Abstract
Background
Ivacaftor, approved for treatment of cystic fibrosis (CF) patients aged ≥6 years with G551D-CFTR or other gating mutations, was evaluated in subjects with R117H-CFTR, a residual function mutation.
Methods
A 24 week, placebo-controlled, double-blind, randomized clinical trial (RCT) enrolled 69 CF subjects aged ≥6 years with R117H-CFTR and percent predicted forced expiratory volume in 1 second (ppFEV1) ≥40. Primary outcome was absolute change from baseline in ppFEV1 through week 24. Secondary outcomes included sweat chloride, CF Questionnaire-Revised (CFQ-R) respiratory domain, and safety. An open-label extension enrolled 65 RCT subjects after washout; after 12 weeks, an interim analysis was performed.
Findings
After 24 weeks, treatment difference in mean absolute change in ppFEV1 between ivacaftor (n=34) and placebo (n=35) was 2.1 percentage points (p=0·20). Ivacaftor treatment resulted in significant RCT treatment differences in sweat chloride (−24.0 mmol/L; p<0.001) and CFQ-R respiratory domain (8.4; p=0.009). In prespecified subgroup analyses, ppFEV1 significantly improved with ivacaftor in subjects aged ≥18 years (treatment difference vs placebo: 5.0 percentage points; p=0.01), but not in subjects aged 6 to 11 years (−6.3 percentage points; p=0.03). In the extension study, both placebo/ivacaftor and ivacaftor/ivacaftor groups showed ppFEV1 improvement (absolute change from postwashout baseline at week 12: 5.5 percentage points; p<0.0001). No new safety concerns were identified.
Interpretation
Although this RCT did not meet the primary outcome, secondary outcomes and subgroup analyses suggest that ivacaftor significantly improves lung function in adult patients with R117H-CFTR and may benefit patients with established disease.
Introduction
Cystic fibrosis (CF) is a life-shortening, autosomal recessive condition caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that result in absence or dysfunction of the CFTR protein,1 a cell-surface localized chloride channel that regulates salt and water absorption and secretion across epithelia.2 CFTR dysfunction affects multiple organs, including the lung, pancreas, and gastrointestinal tract.3
The R117H-CFTR mutation, present in approximately 3% of patients with CF,4 causes impaired CFTR channel conductance and reduced channel gating, with the latter being the primary defect.5 In addition, production of properly spliced CFTR mRNA transcripts is impacted by the length of the cis-localized intron 8 polythymidine (Poly-T) tract.6 As with CFTR mutations, the 3 common alleles at the poly-T locus, 5T, 7T, and 9T, occur with varying geographic frequency,7–13 with the 5T variant in cis with R117H-CFTR associated with greater risk for CF disease.14 Reduced function and variable expression of R117H-CFTR results in residual CFTR ion transport and consequently a variable clinical presentation. CF patients with R117H-CFTR may develop progressive life-limiting pulmonary disease that frequently presents at an older age compared with that of the overall CF population.15 The predicted age of survival in patients with a genotype associated with residual CFTR function, including R117H, is approximately 50 years.16 Many younger patients with R117H-CFTR are being identified through the implementation of newborn screening protocols.4
Ivacaftor, a CFTR potentiator, improves chloride transport through CFTR channels, including R117H, by increasing the channel open probability (or gating).17,18 Ivacaftor was approved based on improved clinical endpoints in patients aged ≥6 years with CF and a CFTR mutation that primarily affects CFTR open probability, such as G551D.3,19,20 This placebo-controlled study, followed by an open-label extension, aimed to evaluate the efficacy and safety of ivacaftor in subjects with CF who have an R117H-CFTR mutation.
Methods
Study design and population
KONDUCT (VX11-770-110) was a multicenter, phase 3, double-blind, placebo-controlled, parallel-group study conducted from July 2012 to October 2013 in subjects aged ≥6 years with a confirmed diagnosis of CF21 and chronic sinopulmonary disease (clinicaltrials.gov identifier NCT01614457). At the screening visit, subjects aged 6–11 years had ppFEV1 ≥40 to ≤105 and subjects aged ≥12 years had ppFEV1 ≥40 to ≤90; all had the R117H mutation on at least one CFTR allele. Eligible subjects were randomized to receive placebo or ivacaftor 150 mg (Kalydeco®; Vertex Pharmaceuticals Incorporated, Boston, MA) every 12 hours (q12h) for 24 weeks (e-Figure 1). Randomization was stratified by age (6–11, 12–17, and ≥18 years) and percent predicted forced expiratory volume in 1 second (ppFEV1; <70, ≥70 to ≤90, and >90). After completing 24 weeks of therapy, subjects underwent an ivacaftor washout period of 3–4 weeks.
Enrollment was planned for a minimum of 40 and a maximum of 80 subjects. It was estimated that a sample size of 60 subjects would provide 80% power at the 5% level of significance to detect a 6.0 percentage point difference in absolute change from baseline in FEV1.
Subjects who completed KONDUCT were eligible to enroll in KONTINUE (VX11-770-112; clinicaltrials.gov identifier NCT01707290) and receive open-label ivacaftor treatment for up to 104 additional weeks. An initial interim analysis of KONTINUE was planned for subjects continuing from KONDUCT after 12 weeks. Optional subject samples were obtained for post hoc determination of R117H poly-T status using allele-specific long-range polymerase chain reaction.
The studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and all applicable local regulations, and were approved by each site’s institutional review board. All subjects provided written informed consent or assent, as appropriate.
Outcome measures
The primary outcome measure in the double-blind, placebo-controlled study KONDUCT was the absolute change from baseline in ppFEV1 through week 24. Secondary outcome measures included the change from baseline through week 24 in body mass index (BMI), the respiratory domain of the CF questionnaire-revised (CFQ-R), and sweat chloride (SwCl). Time to first pulmonary exacerbation and safety, as determined by adverse events (AEs), clinical laboratory values for serum chemistry, hematology, and coagulation, electrocardiograms, and vital signs were also studied. The incidence of pulmonary exacerbations was a tertiary outcome measure. Efficacy measures at week 12 in the open-label extension study KONTINUE included absolute change in ppFEV1, SwCl, and CFQ-R respiratory domain. For a description of the safety analyses in KONTINUE, refer to e-Appendix 1.
Statistical analyses
Analyses were performed for the overall population of all subjects who received study medicationand prespecified subgroups based on age category (6–11, 12–17, and ≥18 years), baseline ppFEV1 category (<70, ≥70 to ≤90, and >90), and sex. Post hoc analyses of the clinical efficacy outcomes were conducted based on poly-T status.
The primary analysis for the absolute change from baseline in ppFEV1 through week 24 was based on a mixed-effects model for repeated measurements (MMRM), with adjustments for continuous baseline values of age and ppFEV1. Change in BMI over 24 weeks was analyzed using a mixed-effects model with random intercept and slope and adjustment for age and categorical ppFEV1 at baseline; the rate of change over all visits was obtained from the model. SwCl and CFQ-R were analyzed with an MMRM similar to ppFEV1. Time to first pulmonary exacerbation was analyzed using a Cox regression model adjusting for age and baseline ppFEV1. Counts of pulmonary exacerbation were analyzed using negative binomial regression, adjusting for age and baseline ppFEV1. Safety results were summarized using descriptive statistics only.
Additional details regarding the study methodology are provided in e-Appendix 1.
Role of the funding source
The funder designed the protocol in collaboration with the authors, analyzed data, participated in data interpretation, and provided editorial/writing assistance. RBM had full access to the study data and made the final decision to submit for publication.
Results
Study population
Enrollment for the double-blind, placebo-controlled KONDUCT study was terminated after 69 subjects were randomized and received ≥1 dose of study medication, which was within the target planned enrollment range. Ninety-four percent (n=32) of ivacaftor and 100% (n=35) of placebo recipients completed their full assigned duration of dosing (Figure 1). Two subjects in the ivacaftor group discontinued prematurely (1 noncompliance and 1 pregnancy). Eight subjects who had not completed 24 weeks of treatment at the time of study termination were considered to have completed their assigned treatment and could enter the rollover study. In total, 65 subjects elected to enroll in the open-label extension study KONTINUE (ivacaftor group, n=30; placebo group, n=35).
Figure 1.
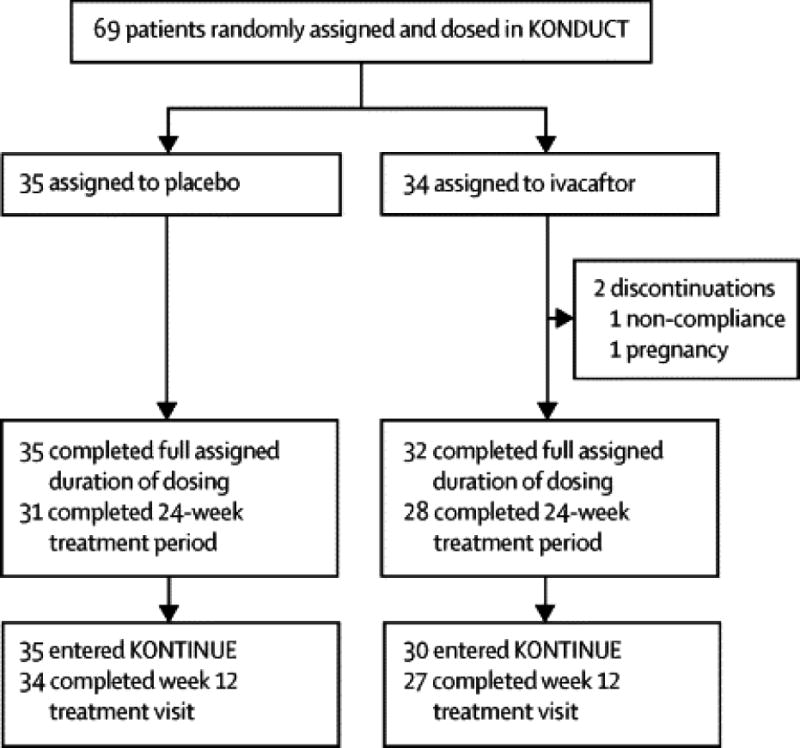
Subject disposition.
At the baseline of KONDUCT, mean age was 31 years and ppFEV1 was 72.9. Most subjects (87%) were confirmed pancreatic sufficient based on baseline fecal elastase levels >200 ug/g. Demographic and baseline characteristics were similar across groups (Table 1). Subjects aged 6–11 years had much higher baseline ppFEV1 (ivacaftor 97.5; placebo 94.0) than subjects aged ≥18 years (ivacaftor 67.0; placebo 62.2). Only 2 subjects were aged 12–17 years; therefore, statistical analyses were not conducted for this age subgroup.
Table 1.
Demographic and baseline characteristics in KONDUCT (full analysis set)
| Overall | 6–11 Years | ≥18 Years | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Placebo (n=35) | Ivacaftor (n=34) | Placebo (n=8) | Ivacaftor (n=9) | Placebo (n=26) | Ivacaftor (n=24) | |
|
| ||||||
| Female, n (%) | 20 (57) | 19 (56) | 3 (38) | 5 (56) | 16 (62) | 13 (54) |
|
| ||||||
| Age, years | 32.7 (17.4) | 29.2 (16.6) | 9.0 (1.6) | 8.8(1.9) | 40.6 (12.6) | 37.5 (12.1) |
| ≥18, n (%) | 26 (74) | 24 (71) | 0 | 0 | 26 (100) | 24 (100) |
| 12–17, n (%) | 1 (3) | 1 (3) | 0 | 0 | 0 | 0 |
| 6–11, n (%) | 8 (23) | 9 (27) | 8 (100) | 9 (100) | 0 | 0 |
|
| ||||||
| Weight, kg | 62.8 (25.4) | 66.1 (25.5) | 34.0 (9.1) | 32.9 (13.3) | 71.7 (22.5) | 77.9 (16.7) |
|
| ||||||
| BMI, kg/m2 | 23.1 (6.0) | 24.5 (6.3) | 17.1 (2.4) | 17.6 (3.3) | 24.9 (5.7) | 26.9 (5.2) |
|
| ||||||
| Percent predicted FEV1 | 70.2 (18.9) | 75.7 (19.3) | 94.0 (8.4) | 97.5 (8.6) | 62.2 (14.4) | 67.0 (15.4) |
| <70%, n (%) | 15 (43) | 13 (38) | 0 | 0 | 15 (58) | 13 (54) |
| ≥70 to ≤90%, n (%) | 14 (40) | 14 (41) | 2 (25) | 3 (33) | 11 (42) | 10 (42) |
| >90%, n (%) | 6 (17) | 7 (21) | 6 (75) | 6 (67) | 0 | 1 (4) |
|
| ||||||
| Sweat chloride, mmol/L | 73.4 (19.7) | 67.3 (23.5) n = 32 |
74.7 (28.6) | 64.2 (22.6) n = 8 |
73.0 (17.3) | 69.3 (24.1) n = 23 |
|
| ||||||
| Respiratory domain of CFQ-R | 66.4 (24.4) n=34 |
75.3 (20.1) n=33 |
91.7 (6.8) n=7 |
92.7 (7.0) n=8 |
59.2 (23.2) n=26 |
68.4 (19.1) n=24 |
|
| ||||||
| Fecal elastase-1 <200 μg/g, n (%) | 5 (14) | 2 (6) | 0 | 0 | 5 (19) | 2 (8) |
|
| ||||||
| RH 7H poly-T status, n (%) | ||||||
|
| ||||||
| 5T | 27 (77) | 21 (62) | 5 (63) | 4 (44) | 21 (81) | 17 (71) |
|
| ||||||
| 7T | 7 (20) | 12 (35) | 3 (38) | 5 (56) | 4 (15) | 6 (25) |
|
| ||||||
| R117H/F508del* | 25 (71) | 28 (82) | 6 (75) | 8 (89) | 19 (73) | 19 (79) |
Represents CFTR mutation, which results in CFTR protein with residual function.
Data are presented as mean (standard deviation) unless otherwise indicated.
The 12–17-year age group only included 2 subjects (ivacaftor, n=1; placebo, n=1) and, therefore, is not represented in subanalyses, but these subjects are included in the overall analyses.
BMI = body mass index; CFQ-R = cystic fibrosis questionnaire-revised; FEV1 = forced expiratory volume in 1 second.
Poly-T analysis showed 62% (n=21) of subjects in the ivacaftor group and 77% (n=27) of subjects in the placebo group had R117H-5T, while 35% (n=12) in the ivacaftor group and 20% (n=7) in the placebo group had R117H-7T (Table 1). There were no significant differences in the proportion of 5T versus 7T across the treated and placebo groups for the overall study population or within age subgroups (Chi-squared test p>0.05 for all). Overall, 77% (n=53) of subjects had the F508del mutation on the non-R117HCFTR allele (28/34 [82%] in the ivacaftor group; 25/35 [71%] in the placebo group). For most other subjects, the non-R117H CFTR allele had a protein null mutation.
Compliance with study medication during KONDUCT was 99.0% in the ivacaftor group and 98.4% in the placebo group.
Overall efficacy
Through week 24 of the double-blind, placebo-controlled KONDUCT study, the model-adjusted mean absolute increase from baseline in ppFEV1 was 2.6 percentage points in the ivacaftor group versus 0.5 percentage points in the placebo group, resulting in a treatment difference of 2.1 (p=0.20; Table 2; Figure 2a). During the washout period, mean ppFEV1 values returned to their pretreatment baseline levels. When subjects received open-label ivacaftor for 12 weeks in the KONTINUE extension study, ppFEV1 increased from the postwashout baseline by a mean 5.0 and 6.0 percentage points for those previously in the placebo and ivacaftor arms (p=0.0005 and p=0.006, respectively; e-Figure 2). Additionally, when assessed by poly-T status, most subjects in the ivacaftor group with 5T experienced improvements from baseline in ppFEV1 at week 24, as did a few subjects with 7T (e-Figure3).
Table 2.
Clinical efficacy outcomes through week 24 in KONDUCT (full analysis set)
| Overall | 6–11 Years | ≥18 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=35) |
Ivacaftor (n=34) |
Treatment Difference (P value) |
Placebo (n=8) |
Ivacaftor (n=9) |
Treatment Difference (P value) |
Placebo (n=26) |
Ivacaftor (n=24) |
Treatment Difference (P value) |
||
| Percent predicted FEV1 | ||||||||||
| Baseline value | 70.2 (18.9) | 75.7 (19.3) | – | 94.0 (8.4) | 97.5 (8.6) | – | 62.2 (14.4) | 67.0 (15.4) | – | |
| Absolute change from baseline, percentage points | 0.5 (1.1) | 2.6 (1.2) | 2.1 (0.20) | 3.5 (1.9) | −2.8 (1.8) | −6.3 (0.03) | −0.5 (1.3) | 4.5 (1.4) | 5.0 (0.01) | |
| Relative change from baseline, % | −0.2 (1.8) | 4.8 (1.9) | 5.0 (0.06) | 3.8 (2·2) | −3.0 (2.0) | −6.8 (0.04) | −1.5 (2.3) | 7.7 (2.4) | 9.1 (0.008) | |
| Absolute change in BMI, kg/m2 | Baseline value | 23.1 (6.0) | 24.5 (6.2) | – | 17.1 (2·4) | 17.6 (3.3) | – | 24.9 (5.7) | 26.9 (5.2) | – |
| Change from baseline | 0.23 (0.65) | 0.49 (0.67) | 0.26 (0.78) | 0.51 (0.80) | 0.33 (0.76) | −0.18 (0.87) | 0.22 (0.78) | 0.53 (0.80) | 0.31 (0.78) | |
| Absolute change in sweat chloride, mmol/L | Baseline value | 73.4 (19.7) | 67.3 (23.5) | – | 74.7 (28.6) | 64.2 (22.6) | – | 73.0 (17.3) | 69.3 (24.1) | – |
| Change from baseline | −2.3 (1.4) | −26.3 (1.5) | −24.0 (<0.0001) | 1.0 (3.0) | −26.6 (3.0) | −27.6 (<0.0001) | −4.0 (1.5) | −25.9 (1.6) | −21.9 (<0.0001) | |
| Change in respiratory domain of CFQ-R, pooled | Baseline value | 66.4 (24.4) | 75.3 (20.1) | – | 91.7 (6.8) | 92.7 (7.0) | – | 59.9 (23.2) | 68.4 (19.1) | – |
| Change from baseline | −0.8 (2.2) | 7.6 (2.2) | 8.4 (0.009) | −1.6 (3.1) | −7.7 (3.0) | −6.1 (.19) | −0.5 (2.6) | 12.2 (2.7) | 12.6 (0.002) | |
Baseline values are reported as mean (standard deviation).
Change from baseline values are reported as least squares mean (standard error).
The 12–17-year age group only included 2 subjects (ivacaftor, n=1; placebo, n=1) and, therefore, is not represented in subanalyses, but subjects are included in the overall analyses.
BMI = body mass index; CFQ-R = Cystic Fibrosis Questionnaire-revised; FEV1 = forced expiratory volume in 1 second.
Figure 2. Absolute change from baseline over 24 weeks in KONDUCT and over 12 weeks in KONTINUE in (A) percent predicted FEV1 and (B) CFR-R respiratory domain score (overall population).
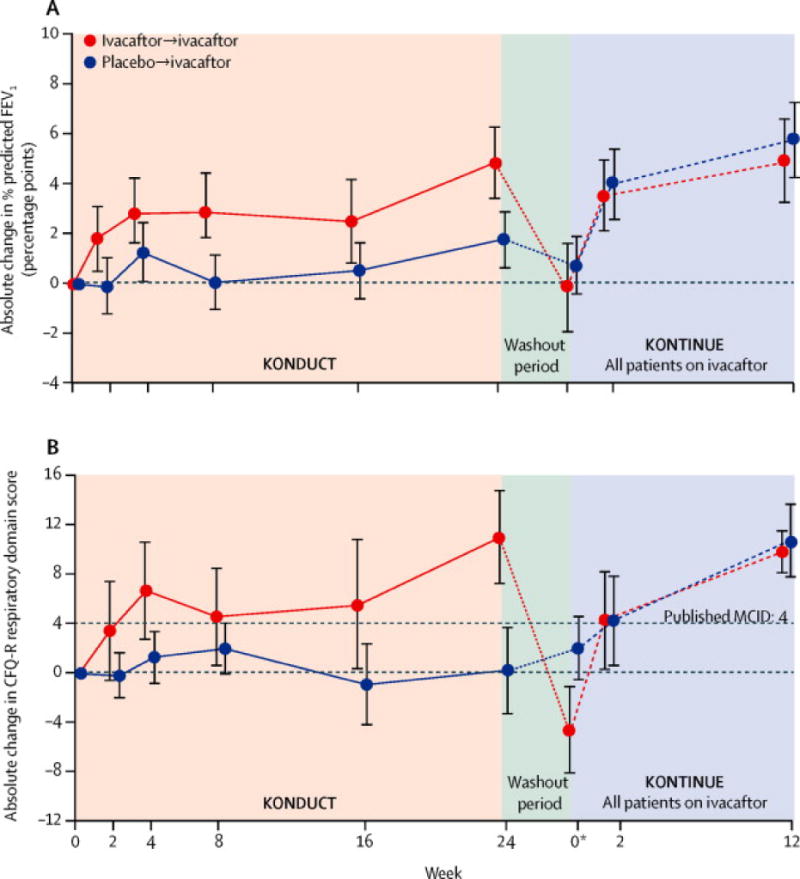
aAtweek 0 of KONTINUE, data presented are mean change (±SE) from KONDUCT baseline at KONDUCT follow-up visit. Line graphs plot summary statistics (mean change ±SE) from KONDUCT baseline at each time point for KONDUCT and KONTINUE CFQ-R, Cystic Fibrosis Questionnaire-Revised; ppFEV1, percent predicted forced expiratory volume in 1 second; SE, standard error.
Significant improvement from baseline of 8.4 points in the CFQ-R was observed in the ivacaftor versus placebo group (p=0.009) (Table 2; Figure 2b). The percentage of subjects who achieved the minimal clinically important difference of ≥4 points for respiratory domain22 was 41.2% in the ivacaftor group and 28.6% in the placebo group. In KONTINUE, both the placebo/ivacaftor and ivacaftor/ivacaftor groups showed improvement in CFQ-R.
Compared with placebo, SwCl levels were significantly reduced from baseline following 24 weeks of ivacaftor treatment (treatment difference: −24.0 mmol/L; p<0.001) (Table 2; e-Figure 4). A significant reduction from baseline in SwCl was also observed with ivacaftor versus placebo treatment in subjects with R117H-5T (treatment difference: −24.2 mmol/L; p<.0001) and R117H-7T (treatment difference: −24.1 mmol/L; p=0.0003). No change in BMI occurred from baseline through week 24 with ivacaftor treatment in KONDUCT (Table 2). Twenty-four subjects experienced ≥1 protocol-defined pulmonary exacerbation (ivacaftor, n=11 [32%]; placebo, n=13 [37%]) during KONDUCT. No significant between-group difference was noted in the time-to-first pulmonary exacerbation (hazard ratio: 0.93; Table 3), although there was a numerical reduction in the number of pulmonary exacerbations requiring hospitalization (placebo 7 vs ivacaftor 2) or IV antibiotics (placebo 8 vs ivacaftor 2).
Table 3.
Summary of pulmonary exacerbations
| Event Type | Parameter | Placebo (n=35) | Ivacaftor (n=34) |
|---|---|---|---|
| Total days on study | 5485 | 5182 | |
| All pulmonary exacerbationsa | Subjects with events | 13 | 11 |
| Events (event rate) | 17 (0·295) | 13 (0·249) | |
| Requiring hospitalization | Subjects with events | 6 | 2 |
| Events | 7 | 2 | |
| Requiring intravenous antibiotic therapy | Subjects with events | 6 | 2 |
| Events | 8 | 2 |
Pulmonary exacerbation includes events that met the protocol definition of pulmonary exacerbations (ie, treatment with new or changed antibiotic therapy for ≥4 sinopulmonary signs/symptoms).
Subgroup analyses
All subject groups showed significant reductions in SwCl (Figure 3A). Age group (adults and subjects aged 6–11 years) appeared strongly related to the effect of treatment on ppFEV1 (Figure 3B, detailed below). There was also a trend toward greater response to ivacaftor in subjects with or at risk for more advanced disease (i.e., lower baseline ppFEV1, Pseudomonas aeruginosa infection, and R117H-5T; Figure 3B). Pharmacokinetic analyses confirmed that differences in response between age groups were not attributable to differences in drug exposures (e-Figure 5).
Figure 3. Absolute change from baseline through week 24 by MMRM (FAS) in (A) sweat chloride and (B) percent predicted FEV1 in KONDUCT by subject baseline parameters.
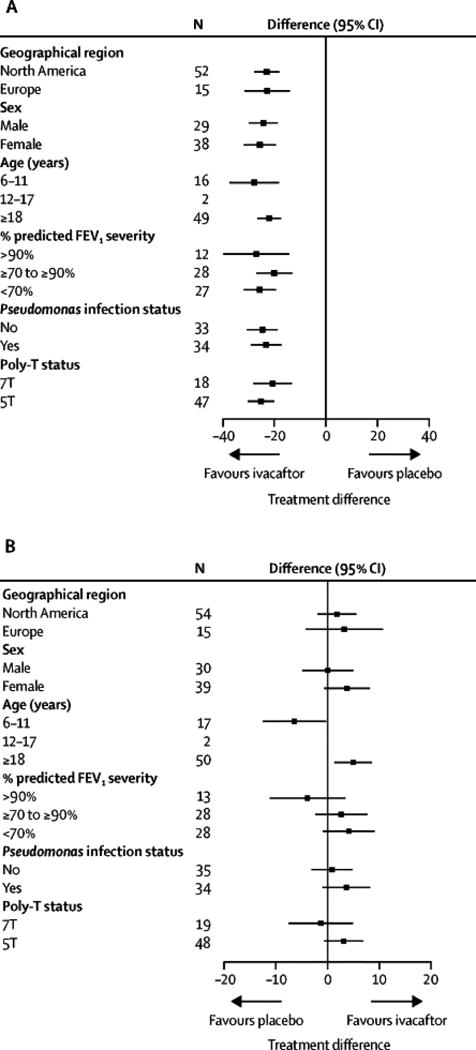
MMRM, mixed-effects model for repeated measures; ppFEV1, percent predicted forced expiratory volume in 1 second.
Figure 5. Absolute change from baseline over 24 weeks in KONDUCT and over 12 weeks in KONTINUE in percent predicted FEV1 (children aged 6–11 years).
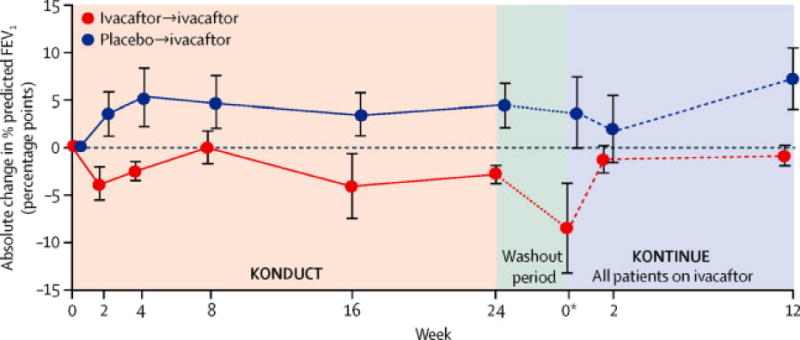
aAtweek 0 of KONTINUE, data presented are mean change (±SE) from KONDUCT baseline at KONDUCT follow-up visit. Line graphs plot summary statistics (mean change ±SE) from KONDUCT baseline at each time point for KONDUCT and KONTINUE. ppFEV1, percent predicted forced expiratory volume in 1 second; SE, standard error.
Efficacy: adults
In the double-blind, placebo-controlled KONDUCT study, a significant improvement from baseline was noted in absolute ppFEV1 in the ivacaftor group versus the placebo group for subjects aged ≥18 years (n=50; treatment difference: 5.0 percentage points; p=0.01; Table 2; Figure 4A). On responder analysis through week 24, 54.2% of ivacaftor group subjects experienced a ≥5% absolute change in ppFEV1 compared with 15.4% of subjects in the placebo group (e-Figure 6). In the open-label extension study KONTINUE, both the placebo/ivacaftor and ivacaftor/ivacaftor groups showed improvement in ppFEV1. For both groups combined, the absolute change from the post-washout baseline at week 12 was 5.1 percentage points (p<.0001).
Figure 4. Absolute change from baseline over 24 weeks in KONDUCT and over 12 weeks in KONTINUE in (A) percent predicted FEV1 and (B) CFQ-R respiratory domain score (adult subjects).
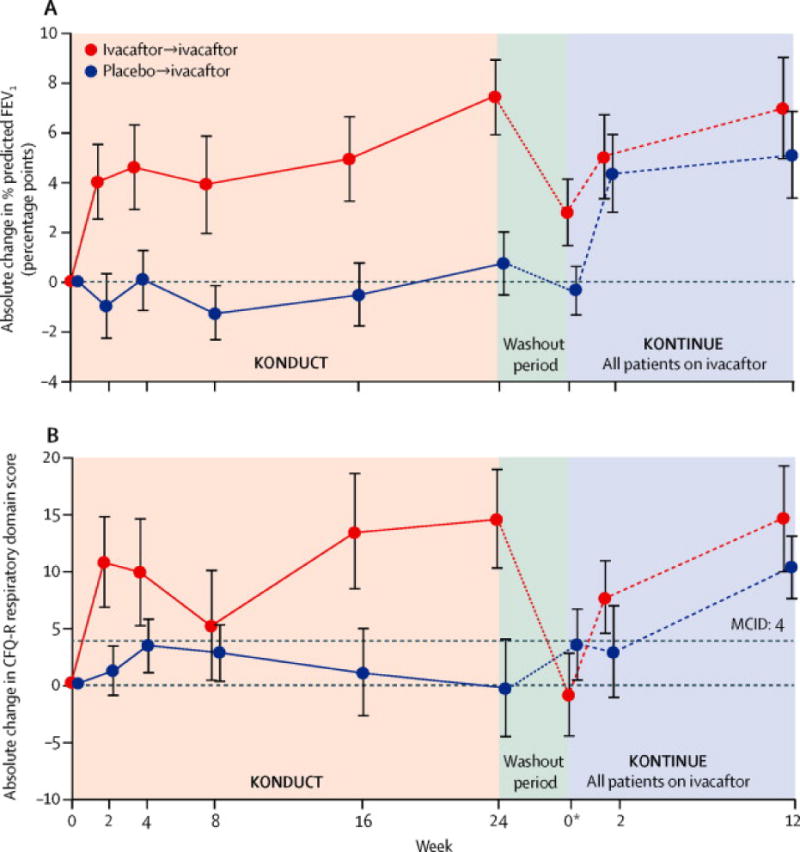
aAtweek 0 of KONTINUE, data presented are mean change (± standard error [SE]) from KONDUCT baseline at KONDUCT follow-up visit. Line graphs plot summary statistics (mean change ±SE) from KONDUCT baseline at each time point for KONDUCT and KONTINUE. CFQ-R, Cystic Fibrosis Questionnaire-Revised; ppFEV1, percent predicted forced expiratory volume in 1 second; SE, standard error.
The increase in CFQ-R in the ivacaftor compared with the placebo group in adults through week 24 in KONDUCT was 12.6 points (p<0.01; Figure 4B). Similarly, in KONTINUE, both the placebo/ivacaftor and ivacaftor/ivacaftor groups showed improvement in CFQ-R; for both groups combined, absolute change from the postwashout baseline at week 12 was 12.3 points.
Efficacy: children aged 6 to 11 years
Mean absolute change from baseline in ppFEV1 favored placebo for the 17 subjects aged 6 to 11 years (treatment difference −6.3; p=0.03); however, the changes remained relatively stable in both treatment groups from week 2 (Table 2 and Figure 5). There was considerable variability in ppFEV1 between screening, run-in, and baseline visits (e-Figure 7).
Safety and tolerability
The incidence of AEs was similar between the ivacaftor and placebo groups in the double-blind, placebo-controlled KONDUCT study (Table 4), with most AEs being mild or moderate in intensity (see e-Table 1 for AEs ≥20% in any group, by age). Six subjects experienced severe AEs. The most commonly reported AEs were pulmonary exacerbation, cough, and headache. Overall, 10 subjects (ivacaftor, n=4; placebo, n=6) experienced a serious AE (SAE). In the ivacaftor group, 5 SAEs occurred in 4 subjects (3 episodes of pulmonary exacerbation, and 1 episode each of cellulitis and constipation). All 6 placebo group subjects who reported an SAE experienced a pulmonary exacerbation. No subject discontinued from the study because of an AE.
Table 4.
Summary of safety (safety set)
| Overall | 6–11 Years | ≥18 Years | ||||
|---|---|---|---|---|---|---|
| Placebo (n=35) |
Ivacaftor (n=34) |
Placebo (n=8) |
Ivacaftor (n=9) |
Placebo (n=26) |
Ivacaftor (n=24) |
|
| Subjects with any AE, n (%) | 35 (100) | 32 (94) | 8 (100) | 9 (100) | 26 (100) | 23 (96) |
| Subjects with an SAE, n (%) | 6 (17) | 4 (12) | 0 | 2 (22) | 6 (23) | 2 (8) |
| Subjects with AE leading to discontinuation, n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs reported in >15% of subjects in any ivacaftor treatment group, n (%) | ||||||
| Pulmonary exacerbation | 14 (40) | 13 (38) | 1 (13) | 2 (22) | 13 (50) | 11 (46) |
| Cough | 9 (26) | 10 (29) | 1 (13) | 1 (11) | 7 (27) | 9 (38) |
| Headache | 5 (14) | 6 (18) | 1 (13) | 2 (22) | 3 (12) | 4 (17) |
| Sputum increased | 4 (11) | 5 (15) | 0 | 0 | 4 (15) | 5 (21) |
| Nasal congestion | 2 (6) | 5 (15) | 1 (13) | 0 | 1 (4) | 5 (21) |
| Oropharyngeal pain | 2 (6) | 5 (15) | 2 (25) | 1 (11) | 0 | 4 (17) |
| Diarrhea | 4 (11) | 5 (15) | 1 (13) | 1 (11) | 3 (12) | 4 (17) |
| Abdominal pain | 0 | 4 (12) | 0 | 2 (22) | 0 | 2 (8) |
| Wheezing | 1 (3) | 4 (12) | 0 | 0 | 1 (4) | 4 (17) |
| CF lung pathogen colonizationa | 1 (3) | 3 (9) | 0 | 2 (22) | 1 (4) | 1 (4) |
The 12–17-year age group only included 2 subjects (ivacaftor, n=1; placebo, n=1) and, therefore, is not represented in subanalyses, but subjects are included in the overall analyses.
Coded as bacterial disease carrier.
AE = adverse event; SAE = serious adverse event.
In the open-label extension study KONTINUE, 12 SAEs occurred in 8 subjects (2 subjects aged 6–11 years, 6 subjects aged ≥18 years). Nine events were pulmonary exacerbations. Other SAEs were influenza (n=1), and angioedema and urticaria in a single subject with a history of environmental allergies.
Discussion
Treatment with ivacaftor, an oral CFTR potentiator, did not significantly improve lung function across the entire study population, as measured by the absolute change from baseline in ppFEV1 through week 24 in CF subjects with R117H-CFTR. However, significant changes were observed in subject-reported respiratory symptoms, as measured by the CFQ-R respiratory domain, and CFTR function, as measured by SwCl. Prespecified subgroup analyses revealed a significant treatment effect based on subject age category, a randomization stratification criterion.
This is the first randomized, controlled study of a CFTR potentiator in patients with the R117H-CFTR mutation—a mutation associated with residual CFTR channel function and variable clinical consequence. The findings extend the in vitro data showing ivacaftor potentiation of R117H-CFTR channels,23 as well as individual case reports.24,25
In adults with the R117H mutation, there was a significant increase in FEV1 of 5.0 absolute percentage points (p=0.01; 9.1% relative improvement, p=0.008). In addition to lung function, adult subjects reported a mean improvement in CF respiratory symptoms (12.6 points on the CFQ-R respiratory domain; p=0.002), well in excess of the minimal clinically important difference (MCID) established for this domain.22 In contrast to adults, children aged 6–11 years who received ivacaftor had a reduction from baseline in FEV1 compared with placebo (−6.3 percentage points, p=0.03) and reported no positive respiratory symptom changes. The contrasting results in adults and children were not due to differences in drug exposure, and SwCl reductions were comparable in both groups (−21.9 mmol/L in adults, −27.6 mmol/L in children; p<0.0001 for both). Examination of intra-individual changes in FEV1 for the children, including multiple pretreatment assessments, showed that lung function was generally stable throughout the study with the exception of a single outlier who experienced a pulmonary exacerbation. Percent predicted FEV1 decreased in both age groups after ivacaftor washout and increased following re-initiation of treatment in the open-label period, consistent with on-off effects of an active drug. The dichotomous results observed by age group in this study highlightthe importance of clinical disease and delayed onset of significant disease involvement in patients with this genotype, rather than arbitrary age, in determining utility and benefit of ivacaftor therapy.
The R117H mutation is associated with variable disease that often presents in adult life.15 Different lung functionresponses with ivacaftor based on age in patients with R117H-CFTR may be primarily a reflection of baseline disease state. This hypothesis is supported by the high ppFEV1 in children at baseline (95.8% vs 64.5% in adults), possibly establishing a ceiling effect for ppFEV1 in the younger population. Analyses of subgroups based on other characteristics associated with baseline disease status showed non-significant trends towards greater response to ivacaftor in groups with more advanced disease or risk thereof (i.e., lower baseline ppFEV1, Pseudomonas aeruginosa infection, and 5T intron 8 variant on the R117H-carrying allele). Prior studies have shown that older patients with R117H-CFTR are more likely to present with respiratory symptoms, including infection with CF pathogens, and have decreased FEV1 compared with younger patients. Likewise, adult populations with R117H-CFTR are enriched for the 5T variant relative to young children more likely to have been diagnosed by newborn screening.26,27 These patterns were seen in the KONDUCT trial population, wherein7 (41%) subjects aged 6–11 years were diagnosed based on newborn screening and several more were diagnosed based on family history rather than phenotypic presentation. In addition, 2 (12%) patients aged 6–11 years had positive Pseudomonas cultures compared with 32(64%) adults, and 53% (n=9) of 6–11 year olds were R117H-5T versus 76% (n=38) of adults.
The effect size of ivacaftor treatment in this R117H population is smaller than observed in other ivacaftor-responsive mutation types, such as G551D-CFTR.3,19 Intrinsic differences in the molecular defects associated with these mutations may be the cause. While the G551D mutation specifically limits CFTR channel opening (gating), the R117H mutation is multifaceted. R117H-CFTR has a primary defect in channel gating, but the reduction is more modest compared with G551D.28 In addition to reduced gating, R117H also modestly reduces channel conductance.
Despite these multiple defects, R117H-CFTR is associated with residual CFTR chloride transport, and the population of patients with this mutation exhibits greater phenotypic variability compared with patients with other common CF-causing mutations.29
Intragenic variation of the CFTR haplotype is a major genetic modifier influencing CF disease phenotype.30,31 Variation in the length of the poly-T tract of the intron 8 acceptor splice site is a well-characterized intragenic modifier of R117H expression as a result of an increased rate of exon 9 skipping that contributes to the partial penetrance observed in people with an R117H-CFTR allele and increases disease risk in some individuals (i.e., 5T variant).6,32 Considering the phenotypic variability associated with the R117H mutation, some countries have moved to eliminate R117H from newborn screening panels, particularly those countries with a high prevalence of the 7T variant in the population.14 Although there are patients with CF and R117H-7T who have significant clinical disease, many others have congenital bilateral absence of the vas deferens or are asymptomatic.6,14,33 The establishment of alternative diagnoses, such as Cystic Fibrosis Metabolic Syndrome, for patients identified through newborn screening who do not meet CF diagnostic criteria (sweat chloride or clinical symptoms) is another approach to the challenge associated with this mutation for phenotypic prediction.34 Even individuals with R117H-5T can have very different clinical courses, which are likely influenced by various other intragenic and extragenic factors.14,32,35,36 The multiple contributors to R117H CFTR expression makes it challenging to predict clinical responsiveness to CFTR modulation solely on the basis of the intron 8 poly-T variant.
The mean baseline SwCl of 70.5 mmol/L in the current study versus baseline values exceeding 100 mmol/L in G551D studies is consistent with the residual chloride transport associated with R117H-CFTR. Ivacaftor’s mechanism of action increases CFTR channel gating and therefore addresses only one aspect of the molecular defects associated with R117H. Consistent with that notion, the magnitude of SwCl change in this study (−24 mmol/L) was not as large as observed in studies in subjects with G551D-CFTR; nevertheless, similar to those prior studies, ivacaftor treatment reduced SwCl below the diagnostic threshold for CF (60 mmol/L) in many subjects. Unlike the G551D and most other gating mutations, the R117H lesion is located in the extracellular loops of the CFTR channel and may promote destabilization of the channel open state.37 Therefore, there may also be a molecular basis for the different magnitude of effect observed in response to CFTR potentiation between patients with the R117H mutation and those with G551D.
These results highlight some of the challenges of assessing standard CF endpoints in subjects with residual CFTR function mutations. In addition to the difficulty in demonstrating FEV1 improvement in individuals with limited pulmonary impairment, exacerbations were infrequent and did not show a difference between treatment and placebo. Similarly, there was no improvement in BMI with treatment; as expected, few (10%) of the study population had fecal elastase levels consistent with exocrine pancreatic insufficiency and the population as a whole had a normal BMI at baseline. But in spite of limited FEV1 changes for the population as a whole, the effect size observed in the CFQ-R respiratory domain in this trial mostly comprising adults was comparable to that of G551D studies. Patients with residual function mutations such as R117H may not develop symptoms until later in life. As a consequence, QOL instruments such as CFQ-R may prove more responsive in these patients compared with younger patients.
Ivacaftor was well tolerated throughout the 24-week study. The safety profile was similar to the placebo group and to that reported in previous ivacaftor clinical trials.3,19 No new safety concerns were identified. Consistent with those prior trials, adverse events were generally mild or moderate.
Conclusions
Ivacaftor treatment improved CFTR function in individuals with CF and the R117H mutation and significantly improved lung function in adults. Trends toward greater pulmonary effects in subgroupswith more advanced and/or symptomatic disease, along with improvements in patient-reported respiratory symptoms and consistent on-off treatment effects, suggest that ivacaftor benefits many patients with R117H-CFTR, particularly those with established disease.
Supplementary Material
Research in Context.
Evidence before this study
We conducted a search of PubMed on March 13, 2015, using the terms “R117H” and “ivacaftor” or “Kalydeco” or “VX770,” with no restrictions on publication date or language. We identified one case study and one in vivo mechanistic study. The case study reported on a patient with CF and an F508del/R117H CFTR genotype and advanced lung disease who improved following initiation of ivacaftor treatment.24 The mechanistic study involved a patient with anI507del/R117H-5T CFTR genotype and provided evidence of increased CFTR-dependent sweat secretion with ivacaftor in this individual.25
There is ample clinical evidence of benefit for the use of ivacaftor in patients with CF and a G551D or other CFTR mutation with a similar gating defect.19,20 Although the R117H-CFTR mutation has often been associated with a conductance defect, the main protein defect is actually reduced channel gating,5 and R117H-CFTR was responsive to ivacaftor potentiation in vitro.38 Nonetheless, beyond these reports,24,25 the effects of ivacaftor had not been evaluated in patients with the R117H-CFTR mutation.
Added value of this study
This report describes the first randomized, controlled clinical trial of a CFTR potentiator in patients with the R117H-CFTR mutation—a mutation associated with residual CFTR channel function and variable clinical consequence. We show that ivacaftor can improve CFTR function in patients with R117H-CFTR. Moreover, pulmonary benefits were evident in subgroups associated with established CF disease—a finding of particular relevance in this genetic subpopulation that is associated with variable CF disease expression.
Implications of all the available evidence
The findings of this study confirm the previous in vitro and in vivo mechanistic results by demonstrating the potential for clinical benefit from CFTR potentiation in this population, particularly those with established disease. These results formed the basis for the approval of ivacaftor as a treatment for patients with the R117H-CFTR mutation in the US and Canada.
Acknowledgments
The authors thank Barry Lubarsky, PhD, for medical writing, editorial coordination, and support. Graphic design support was provided by Jonathan Kirk. Both BL and JK are employees of Vertex Pharmaceuticals and may own stock or options in that company. Editorial assistance was provided by Karen Stauffer, PhD, of AlphaBioCom, LLC, and by Bina J. Patel, PharmD, CMPP, of Peloton Advantage and was funded by Vertex Pharmaceuticals Incorporated.
www.clinicaltrials.gov identifier: NCT01614457; NCT01707290
Funding: Vertex Pharmaceuticals Incorporated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These study results were presented in part at the European Cystic Fibrosis Society Conference, June 11–14, 2014, in Gothenburg, Sweden, and at the 28th Annual North American Conference of the Cystic Fibrosis Foundation, October 9–11, 2014, in Atlanta, GA, USA.
Contributors
The study was designed by the sponsor (Vertex Pharmaceuticals Incorporated, Boston, MA) in collaboration with the investigators. RBM, PAF, JSE, SMR, SAM, and RCR were study investigators, enrolled subjects, and collected the study data. RBM, PAF, JSE, JC, SMR, SAM, RCR, and MH contributed to the interpretation of the data. RBM, PAF, JSE, JC, SMR, SAM, RCR, and MH participated in the critical review and revision of the manuscript and granted final approval for submission.
Declaration of interests
This study was sponsored by Vertex Pharmaceuticals Incorporated. This research publication was also supported by the National Center for Research Resources of the National Institutes of Health (NIH) grant 1UL1 RR025744 to Stanford University; Northwestern University Clinical and Translational Research Institute NIH grant number UL1TR000150; the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH grant number UL1TR000062; University of Alabama Center for Clinical and Translational Science grant number UL1 TR000165; The University of Pennsylvania/Children’s Hospital of Philadelphia CTSA grant numbers UL1RR024134 (NCRR) and UL1TR000003 (NCATS); and by the NICRN (Respiratory Health) in Belfast Health and Social Care Trust. Medical writing and editorial support were funded by Vertex Pharmaceuticals Incorporated. No author received an honorarium or other form of financial support related to the development of this manuscript.
RBM has served as an investigator on Vertex Pharmaceuticals Incorporated, PTC, and N30 clinical studies; has participated in advisory boards or as a consultant for Celtaxys, GSK, Gilead, Novartis, ProQR, Asubio, Proteostasis Therapeutics, and Vertex Pharmaceuticals Incorporated; has received research funding from Genentech, CFF Therapeutics, Inc. PAF has served as an investigator on Vertex Pharmaceuticals Incorporated clinical studies; has participated in advisory boards or as a consultant for Aptalis, Enanta, Gilead, Insmed, Vertex Pharmaceuticals Incorporated, Novartis, Pharmaxis Limited; has received grant support from Aptalis, Gilead, Bayer Healthcare AG, Insmed, Novartis, Vertex Pharmaceuticals Incorporated, Pharmaxis Limited, Boehringer Ingelheim, Savara Pharmaceuticals, KaloBios, CFF. JSE has served as an investigator in Vertex Pharmaceuticals Incorporated clinical studies; has participated in advisory boards or as a consultant for Vertex Pharmaceuticals Incorporated, Novartis, Bayer, Actavis, and Boehringer Ingelheim and has received grant support from Gilead and Novartis. JC is an employee of Vertex Pharmaceuticals (Europe) Limited and may own stock or options in Vertex Pharmaceuticals Incorporated. SMR has served as an investigator on Vertex Pharmaceuticals Incorporated, Novartis, PTC Therapeutics, and Bayer clinical studies; has received grant funding from the National Institutes of Health, Forest Research Institute, CFF, CFF Therapeutics Inc, Bayer Healthcare, Novartis Research Institute, Galapagos and the American Lung Association. SAM has served as an investigator on Vertex Pharmaceuticals Incorporated, PTC Therapeutics, Novartis, SavaraInc, AbbVie Inc., Aptalis Pharma US, Inc. and Janssen Research & Development, LLC studies; served as a consultant for Vertex Pharmaceuticals Incorporated; and has received grant funding from CFF, CFF Therapeutics Inc., and the National Institutes of Health. RCR has served as an investigator on Vertex Pharmaceuticals Incorporated, KaloBios, and N30 clinical studies. MH is an employee of Vertex Pharmaceuticals (Europe) Limited and may own stock or options in Vertex Pharmaceuticals Incorporated.
References
- 1.Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–65. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 2.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–70. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 3.Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–25. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation Patient Registry 2013 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation; 2014. [Google Scholar]
- 5.Sheppard DN, Rich DP, Ostedgaard LS, Gregory RJ, Smith AE, Welsh MJ. Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature. 1993;362:160–4. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- 6.Kiesewetter S, Macek M, Jr, Davis C, et al. A mutation in CFTR produces different phenotypes depending on chromosomal background. Nat Genet. 1993;5:274–8. doi: 10.1038/ng1193-274. [DOI] [PubMed] [Google Scholar]
- 7.Tabaripour R, Niaki HA, Douki MR, Bazzaz JT, Larijani B, Yaghmaei P. Poly thymidine polymorphism and cystic fibrosis in a non-Caucasian population. Dis Markers. 2012;32:241–6. doi: 10.3233/DMA-2011-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations–correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 9.Chavez-Saldana M, Yokoyama E, Lezana JL, et al. CFTR allelic heterogeneity in Mexican patients with cystic fibrosis: implications for molecular screening. Rev Invest Clin. 2010;62:546–52. [PubMed] [Google Scholar]
- 10.Claustres M, Guittard C, Bozon D, et al. Spectrum of CFTR mutations in cystic fibrosis and in congenital absence of the vas deferens in France. Hum Mutat. 2000;16:143–56. doi: 10.1002/1098-1004(200008)16:2<143::AID-HUMU7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Alonso MJ, Heine-Suner D, Calvo M, et al. Spectrum of mutations in the CFTR gene in cystic fibrosis patients of Spanish ancestry. Ann Hum Genet. 2007;71:194–201. doi: 10.1111/j.1469-1809.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- 12.Estivill X, Bancells C, Ramos C. Geographic distribution and regional origin of 272 cystic fibrosis mutations in European populations. The Biomed CF Mutation Analysis Consortium. Hum Mutat. 1997;10:135–54. doi: 10.1002/(SICI)1098-1004(1997)10:2<135::AID-HUMU6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Lucotte G, Hazout S. Geographic and ethnic distributions of the more frequent cystic fibrosis mutations in Europe show that a founder effect is apparent for several mutant alleles. Hum Biol. 1995;67:562–76. [PubMed] [Google Scholar]
- 14.Thauvin-Robinet C, Munck A, Huet F, et al. The very low penetrance of cystic fibrosis for the R117H mutation: a reappraisal for genetic counselling and newborn screening. J Med Genet. 2009;46:752–8. doi: 10.1136/jmg.2009.067215. [DOI] [PubMed] [Google Scholar]
- 15.Comer DM, Ennis M, McDowell C, et al. Clinical phenotype of cystic fibrosis patients with the G551D mutation. QJM. 2009;102:793–8. doi: 10.1093/qjmed/hcp120. [DOI] [PubMed] [Google Scholar]
- 16.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–6. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Burton B, Huang CJ, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11:237–45. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–30. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–80. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998;132:589–95. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 22.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135:1610–8. doi: 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Goor F, Yu H, Burton B, Hoffman BJ. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 2014;13:29–36. doi: 10.1016/j.jcf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Carter S, Kelly S, Caples E, et al. Ivacaftor as salvage therapy in a patient with cystic fibrosis genotype F508del/R117H/IVS8-5T. J Cyst Fibros. 2015 Feb 16; doi: 10.1016/j.jcf.2015.01.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Char JE, Wolfe MH, Cho HJ, et al. A little CFTR goes a long way: CFTR-dependent sweat secretion from G551D and R117H-5T cystic fibrosis subjects taking ivacaftor. PLoS One. 2014;9:e88564. doi: 10.1371/journal.pone.0088564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massie RJ, Poplawski N, Wilcken B, Goldblatt J, Byrnes C, Robertson C. Intron-8 polythymidine sequence in Australasian individuals with CF mutations R117H and R117C. Eur Respir J. 2001;17:1195–200. doi: 10.1183/09031936.01.00057001. [DOI] [PubMed] [Google Scholar]
- 27.Shteinberg M, Downey D, Beattie D, et al. Clinical characteristics of CF patients with R117H mutation and different polythymidine tract variants [abstract]; Presented at: the 28th Annual North American Cystic Fibrosis Conference; Atlanta, GA. October 9–11, 2014. [Google Scholar]
- 28.Sheppard DN, Welsh MJ. Inhibition of the cystic fibrosis transmembrane conductance regulator by ATP-sensitive K+ channel regulators. Ann N Y Acad Sci. 1993;707:275–84. doi: 10.1111/j.1749-6632.1993.tb38058.x. [DOI] [PubMed] [Google Scholar]
- 29.Correlation between genotype and phenotype in patients with cystic fibrosis. The Cystic Fibrosis Genotype-Phenotype Consortium. N Engl J Med. 1993;329:1308–13. doi: 10.1056/NEJM199310283291804. [DOI] [PubMed] [Google Scholar]
- 30.Morral N, Dork T, Llevadot R, et al. Haplotype analysis of 94 cystic fibrosis mutations with seven polymorphic CFTR DNA markers. Hum Mutat. 1996;8:149–59. doi: 10.1002/(SICI)1098-1004(1996)8:2<149::AID-HUMU7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Morral N, Llevadot R, Casals T, et al. Independent origins of cystic fibrosis mutations R334W, R347P, R1162X, and 3849 + 10kbC–>T provide evidence of mutation recurrence in the CFTR gene. Am J Hum Genet. 1994;55:890–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Rave-Harel N, Kerem E, Nissim-Rafinia M, et al. The molecular basis of partial penetrance of splicing mutations in cystic fibrosis. Am J Hum Genet. 1997;60:87–94. [PMC free article] [PubMed] [Google Scholar]
- 33.Peckham D, Conway SP, Morton A, Jones A, Webb K. Delayed diagnosis of cystic fibrosis associated with R117H on a background of 7T polythymidine tract at intron 8. J Cyst Fibros. 2006;5:63–5. doi: 10.1016/j.jcf.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Borowitz D, Parad RB, Sharp JK, et al. Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J Pediatr. 2009;155:S106–S116. doi: 10.1016/j.jpeds.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groman JD, Hefferon TW, Casals T, et al. Variation in a repeat sequence determines whether a common variant of the cystic fibrosis transmembrane conductance regulator gene is pathogenic or benign. Am J Hum Genet. 2004;74:176–9. doi: 10.1086/381001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nissim-Rafinia M, Chiba-Falek O, Sharon G, Boss A, Kerem B. Cellular and viral splicing factors can modify the splicing pattern of CFTR transcripts carrying splicing mutations. Hum Mol Genet. 2000;9:1771–8. doi: 10.1093/hmg/9.12.1771. [DOI] [PubMed] [Google Scholar]
- 37.Cui G, Freeman CS, Knotts T, Prince CZ, Kuang C, McCarty NA. Two salt bridges differentially contribute to the maintenance of cystic fibrosis transmembrane conductance regulator (CFTR) channel function. J Biol Chem. 2013;288:20758–67. doi: 10.1074/jbc.M113.476226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Goor F. Cystic fibrosis drug discovery and personalized medicine [oral presentation]; Presented at: Annual European Cystic Fibrosis Conference; Lisbon, Portugal. June 12–15, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


