Abstract
Intermittent parathyroid hormone (PTH) is a potent anabolic therapy for bone, and several studies have implicated local insulin-like growth factor (IGF) signaling in mediating this effect. The IGF system is complex and includes ligands and receptors, as well as IGF binding proteins (IGFBPs) and IGFBP proteases. Pregnancy-associated plasma protein-A (PAPP-A) is a metalloprotease expressed by osteoblasts in vitro that has been shown to enhance local IGF action through cleavage of inhibitory IGFBP-4. This study was set up to test two specific hypotheses: 1) Intermittent PTH treatment increases the expression of IGF-I, IGFBP-4 and PAPP-A in bone in vivo, thereby increasing local IGF activity. 2) In the absence of PAPP-A, local IGF activity and the anabolic effects of PTH on bone are reduced. Wild-type (WT) and PAPP-A knock-out (KO) mice were treated with 80 μg/kg human PTH 1-34 or vehicle by subcutaneous injection five days per week for six weeks. IGF-I, IGFBP-4 and PAPP-A mRNA expression in bone were significantly increased in response to PTH treatment. PTH treatment of WT mice, but not PAPP-A KO mice, significantly increased expression of an IGF-responsive gene. Bone mineral density (BMD), as measured by DEXA, was significantly decreased in femurs of PAPP-A KO compared to WT mice with PTH treatment. Volumetric BMD, as measured by pQCT, was significantly decreased in femoral midshaft (primarily cortical bone), but not metaphysis (primarily trabecular bone), of PAPP-A KO compared to WT mice with PTH treatment. These data suggest that stimulation of PAPP-A expression by intermittent PTH treatment contributes to PTH bone anabolism in mice.
Keywords: pregnancy-associated plasma protein-A, parathyroid hormone, insulin-like growth factor, insulin-like growth factor binding proteins
1. Introduction
The ability of intermittent parathyroid hormone (PTH) to increase bone formation in several animal models and in humans led to the approval of PTH as an anabolic treatment for osteoporosis [1-3]. However, the underlying mechanisms for the potent anabolic effect of PTH in bone are not fully understood. Early in vitro studies indicated that PTH treatment increased the production of insulin-like growth factor (IGF)-I by bone cells [4] Several in vivo studies followed that highlighted the importance of local IGF-I and IGF-I receptor (IGF-IR) in mediating the anabolic actions of PTH in bone [5-8]. Regulation of local IGF signaling is complex and can be modulated not only by changes in ligand or receptor levels, but also by expression and post-translational modification of IGF binding proteins (IGFBPs) (reviewed in [9]). IGFBP-4 is the most abundant IGFBP in bone, and has been shown to bind and prevent IGF-I from interacting with its receptor [10]. Furthermore, cleavage of IGFBP-4 by the zinc metalloprotease, pregnancy-associated plasma protein-A (PAPP-A), markedly deceases IGFBP-4 affinity for IGF-I, thereby increasing local IGF-I available for receptor activation (reviewed in [11]). Transgenic mice over-expressing PAPP-A in bone show increased bone formation, which can be blocked with simultaneous expression of protease-resistant IGFBP-4 [12, 13].
PTH acts in part through increases in cyclic AMP to stimulate bone formation. In vitro, PTH increases IGFBP-4 and IGF-I expression in bone through a cyclic AMP-mediated pathway [14]. Agents that increase intracellular cyclic AMP also increase PAPP-A expression in cultured osteoblasts [15]. Thus, we hypothesize that PTH increases expression of IGF-I and IGFBP-4 in vivo, thereby creating a pericellular reservoir of IGF-I/IGFBP-4 in the bone microenvironment, and that concerted PTH stimulation of PAPP-A enhances IGF-I action in bone through proteolysis of IGFBP-4. If true, then in the absence of PAPP-A the anabolic effect of PTH on bone would be attenuated. In the study herein, we test this hypothesis in wild-type and PAPP-A knock-out mice.
2. Materials and Methods
2.1 Mice
Mice heterozygous for Pappa (C57BL/6, 129) were bred as previously described to produce wild-type (WT) and PAPP-A knock-out (KO) littermates for these experiments [16]. Genotypes were determined before assignment to groups and confirmed at harvest. Three-month-old female WT and PAPP-A KO mice (n = 10 per group) were treated with human PTH 1-34 (Bachem Americas), at a dose of 80 μg/kg, or with vehicle (0.9% acidified saline with 2% heat-inactivated WT mouse serum) by subcutaneous injection five days per week for six weeks. This study was approved by the Institutional Animal Care and Use Committee of Mayo Clinic.
2.2 Bone morphometry
Dual Energy X-ray absorptiometry (DEXA) and peripheral quantitative computer tomography (pQCT) scans were taken at the start (day 0) and end (day 42) of the treatment period. DEXA measured bone mineral content (BMC) and areal bone mineral density (BMD) in the femur using a PIXImus densitometer (GE-Lunar, Madison, WI). Volumetric BMD was measured by pQCT at the midshaft and distal metaphysis of the femur using a Stratec XCT Research Plus scanner with v 5.40 software (Norland Medical Systems, Fort Atkinson, WI). Each bone at each site was scanned in triplicate using scanning parameters that have been described elsewhere [16]. pQCT was considered inadequate to distinguish trabecular and cortical bone in these small animals, so only total BMD is reported for each site.
2.3. Bone histomorphometry
Fluorescent bone labels (declomycin, calcein) were administered to mice 12 days, 5 days, and 2 days before sacrifice. Femurs were processed and embedded in methyl methacrylate. Analyses were performed by Mayo Clinic's Biomaterials Characterization and Quantitative Histomorphometry Core Laboratory.
2.4 Gene expression analyses
Tibias were rapidly harvested immediately after the second scan and frozen in liquid nitrogen. RNA extraction, reverse transcription, and quantitative real-time PCR have been described previously [16]. Target gene expression was normalized to ribosomal protein L19.
2.5 Statistical analyses
Results are presented as Mean ± SD. Mann-Whitney U test was used to compare results in WT versus PAPP-A KO mice. Significance was set at P < 0.05.
3. Results
3.1 PTH treatment regulates IGF system gene expression in vivo
IGF-I, IGFBP-4 and PAPP-A mRNA expression were significantly increased in bone from WT mice in response to intermittent PTH treatment 80 μg/kg for six weeks (Fig. 1). In particular, PTH increased PAPP-A expression three-fold. Based on in vitro data, coordinated up-regulation of IGF-I/IGFBP-4/PAPP-A would be predicted to create a situation of enhanced local IGF-I available for receptor activation through PAPP-A-induced cleavage of IGFBP-4. It is difficult to measure IGF-I receptor activation in vivo using the phosphorylation assays typically used in vitro due to the transient nature of phosphorylation events. However, IGFBP-5 is an IGF-I responsive gene and elevated mRNA levels have been used as an indicator of IGF-I signaling in vivo in several tissues, including bone [17-19]. PTH treatment of WT mice significantly increased IGFBP-5 mRNA levels in bone (Fig. 2). However, PTH treatment of PAPP-A KO mice did not increase IGFBP-5 expression, consistent with a blunting of local IGF action in the absence of PAPP-A.
Figure 1. IGF system gene expression in response to intermittent PTH treatment.
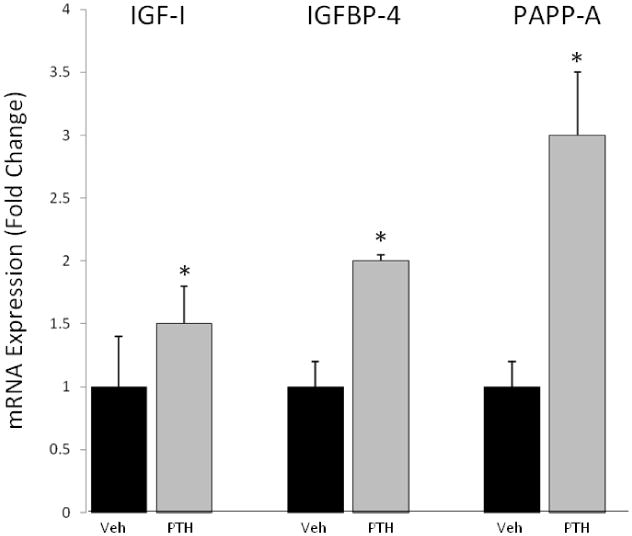
Expression of IGF-I, IGFBP-4, and PAPP-A was assessed by real-time PCR of RNA extracted from the proximal tibia of wild-type mice. Fold change in PTH-treated mice is expressed relative to vehicle-treated mice of the same genotype. N = 10
* Significant effect of PTH treatment, P < 0.05
Figure 2. In vivo biomarker of IGF receptor activation in response to intermittent PTH treatment.
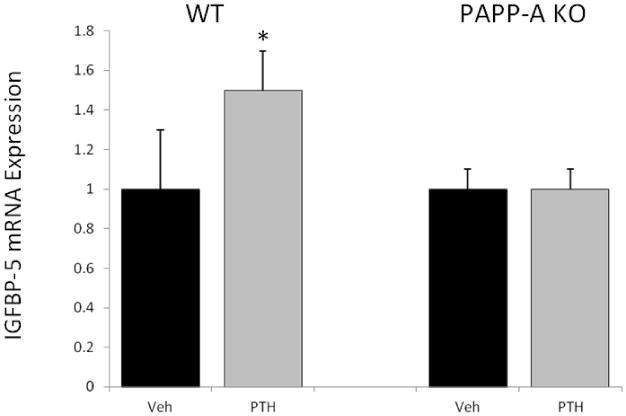
Expression of an IGF-responsive gene, IGFBP-5, was assessed by real-time PCR of RNA extracted from the proximal tibia of WT and PAPP-A KO mice. Fold change in PTH-treated mice is expressed relative to vehicle-treated mice of the same genotype. N = 10
* Significant effect of PTH treatment, P < 0.05
3.2 PAPP-A deficiency attenuates the anabolic effects of PTH in the femur
DEXA of the femur was used to assess changes in BMC and areal BMD over the 42 day treatment period. PAPP-A KO mice are 40% smaller than their WT littermates and have proportionately smaller skeletons and lower BMD [16]. Thus, to directly compare the magnitude of PTH-induced changes in DEXA parameters between WT and PAPP-A KO mice, fold-increases for PTH-treated animals were calculated against vehicle-treated animals of the same genotype. Although femur area was not significantly different between groups due to high variability, BMC was significantly lower in PAPP-A KO than in WT mice, resulting in lower areal BMD (Fig. 3; Supplemental Table 1).
Figure 3. Anabolic effect of intermittent PTH treatment in the femur.
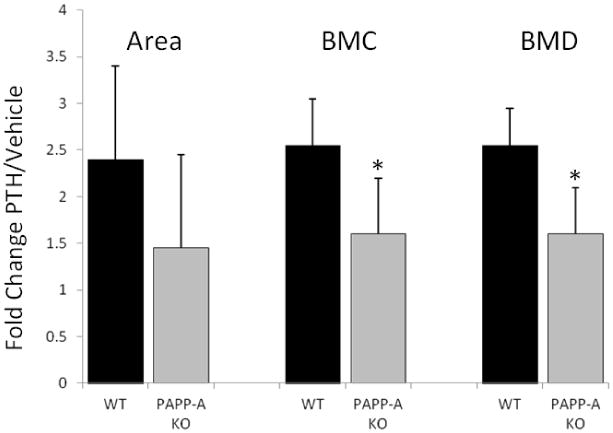
DEXA was used to assess changes in BMC and areal BMD in total femur with intermittent PTH treatment of WT and PAPP-A KO mice. Fold increase for PTH-treated mice was calculated against vehicle-treated mice of the same genotype. N = 10
* Significant difference between WT and PAPP-A KO, P < 0.05
3.3 Differences in WT and PAPP-A KO response to PTH are in cortical bone
pQCT was used to assess volumetric BMD in the midshaft (primarily cortical bone) and distal metaphysis (primarily trabecular bone) of the femur. Total BMD was calculated as percent change to show comparisons between genotypes. As shown in Figure 4 and Supplemental Table 2, PTH treatment significantly increased BMD in the femoral midshaft of WT mice, but had no effect in PAPP-A KO mice. In contrast, responses to PTH were highly significant but equal in magnitude in the distal metaphysis of femurs from WT and PAPP-A KO mice.
Figure 4. Anabolic effect of intermittent PTH treatment in cortical and trabecular bone.
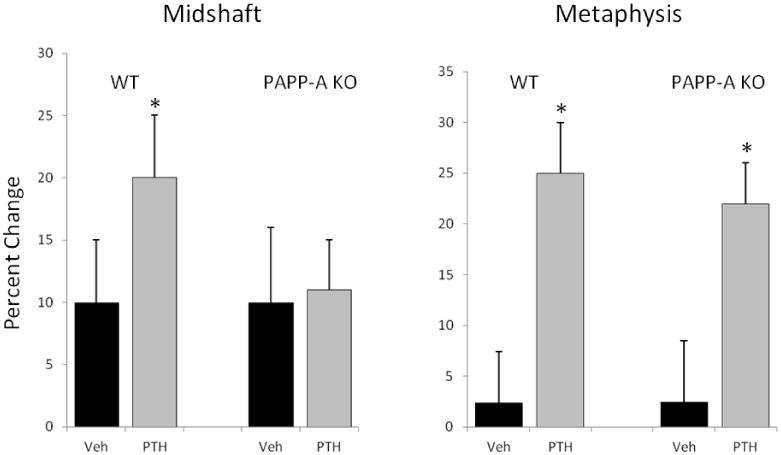
pQCT was used to assess changes in volumetric BMD in the midshaft and distal metaphysis of the femur with intermittent PTH treatment of WT and PAPP-A KO mice. Volumetric BMD was calculated as percent change to allow comparisons between genotypes. N = 10
* Significant effect of PTH treatment, P < 0.05
Bone histomorphometry supported a diminished effect of PTH on bone formation and mineral apposition rates in cortical bone of PAPP-A KO mice (Table 1).
Table 1. Histomorphometry: Cortical bone.
| WT | PAPP-A KO | |||
|---|---|---|---|---|
| Vehicle | PTH (%Δ) | Vehicle | PTH (%Δ) | |
| BV/TV (%) | 4.43 ± .060 | 4.76 ± 0.241 (+7) | 4.36 ± 1.46 | 4.45 ± 0.265 (+2) |
| MS/BS (%) | 17.2 ± 3.97 | 18.8 ± 4.79 (+9) | 20.8 ± 1.50 | 20.0 ± 1.52 (-4) |
| BFR/BS (μm3/ μm2/d) | 0.20 ± 0.035 | 0.27 ± 0.044 (+35) | 0.25 ± 0.009 | 0.23 ± 0.016 (-8) |
| BFR/BV (%/d) | 1.20 ± 0.201 | 1.90 ± 0.047* (+60) | 1.90 ± 0.015 | 1.68 ± 0.212 (-12) |
| MAR (μm/d) | 0.98 ± 0.015 | 1.50 ± 0.165* (+53) | 1.22 ± 0.130 | 1.15 ± 0.078 (-6) |
Results are presented as mean ± SEM of 3-4 mice per treatment group. Percent change with PTH compared to vehicle treatment is in parentheses.
Significant effect of PTH, P < 0.05
BV, bone volume
TV, total volume
BS, bone surface
MS, mineralizing surface
BFR, bone formation rate
MAR, mineral apposition rate
4. Discussion
The major findings of this study were: 1) Intermittent PTH treatment increases the expression of PAPP-A, as well as of IGF-I and IGFBP-4, in bone of mice in vivo, and 2) In the absence of PAPP-A, an in vivo marker of IGF-I activity is reduced and the anabolic effects of PTH are attenuated in cortical bone.
PTH has been shown to increase IGF-I and IGFBP-4 in bone cell cultures [4, 14], and IGFBP-4 was first identified in osteoblast cell conditioned medium as ‘inhibitory IGFBP’ [20]. In addition, PTH mimetics, such as forskolin and dibutyrl cAMP, stimulated osteoblast production of PAPP-A in vitro [15]. This study is the first to show that PTH could coordinately enhance all three components in bone in vivo. Intermittent PTH treatment increases bone cell proliferation, differentiation and survival, and many of these effects appear to be mediated by IGF signaling [5-8]. Thus, IGF-I KO mice, osteoblast-specific IGF-IR KO mice, and insulin receptor substrate-1 KO mice (IRS-1 Is an intracellular transducer of IGF-I receptor signaling) all show blunted anabolic responses to PTH. PAPP-A KO mice are a model of local attenuation of IGF action; circulating levels of IGF-I are the same as in WT mice. The anabolic effects of PTH were inhibited in cortical bone of PAPP-A KO mice but not in trabecular bone. The reason for this differential effect is unclear, but suggests that much of PTH's effect on trabecular bone is not IGF-dependent. In osteoblast-specific IGF-IR KO mice, the PTH effect was blocked in cortical bone but only partially inhibited in trabecular bone [8]. PTH's anabolic effect is stronger on trabecular bone than on cortical bone [7]. However, it does not appear that the 80 μg/kg dose of PTH was too high, because a 20 μg/kg dose had similar effects on gene expression and BMD in WT and PAPP-A KO mice (Supplemental Table 3).
There have been several studies indicating an anabolic role for PAPP-A in bone through protease-dependent regulation of local IGF bioavailability [12, 13, 16, 21]. Probably the most compelling evidence comes from the work of Phang et al. [13] with bone-specific PAPP-A transgenic and double PAPP-A/protease-resistant IGFBP-4 transgenic mice. Co-expression of PAPP-A and protease-resistant IGFBP-4 effectively blocked the increase in bone formation seen with PAPP-A over-expression alone. However, ours is the first study to show that stimulation of PAPP-A expression by intermittent PTH contributes to PTH effects on bone anabolism.
In conclusion, gene expression data support our hypothesis that PTH increases expression of PAPP-A, an IGFBP-4 protease, in vivo and that loss of PAPP-A reduces the pool of bioavailable IGF-I, measured indirectly by the expression of an IGF-responsive gene. Bone densitometry data indicated that while the anabolic effects of PTH were observed in both trabecular and cortical compartments, loss of PAPP-A attenuated the anabolic effects only in cortical bone in this model. Further studies will be necessary to establish mechanism.
Supplementary Material
Highlights.
Intermittent PTH treatment coordinately increased the expression of IGF-I, IGFBP-4, and PAPP-A in bone of mice
In PAPP-A knock-out mice treated with PTH, an in vivo marker of IGF-I activity was reduced compared to wild-type mice
In PAPP-A knock-out mice, the anabolic effects of PTH were attenuated in cortical bone
Acknowledgments
This work was supported, in part, by NIH grant T32 DK07352 to KBC.
Abbreviations
- PTH
parathyroid hormone
- PAPP-A
pregnancy-associated plasma protein-A
- IGF-I
insulin-like growth factor-I
- IGFBP
insulin-like growth factor binding protein
- IGF-IR
IGF-I receptor
- WT
wild-type
- KO
knock-out
- DEXA
dual energy X-ray absorptiometry
- pQCT
peripheral quantitative computer tomography
- BMC
bone mineral content
- BMD
bone mineral density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander JM, Bab I, Fish S, et al. Human parathyroid hormone 1-34 reverses bone loss in ovariectomized mice. J Bone Min Res. 2001;16:1665–1673. doi: 10.1359/jbmr.2001.16.9.1665. [DOI] [PubMed] [Google Scholar]
- 2.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 3.Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 4.Canalis E, Centrella M, Burch W, McCarthy TL. Insulin-like growth factor I mediates selective anabolic effects of parathyroid hormone in bone cultures. J Clin Invest. 1989;83:60–65. doi: 10.1172/JCI113885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyakoshi N, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that anabolic effects of PTH on bone require IGF-I in growing mice. Endocrinology. 2001;142:4349–4356. doi: 10.1210/endo.142.10.8436. [DOI] [PubMed] [Google Scholar]
- 6.Bikle DD, Sakata T, Leary C, et al. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi M, Ogata N, Shinoda Y, et al. Insulin receptor substrate-1 is required for bone anabolic function of parathyroid hormone in mice. Endocrinology. 2005;146:2620–2628. doi: 10.1210/en.2004-1511. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Nishida S, Boudignon BM, et al. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holly J, Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83:154–160. doi: 10.1159/000095523. [DOI] [PubMed] [Google Scholar]
- 10.Zhou R, Diehl D, Hoeflich A, Lahm H. Wolf E, IGF-binding protein-4: biochemical characteristics and functional consequences. J Endocrinology. 2003;178:177–193. doi: 10.1677/joe.0.1780177. [DOI] [PubMed] [Google Scholar]
- 11.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin X, Wergedal JE, Rehage M, et al. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology. 2006;147:5653–5661. doi: 10.1210/en.2006-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phang D, Rehage M, Bonafede B, et al. Inactivation of insulin-like-growth factors diminished the anabolic effects of pregnancy-associated plasma protein-A (PAPP-A) on bone in mice. GH & IGF Res. 2010;20:192–200. doi: 10.1016/j.ghir.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 14.LaTour D, Mohan S, Linkhart TA, Baylink DJ, Strong DD. Inhibitory insulin-like growth factor-binding protein: cloning, complete sequence, and physiological regulation. Molecular Endocrinology. 1990;4:1806–1814. doi: 10.1210/mend-4-12-1806. [DOI] [PubMed] [Google Scholar]
- 15.Conover CA, Chen BK, Resch ZT. Regulation of pregnancy-associated plasma protein-A expression in cultured human osteoblasts. Bone. 2004;34:297–302. doi: 10.1016/j.bone.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of pregnancy-associated plasma protein-A deletion on the adult murine skeleton. J Bone Miner Res. 2008;23:655–662. doi: 10.1359/JBMR.071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamo ML, Ma X, Ackert-Bicknell CL. Donahue LR, Beamer WG, Rosen CJ, Genetic increase in serum insulin-like growth factor-I (IGF-I) in C3H/HeJ compared with C57BL/6J mice is associated with increased transcription from the IGF-I exon 2 promoter. Endocrinology. 2006;147:2944–2955. doi: 10.1210/en.2005-0742. [DOI] [PubMed] [Google Scholar]
- 18.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100:1696–1702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- 19.Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology. 2006;147:5634–5340. doi: 10.1210/en.2006-0493. [DOI] [PubMed] [Google Scholar]
- 20.Mohan S, Bautista CM, Wergedal J. Baylink DJ, Isolation of an inhibitory insulin-like growth factor (IGF) binding protein from bone cell-conditioned medium: a potential local regulator of IGF action. Proc Natl Acad Sci USA. 1989;86:8338–8342. doi: 10.1073/pnas.86.21.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyakoshi N, Qin X, Kasukawa Y, et al. Systemic administration of insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) increases bone formation parameters in mice by increasing IGF bioavailability via an IGFBP-4 protease-dependent mechanism. Endocrinology. 2001;142:2641–2648. doi: 10.1210/endo.142.6.8192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


