Abstract
After nerve injury, Schwann cells (SCs) dedifferentiate, proliferate, and support axon regrowth. If axons fail to regenerate, denervated SCs eventually undergo apoptosis due, in part, to increased expression of the low-affinity neurotrophin receptor, p75NTR. Merlin is the protein product of the NF2 tumor suppressor gene implicated in SC tumorigenesis. Here we explore the contribution of merlin to SCs responses to nerve injury. We find that merlin becomes phosphorylated (growth permissive) in SCs following acute axotomy and following gradual neural degeneration in a deafness model, temporally correlated with increased p75NTR expression. p75NTR levels are elevated in P0SchΔ39-121 transgenic mice that harbor a Nf2 mutation in SCs relative to wild-type mice before axotomy and remain elevated for a longer period of time following injury. Replacement of wild-type, but not phosphomimetic (S518D), merlin isoforms suppresses p75NTR expression in primary human schwannoma cultures which otherwise lack functional merlin. Despite elevated levels of p75NTR, SC apoptosis following axotomy is blunted in P0SchΔ39-121 mice relative to wild-type mice suggesting that loss of functional merlin contributes to SC resistance to apoptosis. Further, cultured SCs from mice with a tamoxifen-inducible knock-out of Nf2 confirm that SCs lacking functional merlin are less-sensitive to p75NTR-mediated cell death. Taken together these results point to a model whereby loss of axonal contact following nerve injury results in merlin phosphorylation leading to increased p75NTR expression. Further, they demonstrate that merlin facilitates p75NTR-mediated apoptosis in SCs helping to explain how neoplastic SCs that lack functional merlin survive long-term in the absence of axonal contact.
Introduction
Peripheral nerve injury results in axon degeneration distal to the site of injury (Wallerian degeneration) (Stoll and Muller, 1999; Lorenzetto et al., 2008). Following loss of axonal contact, denervated Schwann cells (SCs) undergo a series of events, including dedifferentiation and proliferation, and provide support for eventual axonal regrowth (Chen et al., 2007). They then redifferentiate and remyelinate regenerated axons as part of the repair process (Chen et al., 2007). However, SCs that remain isolated from neural elements following nerve injury eventually die. This SC loss, among other factors, complicates attempts to restore neural function after injury (Hoffman, 1992). Following denervation, SCs dramatically increase expression of the low affinity neurotrophin receptor, p75NTR, which promotes SC apoptosis (Taniuchi et al., 1986; Ferri and Bisby, 1999).
p75NTR promotes SC apoptosis following denervation
p75NTR is a single pass transmembrane receptor implicated in a wide variety of cellular responses including differentiation, growth, apoptosis and survival depending on the context and co-receptors (Parkhurst et al., 2010). In neurons, it frequently functions as a co-receptor with Trks to bind mature neurotrophins and promote neuronal survival (Chao and Hempstead, 1995). However, in the absence of Trk receptors, p75NTR often interacts with other co-receptors, including sortilin or Nogo, to mediate cell death (Bandtlow and Dechant, 2004; Barker, 2004). Although p75NTR binds with relative low affinity to mature neurotrophins in the absence of Trk receptors, it binds proforms of neurotrophins with high affinity (Barker, 2004). Following ligand binding, p75NTR undergoes intramembrane cleavage by γ-secretase to generate an intracellular domain (ICD) fragment (Jung et al., 2003; Kanning et al., 2003; Kenchappa et al., 2006). The ICD contains a death domain that functions as docking site necessary for the activation of TNF and Fas ligand and leads to c-Jun N-terminal kinase (JNK) activation (Haase et al., 2008). Further, γ-secretase-mediated cleavage results in nuclear translocation of NRIF, a DNA binding protein essential for p75-mediated apoptosis (Kenchappa et al., 2006). Recent data indicate that schwannoma cells express high levels of p75NTR yet, in contrast to non-neoplastic SCs, are resistant to p75NTR-mediated apoptosis (Ahmad et al., 2014).
The tumor suppressor, merlin, regulates SC proliferation and neoplasia
Merlin is the protein product of the NF2 tumor suppressor gene. Loss of NF2 gene function underlies development of neurofibromatosis type 2 (NF2)-associated and sporadic schwannomas (Rouleau et al., 1993; Trofatter et al., 1993; Irving et al., 1994) Merlin mediates cell-cell contact to suppress cell proliferation. The N- and C-termini of merlin interact with each other as merlin alternates between growth permissive and growth suppressive conformations depending on the phosphorylation of serine residues. For example, S518 phosphorylation leads to a conformation that facilitates cell growth (Gutmann et al., 1999). The tumor suppressor function becomes active after S518 dephosphorylation (Okada et al., 2007). Merlin regulates a wide variety of signaling events to suppress cell growth (Li et al., 2010). However, the function of merlin in normal SCs and their response to injury remains largely unknown. Here we explore the possibility that merlin plays a fundamental role in p75NTR-mediated SCs responses to loss of axonal contact. We find that merlin suppresses p75NTR expression in a phosphorylation dependent fashion and that merlin facilitates SC cell death in response to p75NTR ligands.
Materials and Methods
Mice strains
P0SchΔ(39-121) and Nf2f/f mice were obtained from Riken BioResource Center (Tsukuba, Japan) (Giovannini et al., 1999; Giovannini et al., 2000) and RosaCRE-ERT2 and FVB mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The RosaCRE-ERT2 mice contain a tamoxifen-inducible Cre recombinase system. We crossed the Nf2f/f mice with RosaCRE-ERT2 mice, producing an F1 generation of mice carry a Cre-inducible loxP sequence that is site specific for the Nf2 gene. Animals of either sex were used for all experiments. All animal work was approved by the University of Iowa Institutional Animal Care and Use Committee.
Sciatic nerve axotomies and protein lysates
Sciatic nerve (SN) axotomies were performed in adult rats and adult FVB control (WT) and P0SchΔ39-121 mice as previously described (Brown and Hansen, 2008). In short, a small horizontal skin incision was made over the quadriceps muscle. The muscle was then bluntly dissected and the SN was identified. The SN was dissected proximally and transected approximately 1 cm from the spinal cord and the cut ends of the nerve were displaced into separate planes of tissue to prevent reattachment. The contralateral SN remained uncut as the control. The distal portion of the SN and the contralateral intact nerve were excised and on post-axotomy (PA) days 7, 21, and 180. These time points were chosen to correlate with periods of increased p75NTR expression and cellular proliferation (PA7-21) as well as a late phase when SCs are no longer being replenished (see Figs 3, 5, 6). Three nerve samples at each time point were pooled and immediately placed in a modified RIPA lysis buffer solution on ice. A sterilized ground glass mortar and pestle was used to crush the nerve. The crude lysate was sonicated (1 second pulses for 30 seconds) and then incubated on ice for 30 min. The homogenate was cleared by centrifugation at 18,000 × g for 10 min at 4°C. The lysate was then aliquoted and stored at −80°C.
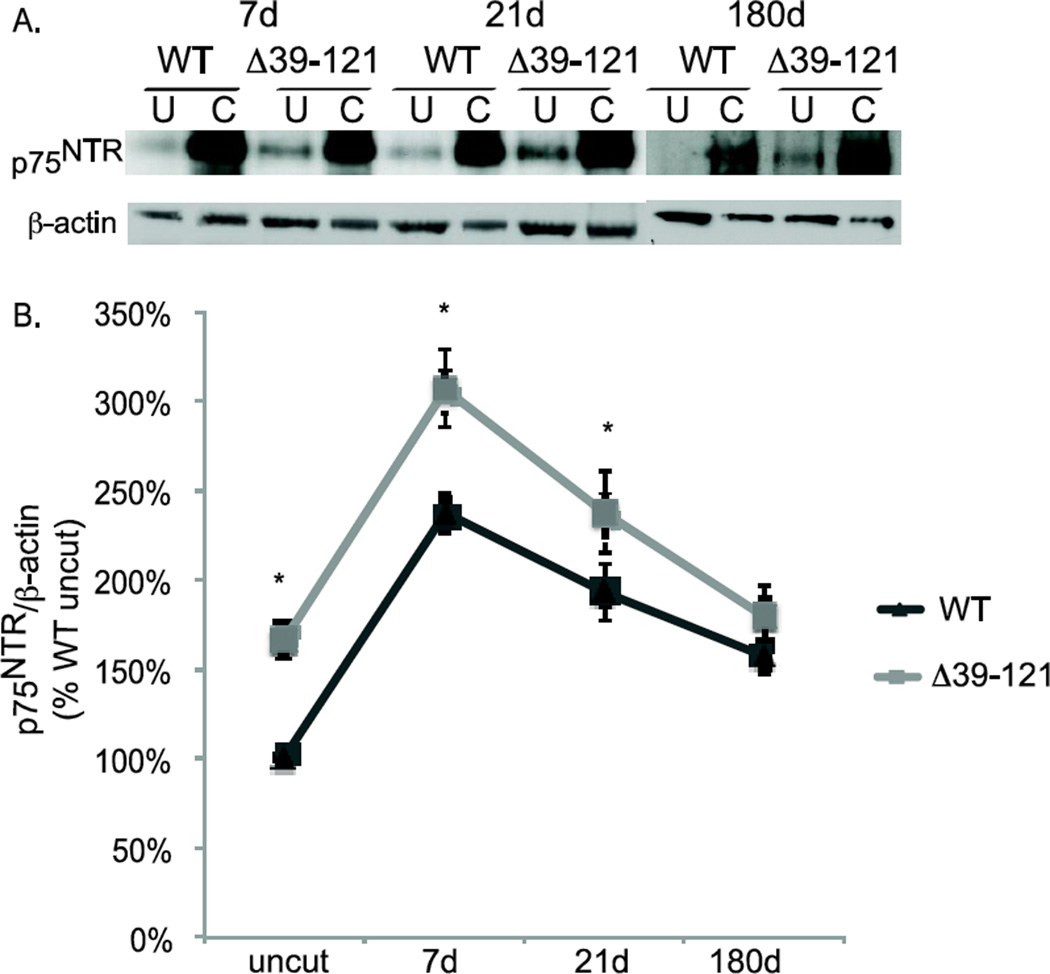
Figure 3.
Merlin status regulates p75NTR expression. A. Protein lysates from cut and uncut sciatic nerves were collected 7,21, and 180 days following unilateral axotomy in wild type and P0SchΔ39-121 mice. Blots were probed with anti-p75NTRantibody and then stripped and re-probed with anti-β-actin antibodies. B. Average p75NTR/β-actin levels based on densitometry. Blots are from 3 nerve samples pooled for each condition and averaged from 4 separate repetitions for each time point. Error bars present SEM. *p<0.05 by two-tailed Student t-test.
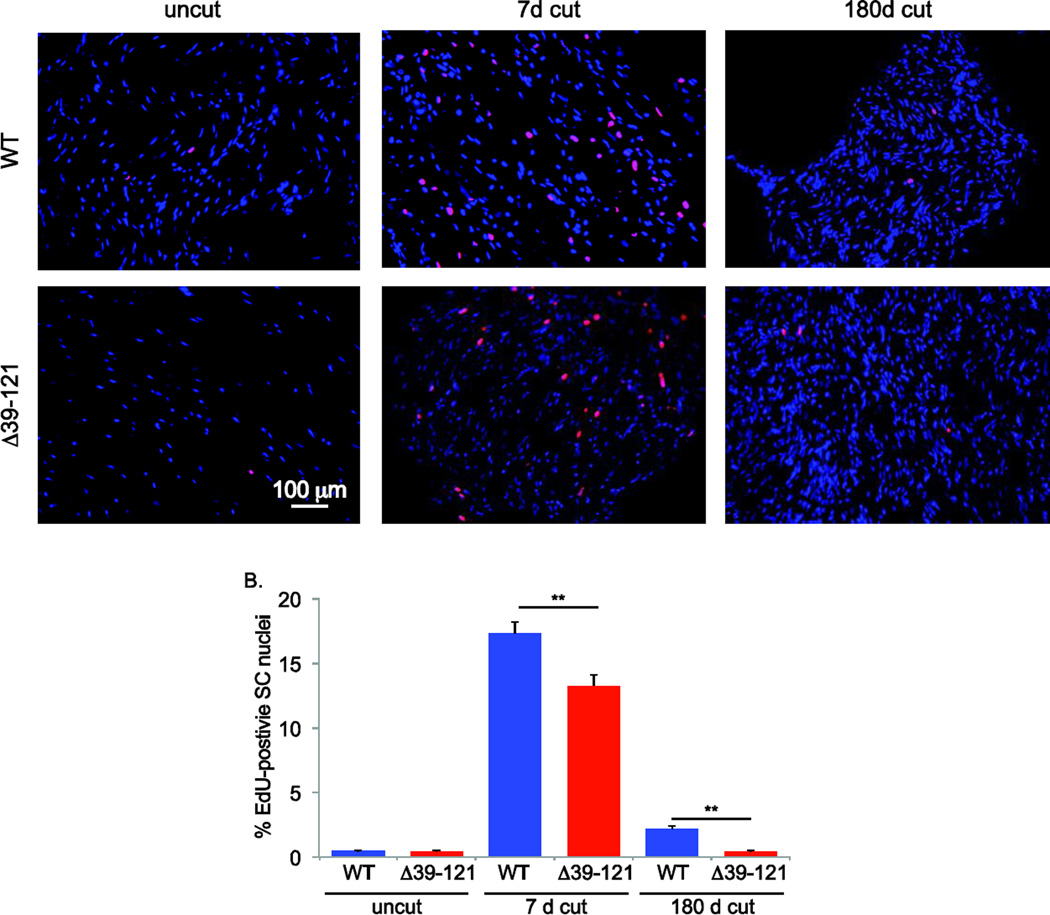
Figure. 5.
Lack of functional merlin results in decreased Schwann cell proliferation following axotomy. A. Cut and uncut sciatic nerves from wild type (WT) and P0SchΔ39-121 mice treated with EdU were collected at 7 and 180 days following unilateral axotomy and frozen sections were labeled for EdU using the Click-IT reaction (red). Nuclei are labeled with DAPI (blue). Scale bar=100 µm. B. The number of EdU-positive SC nuclei was scored. Counts represent the mean from 4 animals per group. Error bars present SEM. One way ANOVA with post hoc Holm-Sidak was used to test for significance of differences *p< 0.05, ** p<0.001.
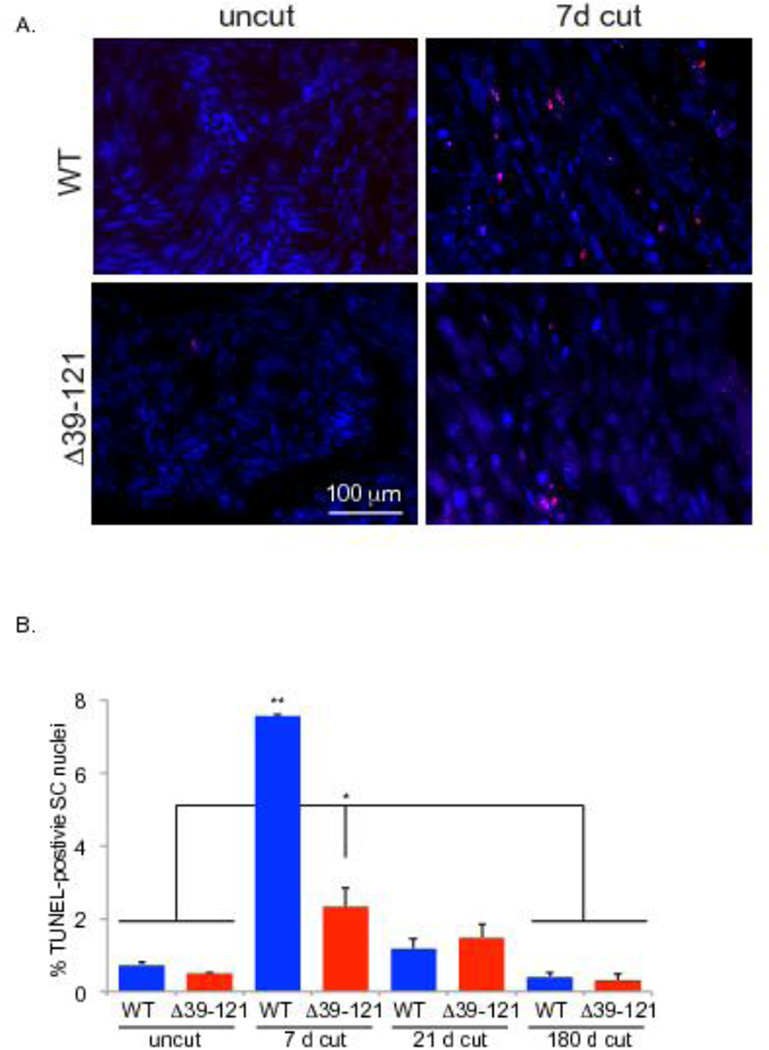
Figure 6.
Lack of functional merlin blunts Schwann cell apoptosis following axotomy. A. Cut and uncut sciatic nerves from wild type (WT) and P0SchΔ39-121 mice were collected at 7, 21, and 180 days following unilateral axotomy and frozen sections were labeled with TUNEL (red). Nuclei are labeled with DAPI (blue). Scale bar=100 µm. B. The average number of TUNEL-positive SC nuclei was scored. Counts represent the mean from 4 animals per group. Error bars present SEM. One way ANOVA with post hoc Holm Sidak was used to test significance of differences *p< 0.05. The greatest difference between wild-type and mutant mice occurred at post-axotomy day 7 (p< 0.001).
Kanamycin deafening
Sprague Dawley rats were obtained from Charles River. Deafening was performed as previously described by injecting kanamycin, which is toxic to hair cells, from P8–16 (Provenzano et al., 2011). Deafness was confirmed by elevated auditory brainstem response thresholds in a subset of animals and by a lack of MyoVIIA-positive hair cells in all animals.
Human vestibular schwannoma and mouse Schwann cell cultures
Primary human VS cultures were prepared from acutely resected tumors as previously described (Hansen et al., 2006; Yue et al., 2011). None of the cultures were derived from neurofibromatosis type II-associated tumors. The cultures were not passaged prior to experimental manipulation. Adenoviral-mediated gene transfer was also performed as previously described with Ad5.Emptyvector-GFP, Ad5-wild-type merlin-GFP, Ad5-merlinS518A-GFP, Ad5-merlinS518D-GFP (Yue et al., 2011; Ahmad et al., 2014). Live cultures were monitored for GFP fluorescence to ensure that over 80% of the cells had been successfully transduced. After 48h, protein lysates were prepared and immunoblotted.
SC cultures from the sciatic nerves of P3–4 neonatal RosaCre:NF2f/f mice were prepared as previously described (Provenzano et al., 2008). Once the cultures were 70–80% confluent, they were treated with tamoxifen (500 nM, Sigma-Aldrich, St. Louis, MO) or vehicle. The cultures were washed and the tamoxifen was removed with a media change after 72 hrs. Cultures were then maintained for another 24 hr with or without proNGF (3 nM) and then fixed for 10 min with 4% paraformaldehyde.
Western blot
Western blots from the nerve lysates or culture lysates were performed as previously described (Hansen et al., 2006). The blot was probed with anti-p75NTR antibody (generous gift of Dr. Moses Chao) and stripped and reprobed with anti-β-actin antibodies (Sigma-Aldrich) to confirm equal protein loading and to determine relative p75NTR levels. Parallel blots were probed with anti-phosphomerlin (p-merlin) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and stripped and reprobed with non phosphospecific anti-merlin antibodies (Santa Cruz Biotechnology) to determine the relative phosphorylation level of merlin. The experiment was repeated at least 4 times (total of 12 animals) for each time point. Western blots were quantified as previously described using ImageJ software (NIH) (Yue et al., 2011; Ahmad et al., 2014)
Immunohistochemistry
Following axotomy a small portion of each nerve was resected and placed in 4% paraformaldehyde, cryoprotected in a serial sucrose gradient, embedded and frozen in OCT, and finally cryosectioned as previously described (Provenzano et al., 2008). We performed immunostaining of frozen sections (~10 µm thick) with anti-p75NTR and anti-phosphomerlin antibodies to determine the spatial distribution of SCs that express p75NTR and phosphorylated merlin. Subsets of sections were immunostained with anti-neurofilament 200 (NF200) antibodies (Sigma, St. Louis, MO) to confirm loss of axons and correlate p75NTR and phosphorylated merlin expression with axonal contact. Sections were mounted with Prolong Gold + DAPI (Life Technologies, Carlsbad, CA) prior to coverslipping. Images were captured on a Leica DMIRE 2 microscope (Leica Microsystems, Bannockburn, IL) equipped with epifluorescent filters and a cooled CCD camera using Metamorph software (Molecular Devices, LLC, Downington, PA) or with a Leica TCS SP5 confocal microscope (Leica Microsystems, Bannockburn, IL).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
Frozen nerve sections and SC cultures were labeled with dUTP for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) to detect apoptotic nuclei as previously described (Provenzano et al., 2008; Provenzano et al., 2011). All samples were counterlabeled with S100 and nuclei were labeled with DAPI prior to coverslipping. Criteria for scoring apoptotic cells included: S100-positive, TUNEL-positive nucleus, and a condensed or fragmented nucleus. The percent of TUNEL–positive SCs was scored from 10 randomly selected 20X fields as previously described for each culture condition (Hansen et al., 2008; Yue et al., 2011). For cultures, the percent of TUNEL-positive cells was expressed as a percentage of the control condition, defined as 100%. Each condition was performed in duplicate and was repeated on ≥3 cultures Protein lysates were prepared from parallel cultures and immunoblotted with anti-cleaved caspase 3 antibodies (Cell Signaling) to confirm apoptosis. For nerve sections a total of 10 microscopic fields per section and 6 sections per nerve were counted. Nerves were derived from 4 animals per group. Statistical significance of differences in the average percent of apoptotic cells among the various conditions was determined by one way ANOVA followed by Holm-Sidak method using SigmaStat software (Systat Software Inc, Richmond, CA).
EdU labeling
Starting 24 hours prior to euthanasia, mice were injected intraperitoneally 4 times at regular intervals with EdU (10 µM, Life Technologies, Carlsbad, CA) in order to label cells undergoing karyokinesis. SNs were dissected and prepared as described above. EdU was detected in SN nuclei of WT and P0SchΔ(39-121) using the Click-IT reaction per manufacturer’s instructions (Life Technologies). Sections were counterstained with S100, mounted with Prolong-Gold +DAPI (Life Technologies), and coverslipped. Percentage of SN cells undergoing nuclear division was determined by dividing the total number of SN nuclei (S100-positive + DAPI-positive) by the number of EdU positive SN nuclei (S100-positive cells + EdU-positive nuclei). A total of 10 microscopic fields per section and 6 sections per nerve were counted. Nerves were derived from 4 animals per group. Statistical significance of differences in the average percent of EdU-positive cells among the various conditions was determined by one way ANOVA followed by Holm-Sidak method using SigmaStat software (Systat Software Inc, Richmond, CA).
Results
Merlin is phosphorylated in SCs following axotomy
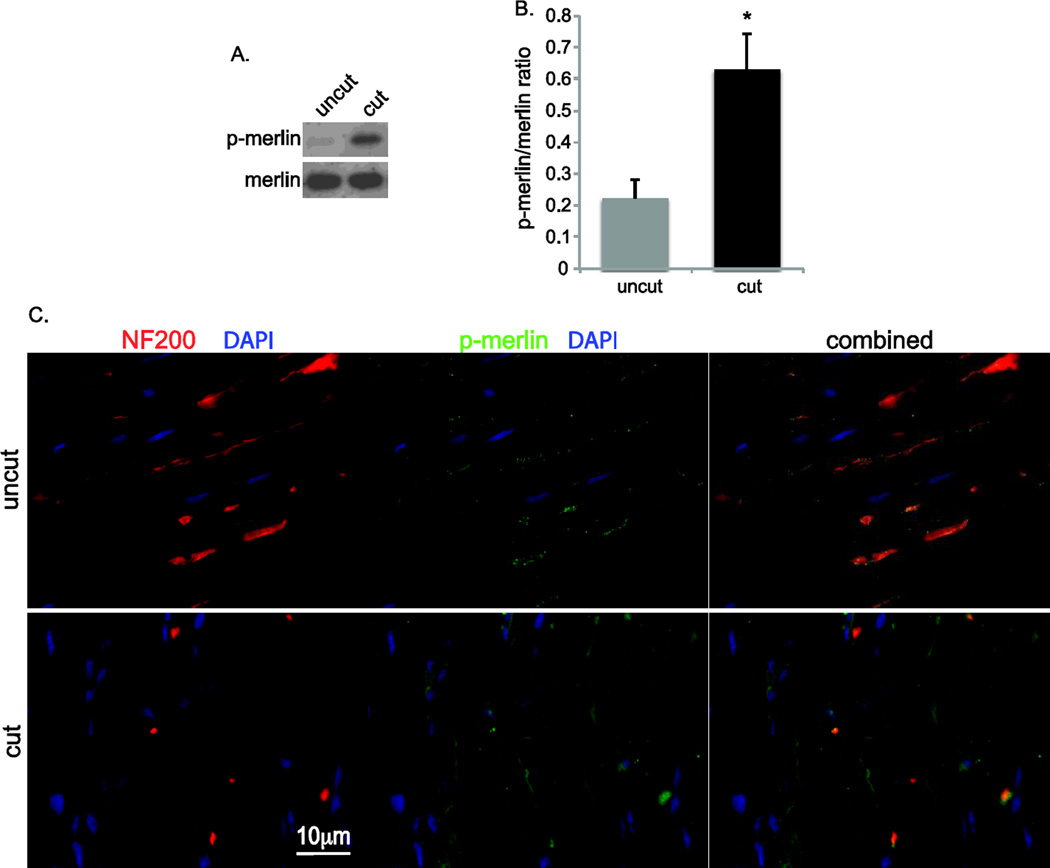
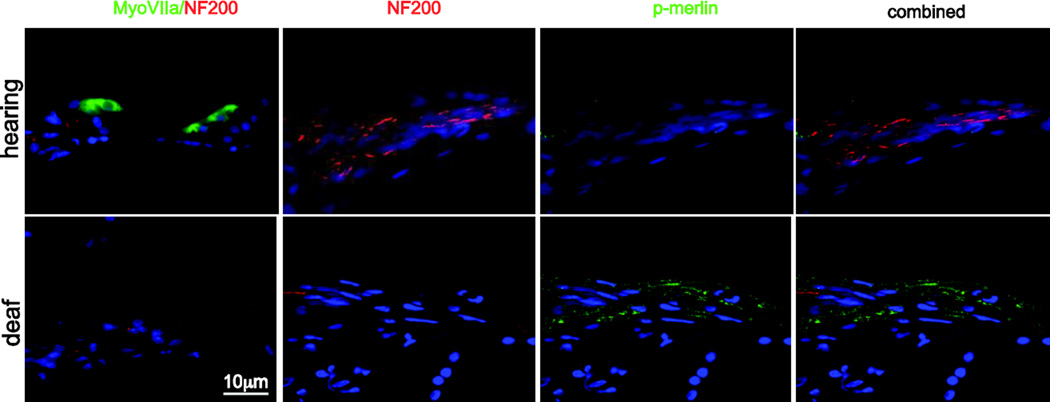
To examine the effect of nerve injury on merlin phosphorylation in SCs, we immunoblotted protein lysates from cut and uncut rat sciatic nerves with anti-phospho-merlin (p-merlin) antibodies (Fig. 1A,B). Blots were stripped and reprobed with non-phospho-specific anti-merlin antibodies. Densitometry was performed to quantify the level of p-merlin expression relative to overall merlin levels. Axotomy resulted in a significant increase of merlin phosphorylation. Immunolabeling of frozen nerve sections with anti-p-merlin and anti-NF200 antibodies demonstrated punctate labeling of p-merlin that was confined to the NF200-positive axons prior to nerve injury with no significant labeling in SCs. Following axotomy, there was evidence of axonal degeneration evidenced by loss of intact NF200-positive nerve fibers with a parallel increase in p-merlin labeling in the SCs that remain following axotomy (Fig. 1C). We also examined merlin phosphorylation in a different model of nerve injury. Kanamycin treatment causes hair cell loss leading to a gradual degeneration of spiral ganglion distal axons and denervation of the SCs in the osseous spiral lamina (Alam et al., 2007). We have previously shown that these SCs that have undergone a gradual denervation increase p75NTR expression and re-enter the cell cycle similar to SCs following acute axotomy (Provenzano et al., 2011). Frozen sections from cochleae were immunolabeled with anti-p-merlin and anti-NF200 antibodies. Parallel sections were immunolabeled with anti-myosin VII (MyoVII) to verify loss of hair cells in kanamycin-treated animals. Kanamycin treatment resulted in loss of the NF200-positive peripheral axons in the osseous spiral lamina leading to the organ of Corti and increased p-merlin labeling in the SCs that had lost axonal contact and remain in the osseous spiral lamina (Fig. 2) (Provenzano et al., 2011). These data demonstrate that merlin becomes phosphorylated in SCs following acute or gradual loss of axonal contact.
Figure 1.
Merlin is phosphorylated in Schwann cells following nerve injury. A. Immunoblots of protein lysate from cut and uncut rat sciatic nerves probed with anti-phospho-merlin (p-merlin) and merlin antibodies. B. Average p-merlin/merlin levels based on densitometry based on samples from 3 nerves pooled for each condition and averaged from 4 separate repetitions. Error bars present SEM. *p=0.0165, Student’s unpaired t-test. C. Frozen sections of cut and uncut sciatic nerves were immunolabeled with anti-neurofilament 200 (NF200, red) and anti-p-merlin (@green) antibodies. Nuclei were labeled with DAPI (blue). Right column demonstrates combined image with superimposed staining showing presence of p-merlin confined to the axons in in uncut nerve and increased diffuse p-merlin labeling of denervated SCs following axotomy. Scale bar=10 µm.
Figure 2.
Merlin is phosphorylated spiral ganglion Schwann cells after kanamycin-induced hair cell loss. Cochlear frozen sections were labeled with anti-myosin VII, (green), anti-NF200 (red), and phospho-merlin (p-merlin, green) antibodies.
Merlin suppresses p75NTR expression in Schwann cells
Denervated SCs with phosphorylated merlin and VS cells that lack functional merlin express high levels of p75NTR raising the possibility that merlin status regulates p75NTR expression in SCs. To test this possibility, we performed sciatic nerve axotomies in wild-type (WT) and P0SchΔ39-121 mice. P0SchΔ39-121 mice harbor a dominant negative Nf2 mutation restricted to SCs (Giovannini et al., 1999). Protein lysates from cut and uncut nerves, collected 7, 21, and 180 days following axotomy, were immunoblotted with anti-p75NTR antibodies (Fig. 3A). The blots were probed with anti-β-actin antibodies to verify protein loading. Densitometry was performed to quantify the level of p75NTR expression relative to β-actin (Fig. 3B). As expected, axotomy led to a significant increase in p75NTR levels. Consistent with the notion that merlin regulates p75NTR expression, p75NTR levels were elevated in uncut nerves from P0SchΔ39-121 mice compared to WT mice. Further, comparison of p75NTR levels following axotomy demonstrates that p75NTR levels were significantly elevated in P0SchΔ39-121 mice compared to WT mice at 7 and 21 days post-axotomy (PA) (Fig. 3B). This difference was no longer statistically significant 180 days PA (p=0.228). Thus, loss of merlin function increases p75NTR expression, even in SCs that remain in contact with axons.
Merlin status regulates p75NTR expression in SCs
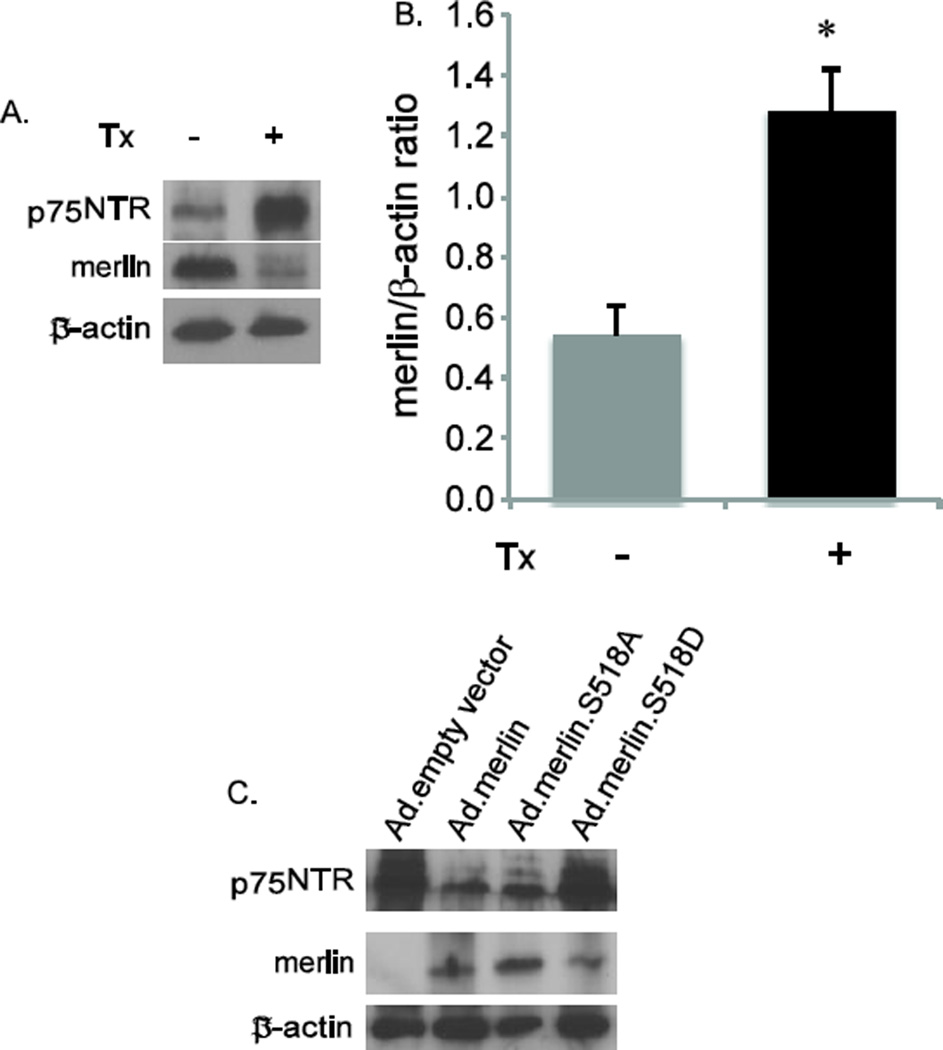
To further explore the relationship between p75NTR expression levels and merlin status, we cultured sciatic nerve SCs derived from a transgenic mouse line with floxed Nf2 and a tamoxifen (Tx)-inducible Cre (RosaCre:Nf2f/f) (Giovannini et al., 2000), allowing for conditional knock-out of Nf2. The estrogen receptor T2 (ERT2) moiety fused to Cre retains the recombinase in the cytosol until Tx administration releases this inhibition, thus permitting inducible recombination of LoxP sites. Sciatic nerve cultures were prepared from these mice. Treatment of cultures with Tx (500 nM) reduced merlin and significantly elevated p75NTR expression levels consistent with the notion that merlin suppresses p75NTR expression in SCs (Fig. 4A,B).
Figure 4.
Merlin regulates p75NTR expression in cultured Schwann and schwannoma cells. A. SC cultures from RosaCre:Nf2f/f mice were treated with (Tx +) or without (Tx −) tamoxifen. Protein lysates were probed with anti-p75NTR and merlin antibodies. The blots were stripped and reprobed with anti-β-actin antibodies. B. Average p75NTR/β-actin levels based on densitometry based on blots from 3 separate repetitions. Error bars present SEM. *p=0.012 by two-tailed Student t-test. C. Primary VS cultures were transduced with Ad-empty vector, Ad-merlin (wild-type), Ad-merlin S518A (unphosphorylatable), or Ad-merlin S518D (phospho-mimetic). Protein lysates were probed with anti-p75NTR and merlin antibodies. The blots were stripped and reprobed with anti-β-actin antibodies.
The fact that elevated p75NTR expression in SCs following axotomy correlates with merlin phosphorylation raises the possibility that merlin phosphorylation hinders its ability to suppress p75NTR expression. To address this possibility we performed merlin replacements experiments using primary VS schwannoma cultures. These cultures lack functional merlin expression thereby allowing us to introduce merlin isoforms with specified serine 518 (S518) phosphorylation status (Yue et al., 2011; Ahmad et al., 2014). Cultures were transduced with an adenoviral vector that expresses wild-type merlin, S518-mutated merlin isoforms, or an empty, control vector. The S518A mutation renders merlin unable to be phosphorylated on this residue while the S518D functions as a phospho-mimetic. Protein lysates from the cultures were immunoblotted with anti-p75NTR antibodies followed by anti-merlin and then anti-β-actin antibodies. Replacement of merlin reduced p75NTR expression in primary VS cultures (Fig. 4C). Further, p75NTR expression was suppressed in cultures transduced with S518A merlin isoform whereas transduction with the S518D isoform resulted in increased in p75NTR expression. Taken together these results indicate that S518 phosphorylation of merlin reduces its ability to suppress p75NTR expression.
Merlin mutation decreases Schwann cell proliferation following axotomy
Next we sought to determine the effect of merlin on SC survival and proliferation following axotomy. Sciatic nerve axotomies were performed in FVB control and P0SchΔ39-121 mice and the mice were treated with EdU to label proliferating nuclei. Frozen sections of the portion of the nerves distal to the axotomy were labeled with the Click-IT reaction to identify EdU-positive nuclei and EdU-positive SC nuclei were counted as before (Provenzano et al., 2008; Provenzano et al., 2011). Axotomy resulted in a significant increase in the percent of EdU-positive SC nuclei 7 days following injury. The percent of EdU-positive SCs diminished by 180 days after axotomy but remained elevated above the level in uncut nerves. Remarkably, the percent of EdU-positive SC nuclei was significantly less in nerves from P0SchΔ39-121 mice 7 and 180 days following injury compared to wild-type mice (Fig. 5A,B). Further, there was no difference in the percent of EdU-positive SC nuclei in uncut nerves from P0SchΔ39-121 and wild-type mice. Thus, merlin mutation blunts the proliferative capacity of SCs following injury.
Merlin mutation reduces SC apoptosis following axotomy
To determine the effect of merlin on SC death following axotomy frozen sections from the portion of the nerve distal to the axotomy were labeled with TUNEL and the number of apoptotic SC nuclei were counted as before (Provenzano et al., 2008; Provenzano et al., 2011). Axotomy resulted in a significant increase in the percent of TUNEL-positive SC nuclei 7 days following injury. This apoptotic response diminished over time, remaining elevated above baseline at 21 days and returning to baseline by 180 days following axotomy (Fig. 6). The percent of TUNEL-positive SC nuclei was significantly less in nerves from P0SchΔ39-121 mice 7 and 21-days following injury as well as in the uncut nerves (Fig. 6). Thus, merlin mutation renders SCs less sensitive apoptosis following nerve injury.
Merlin is necessary for p75NTR-induced Schwann cell apoptosis
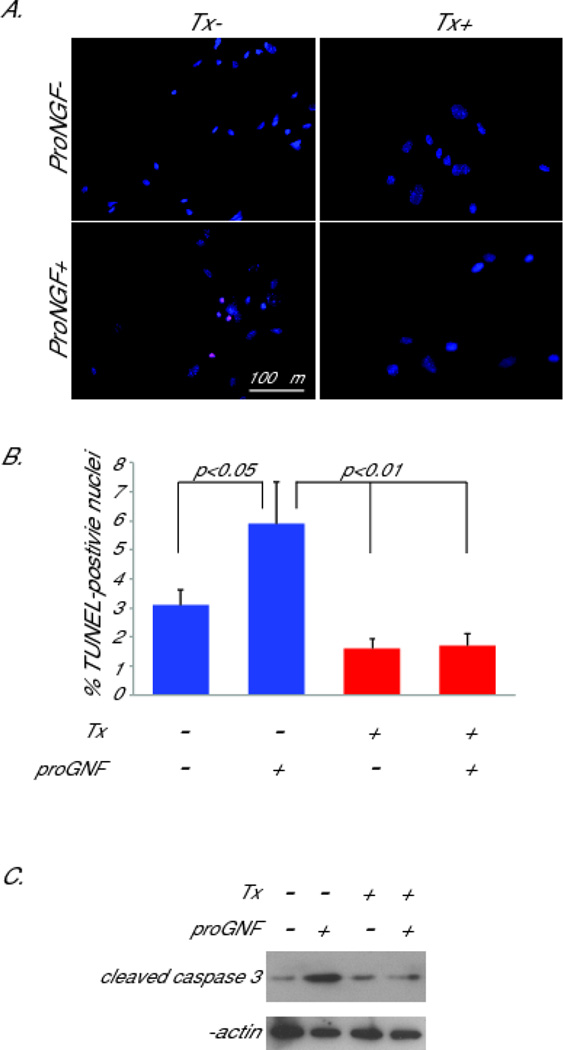
SCs from P0SchΔ39-121 mice that lack functional merlin express high levels of p75NTR yet are resistant to apoptosis before and following axotomy (Fig. 6). This observation raises the possibility that functional merlin is necessary for p75NTR-induced SC apoptosis. To test this possibility we cultured SCs from RosaCre:Nf2f/f mice. Cultures were either exposed to or not exposed to Tx as above. Subsets of cultures were treated with the p75NTR ligand, proNGF (3 nM) to induce apoptosis. The number of TUNEL-positive SC nuclei were scored as before. proNGF induced apoptosis in SCs that were not exposed to Tx (merlin present) reflected as a significant increase in the percent of TUNEL-positive SC nuclei. This apoptotic response was significantly blunted in SCs that had been exposed to Tx (Fig. 7 A,B). To confirm that the increase in TUNEL-positive nuclei represented an apoptotic response, we blotted protein lysates from the cultures with anti-cleaved caspase 3 antibody. Consistent with the TUNEL results, there was an increase in cleaved caspase 3 in cultures treated with proNGF that was diminished by pretreatment with Tx (Fig. 7C). These results confirm that SCs lacking functional merlin are less sensitive to apoptosis, particularly cell death initiated by activation of p75NTR.
Figure 7.
Merlin is necessary for p75NTR-mediated Schwann cell apoptosis. A. SC cultures from RosaCre:Nf2f/f mice were treated with tamoxifen (Tx+) or carrier (Tx−) and subsequently maintained in the presence or absence of proNGF (3nM). Cultures were labeled with TUNEL and the number of TUNEL-positive SC nuclei was scored. B. Average number of TUNEL-positive SC nuclei from 3 separate cultures for each condition. Error bars present SEM. One way ANOVA with post hoc Holm Sidak was used to test for significance of differences. Scale bar=100 µm. C. Protein lysates from cultures were immunoblotted wth anti-cleaved caspase3 antibody. Blots were stripped and reprobed with anti-β-actin antibody.
Discussion
In a normal nerve, SCs are in contact with axons and remain in a quiescent state. Following axon degeneration due to nerve injury SCs lose contact with axons and initially dedifferentiate, proliferate and provide axonal support. Loss of axonal contact results in a rapid and significant increase in p75NTR expression (Taniuchi et al., 1986; Taniuchi et al., 1988); the mechanisms leading to this elevated expression have remained unknown. Eventually in the absence of reinnervation, denervated SC’s undergo p75NTR-mediated apoptosis (Ferri and Bisby, 1999). SCs are essential for axon regeneration and loss of supportive SCs is a barrier to late neural regeneration (Hoffman, 1992). The data presented here implicate the tumor suppressor merlin as a key mediator of p75NTR expression and apoptotic signaling in SCs following nerve injury.
Merlin phosphorylation after axotomy
Merlin’s molecular conformation is altered by its phosphorylation status and this determines its ability to bind other proteins and regulate cell growth (Gutmann et al., 1999; Rong et al., 2004; Ye, 2007; Sher et al., 2012). Phosphorylation of S518 by PKA, p21-activated kinases 1 and 2 (PAK1/2), or Akt, leads to a growth-permissive (inactive) conformation (Gutmann et al., 1999; Kissil et al., 2002; Alfthan et al., 2004; Okada et al., 2007; Thaxton et al., 2007; Sher et al., 2012). Besides S518, other residues including S10, S66, T230 and S315 are also targets for phosphorylation; the extent to which phosphorylation of these residues regulates merlin ability to interact with other proteins and control cell growth has not been firmly established. However, Akt-mediated T230 and S315 phosphorylation results in ubiquitination of merlin marking it for degradation (Tang et al., 2007). Using models of both acute, primary (axotomy) and gradual, secondary (deafening by aminoglycosides) neural degeneration, we demonstrate that merlin is phosphorylated in SCs following axon degeneration. Coincident with this phosphorylation, the denervated SCs dedifferentiate and re-enter the cell cycle. These data fit a general model of merlin functioning as a molecular switch responsive to cell-cell contact cues that is able to suppress cell proliferation when it remains in a dephosphorylated conformation (Sher et al., 2012). In this model, merlin phosphorylation in SCs that have lost axonal contact relieves this inhibition allowing cells to re-enter the cell cycle.
Merlin status regulates p75NTR expression following nerve injury
Merlin has been shown to suppress the expression of transmembrane receptors in cultured cells, particularly the ErbB and PDGFR, receptor tyrosine kinase (RTK) families that promote SC proliferation (Lallemand et al., 2009b; Zhou et al., 2011). The data presented here demonstrate that loss of merlin function results in increased p75NTR expression in SCs. Nerve lysates from P0SchΔ39-121 mice prior to injury and following axotomy revealed an increase in p75NTR levels in comparison to wild-type mice. Further, suppression of merlin expression in cultured SCs increased p75NTR expression while replacement of wild-type merlin reduced p75NTR expression in primary VS cells. Taken together these data confirm that merlin suppresses p75NTR levels in SCs in vitro and in vivo and are consistent with the observation of elevated p75NTR levels in neoplastic VS cells (Ahmad et al., 2014). While p75NTR levels were elevated in nerve lysates from P0SchΔ39-121 mice compared to wild-type mice prior to injury, there was a significant increase in p75NTR levels in nerves from P0SchΔ39-121 following axotomy indicating that other factors, in addition to merlin status, contribute to the increase in p75NTR expression following nerve injury.
Following nerve injury, merlin becomes phosphorylated which is temporally correlated with an increase in p75NTR expression. To determine whether merlin phosphorylation promotes p75NTR expression we used a merlin replacement strategy in primary VS cells that allowed us to define the status of the S518 residue. The S518A mutation is unable to be phosphorylated and results in suppressed p75NTR expression. By contrast, the S518D mutation functions as a phospho-mimetic and failed to suppress p75NTR expression. Taken together, these data suggest that merlin inactivation by phosphorylation facilitates increased p75NTR expression in SCs following nerve injury.
Merlin is necessary for p75NTR-induced SC apoptosis
Activation of p75NTR leads to apoptosis of SCs in vivo following nerve injury and in vitro (Ferri and Bisby, 1999; Provenzano et al., 2011). However, neoplastic VS cells, which lack functional merlin, express high levels of p75NTR and resist apoptosis in response to the p75NTR ligands, proNGF and proBNDF (Ahmad et al., 2014). Further, the data presented here demonstrate that SCs from P0SchΔ39-121 mice are less sensitive to apoptosis after nerve injury, despite elevated levels of p75NTR. These observations raise the possibility that merlin facilitates p75NTR apoptotic signaling in SCs. To test this possibility we suppressed merlin expression in cultured SCs using an inducible knock-out gene system. Indeed, SCs with suppressed merlin expression were less sensitive to apoptosis in response to proNGF, confirming that merlin facilitates pro-apoptotic p75NTR signaling in SCs. The observation that SCs lacking functional merlin are less sensitive to p75NTR-apoptosis may explain, at least in part, the ability of neoplastic schwannoma cells to grow and survive long-term in the absence of axonal contact. This is in contrast to the eventual death of non-neoplastic SCs following denervation. Thus, p75NTR represents a potential therapeutic target that would specifically target neoplastic schwannoma cells, but not their non-neoplastic counterparts.
Although p75NTR signaling is often associated with apoptosis, activation of p75NTR has also been shown to promote survival of several other cell types, particularly neurons that co-express Trk receptors and some breast, melanoma, and glioma cells (Chao and Hempstead, 1995). In the case of breast cancer cells, the pro-survival response is linked to the carboxyl-terminal fragment of p75NTR (Verbeke et al., 2013). Other malignant cell lines (e.g. colorectal) retain sensitivity to p75NTR-mediated apoptosis (Yang et al., 2014). What determines whether p75NTR activation leads to cell death or survival remains unknown; however, p75NTR activation of the nuclear transcription factor κB (NF-κB) has been implicated in the pro-survival response (Gentry et al., 2000; Ahmad et al., 2014), whereas activation of JNK is required for the pro-death signal (Yoon et al., 1998; Friedman, 2000; Harrington et al., 2002). The data here suggest that p75NTR-mediated apoptosis in SCs depends, at least in part, on functional merlin. Whether sensitivity to p75NTR-mediated apoptosis is likewise merlin-dependent in these other cell types remains to be determined.
Beyond failing to induce apoptosis, p75NTR ligands appear to provide prosurvival signaling in neoplastic schwannoma cells (Ahmad et al., 2014). This prosurvival effect is not due to co-expression of Trk receptors and involves activation of c-Jun N-terminal kinase (JNK) and the transcription factor, NF-kB (Ahmad et al., 2014). Thus, proNGF and proBDNF reduce apoptosis in VS cells treated with JNK inhibitors (Ahmad et al., 2014). Those observations coupled with the data from this study raise the possibility that activation of p75NTR represents a mechanism whereby VSs are resistant to chemotherapeutics that target kinase signaling (Karajannis et al., 2012; Ahmad et al., 2014; Karajannis et al., 2014).
Loss of functional merlin reduces peripheral nerve SC proliferation in vivo
Several studies confirm that merlin suppresses cell proliferation in vitro by inhibiting signaling pathways at the cell membrane and in the nucleus (Cooper and Giancotti, 2014). As noted above, merlin regulates the expressio n, subcellular localization, and activity of RTKs, including ErbB2 and PDGFR (Fraenzer et al., 2003; Brown and Hansen, 2008; Lallemand et al., 2009b; Schulz et al., 2014). It likewise suppresses the activity of several downstream pro-growth signaling cascades including Ras, Rac1/Cdc42, Raf, PAK1/2, extracellular regulated kinase/mitogen activated protein kinase (ERK/MAPK), phosphatidyl-inositol 3-kinase (PI3-K)/Akt/mTORC, and JNK (Guo et al., 2012; Zhou and Hanemann, 2012). Merlin also has an impact on intranuclear signaling by entering the nucleus and inhibiting the E3 ubiquitin ligase CRL4DCAF1 to suppress proliferation (Li et al., 2014).
Experiments using SC cultures from mice with an Nf2 deletion confirm that merlin functions to suppress SC proliferation in vitro, particularly when the cells are in contact inhibition (Lallemand et al., 2009a). Interestingly, EdU-uptake was not significantly greater in normal, uninjured SCs from P0SchΔ39-121 mice compared with wild-type mice suggesting that loss of merlin function alone is not sufficient to drive peripheral nerve SCs into a significant hyperplastic response. Indeed, immmunolabeling and ultrastructural studies identified myelination abnormalities in sciatic nerves from P0SchΔ39-121 mice but did not reveal significant SC hyperplasia within the peripheral nerve (Denisenko et al., 2008). Rather, SC hyperplasia in P0SchΔ39-121 mice is found in foci near or within sensory ganglia and skeletal muscle (Giovannini et al., 2014), consistent with the observation that most schwannomas arise within sensory ganglia (Tryggvason et al., 2012), and suggesting that intraganglionic SCs are particularly sensitive to merlin status. Further, SC proliferation in peripheral nerves following axotomy was reduced in P0SchΔ39-121 mice compared with wild-type mice. These data suggest that merlin paradoxically facilitates peripheral nerve SC proliferation in vivo following nerve injury, similar to the pro-growth function of other members of the ERM family of proteins (Bretscher et al., 2002). As demonstrated here, merlin is phosphorylated following nerve injury suggesting that phosphorylated merlin not only allows for, but also facilitates, SC proliferation. These data are consistent with the observation that primary neoplastic schwannoma cells, which lack functional merlin, proliferate very slowly in vitro particularly in the absence of exogenous mitogens and are also consistent with the slow growth rate of most schwannomas in vivo (Stangerup et al., 2006; Hansen et al., 2008).
In summary, the results of these studies demonstrate that the tumor suppressor, merlin, decreases p75NTR expression and apoptotic signaling in peripheral nerve SCs. The ability to decrease p75NTR expression depends on the phosphorylation state of merlin and points to a model whereby loss of axonal contact following nerve injury results in merlin phosphorylation leading to increased p75NTR expression. Interestingly, loss of functional merlin also decreases peripheral nerve SC proliferation. Taken together, these data suggest that loss of merlin function reduces SCs sensitivity to apoptosis allowing for long-term survival in the absence of axonal contact consistent with the biology of neoplastic schwannoma cells which are very slow-growing and are able maintain long-term survival in the absence of axons.
Highlights.
Acute or gradual nerve injury increases merlin phosphorylation in SCs.
Loss of functional merlin increases p75NTR expression in SCs.
The absence of functional merlin blunts SC proliferation following axotomy.
SCs lacking functional merlin are less sensitive to p75NTR-mediated apoptosis.
Acknowledgments
Support: NIDCD R01DC009801, P30DC010362, T32DC000040, CDMRP NF130072 and grants from the PSF/AAO-HNSF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad I, Yue WY, Fernando A, Clark JJ, Woodson EA, Hansen MR. p75NTR is highly expressed in vestibular schwannomas and promotes cell survival by activating nuclear transcription factor kappaB. Glia. 2014;62:1699–1712. doi: 10.1002/glia.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. The Journal of comparative neurology. 2007;503:832–852. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O. Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem. 2004;279:18559–18566. doi: 10.1074/jbc.M313916200. [DOI] [PubMed] [Google Scholar]
- Bandtlow C, Dechant G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Science's STKE : signal transduction knowledge environment. 2004;2004:pe24. doi: 10.1126/stke.2352004pe24. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nature reviews Molecular cell biology. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Brown KD, Hansen MR. Lipid raft localization of erbB2 in vestibular schwannoma and Schwann cells. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29:79–85. doi: 10.1097/mao.0b013e31815dbb11. [DOI] [PubMed] [Google Scholar]
- Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends in neurosciences. 1995;18:321–326. [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Cooper J, Giancotti FG. Molecular insights into NF2/Merlin tumor suppressor function. FEBS letters. 2014;588:2743–2752. doi: 10.1016/j.febslet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko N, Cifuentes-Diaz C, Irinopoulou T, Carnaud M, Benoit E, Niwa-Kawakita M, Chareyre F, Giovannini M, Girault JA, Goutebroze L. Tumor suppressor schwannomin/merlin is critical for the organization of Schwann cell contacts in peripheral nerves. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10472–10481. doi: 10.1523/JNEUROSCI.2537-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri CC, Bisby MA. Improved survival of injured sciatic nerve Schwann cells in mice lacking the p75 receptor. Neuroscience letters. 1999;272:191–194. doi: 10.1016/s0304-3940(99)00618-7. [DOI] [PubMed] [Google Scholar]
- Fraenzer JT, Pan H, Minimo L, Jr, Smith GM, Knauer D, Hung G. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–1500. [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry JJ, Casaccia-Bonnefil P, Carter BD. Nerve growth factor activation of nuclear factor kappaB through its p75 receptor is an anti-apoptotic signal in RN22 schwannoma cells. J Biol Chem. 2000;275:7558–7565. doi: 10.1074/jbc.275.11.7558. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, Niwa-Kawakita M, van der Valk M, Woodruff JM, Goutebroze L, Merel P, Berns A, Thomas G. Schwann cell hyperplasia and tumors in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes Dev. 1999;13:978–986. doi: 10.1101/gad.13.8.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- Giovannini M, Bonne NX, Vitte J, Chareyre F, Tanaka K, Adams R, Fisher LM, Valeyrie-Allanore L, Wolkenstein P, Goutagny S, Kalamarides M. mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma. Neuro-oncology. 2014;16:493–504. doi: 10.1093/neuonc/not242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Moon C, Niehaus K, Zheng Y, Ratner N. Rac1 controls Schwann cell myelination through cAMP and NF2/merlin. J Neurosci. 2012;32:17251–17261. doi: 10.1523/JNEUROSCI.2461-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Haipek CA, Hoang Lu K. Neurofibromatosis 2 tumor suppressor protein, merlin, forms two functionally important intramolecular associations. J Neurosci Res. 1999;58:706–716. [PubMed] [Google Scholar]
- Haase G, Pettmann B, Raoul C, Henderson CE. Signaling by death receptors in the nervous system. Current opinion in neurobiology. 2008;18:284–291. doi: 10.1016/j.conb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MR, Roehm PC, Chatterjee P, Green SH. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53:593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Clark JJ, Gantz BJ, Goswami PC. Effects of ErbB2 signaling on the response of vestibular schwannoma cells to gamma-irradiation. Laryngoscope. 2008;118:1023–1030. doi: 10.1097/MLG.0b013e318163f920. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. J Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WY. Reanimation of the paralyzed face. Otolaryngologic clinics of North America. 1992;25:649–667. [PubMed] [Google Scholar]
- Irving RM, Moffat DA, Hardy DG, Barton DE, Xuereb JH, Maher ER. Somatic NF2 gene mutations in familial and non-familial vestibular schwannoma. Human molecular genetics. 1994;3:347–350. doi: 10.1093/hmg/3.2.347. [DOI] [PubMed] [Google Scholar]
- Jung KM, Tan S, Landman N, Petrova K, Murray S, Lewis R, Kim PK, Kim DS, Ryu SH, Chao MV, Kim TW. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. The Journal of biological chemistry. 2003;278:42161–42169. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karajannis MA, Legault G, Hagiwara M, Ballas MS, Brown K, Nusbaum AO, Hochman T, Goldberg JD, Koch KM, Golfinos JG, Roland JT, Allen JC. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-oncology. 2012;14:1163–1170. doi: 10.1093/neuonc/nos146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karajannis MA, Legault G, Hagiwara M, Giancotti FG, Filatov A, Derman A, Hochman T, Goldberg JD, Vega E, Wisoff JH, Golfinos JG, Merkelson A, Roland JT, Allen JC. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-oncology. 2014;16:292–297. doi: 10.1093/neuonc/not150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–10399. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Saint-Amaux AL, Giovannini M. Tumor-suppression functions of merlin are independent of its role as an organizer of the actin cytoskeleton in Schwann cells. Journal of cell science. 2009a;122:4141–4149. doi: 10.1242/jcs.045914. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, Lampin A, Niwa-Kawakita M, Kalamarides M, Giovannini M. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009b;28:854–865. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, Snuderl M, Ladanyi M, Hanemann CO, Zhou P, Karajannis MA, Giancotti FG. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26:48–60. doi: 10.1016/j.ccr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, Erdjument-Bromage H, Zhou P, Tempst P, Giancotti FG. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetto E, Panteri R, Marino R, Keller F, Buffelli M. Impaired nerve regeneration in reeler mice after peripheral nerve injury. The European journal of neuroscience. 2008;27:12–19. doi: 10.1111/j.1460-9568.2007.05978.x. [DOI] [PubMed] [Google Scholar]
- Okada T, You L, Giancotti FG. Shedding light on Merlin's wizardry. Trends in cell biology. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Provenzano MJ, Xu N, Ver Meer MR, Clark JJ, Hansen MR. p75NTR and sortilin increase after facial nerve injury. Laryngoscope. 2008;118:87–93. doi: 10.1097/MLG.0b013e31814b8d9f. [DOI] [PubMed] [Google Scholar]
- Provenzano MJ, Minner SA, Zander K, Clark JJ, Kane CJ, Green SH, Hansen MR. p75(NTR) expression and nuclear localization of p75(NTR) intracellular domain in spiral ganglion Schwann cells following deafness correlate with cell proliferation. Molecular and cellular neurosciences. 2011;47:306–315. doi: 10.1016/j.mcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–8454. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Schulz A, Kyselyova A, Baader SL, Jung MJ, Zoch A, Mautner VF, Hagel C, Morrison H. Neuronal merlin influences ERBB2 receptor expression on Schwann cells through neuregulin 1 type III signalling. Brain : a journal of neurology. 2014;137:420–432. doi: 10.1093/brain/awt327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher I, Hanemann CO, Karplus PA, Bretscher A. The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Developmental cell. 2012;22:703–705. doi: 10.1016/j.devcel.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2006;27:547–552. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain pathology. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, Brat D, Gutmann DH, Ye K. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nature cell biology. 2007;9:1199–1207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Johnson EM., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Schweitzer JB, Johnson EM., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Lopera J, Bott M, Baldwin ME, Kalidas P, Fernandez-Valle C. Phosphorylation of the NF2 tumor suppressor in Schwann cells is mediated by Cdc42-Pak and requires paxillin binding. Molecular and cellular neurosciences. 2007;34:231–242. doi: 10.1016/j.mcn.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Tryggvason G, Barnett A, Kim J, Soken H, Maley J, Hansen MR. Radiographic association of schwannomas with sensory ganglia. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:1276–1282. doi: 10.1097/MAO.0b013e318263d315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke S, Tomellini E, Dhamani F, Meignan S, Adriaenssens E, Xuefen le B. Extracellular cleavage of the p75 neurotrophin receptor is implicated in its pro-survival effect in breast cancer cells. FEBS letters. 2013;587:2591–2596. doi: 10.1016/j.febslet.2013.06.039. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen H, Huo L, Yang Z, Bai Y, Fan X, Ni B, Fang L, Hu J, Peng J, Wang L, Wang J. Epigenetic Inactivation and Tumor Suppressor Behavior of NGFR in Human Colorectal Cancer. Molecular cancer research : MCR. 2014 doi: 10.1158/1541-7786.MCR-13-0247. [DOI] [PubMed] [Google Scholar]
- Ye K. Phosphorylation of merlin regulates its stability and tumor suppressive activity. Cell adhesion & migration. 2007;1:196–198. doi: 10.4161/cam.1.4.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue WY, Clark JJ, Fernando A, Domann F, Hansen MR. Contribution of persistent C-Jun N-terminal kinase activity to the survival of human vestibular schwannoma cells by suppression of accumulation of mitochondrial superoxides. Neuro-oncology. 2011;13:961–973. doi: 10.1093/neuonc/nor068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Hanemann CO. Merlin, a multi-suppressor from cell membrane to the nucleus. FEBS Lett. 2012;586:1403–1408. doi: 10.1016/j.febslet.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 2011;13:1101–1112. doi: 10.1593/neo.111060. [DOI] [PMC free article] [PubMed] [Google Scholar]