Abstract
This study was done to evaluate respiratory syncytial virus (RSV) related readmission (RRR) and risk factors of RRR in preterm infants < 34 weeks gestational age (GA) within 1 yr following discharge from the neonatal intensive care unit (NICU). Infants (n = 1,140) who were born and admitted to the NICUs of 46 hospitals in Korea from April to September 2012, and followed up for > 1 yr after discharge from the NICU, were enrolled. The average GA and birth weight of the infants was 30+5 ± 2+5 weeks and 1,502 ± 474 g, respectively. The RRR rate of enrolled infants was 8.4% (96/1,140), and RSV accounted for 58.2% of respiratory readmissions of infants who had laboratory tests confirming etiological viruses. Living with elder siblings (odd ratio [OR], 2.68; 95% confidence interval [CI], 1.68-4.28; P < 0.001), and bronchopulmonary dysplasia (BPD) (OR, 2.95; 95% CI, 1.44-6.04; P = 0.003, BPD vs. none) increased the risk of RRR. Palivizumab prophylaxis (OR, 0.06; 95% CI, 0.03-0.13; P < 0.001) decreased the risk of RRR. The risk of RRR of infants of 32-33 weeks' gestation was lower than that of infants < 26 weeks' gestation (OR, 0.11; 95% CI, 0.02-0.53; P = 0.006). This was a nationwide study that evaluated the rate and associated risk factors of RRR in Korean preterm infants. Preterm infants with BPD or living with siblings should be supervised, and administration of palivizumab to prevent RRR should be considered.
Keywords: Infants, Premature, Patient Readmission, Respiratory Syncytial Virus, Bronchopulmonary Dysplasia, Palivizumab
INTRODUCTION
Respiratory syncytial virus (RSV) is the most common virus of causing lower respiratory tract infections in infants (1,2) and accounts for 74.8% of admissions for bronchiolitis (3). Although infections caused by RSV usually have a mild and self-limiting course, it can be severe enough to require hospital admission of high risk infants. Bronchopulmonary dysplasia (BPD), congenital heart disease, and premature birth are known risk factors for complications from an RSV infection, such as hospital admission, need for intensive care, and a poor prognosis (4,5).
The monoclonal antibody, palivizumab (Synagis®; MedImmune LLC, Gaithersburg, MD, USA) is currently being used for prophylaxis of severe RSV infection in preterm infants (6,7). Palivizumab is also indicated in Korean infants who are diagnosed with BPD within 6 months and are < 2 yr of age, or are born at < 32 weeks' gestation and < 6 months of age at the beginning of the RSV season.
Several reports have been published on readmission of preterm infants with RSV in Korea (8,9,10). However, they were not multicenter-based studies or did not evaluate the effect of palivizumab prophylaxis on the readmission with RSV in Korea.
Hence, the aim of this study was to evaluate the rate of RSV related readmissions (RRR) and risk factors in preterm infants less than 34 weeks gestational age (GA) within 1 yr following discharge from the neonatal intensive care unit (NICU) in Korea.
MATERIALS AND METHODS
This study was performed as part of a project "Retrospective Study to Evaluate Rehospitalization & Health Care Utilization after NICU Discharge in Preterm Infants (less than 34 weeks' gestation) II" (RHANPI II) conducted by the Committee on Data Collection and Statistical Analysis of the Korean Society of Neonatology.
Patient population
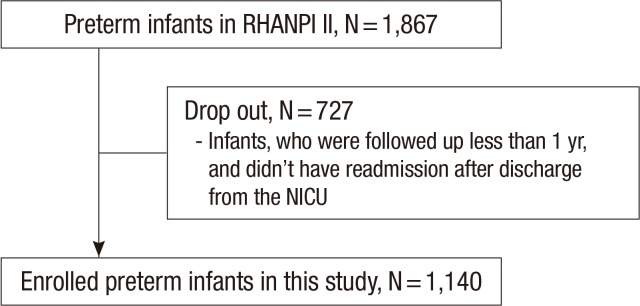
The cohort of RHANPI II included all preterm infants less than 34 weeks' gestation who were born and survived to discharge at the NICUs of 46 hospitals in Korea from April 2012 to September 2012 (n=1,867). The overall readmission rate of this cohort was 28.4% after discharge from the NICU. Out of the cohort, the infants who were followed up for >1 yr after discharge from the NICU, or who were readmitted after discharge from the NICU were enrolled in this study (n=1,140) (Fig. 1).
Fig. 1. Study population. Out of the cohort that included all preterm infants less than 34 weeks' gestation who were born and survived at the NICUs of 46 hospitals in Korea from April 2012 to September 2012 (n = 1,867), the infants who were followed up for > 1 yr after discharge from the NICU, or who had readmission after discharge from the NICU were enrolled in this study (n = 1,140).
Data collection
The Committee on Data Collection and Statistical Analysis of the Korean Society of Neonatology chose the 46 Korean NICUs. The data were collected by the neonatologists of the 46 NICUs retrospectively through chart review based on standard study formats accompanied with a manual defining the variables. All data were entered electronically into a central database during the study period from January to March 2014. The data were checked repeatedly for quality and completeness. Missing information or errors were returned to the neonatologists to verify the data. We modified the database of RHANPI I that was created in collaboration with the Clinical Research Center of Samsung Medical Center, which used the Oracle Korea electronic case reporting system (Oracle corp., Seoul, Korea). The data included perinatal and neonatal characteristics such as major morbidities in the NICU, and data obtained at readmission including the cause for the readmission, need for oxygen supplementation, need for ventilator support, and need for intensive care. Readmission included the events of readmission in each hospital or another hospital stated in the medical records.
Definitions of variables
BPD was defined as the need for supplemental oxygen for at least 28 days after birth and severity was graded according to the respiratory support required at 36 postmenstrual weeks or discharge, whichever came first (11). Necrotizing enterocolitis was defined as Bell's stage II or greater (12). Stage III or IV intraventricular hemorrhage (13) and cystic periventricular leukomalacia on a cranial ultrasonogram based on the Papile grading system. Sepsis was defined according to the Centers for Disease Control and Prevention/National Nosocomial Infection Surveillance definitions for infants ≤12 months (14). Palivizumab prophylaxis was defined as to whether the protective effects of injecting palivizumab were noted at the time of RRR.
Statistical analysis
Statistical analysis for readmission rate including RRR, Comparison of RRR based on GA, and risk factors for RRR were performed targeting all infants (n=1,140) and infants who were diagnosed with BPD which is a well-known high risk morbidity (n=326). Categorical data are presented as numbers (%), and continuous data are presented as the mean±standard deviation. The cumulative probability of first RRR was calculated with the Kaplan-Meier product limit method and the trend for rate of RRR according to categorized GA was estimated with the log-rank test. The multivariable logistic regression was used for assessing association between risk factor and the RRR following discharge from the NICU. The risk factors were selected for adjusting potential confounding effects of clinically plausible and statistically reasonable covariates (GA, birth weight, elder siblings, BPD, palivizumab prophylaxis). To compare RRR and analyze risk factors of RRR based on GA, subgroups were categorized as; 25 weeks' gestation or less, 26-27 weeks' gestation, 28-29 weeks' gestation, 30-31 weeks' gestation, and 32-33 weeks' gestation. All statistical tests were two-sided and P<0.05 was considered significant. Data was analyzed with SAS software ver. 9.4 (SAS Institute, Cary, NC, USA).
Ethics statement
This study protocol was reviewed and approved by the institutional review board (IRB) of Samsung Medical Center (IRB No. 2012-12-032) and the other 45 hospitals. Permission was given to waive parental consent.
RESULTS
Characteristics of study population
Mean weeks of GA for all infants (n=1,140) was 30+5±2+5 weeks, and mean birth weight was 1,502±474 g. Of all infants that include eight infants whose data on BPD were unavailable, 28.6% (326/1,140) were diagnosed with BPD in the NICU. Mean weeks of GA for the infants with BPD (n=326) was 27+6±2+3 weeks and mean birth weight was 1,069±324 g. The perinatal and familial characteristics and major outcomes of enrolled infants in the NICU are shown in Tables 1 and 2. A total of 47.5% of the infants and 89.3% of the infants with BPD were put on a course of palivzumab.
Table 1. Perinatal and family history of enrolled infants.
| Parameters | All infants* (n = 1,140) | Infants with BPD (n = 326) | Infants without BPD (n = 806) |
|---|---|---|---|
| Gestational age (week) | 30+5 ± 25/7 | 27+6 ± 2+3 | 31+5 ± 1+6 |
| Birth weight (g) | 1,502 ± 474 | 1,069 ± 324 | 1,678 ± 407 |
| Male | 54.5 (621/1,140) | 54.6 (178/326) | 54.5 (439/806) |
| Multiple pregnancy | 31.4 (358/1,140) | 32.5 (106/326) | 30.8 (248/806) |
| Stay at NICU (day) | 49.3 ± 34.3 | 84.2 ± 36.1 | 35.5 ± 20.9 |
| Antenatal corticosteroid | 67.8 (736/1,086) | 75.6 (232/307) | 65.0 (502/772) |
| Caesarian section | 70.0 (798/1,139) | 72.7 (237/326) | 69.1 (556/805) |
| SGA | 10.1 (115/1,137) | 10.8 (35/325) | 9.8 (79/804) |
| Maternal age (yr) | 32.6 ± 4.0 | 33.2 ± 3.7 | 32.4 ± 4.1 |
| Elder siblings | 42.4 (466/1,099) | 42.3 (133/314) | 42.6 (331/777) |
Values are mean±standard deviation or % (No./total No.). *Includes eight infants whose data on BPD were unavailable. BPD, bronchopulmonary dysplasia; SGA, small for gestational age; C/S, caesarian section; NICU, neonatal intensive care unit.
Table 2. Major outcomes in the NICU of the enrolled infants.
| Outcomes | All infants* (n = 1,140) | Infants with BPD (n = 326) | Infants without BPD (n = 806) |
|---|---|---|---|
| Use of surfactant | 56.9 (647/1,138) | 92.6 (302/326) | 42.4 (342/806) |
| PDA | 34.4 (381/1,109) | 63.4 (206/325) | 21.9 (171/780) |
| NEC ( ≥ stage 2) | 2.4 (27/1,135) | 5.5 (18/325) | 1.1 (9/806) |
| IVH ( ≥ grade 3) | 2.7 (31/1,135) | 7.1 (23/326) | 1.0 (8/805) |
| Cystic PVL | 4.9 (55/1,126) | 10.5 (34/325) | 2.6 (21/797) |
| ROP ( ≥ stage 2) | 12.9 (143/1,108) | 33.4 (109/326) | 4.1 (32/779) |
| BPD | 28.8 (326/1,132) | 100.0 (326/326) | 0.0 (0/806) |
| Mild | 49.4 (161/326) | ||
| Moderate | 31.6 (103/326) | ||
| Severe | 19.0 (62/326) | ||
| Sepsis | 14.5 (165/1,135) | 32.3 (105/325) | 7.3 (59/804) |
Values are mean±standard deviation or % (No./total No.). *Includes eight infants whose data on BPD were unavailable. PDA, patent ductus arteriosus; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia, ROP, retinopathy of prematurity; BPD, bronchopulmonary dysplasia.
Overall readmission, respiratory readmission, and RSV related readmission
Out of all infants (n=1,140), 531 (46.6%) experienced overall readmission. Three hundred thirty (28.9%) experienced respiratory readmission that accounted for 58.8% of the infants who experienced overall readmission. Ninety-six infants (8.4%) experienced RRR that accounted for 29.1% of the infants who experienced respiratory readmission.
Out of the infants with BPD (n=326), 195 (59.8%) experienced overall readmission. 130 (39.9%) experienced respiratory readmission that accounted for 66.7% of the infants who experienced overall readmission. Thirty-two infants (9.8%) experienced RRR that accounted for 24.6% of the infants who experienced respiratory readmission. A summary of the readmission rates following discharge from the NICU is shown in Table 3.
Table 3. Readmission rate of infants after discharge from the neonatal intensive care unit.
| Readmission | All infants* (n=1,140) | Infants with BPD (n=326) | Infants without BPD (n=806) |
|---|---|---|---|
| Overall readmission | 46.6% (531/1,140) |
59.8% (195/326) |
41.7% (336/806) |
| Respiratory readmission | 28.9% (330/1,140) |
39.9% (130/326) |
24.8% (200/806) |
| RSV related readmission | 8.4% (96/1,140) |
9.8% (32/326) |
7.9% (64/806) |
*Includes eight infants whose data on BPD were unavailable. RSV, respiratory syncytial virus.
Out of the infants who experienced respiratory readmission, 50% had laboratory tests for confirming etiological viruses. RSV accounted for 58.2% of respiratory readmissions of infants who had laboratory tests for confirming etiological viruses.
The mean frequency of RRR in all infants was 1.0±0.1. Intensive care was required for 15.5% of the infants with RRR, and ventilator support was required for 6.2% of the infants with RRR.
Comparison of RSV related readmission based on GA
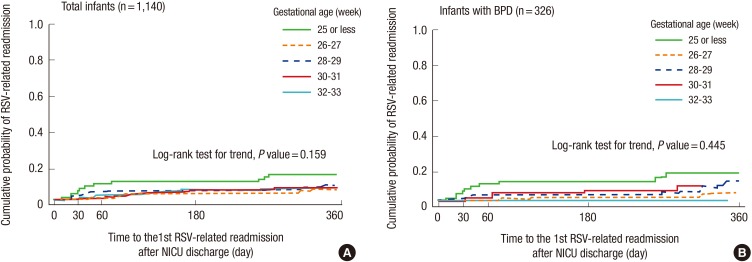
No significant difference in RRR was observed when the GA was divided into five groups in all infants (P=0.159, Log-rank test for trend) (Fig. 2A) and in the infants with BPD (P=0.445, Log-rank test for trend) (Fig. 2B). The rates of RRR and 95% confidence intervals at the time-points after discharge from the NICU are shown in Table 4.
Fig. 2. Days to first respiratory syncytial virus related readmission (RRR) following discharge from the neonatal intensive care unit (Kaplan-Meier curve). No significant difference in RRR was observed when the GA was divided into five groups in all infants (n = 1,140, P = 0.159, Log-rank test for trend) (A) and in the infants with BPD (n = 326, P = 0.445, Log-rank test for trend) (B).
Table 4. The rate of respiratory syncytial virus related readmission at the time-points after discharge from the neonatal intensive care unit.
| Infants by GA | No. of infants | No. of 1st RSV-related readmission (%) | No. of censored† (%) | RSV-related readmission rate* | ||
|---|---|---|---|---|---|---|
| at 60 days (95% CI) |
at 180 days (95% CI) |
at 360 days (95% CI) |
||||
| All infants (n = 1,140) | ||||||
| GA (week) | ||||||
| 25 or less | 77 | 11 (14.3) | 66 (85.7) | 9.2 (4.5 to 18.4) | 10.5 (5.4 to 20.0) | 15.0 (8.6 to 25.4) |
| 26-27 | 124 | 7 (5.6) | 117 (94.4) | 0.8 (0.1 to 5.6) | 4.0 (1.7 to 9.4) | 5.8 (2.8 to 11.9) |
| 28-29 | 203 | 17 (8.4) | 186 (91.6) | 4.5 (2.4 to 8.5) | 5.0 (2.7 to 9.1) | 8.3 (5.2 to 13.2) |
| 30-31 | 289 | 28 (9.7) | 261 (90.3) | 1.4 (0.5 to 3.7) | 5.7 (3.5 to 9.1) | 6.8 (4.4 to 10.4) |
| 32-33 | 447 | 33 (7.4) | 414 (92.6) | 2.7 (1.6 to 4.7) | 6.2 (4.3 to 8.9) | 7.1 (5.1 to 10.0) |
| Total | 1,140 | 96 (8.4) | 1,044 (91.6) | 2.9 (2.1 to 4.1) | 5.9 (4.7 to 7.4) | 7.6 (6.2 to 9.4) |
| Infants with BPD (n = 326) | ||||||
| GA (week) | ||||||
| 25 or less | 72 | 11 (15.3) | 61 (84.7) | 9.7 (4.8 to 19.3) | 11.1 (5.7 to 21.0) | 15.8 (9.1 to 26.8) |
| 26-27 | 94 | 4 (4.3) | 90 (95.7) | 0.0 (0.0 to 0.0) | 2.1 (0.5 to 8.2) | 4.5 (1.7 to 11.6) |
| 28-29 | 96 | 10 (10.4) | 86 (89.6) | 3.2 (1.0 to 9.5) | 3.2 (1.0 to 9.5) | 10.0 (5.3 to 18.4) |
| 30-31 | 49 | 7 (14.3) | 42 (85.7) | 2.1 (0.3 to 13.9) | 6.3 (2.1 to 18.3) | 8.6 (3.3 to 21.3) |
| 32-33 | 15 | 0 (0.0) | 15 (100.0) | - | - | - |
| 326 | 32 (9.8) | 294 (90.2) | 3.4 (1.9 to 6.1) | 5.0 (3.1 to 8.0) | 9.2 (6.4 to 13.1) | |
*Cumulative probability using the Kaplan-Meier product limit method; †No. of censored, the number of infants who were not readmitted after discharge from the neonatal intensive care unit. GA, gestational age; CI, confidence interval; RSV, respiratory syncytial virus.
Risk factor of RSV related readmission
Compared with the infants 25 or less weeks' gestation, the infants 32-33 weeks' gestation had a decreased risk of RRR (odd ratio, [OR], 0.11; 95% confidence interval [CI], 0.02-0.53; P=0.006) but the infants 26-31 weeks' gestation did not differ in the risk of RRR. Compared with the infants who were not diagnosed with BPD, the infants with BPD had an increased risk of RRR (OR, 2.95; 95% CI, 1.44-6.04; P=0.003). Living with elder siblings increased the risk of RRR (OR, 2.68; 95% CI, 1.68-4.28; P<0.001); however palvizumab prophylaxis decreased the risk of RRR (OR, 0.06; 95% CI, 0.03-0.13; P<0.001) (Table 5).
Table 5. Analysis of the risk factors of respiratory syncytial virus related readmission after discharge from the NICU.
| Parameters | RSV-related readmission | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | Adjusted OR | 95% CI | P value | ||
| All infants (n = 1,140) | |||||||
| GA (week) | |||||||
| 25 or less | 11/77 (14.3%) | Ref. | Ref. | ||||
| 26-27 | 7/124 (5.6%) | 0.36 | (0.13-0.97) | 0.044 | 0.35 | (0.09-1.34) | 0.125 |
| 28-29 | 17/203 (8.4%) | 0.55 | (0.24-1.23) | 0.145 | 0.30 | (0.07-1.23) | 0.094 |
| 30-31 | 28/289 (9.6%) | 0.64 | (0.30-1.36) | 0.248 | 0.26 | (0.06-1.25) | 0.094 |
| 32-33 | 33/447 (7.4%) | 0.48 | (0.23-0.99) | 0.048 | 0.11 | (0.02-0.53) | 0.006 |
| Birth weight (g) | |||||||
| < 750 | 5/56 (8.9%) | 1.01 | (0.39-2.66) | 0.978 | 0.44 | (0.08-2.36) | 0.338 |
| 750-999 | 12/133 (9.0%) | 0.60 | (0.30-1.20) | 0.150 | 0.67 | (0.26-1.72) | 0.403 |
| 1,000-1,249 | 10/183 (5.5%) | 1.08 | (0.62-1.88) | 0.787 | 1.03 | (0.53-2.00) | 0.939 |
| 1,250-1,499 | 19/201 (9.5%) | 1.03 | (0.53-1.98) | 0.941 | 0.71 | (0.21-2.46) | 0.589 |
| ≥ 1,500 | 50/567(8.8%) | Ref. | Ref. | ||||
| Elder siblings | |||||||
| 0 (none) | 33/633 (5.2%) | Ref. | Ref. | ||||
| ≥1 | 63/466 (13.5%) | 2.84 | (1.83-4.41) | < 0.001 | 2.68 | (1.68-4.28) | < 0.001 |
| BPD | |||||||
| No | 64/806 (7.9%) | Ref. | Ref. | ||||
| Yes | 32/326 (9.8%) | 1.26 | (0.81-1.97) | 0.306 | 2.95 | (1.44-6.04) | 0.003 |
| Palivizumab prophylaxis | |||||||
| Yes | 15/507 (3.0%) | 0.21 | 0.06 | ||||
| No | 81/633 (12.8%) | Ref. | (0.12-0.37) | < 0.001 | Ref. | (0.03-0.13) | < 0.001 |
| Infants with BPD (n = 326) | |||||||
| GA (week) | |||||||
| 25 or less | 11/72 (15.3%) | Ref. | Ref. | ||||
| 26-27 | 4/94 (4.2%) | 0.25 | (0.08-0.81) | 0.021 | 0.36 | (0.07-1.95) | 0.237 |
| 28-29 | 10/96 (10.4%) | 0.64 | (0.26-1.61) | 0.348 | 0.51 | (0.10-2.69) | 0.425 |
| 30-31 | 7/49 (14.3%) | 0.92 | (0.33-2.58) | 0.880 | 0.47 | (0.06-3.96) | 0.487 |
| 32-33 | 0/15 (0%) | - | - | - | - | - | - |
| Birth weight (g) | |||||||
| < 750 | 5/51 (9.8%) | 0.42 | (0.12-1.45) | 0.170 | 0.24 | (0.03-2.35) | 0.223 |
| 750-999 | 11/101 (10.9%) | 0.18 | (0.05-0.67) | 0.010 | 0.18 | (0.03-1.23) | 0.080 |
| 1,000-1,249 | 4/89 (4.5%) | 0.42 | (0.12-1.45) | 0.170 | 0.20 | (0.03-1.18) | 0.075 |
| 1,250-1,499 | 5/51 (9.8%) | 0.47 | (0.17-1.33) | 0.157 | 0.55 | (0.08-3.91) | 0.550 |
| ≥ 1,500 | 7/34 (20.6%) | Ref. | Ref. | ||||
| Elder siblings | |||||||
| 0 (none) | 10/181 (5.5%) | Ref. | Ref. | ||||
| ≥1 | 22/133 (16.5%) | 3.39 | (1.55-7.43) | 0.002 | 5.82 | (1.95-17.32) | 0.002 |
| Severity of BPD | |||||||
| Mild | 15/161 (9.9%) | Ref. | Ref. | ||||
| Moderate | 6/103 (5.8%) | 0.60 | (0.23-1.61) | 0.311 | 0.72 | (0.21-2.41) | 0.591 |
| Severe | 11/62 (17.7%) | 2.10 | (0.91-4.87) | 0.084 | 1.19 | (0.34-4.17) | 0.786 |
| Palivizumab prophylaxis | |||||||
| Yes | 9/270 (3.3%) | 0.05 | (0.02-0.12) | < 0.001 | 0.03 | (0.01-0.08) | < 0.001 |
| No | 23/56 (41.1%) | Ref. | Ref. | ||||
A logistic regression model was used for the multivariate analysis. OR, odds ratio; CI, confidence interval; RSV, respiratory syncytial virus; GA, gestational age; NICU, neonatal intensive care unit; BPD, bronchopulmonary dysplasia.
In an analysis of infants with BPD, living with elder siblings increased the risk of RRR (OR, 5.82; 95% CI, 1.95-17.32; P=0.002); however, palivizumab prophylaxis decreased the risk of RRR (OR, 0.03; 95% CI, 0.01-0.008; P<0.001). GA, birth weight, and severity of BPD did not influence the risk of RRR (Table 5).
DISCUSSION
In this study, 8.4% of preterm infants less than 34 weeks' gestation were readmitted with an RSV infection within 1 yr following discharge from the NICU. Living with elder siblings or BPD increased the risk of RRR but birth at 32-33 weeks' gestation (vs. birth at <26 weeks' gestation) or palivizumab prophylaxis significantly decreased the risk of RRR.
RRR of preterm infants in previous studies varied considerably (15,16,17). Resch et al. (18) analyzed 18 previous studies and reported the mean RRR rate of 9.3% in preterm infants without BPD. The rate of RRR of preterm infants was 3.1%-9.3% in Korean studies (8,9,10,19,20). The rate of RRR of this study was 8.4% and was similar with other Korean studies. The variations in the readmission rates among these studies may be due to seasonal fluctuations in RSV infection, RSV virulence, compliance to palivizumab prophylaxis or variable indications of laboratory tests for diagnosing RSV infection during admission. In this study, only 50% of infants who were readmitted with respiratory problems had laboratory tests done for diagnosing RSV infection during their readmission. Paes et al. (21) reported that 20.9% of preterm infants who had laboratory tests for RSV during their readmission were diagnosed with RSV infection. In this study, 58.2% of preterm infants who had laboratory tests for RSV infection were confirmed to have an RSV infection. The difference between Paes's and this study may be due to because in this study, laboratory tests were done mainly on infants who presented with a more serious condition.
In this study, 46.6% of enrolled infants experienced overall readmission, 28.9% were readmitted due to respiratory problems, and 8.4% experienced RRR. These rates of readmission may seem to be higher than previous studies (8,9,10,19,22,23,24). However, if the infants (N=727), who dropped out from this study were included in readmission calculation, the rates of overall readmission, respiratory readmission, and RRR would have been 28.4%, 17.7%, and 5.1%, respectively, and these rates of readmission would have been comparable with previous studies.
Prematurity is a known risk factor of RRR (25,26). The risk results from characteristics of their lung, such as a small lung volume, a reduced lung surface area, small airway and increased air space wall thickness (27). In addition, they have insufficient humoral and cellular immunity to clear viral load (28). Carbonell-Estrany et al. (29) reported that the risk of RRR decreased with older GA (OR, 0.85; 95% CI, 0.72-0.99; P=0.047). Joffe et al. (16) reported that infants 23 to 32 weeks' gestation were at greater risk of RRR than those 33-36 weeks' gestation (OR 2.6; 95% CI, 1.4-5; P=0.003). In contrast, GA was not a significant independent risk factor of RRR in the study of Paes et al. (21). In this study, there was no significant difference of RRR among the infants according to categorized GA except the comparison between infants <26 weeks' gestation and infants 32-33 weeks' gestation. The differences among these studies may be due to differences in the enrolled study population or grouping of categorized GA.
Resch et al. (18) reported that the rate of RRR of infants with BPD was 19.8% in the analysis of eight studies of RRR. Since the Impact-RSV (5), many studies have reiterated that BPD is a risk factor of RRR. Carbonell-Estrany et al. (2) reported that BPD was an independent risk factor of RRR in study populations who were born at <33 weeks' gestation (OR, 1.86; 95% CI, 1.22-7.91; P=0.048). This study also showed a similarity in analysis between the RRR and BPD with previous studies.
In a subgroup analysis, 9.8% of infants with BPD were readmitted with RSV and 89.1% of them were administered with palivizumab. Additionally, living with elder siblings increased the risk of RRR; however, palivizumab prophylaxis decreased the risk of RRR, significantly. GA at birth, birth weight, and BPD severity did not influence the risk of RRR in infants with BPD. Chang et al. (9) reported that the rate of RRR was 12.6% in infants with BPD who were not administered with palivizumab and 4.0% in infants with BPD who were administered with palivizumab in a single center study in Korea. Lacaze-Masmonteil et al. (30) reported that the rate of RRR was 8.1% in a French cohort study where 81% of infants were diagnosed with BPD. Palivizumab prophylaxis reduced the rate of RRR in infants with BPD from 12.8% to 7.9% in Impact-RSV Study (5).
Paes's et al. (21) reported that infants who lived with siblings had a higher risk of RRR than infants who did not (hazard ratio 2.1; 95% CI, 1.4-3.3; P<0.001). In this study, living with elder siblings was also an independent risk factor of RRR. This study was conducted in a retrospective design that aimed at preterm infants during one RSV season which may be a limitation to represent the epidemiological characteristics associated with RRR in Korea.
The main outcome of this study is that it is the first multicenter-based, nation-wide study to evaluate the readmission rate and associated risk factors of RSV in preterm infants less than 34 weeks' gestation within 1 yr following discharge from the NICU in Korea. This study suggests that preterm infants with BPD or those living with siblings should be supervised closely and considered for administration of palivizumab to prevent RRR following discharge from the NICU.
ACKNOWLEDGMENTS
We thank Hae Sook Bok and Soo Hee Kim for creating report form, and validating the data. We thank Hye Kyoung Yoon and Seon Woo Kim for statistical analysis and Ye Kyuong Kwon (Oracle Korea) for developing the electronic case reporting system. We express our profound gratitude to the physicians of the institutions that participated in this study. These institutions were: Ajou University Hospital, Asan Medical Center, Bundang Cha Hospital, Busan National University Hospital, Busan National University Children's Hospital, Gachon University Gil Medical Center, Cheil General Hospital & Women's Healthcare Center, Chonbuk National University Hospital, Chonnam National University Hospital, Chosun University Hospital, Chungnam National University Hospital, Daegu Fatima Hospital, Dankook University Hospital, Dong-A University Hospital, Dongguk University Ilsan Hospital, Eulji General Hospital, Eulji University Hospital, Ewha Woman's University Mokdong Hospital, Gangneung Asan Hospital, Good Moonhwa Hospital, Gyeongsang National University Hospital, Hallym University Kangnam Sacred Heart Hospital, Hanyang University Guri Hospital, Inje University Busan-Paik Hospital, Inje University Ilsan-Paik Hospital, Inje University Sanggye-Paik Hospital, Jeju National University Hospital, Kangnam Cha Hospital, Keimyung University Dongsan Medical Center, Konkuk University Medical Center, Konyang Unnivervity Hospital, Korea University Ansan Hospital, Korea University Guro Hospital, Kyunghee University Medical Center at Gangdong, Kyungpook National University Hospital, Samsung Changwon Hospital, Samsung Medical Center, Seoul National University Bundang Hospital, Seoul National University Children's Hospital, Seoul St. Mary's Hospital of the Catholic University, Soon Chun Hyang University Seoul Hospital, St. Mary's Hospital of the Catholic University, Wonkwang University Hospital, Yonsei University Gangnam Severance Hospital, Yonsei University Severance Hospital and Yonsei University Wonju Christian Hospital.
Footnotes
Funding: This study was supported by a grant (PHO1125915) from the Committee on Data Collection and Statistical Analysis of the Korean Society of Neonatology.
DISCLOSURE: All of the authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design of the study: Lee JH, Chang YS, Choi JH. Acquisition of data: Lee JH, Kim CS. Statistical analysis: Lee JH, Kim CS, Chang YS. First draft of the manuscript: Lee JH, Kim CS. Revision and ciritical review of the manuscript: all authors.
References
- 1.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.The PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Pebody B, Edmunds WJ, Zambon MC, Gay NJ, Crowcroft NS. Contribution of RSV to bronchiolitis and pneumonia-associated hospitalizations in English children, April 1995-March 1998. Epidemiol Infect. 2002;129:99–106. doi: 10.1017/s095026880200729x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, Jr, Connor EM, Sondheimer HM Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 5.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande SA, Northern V. The clinical and health economic burden of respiratory syncytial virus disease among children under 2 years of age in a defined geographical area. Arch Dis Child. 2003;88:1065–1069. doi: 10.1136/adc.88.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HW, Lee BS, Kim AR, Yoon HS, Kim BI, Song ES, Kim WT, Lim J, Kim S, Jin HS, et al. Epidemiology of respiratory syncytial virus infection in infants born at less than thirty-five weeks of gestational age. Pediatr Infect Dis J. 2012;31:e99–e104. doi: 10.1097/INF.0b013e318257f619. [DOI] [PubMed] [Google Scholar]
- 9.Chang SG, Park MS, Yu JE. Outcomes of palivizumab prophylaxis for respiratory syncytial virus infection in preterm children with bronchopulmonary dysplasia at a single hospital in Korea from 2005 to 2009. J Korean Med Sci. 2010;25:251–256. doi: 10.3346/jkms.2010.25.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SK, Jung YJ, Yoo HS, Ahn SY, Seo HJ, Choi SH, Kim MJ, Jeon GW, Koo SH, Lee KH, et al. Effect of Synagis(R) (palivizumab) prophylaxis on readmission due to respiratory syncytial virus in very low birth weight infants. Korean J Pediatr. 2010;53:358–363. [Google Scholar]
- 11.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 12.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 14.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 15.Parnes C, Guillermin J, Habersang R, Nicholes P, Chawla V, Kelly T, Fishbein J, McRae P, Goessler M, Gatti A, et al. Palivizumab Outcomes Registry Study Group. Palivizumab Outcomes Registry Study Group. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000-2001: results from The Palivizumab Outcomes Registry. Pediatr Pulmonol. 2003;35:484–489. doi: 10.1002/ppul.10288. [DOI] [PubMed] [Google Scholar]
- 16.Joffe S, Escobar GJ, Black SB, Armstrong MA, Lieu TA. Rehospitalization for respiratory syncytial virus among premature infants. Pediatrics. 1999;104:894–899. doi: 10.1542/peds.104.4.894. [DOI] [PubMed] [Google Scholar]
- 17.Geskey JM, Ceneviva GD, Brummel GL, Graff GR, Javier MC. Administration of the first dose of palivizumab immunoprophylaxis against respiratory syncytial virus in infants before hospital discharge: what is the evidence for its benefit? Clin Ther. 2004;26:2130–2137. doi: 10.1016/j.clinthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Resch B, Resch E, Müller W. Should respiratory care in preterm infants include prophylaxis against respiratory syncytial virus infection? The case in favour. Paediatr Respir Rev. 2013;14:130–136. doi: 10.1016/j.prrv.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Chae SH, Lee JH, Kim YJ, Koo SH, Jeon GW, Chang YS, Park WS. Rehospitalization of Very Low Birth Weight Infants in the First Year after Discharge from NICU and Risk Factor of Rehospitalization caused by Respiratory Illness. J Korean Soc Neonatol. 2006;13:17–23. [Google Scholar]
- 20.Lee EA, Jeong JH, Yu ST, Lee CW, Yoon HS, Park DS, Oh YK. Incidence and Risk Factors of Rehospitalization with Respiratory Syncytial Virus Infection in Premature Infants. Korean J Pediatr. 2004;47:510–514. [Google Scholar]
- 21.Paes B, Mitchell I, Li A, Harimoto T, Lanctôt KL. Respiratory-related hospitalizations following prophylaxis in the Canadian registry for palivizumab (2005-2012) compared to other international registries. Clin Dev Immunol. 2013;2013:917068. doi: 10.1155/2013/917068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underwood MA, Danielsen B, Gilbert WM. Cost, causes and rates of rehospitalization of preterm infants. J Perinatol. 2007;27:614–619. doi: 10.1038/sj.jp.7211801. [DOI] [PubMed] [Google Scholar]
- 23.Ralser E, Mueller W, Haberland C, Fink FM, Gutenberger KH, Strobl R, Kiechl-Kohlendorfer U. Rehospitalization in the first 2 years of life in children born preterm. Acta Paediatr. 2012;101:e1–e5. doi: 10.1111/j.1651-2227.2011.02404.x. [DOI] [PubMed] [Google Scholar]
- 24.Resch B, Pasnocht A, Gusenleitner W, Müller W. Rehospitalisations for respiratory disease and respiratory syncytial virus infection in preterm infants of 29-36 weeks gestational age. J Infect. 2005;50:397–403. doi: 10.1016/j.jinf.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Kristensen K, Dahm T, Frederiksen PS, Ibsen J, Iyore E, Jensen AM, Kjaer BB, Olofsson K, Pedersen P, Poulsen S. Epidemiology of respiratory syncytial virus infection requiring hospitalization in East Denmark. Pediatr Infect Dis J. 1998;17:996–1000. doi: 10.1097/00006454-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Resch B, Gusenleitner W, Mandl C, Müller W. Epidemiology of respiratory syncytial virus infection in Southern Austria. Pediatr Infect Dis J. 2000;19:587–588. doi: 10.1097/00006454-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 27.Wert SE. The lung: normal and abnormal structural development of lung. In: Polin RA, Fox WW, Abman SH, editors. Fetal and neonatal physiology. 3rd ed. Philadelphia, Pa.: W.B. Saunders; 2004. pp. 784–794. [Google Scholar]
- 28.Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res. 1986;20:899–904. doi: 10.1203/00006450-198609000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Carbonell-Estrany X, Quero J, Bustos G, Cotero A, Domenéch E, Figueras-Aloy J, Fraga JM, Garcia LG, Garcia-Alix A, Del Río MG, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr Infect Dis J. 2000;19:592–597. doi: 10.1097/00006454-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Lacaze-Masmonteil T, Roze JC, Fauroux B French Pediatricians' Group of Sunagis Patients' Name-Based Programs. Incidence of respiratory syncytial virus-related hospitalizations in high-risk children: follow-up of a national cohort of infants treated with Palivizumab as RSV prophylaxis. Pediatr Pulmonol. 2002;34:181–188. doi: 10.1002/ppul.10175. [DOI] [PubMed] [Google Scholar]