Abstract
The complex pathogenesis of psoriasis is still not fully understood. The study by Sun et al. (Sun et al., 2015) in this issue of the journal suggests that CYR61(now named CCN1), a secreted matricellular protein, plays a role in the pathogenesis of psoriasis, and thus targeting CCN1 represents a potential therapeutic strategy in its treatment.
CCN1 in psoriasis: new findings and clinical implications
Psoriasis is a chronic inflammatory skin disease characterized by hyperproliferation of epidermal keratinocytes and resulting in scaly, red and well-demarcated skin lesions. Hyperproliferation is driven by the additive and synergistic activities of a plethora of cytokines and growth factors (Baliwag et al., 2015) acting on, and driving changes in the architecture of the epidermis, keratinocytes, resident and infiltrating immune cells as well as fibroblasts and vascular endothelium in the dermis (Elder et al., 2010). In this issue of the journal, Sun et al. (Sun et al., 2015) add another ingredient to the inflammatory recipe responsible for driving this disease; they report that the expression of CCN1, a multifunctional non-structural protein found in the extracellular matrix (ECM), is elevated in the skin of patients with psoriasis, as well as in psoriasis-like mouse models. Importantly, blocking CCN1 function by either lentiviral-based sh-RNA knockdown of CCN1 or anti-CCN1 neutralizing antibodies attenuated the epidermal hyperplasia and inflammation seen in the psoriasis-like mouse models. Thus, they concluded that CCN1 participates in the pathogenesis of psoriasis and that targeting CCN1 could be an important therapeutic strategy. Although the mechanisms by which CCN1 promotes epidermal hyperplasia and inflammation are not fully understood, Sun et al. present findings that CCN1 acts through integrin α6β1 on keratinocytes. This interaction activates downstream PI3K/Akt/NF-κB signaling pathways. This finding has clinical implication by suggesting α6β1 integrin as a relevant target.
CCN1 is a secreted protein that interacts with the ECM, making it a member of a large family of matricellular proteins. Based on structural homology, CCN proteins comprise a family of six members, CCN1-6 (Perbal, 2004). The CCN acronym is taken from the names of the first three members of the family to be discovered: CYR61/CCN1 (cysteine-rich protein 61), CTGF/CCN2 (connective tissue growth factor) and NOV/CCN3 (nephroblastoma overexpressed gene). Members of the CCN family exhibit diverse cellular functions, such as regulation of cell proliferation, chemotaxis, apoptosis, adhesion, motility, ion transport, and ECM regulation (Chen and Lau, 2009).
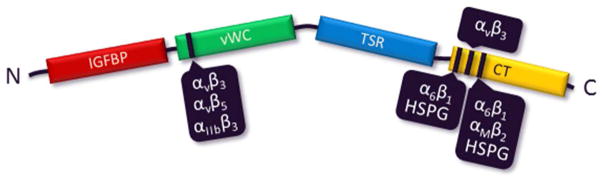
CCN1 has been reported to regulate cell adhesion, migration, chemotaxis, inflammation, cell-matrix interactions, synthesis of ECM proteins, and wound healing in a variety of cells in culture (Jun and Lau, 2010; Lau, 2011). CCN1 exerts this range of functions through interaction with multiple integrins in a cell-type and context-dependent manner [Figure 1] (Lau, 2011). During embryonic development, CCN1 plays a critical role in vascular development and blood vessel formation in the placenta. CCN1 knockout in mice is embryonic lethal primarily due to a failure in vascular development caused by impaired ECM homeostasis. Studies in animal models and in patients have confirmed that deregulation of CCN1 protein is found in several diseases associated with chronic inflammation and/or tissue injury, including rheumatoid arthritis, atherosclerosis, diabetes-related nephropathy and retinopathy, and many forms of cancer (Chen and Lau, 2009; Kular et al., 2011).
Figure 1.
Full-length CCN1 contains 381 amino acids with an N-terminal signal peptide followed by four structurally distinct domains (Lau, 2011; Perbal, 2004): an insulin-like growth factor binding protein (IGFBP) domain, a von Willebrand type C repeats (vWC) domain, a thrombospondin type 1 repeat (TSR) domain, and a C-terminal (CT) cysteine-knot motif domain. CCN family proteins have an unusually high cysteine content (~10% of residues) and the number and spacing of these cysteine residues are fully conserved in CYR61/CCN1, CTGF/CCN2, NOV/CCN3, and WISP1/CCN4, while being largely conserved in WISP2/CCN5, which lacks the CT domain, and WISP3/CCN6, which lacks 4 cysteine residues in the vWC domain. CCN1 promotes diverse and sometimes opposing cellular responses, which can be assigned as least in part, to disparate activities mediated through distinct integrins expressed by different cell types. Accordingly, CCN1 promotes cell proliferation, survival, and angiogenesis by binding to αvβ3 integrin, and it induces apoptosis and senescence through α6β1 integrin and heparin sulfate proteoglycans (HSPG).
What drives sustained elevation of CCN1 in psoriatic skin?
The study by Sun et al. reveals elevated CCN1 in the skin of patients with psoriasis and its potential role as a driving force for epidermal hyperplasia and inflammation. Obviously, their findings raise the question of what drives the sustained elevation of CCN. Several recent studies have shown that the CCN1 expression is regulated directly by the transcriptional co-activator Yes-associated protein (YAP), a major downstream effector of the hippo signaling pathway. In mouse skin, YAP plays a critical role in regulating keratinocyte proliferation, differentiation, and survival through up-regulation of CCN1 (Zhang et al., 2011). In the human skin cancer, basal cell carcinoma (BCC), YAP and its downstream transcriptional target CCN1 are elevated markedly in tumor islands. In human keratinocytes, knockdown of YAP significantly reduces the expression of CCN1 and represses proliferation and survival. This inhibition of proliferation and survival is rescued by restoration of CCN1 expression (Quan et al., 2014). These data provide evidence that up-regulation of YAP promotes aberrant keratinocyte proliferation through up-regulation of CCN1 in BCC. Although the expression of YAP in psoriasis is unknown, these data suggest that YAP may be involved in up-regulating CCN1 in psoriasis.
Additionally, a positive feedback loop between CCN1 and the cytokine network may also contribute to the sustained elevation of CCN1 in psoriatic skin. CCN1 not only promotes the production of cytokines (Qin et al., 2014), it is also induced by cytokines, growth factors and environmental stressors (Chen and Lau, 2009), suggesting that a positive feedback loop may contribute to sustained elevation of CCN1 found in psoriatic skin. Therefore, targeting CCN1 could be an effective therapeutic strategy by inhibiting such a positive feedback loop in psoriasis
Important role of CCN1 in skin biology
CCN1 could be an important regulator in skin biology since it impacts both epidermal keratinocytes and dermal fibroblasts. In normal adult human skin, CCN1 is substantially elevated in the dermis by aging, as well as under the influence of ultraviolet (UV)-irradiation (Quan and Fisher, 2014). Elevated expression of CYR61/CCN1 in the dermis leads to reduced collagen production by impairing TGF-β signaling (Quan and Fisher, 2014) and increasing collagen fibril fragmentation through the production of multiple matrix metalloproteinase proteins (MMPs) (Quan and Fisher, 2014). Thus, elevated CCN1 promotes skin thinning and fragility, two prominent features of aged skin, but interestingly this is not seen in long-standing psoriasis, suggesting the involvement of other mitigating factors, as well.
Abnormal regulation of CCN1 expression has been observed in several skin diseases, including wound healing and fibrosis (Jun and Lau, 2010). Studies in a mouse model have demonstrated that CCN1 exerts anti-fibrotic activity via induction of dermal fibroblast senescence during cutaneous wound healing. Elevated CCN1 may also have significant impact on the delayed wound healing seen in elderly patients. CO2 laser ablation, which causes partial thickness wounding, rapidly and markedly induces CCN1 in human skin dermis in vivo (Quan and Fisher, 2014). Timing of the elevated CCN1 levels corresponds to the inflammatory phase (ECM breakdown), and the timing of CYR61/CCN1 diminution corresponds to the ECM remodeling phase (collagen production) of wound healing responses. Also of note, CCN1-regulated factors such as proinflammatory cytokine (IL-1β), collagen-degrading MMPs (MMP-1, MMP-3, and MMP-9), and type I procollagen are well-known components of the wound healing response. These data suggest that CCN1 may play a critical role in regulating wound repair in human skin. Further support comes from mouse studies, which have reported that CCN1 is induced by cutaneous wounding and that it participates in both the inflammatory and remodeling phases of repair (Jun and Lau, 2010). Therefore, the constitutive elevation of CCN1 in aged human skin may delay wound healing by extending the inflammatory phase and delaying the remodeling phase. These findings highlight CCN1 as a possible therapeutic target, not only for psoriasis, but also for improving wound healing in the elderly and in skin cancer.
Concluding remarks
The results reported by Sun et al. in this issue reveal the potential role of CCN1 in the pathogenesis of psoriasis. This study, together with other emerging data, suggests that CCN1 may function as an important regulator in skin biology, by modulating key functions of keratinocytes and fibroblasts. Clearly, there remains much to be learned about the regulation of CCN1 and its functions in skin physiology and pathology. It should be noted that CCN1 is one of six members of the CCN family. CCN2 is a well-established mediator of fibrosis. However, little is known regarding the roles of CCN3-6 in skin biology. Certainly the article by Sun et al. should spur new appreciation of the importance of the CCN family of proteins in skin biology.
Bullet points.
CCN1 is a potential new ingredient in the recipe of inflammatory mediators that drive psoriasis
Targeting the interaction of CCN1 with integrin α6β1 represents a potential new strategy to treat psoriasis
CCN1 may function as an important regulator in skin biology, by modulating key functions of keratinocytes and fibroblasts.
Acknowledgments
This article was partially funded by grants from National Institutes of Health RO1-AG025186 (GF), RO1-AG031452 (GF), R01-ES-ES014697 (TQ), and RO1-AG019364 (GF).
Footnotes
The authors state no conflict of interest.
References
- Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73:342–50. doi: 10.1016/j.cyto.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–83. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JT, Bruce AT, Gudjonsson JE, et al. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–26. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–85. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kular L, Pakradouni J, Kitabgi P, et al. The CCN family: a new class of inflammation modulators? Biochimie. 2011;93:377–88. doi: 10.1016/j.biochi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68:3149–63. doi: 10.1007/s00018-011-0778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Qin Z, Okubo T, Voorhees JJ, et al. Elevated cysteine-rich protein 61 (CCN1) promotes skin aging via upregulation of IL-1beta in chronically sun-exposed human skin. Age. 2014;36:353–64. doi: 10.1007/s11357-013-9565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Fisher G. Age-Associated Dermal Microenvironment (AADM) Promotes Human skin Connective Tissue Aging - A Mini Review. Gerontology. 2014 doi: 10.1159/000371708. Invited Review Article. [DOI] [Google Scholar]
- Quan T, Xu Y, Qin Z, et al. Elevated YAP and its downstream targets CCN1 and CCN2 in basal cell carcinoma: impact on keratinocyte proliferation and stromal cell activation. Am J Pathol. 2014;184:937–43. doi: 10.1016/j.ajpath.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Zhou Z, et al. Cyr61, a novel pro-inflammatory factor, aggravates psoriasis skin lesions by promoting keratinocytes activation. J Invest Dermatol. 2015 doi: 10.1038/jid.2015.231. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108:2270–5. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]