder, substance use disorder, anxiety disorder (excluding simple phobia) or psychosis as determined by the relevant modules of the Structured Clinical Interview for DSM-IV Axis I Disorders-Non-Patient Edition (Cooper et al., 1996).
The study protocol was approved by the Institutional Review Board of Women & Infants Hospital (WIH), Providence, Rhode Island, and was registered at clinicaltrials.gov (NCT00601757) on January 22, 2008.
2.3. Procedures
A research assistant approached women privately in the clinic exam room while waiting for their medical appointment and described the study. Participants completed an assessment that included demographic and obstetric information and the self-report Cooper Survey Questionnaire (CSQ), a 17-item risk validated predictive index for PPD (First et al., 2002), which assesses factors that have been shown to be related to PPD such as current and previous postpartum mood disturbances and relationship discord (e.g., “How have you and your partner been getting along in recent months?”). Possible scores on the index range from 0 to 63, with higher scores indicating higher risk. Assessments were conducted at 3-, 6- and 12-months after delivery. The Treatment Services Review (TSR) was used to assess if mental health treatment was received within a 90 day period at the 3, 6, and 12-months post-partum assessment points (McLellan et al., 1992).
2.4. Intervention
The IPT–based intervention, ROSE (Reach Out, Stand strong, Essentials for new mothers) Program, is designed to be administered antenatally to women in small groups (2–5 women), is highly structured, contains psychoeducational components, and IPT-based skills for improving relationships and building social support, that includes role plays and homework with feedback. The intervention consists of four, 90-minute group sessions over a 4-week period and a 50-minute individual booster session within 2 weeks after delivery. The content of the intervention focuses on managing role transitions with an emphasis on transition to motherhood, developing a support system, developing effective communication skills to manage relationship conflicts before and after the birth of their baby, goal setting, and psychosocial resources for new mothers. Due to the highly structured nature, training of interventionists consisted of a “mock” trial of the intervention with supervision by the first author (Zlotnick). Interventionists were monitored for adherence and competency. Trained interventionists consisted of a health educator (a registered nurse), and two individuals with bachelor’s degrees. Independent raters found that all three interventionists had average ratings of 3 or higher (adequate adherence and competence) for all adherence and competence scales (n=101).
2.5. Statistical methods
Urn randomization, a procedure to help produce better-balanced treatment groups (Stout et al., 1994), was used to assign participants to treatment, taking into account whether the participant had a previous major depressive episode. After a participant had completed the baseline assessment, and met eligibility criteria for the study, a computer-based urn randomization program was used and the study group was unveiled. The group allocation was not revealed to the research team members who conducted the follow up assessments. Women were randomly assigned to receive either the ROSE Program in addition to standard antenatal care, or treatment as usual (standard antenatal care alone). All randomized participants with at least one postpartum assessment were included in the primary analysis and were classified according to their randomly assigned study group (intention-to-treat).
Data analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC). Two-tailed p-values less than 0.05 were considered statistically significant. The primary outcome analysis used Cox proportional hazards regression for time to onset of a major depressive episode (MDE) (Cox, 1972). Time to onset was determined from psychiatric status ratings (PSRs). These ratings are captured on a weekly calendar, ranging from a PSR=1 for no symptoms to PSR=6 for severe, full-criteria depression. The onset of a MDE was defined by a minimum of two weeks of symptoms at full DSM-IV criteria (PSR levels 5 or 6). The median inter-rater kappa for PSR ratings was.94.
2.6. Outcomes
The primary outcome measure was the time to onset of a MDE. Postpartum Depression (PPD) was defined as a MDE occurring within the first 6 months after delivery, which is generally consistent with experts in the field who regard a duration to 6 months as the time frame that should be used to define a postpartum depressive episode (Jones et al., 2010).
Interviewers gathered Longitudinal Interval Follow-up Examination (LIFE) (Keller et al.) data from participants to determine the time to onset of an episode of major depression up to twelve months after delivery and from baseline to delivery (to determine that the onset of the MDE was after delivery). The LIFE is an interviewer-based assessment used to determine the longitudinal course of disorders such as major depression. Unlike the SCID, which provides only a cross-sectional measure, the LIFE tracks the severity and course of the disorder utilizing diagnostic criteria. Psychiatric Status Ratings (PSRs), a 6-point measure of symptomatic status is recorded for each week of follow-up based on DSM-IV criteria (from asymptomatic at PSR=1 to incapacitated at PSR=6).
To account for variability in gestational age at birth among the participants, we used delivery in the primary analyses as a clear and consistent marker for measuring the onset of the condition (PPD) as indicated by two successive weeks of PSR scores of 5 or higher, though we also conducted a sensitivity analysis including pre-delivery cases as described below. All assessments were administered by research assistants blinded to study group assignment from the initial contact through follow-up. All research assistants participated in a training protocol and reliability program for the LIFE and SCID at the Clinical Assessment and Training Unit of Brown University Department of Psychiatry and Human Behavior, an established training program for these measures.
3. Results
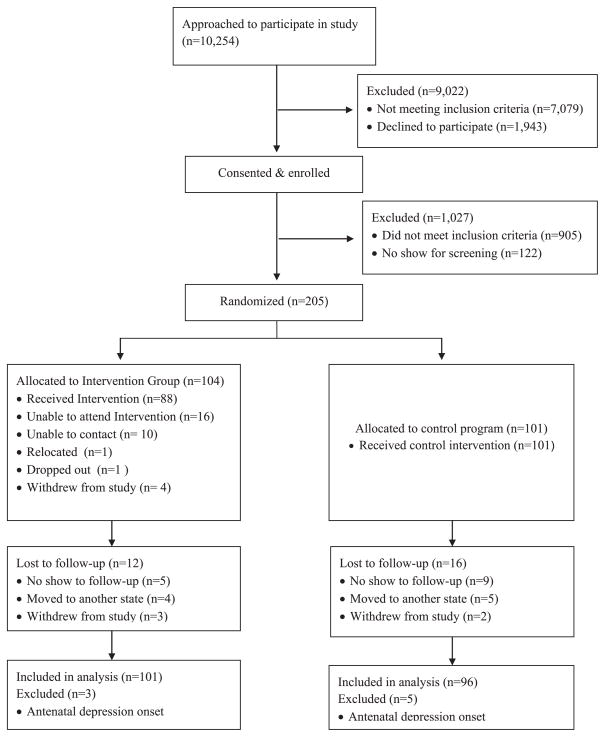
Participant recruitment was conducted between 8/8/06 and 7/ 20/11, and follow up assessments were conducted between 11/17/ 06 and 11/21/12. Of the 10,254 women approached, 1943 (18%) declined to participate in the study, and 1232 women consented and enrolled in the study and completed the risk survey (see Fig. 1). Of these women, 205 met inclusion criteria and were randomized either to the intervention (n=104) or the control condition (n=101). The remaining women (n=9022) were excluded because they were ineligible (reasons included not being pregnant, not between 20 and 35 weeks pregnant, not on public assistance, had been approached before, and not English speaking). Eight percent (n=16) dropped out of this phase of the study (i.e., did not show for their baseline assessment). On average, 3.5 sessions (out of a total of 5 sessions) were attended by women randomized to the intervention condition. Regarding postpartum follow-up assessments, 160 (78%) completed the 3-month, 171 (83%) the 6-month, and 173 (84%) the 12-month follow up. Overall, 32 (16%) women dropped out of the study (9 women withdrew from the study and 23 could not be reached for their follow-up assessments).
Fig. 1.
Participant selection and follow-up evaluation.
Within 3 months postpartum, participants randomized to the control condition used significantly more mental health treatment than those who received the intervention (23% to 10%); p=.0229 (Fisher’s exact test). Likewise there was a significant difference between the two conditions (23% vs. 11%); p=.0415 (exact test) across months 4–6 postpartum. There was no significant group difference for months 7–12 postpartum, although participants in the control condition used more mental health treatment than those randomized to ROSE (25% vs. 20%); p=.59 (exact test).
3.1. Baseline data
Participant characteristics are reported in Table 1. The average age for participants was 22.7 (4.4) years and the majority of participants were single. The groups were equivalent on all characteristics (including age, race, ethnicity, educational level, employment status, comorbid SCID diagnoses, prior pregnancies, gestational week, prior history of depression and age of onset of depressive episode), except how many participants reported being single. Categorical variables were compared by Fisher’s exact test and continuous variables were compared by the nonparametric Kruskal–Wallis test. Of the control group participants, 47% were single, vs. 64% of the intervention group (exact p=0.012). We therefore decided to covary this variable in our outcome analyses. We tested for effects of treatment site, but did not find significant effects for site either as a main effect or in interaction with treatment, and therefore did not use this variable in our analyses.
Table 1.
Characteristics of the study sample (N=205).
| Variable (n%) | Control (n=101) | Intervention (n=104) |
|---|---|---|
| Race/ethnicity | ||
| Latina | 35 (35%) | 43 (41%) |
| Black or African American | 27 (27%) | 21 (20%) |
| Caucasian | 29 (29%) | 28 (27%) |
| Asian | 1 (1.0%) | 3 (2.9%) |
| American Indian/Alaska Native | 4 (4.0%) | 5 (4.8%) |
| Other/multiracial | 7 (7%) | 5 (4.8%) |
| Marital status | ||
| Single* | 46 (46%) | 62 (60%) |
| Married | 9 (8.9%) | 7 (6.7%) |
| Divorced | 0 (0) | 4 (3.8%) |
| Separated | 1 (1.0%) | 1 (1.0%) |
| Widowed | 0 (0) | 0 (0%) |
| Remarried | 4 (4.0%) | 0 (0%) |
| Living together | 41 (41%) | 30 (29%) |
| Employment status | ||
| Full-time | 20 (20%) | 20 (19%) |
| Part-time | 20 (20%) | 16 (15%) |
| Student | 7 (6.9%) | 11 (11%) |
| Housewife | 8 (7.9%) | 2 (1.9%) |
| Retired | 0 (0) | 0 (0) |
| Unemployed | 46 (46%) | 55 (53%) |
| Education | ||
| 8th grade or less | 4 (4.0%) | 0 (0) |
| Some high school | 25 (25%) | 29 (28%) |
| High school graduate | 44 (44%) | 42 (40%) |
| Some college/training program | 24 (24%) | 31 (30%) |
| College graduate | 4 (4.0%) | 2 (1.9%) |
| Parity | ||
| 0 | 46 (46%) | 57 (55%) |
| 1 | 24 (24%) | 31 (30%) |
| 2 | 14 (14%) | 9 (8.7%) |
| 3 or more | 17 (17%) | 7 (7%) |
| Gestational period at enrollment in weeks | ||
| Mean, SD | 27.4 (4.38) | 26.9 (4.52) |
denotes a significant difference at p<0.05.
3.2. Outcomes
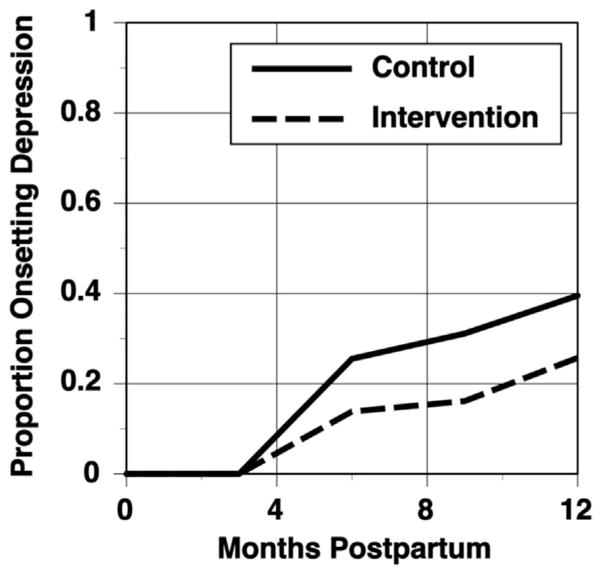
Based on 197 participants who had outcome data and were not in an episode of major depression at delivery, Kaplan–Meier Life-table estimates of the rate of a depressive episode by our primary outcome point six months after delivery found that 31% of control participants had an onset of PPD, compared to 16% for the intervention group (see Fig. 2). The Cox regression analysis indicated that after covarying for being single, the effect of the intervention was statistically significant at p=0.041. Whether or not participants were single did not significantly affect time to onset of PPD. Over the full 12 months of follow-up, 40% of controls and 26% of intervention participants had an onset of MDE, with the difference between the groups being marginally significant (p=0.052). The power analysis for the study used a two-sided alpha level of .05 and pilot data on the intervention effect to project that with an intake N of 188 and an attrition rate of 20%, power would be 87%. Because of the uncertainty of the pilot effect size, the ultimate recruited N was 205. We conducted a sensitivity analysis with the 6 month postpartum outcomes to ascertain whether our results remained robust by replicating the analyses including the 8 participants who we excluded because they were in episode for depression at delivery. These participants were counted as having onset before the other participants. This analysis had an N of 205, and the group difference remained statistically significant at p=0.045 at six months postpartum.
Fig. 2.
Rates of onset of Major Depressive Disorder (MDD) across conditions.
4. Discussion
The present study found that among a sample of pregnant women on public assistance who were assessed to be at risk for PPD, those who were assigned to receive an IPT-based intervention in addition to standard antenatal care were significantly less likely to develop PPD compared to those receiving standard antenatal care alone within a 6 month period after delivery. At the full 12 months after delivery, the onset of MDE was lower in the intervention group as compared to those receiving standard antenatal care, with this difference being marginally significant (p=0.052).
Using a larger sample and a longer follow-up period, the present study replicated the results of our previous pilot trials Zlotnick et al. (2001, 2006) demonstrating the efficacy of the ROSE Program in the prevention of PPD within a 6 months period after delivery in a sample of at risk, low-income, pregnant women. Although the intervention was marginally significant in reducing a depressive episode within 12 months after delivery, the first 6 months after delivery is considered a time of elevated risk and vulnerability for unipolar depression (O’Hara and McCabe, 2013). Hence reducing the risk of a depressive episode in this time period is potentially impactful in relation to the social and economic burden of the disease (i.e., the personal cost of depression for women and the likely enduring negative outcomes for the child that often occurs within the first 6 months postpartum (Grace et al., 2003; Murray and Cooper, 1997). Further, the cost of the delivery of our preventative intervention in addition to the cost of mental health treatment for participants who completed the intervention and had an onset of MDE over the 12-month period after delivery would have likely been far less than the cost of mental health treatment for participants in the control condition who had an onset of PPD over the same time period. The delivery of intervention by paraprofessionals may improve access to care, reduce the burden for treatment providers, particularly in busy prenatal or primary care settings, and reduce the implementation costs of the intervention. Nurses and social workers are typically used as interventionists in primary care settings.
The current findings in addition to our prior significant effect of the ROSE Program on the onset of PPD suggests that the content and delivery of the IPT-based intervention is efficacious in reducing the onset of PPD within a six month period among this sample of women. The content of the ROSE Program, consistent with the principles of IPT, focused on issues which have been identified as psychosocial risk factors for PPD depression Beck (2001); Milgrom et al. (2008); Nunes and Phipps (2013); O’Hara and Swain (1996); O’Hara and McCabe (2013) including increasing social support, both formal and informal resources, and developing effective communication skills to reduce conflict in significant relationships (Robertson et al., 2004; Stewart et al., 2003). A recent qualitative study of 37 underserved, pregnant women described a range of mental health needs, including addressing emotional needs and ways to overcome social and economic stressors (Raymond et al., 2014). Women desired interventions that were delivered using an educational format, with the support of peers in group settings, and included support for accessing services relevant to their needs (e.g., housing/transportation services, counseling/therapy, and faith-based support services). The delivery of the ROSE Program during pregnancy may be optimal timing given that women are in regular contact with health professionals and are motivated to make changes to benefit the health outcome of the baby (Johnston and Moreno, 2014). Further, unlike the current study, many positive effects of preventive interventions delivered during pregnancy do not extend beyond into the post-partum period (Dennis and Dowswell, 2013a).
Limitations of the current study are that it is unknown if findings will generalize to a more heterogeneous sample of women than the current study, such as women from a range of socio-economic and cultural backgrounds, marital status, or a more homogenous sample of women, such as specific ethnic/racial groups of women. There was a differential amount of contact between the treatment as usual (TAU) and intervention conditions, which did not allow for differences between the effects of contact alone and the specific effects of our intervention to be determined. However, the majority of extant studies to examine the efficacy of an intervention to reduce the risk of PPD or symptoms of PPD have used TAU as a control condition and over half of these studies found no significant differences between the conditions (Werner et al., 2015). This suggests that the additional attention received by the intervention participants did not produce a significant bias, although some interventions consisted of 10 or more sessions. The current study screened a large number of women for inclusion; however, the enrollment rate (12%) is comparable to other prevention trials, including PPD studies (Dennis and Dowswell, 2013b), and is appropriate given that the ideal characteristics for screening of PPD (e.g., sensitivity/specificity of screening tests, timing, and frequency) continues to be undefined within the field (U.S. Department of Health and Human Services, 2013). The study design includes several important strengths. First, with this longitudinal study, we were able to replicate positive findings of our previous trials of the ROSE Program extended to 6 months. Moreover, the current study included an interviewer-based, longitudinal assessment that was sensitive to changes in depressive symptoms within the 6 and 12 months postpartum period. Second, our study included a more racially and ethnically diverse, community-based sample of pregnant women than most extant postpartum prevention studies. Third, the outcome variable was assessed using a reliable and valid diagnostic measure, the LIFE (Keller et al., 1987), with PPD onset assessed using a clear and consistent marker. And finally, following recommendations from the field (U.S. Department of Health and Human Services, 2013), the current study included a longer follow up (12 months) than the vast majority of previous prevention studies, and importantly, demonstrated better retention rates than most prevention studies in this area (Dennis and Dowswell, 2013b).
This study provides support for an IPT-based, group intervention that paraprofessionals can deliver to at risk, financially disadvantaged pregnant women to reduce the risk of PPD. Pregnancy provides an opportunity to initiate a preventive intervention that could impact the health and well-being of both mother and infant, reducing the burden of disease. Future trials are needed to test whether the ROSE Program can be successfully implemented as part of routine prenatal care.
Footnotes
Conflict of interest
The authors report no competing interests. This work was supported by the National Institute of Mental Health (R01), MH071766-A2, Depression Prevention for Poor Pregnant Women.
References
- Abrams LS, Dornig K, Curran L. Barriers to service use for postpartum depression symptoms among low-income ethnic minority mothers in the United States. Qual Health Res. 2009;19:535–551. doi: 10.1177/1049732309332794. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Callister JC, Beckstrand RL, Corbett C. Postpartum depression and help-seeking behaviors in immigrant Hispanic women. J Obstet Gynecol Neonatal Nurs. 2011;40:440–449. doi: 10.1111/j.1552-6909.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Chon JF, Myers T. Depression in first-time mothers: Mother–Infant interaction and depression chronicity. Dev Psychol. 1995;31:349–357. [Google Scholar]
- Cooper PJ, Murray L, Hooper R, West A. The development and validation of a predictive index for postpartum depression. Psychol Med. 1996;26:628–634. doi: 10.1017/s0033291700035698. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables (with discussion) J R State Soc Ser B. 1972:187–220. [Google Scholar]
- Dave S, Petersen I, Sheer L, Nazateth I. Incidence of maternal and paternal depression in primary care. Arch Pediatr Adolesc Med. 2010;164:1038–1044. doi: 10.1001/archpediatrics.2010.184. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev. 2013a:1–206. doi: 10.1002/14651858.CD001134.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev. 2013b;2:CD001134. doi: 10.1002/14651858.CD001134.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djulus J, Koren G, Einarson T, Wilton L, Shakir S, Diav-Citrin O, Kennedy D, Voyer Lagivne S, DeSantis M, Einarson A. Exposure to mirtzapine during pregnancy: a prospective, comparative study of birth outcomes. J Clin Psychiatry. 2006;67:1280–1284. doi: 10.4088/jcp.v67n0817. [DOI] [PubMed] [Google Scholar]
- Elkin I, Shea MT, Watkins JT, Imber SD. National Institute of Mental Health Treatment of Depression Collaborative Research Program: general effectiveness of treatments. Arch Gen Psychiatry. 1989;46:971–982. doi: 10.1001/archpsyc.1989.01810110013002. [DOI] [PubMed] [Google Scholar]
- Ferreira E, Carceller A, Agogue C, Martin B, St-Andre M, Francoeur D, Verard A. Effects of selective serotonin reuptake inhibitors and venlafaxine during pregnancy in term and preterm neonates. Pediatrics. 2007;119:52–59. doi: 10.1542/peds.2006-2133. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Research, B, editor. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York Psychiatric Institute; New York: 2002. [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Hobfoll S, Ritter C, Lavin J, Hulsizer M, Cameron R. Depression prevalence and incidence among inner-city pregnant and postpartum women. J Consult Clin Psychol. 1995;63:445–453. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- Horowitz JA, Murphy CA, Gregory KE, Wojcik J. A community-based screening initiative to identify mothers at risk for postpartum depression. J Obstet Gynecol Neonatal Nurs. 2011;40:52–61. doi: 10.1111/j.1552-6909.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Moreno J. Pregnancy: a time of increased focus on health. Am J Lifestyle Med. 2014;8:93–96. [Google Scholar]
- Jones I, Cantwell R Nosology Working Group. The classification of perinatal mood disorders-suggestions for DSMV and ICD11. Arch Womens Ment Health. 2010;13:33–36. doi: 10.1007/s00737-009-0122-1. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott NC, Andreasen NC. The longitudinal interval follow-up evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. J Nerv Ment Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, Ericksen J, Ellwood D, Buist A. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–157. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev. 1996:67. [PubMed] [Google Scholar]
- Murray L, Cooper PJ. Postpartum depression and child development (Editorial) Psychol Med. 1997;27:253–260. doi: 10.1017/s0033291796004564. [DOI] [PubMed] [Google Scholar]
- Muzik M, Marcus S, Flynn H, Rosenblum K. Depression during pregnancy: Detection, comorbidity and treatment. Asia Pac Psychiatry. 2010;2:7–18. [Google Scholar]
- Nunes AP, Phipps MG. Postpartum depression in adolescent and adult mothers: comparing prenatal risk factors and predictive models. Matern Child Health J. 2013;17:1071–1079. doi: 10.1007/s10995-012-1089-5. [DOI] [PubMed] [Google Scholar]
- O’Hara MS, Swain AM. Rates and risk of postpartum depression; a meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- O’Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- O’Mahen HA, Flynn HA. Preferences and perceived barriers to treatment for depression during the perinatal period. J Women’s Health. 2008;17:1301–1309. doi: 10.1089/jwh.2007.0631. [DOI] [PubMed] [Google Scholar]
- Raymond NC, Pratt RJ, Godecker A, Harrison PA, Kim H, Kuendig J, O’Brien JM. Addressing perinatal depression in a group of underserved urban women: a focus group study. BMC Pregnancy Childbirth. 2014;14:336. doi: 10.1186/1471-2393-14-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Scholle SH, Haskett RF, Hanusa BH, Pincus HA, Kupfer DJ. Addressing depression in obstetrics/gynecology practice. Gen Hosp Psychiatry. 2003;25:83–90. doi: 10.1016/s0163-8343(03)00006-9. [DOI] [PubMed] [Google Scholar]
- Stewart DE, Robertson E, Dennis CL, Grace S, Wallington T. Postpartum Depression: Literature Review of Risk Factors and Interventions 2003 [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Drugs Suppl. 1994;12:S70–S75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Sword W, Busser D, Ganann R, McMillan T, Swinton M. Women’s care-seeking expereinces after referral for postpartum depression. Qual Health Res. 2008;18:1161–1173. doi: 10.1177/1049732308321736. [DOI] [PubMed] [Google Scholar]
- Tzilos G, Davis K, Zlotnick C. Prevention of postpartum psychopathology. In: Wenzel AAS, editor. Oxford Handbook of Perinatal Psychopathology. Oxford Library of Psychology. Oxford University Press; New York, New York: 2015. [Google Scholar]
- U.S. Department of Health and Human Services. Efficacy and Safety of Screening for Postpartum Depression. 2013. [Google Scholar]
- Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, Miller M, Osborne LM, Kuzava S, Monk C. Preventing post-partum depression: review and recommendations. Arch Womens Ment Health. 2015;18:41–60. doi: 10.1007/s00737-014-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Smith MV, Lin H, Howell HB, Shao L, Rosenheck RA. Depression screening of perinatal women: an evaluation of the healthy start depression initiative. Psychiatr Serv. 2009;60:322–328. doi: 10.1176/appi.ps.60.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick C, Ivan W, Pearlstein T, Howard M, Sweeney P. A preventive intervention for pregnant women on public assistance at risk for postpartum depression. Am J Psychiatry. 2006;163:1443–1445. doi: 10.1176/appi.ajp.163.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick C, Johnson SL, Miller IW, Pearlstein T, Howard M. Postpartum depression in women receiving public assistance: pilot study of an interpersonal-therapy-oriented group intervention. Am J Psychiatry. 2001;158:638–640. doi: 10.1176/appi.ajp.158.4.638. [DOI] [PubMed] [Google Scholar]