Abstract
Currently, in the Republic of Korea, despite the very-low-birth rate, the birth rate and number of preterm infants are markedly increasing. Neonatal deaths and major complications mostly occur in premature infants, especially very-low-birth-weight infants (VLBWIs). VLBWIs weigh less than 1,500 g at birth and require intensive treatment in a neonatal intensive care unit (NICU). The operation of the Korean Neonatal Network (KNN) officially started on April 15, 2013, by the Korean Society of Neonatology with support from the Korea Centers for Disease Control and Prevention. The KNN is a national multicenter neonatal network based on a prospective web-based registry for VLBWIs. About 2,000 VLBWIs from 60 participating hospital NICUs are registered annually in the KNN. The KNN has built unique systems such as a web-based real-time data display on the web site and a site-visit monitoring system for data quality surveillance. The KNN should be maintained and developed further in order to generate appropriate, population-based, data-driven, health-care policies; facilitate active multicenter neonatal research, including quality improvement of neonatal care; and ultimately lead to improvement in the prognosis of high-risk newborns and subsequent reduction in health-care costs through the development of evidence-based neonatal medicine in Korea.
Keywords: Infant, Premature; Infant, Very-Low-Birth-Weight; Quality Improvement
Graphical Abstract

INTRODUCTION
Recently, in the Republic of Korea, the extremely low birth rate has become a serious national issue. Various social changes such as far advanced maternal age and the subsequently increased infertility rate, and recent development of assisted reproductive technologies lead to increased multiple and preterm births in the nation (1,2,3,4). These result in an absolute increase in high-risk newborn infants, including preterm infants. Neonatal deaths and major complications mostly occur in premature infants, especially in very-low-birth-weight infants (VLBWI; less than 1,500 g of birth weight), who require intense care in a neonatal intensive care unit (NICU). Internationally, the outcomes of VLBWI are known as a reflection of the quality of neonatal care provided in NICUs in the nation (5,6).
For the first time in Korea, a nationwide prospective Web-based registration system for VLBWIs, the Korean Neonatal Network (KNN), was organized (7). Operation of the KNN was started officially on April 15, 2013, by the Korean Society of Neonatology (KSN), with support from the Korea Centers for Disease Control and Prevention (CDC). The KNN is not only a national registry for VLBWIs but also an infrastructure for multicenter clinical trials and quality improvement of neonatal care, which leads to the ultimate development of evidence-based neonatal medicine in Korea.
The purpose of this review is to provide a systematic overview of the KNN, including its background and purpose, organizational process, unique systems, and future perspectives.
BACKGROUND AND PURPOSES OF THE KNN
Background
The total fertility rate in Korea was only 1.19 in 2013, which is one of the lowest in the world and a markedly reduced level compared with 1.65 in 1993, 20 yr ago. According to birth statistics (8), the total number of live births in Korea has decreased from 715,826 in 1993 to 436,455 in 2013, equivalent to a 40% decrease in 20 yr. In contrast, the number of low-birth-weight infants (less than 2,500 g of birth weight) was increased from 18,532 (2.6% of total live births) in 1993 to 24,189 (5.5% of total live births) in 2013, indicating a twofold increase in 20 yr. Moreover, the number of VLBWIs was 929 in 1993 and 2,961 in 2013, indicating a numeric increase of 318%. The prevalence of VLBWIs among the total live births increased from 0.13% to 0.68%, equivalent to a 523% increase during the past 20 yr. Such high increases in the numbers of low-birth-weight and premature infants are derived from various social changes, including far advanced maternal age (1,3), subsequently increased infertility rate, and increased number of artificial pregnancies (4).
In other developed countries such as the United States and Japan, which have already experienced these processes, the current prevalence of low-birth-weight or preterm infants is as high as 8%-10% (9). Therefore, the number of premature infants in Korea is expected to increase even further in the future.
Meanwhile, owing to recent advances in perinatal and neonatal medicine, the survival rate of high-risk neonates, especially VLBWIs, has significantly increased recently in Korea. However, it is still lower than that in other developed countries (10). Moreover, the survival rate (11) and incidence of complications of VLBWIs such as bronchopulmonary dysplasia vary significantly between NICUs (12). Therefore, new evidence-based strategies to improve neonatal intensive care quality in Korea are urgently needed. Because the number of VLBWIs is only less than 1% of the total live births in the nation, the available data are insufficient to develop a reliable evidence-based strategy in a single center or in several centers. Collected data from a large number of patient groups are more accurate and credible than those collected from a single institution or several institutions. Therefore, to obtain a large collection of data for high-risk newborn infants such as VLBWIs, a national multicenter neonatal network is obligatorily needed.
Neonatal network refers to the group of NICUs in different hospitals that participate in the registration system that collects data prospectively and persistently based on the definition agreed upon (13). Its core function is not only to improve the level of care in participating institutions by providing evidence-based feedback and facilitating collaborative neonatal researchers but also to develop national health-care policies based on their own population statistics. Other developed countries have already organized and implemented a neonatal network, thereby contributing to a large population-based data collection and health-care statistics at a national or international level, providing a tool for quality improvement, and facilitating multicenter clinical trials beyond differences between hospitals, areas, and countries. In addition, they actively publish numerous research articles by using network data. Table 1 lists well-known major international neonatal networks, actively operating with the generation of large-scale national or international data for high-risk newborn infants and contributing to the development of national health-care policies and evidence-based neonatal medicine.
Table 1. Major international neonatal networks.
| Name | Country | Year | Participating units | Registry inclusion at birth |
|---|---|---|---|---|
| NICHD Neonatal Research Network14) | United States | 1986~ | 21 tertiary care center | - <29 w or 401-1,000 g |
| - Follow-up for <27 w | ||||
| Vermont Oxford Neonatal Network (VON)15) | United States, Asia, Europe, Africa | 1988~ | 900 units | - 401-1,500 g or 22 w-29 w |
| - Others | ||||
| Australian and New Zealand Neonatal Network (ANZNN)16) | Australia, New Zealand | 1994~ | 29 level 3 units | - < 32 w or < 1,500 g or received assisted ventilation ≥ 4 hr, or major or therapeutic hypothermia |
| 26 level 2 units | ||||
| Canadian Neonatal Network (CNN)17) | Canada | 1995~ | 30 units from | - Total admitted |
| 17 universities | - <33 w or <1,500 g, HIE | |||
| Neonatal Research Network Japan (NRNJ)18) | Japan | 2003~ | 171 units | - <1,500g |
| - Hypothermia registry | ||||
| - Clinical trials | ||||
| National Neonatal Audit Programme (NNAP)19) | England | 2006~ | 179 units | - Various audit questions for < 32 w or < 1,501 g, or others |
| EuroNeoNet20) | 16 countries in Europe | 2006~ | 60 NICUs | - < 1,501 g or < 32 w |
| Korean Neonatal Network (KNN)23) | The Republic of Korea | 2013~ | 60 NICUs | - < 1,500 g |
gNICHD, National Institute of Child Health and Human Development; HIE, hypoxic-ischemic encephalopathy.
Purposes
The mission of the KNN is to provide a population-based national core data to plan appropriate health-care policies for high-risk newborn infants by establishing a national Web-based registry. The KNN offers data-driven interactive tools for quality improvement to participating NICUs for improving neonatal outcomes. The KNN also provide the infrastructure to facilitate collaborative multicenter clinical research. The KNN would generate evidence-based effective strategies to improve the survival rate and decrease the complication rate of high-risk newborn infants that finally result in a decrease in socioeconomic burden and an increase in effective economic population in Korea, a nation with an extremely low birth rate.
LAUNCHING OF THE KNN
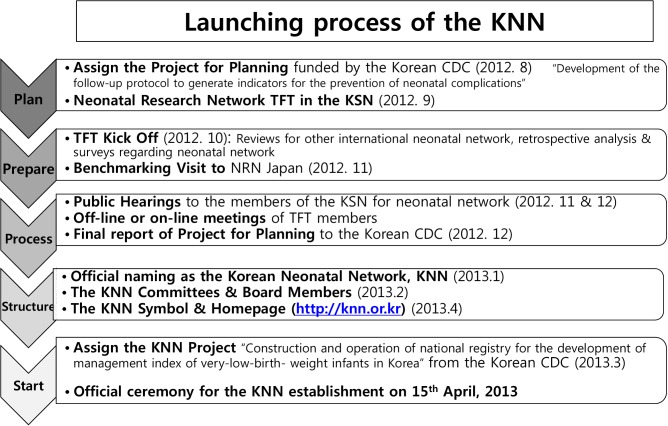
The KSN had been considering creating a national-based neonatal network for the reasons mentioned above since its establishment on 1993 (Fig. 1). Eventually, the task force team (TFT) of "Evidence Producing Clinical Research by Funding from the Government" was launched by the KSN in November 2011 and was assigned a planning project called "Development of follow-up protocol to generate indicators for the prevention of neonatal complication," funded by the Division of Cardiovascular and Rare Diseases of the Korea CDC, in August 2012. Tentatively named "Neonatal Research Network (NRN)," the TFT was started and operated with an advisory committee from the KSN since September 2012. A kickoff meeting with the members of the Korea CDC was held in October 2012. TFT members reviewed actively other international neonatal networks such as the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network in the United States (14), the Vermont Oxford Neonatal Network in North America (15), the Australian and New Zealand Neonatal Network (16), the Canadian Neonatal Network (17), the Neonatal Research Network Japan (18), the National Neonatal Audit Programme in England (19), and EuroNeoNet (20). They also analyzed pre-existing domestic retrospective multicenter studies and surveys regarding systems and organization for the neonatal network. Some of the members of the NRN TFT visited the Neonatal Research Network Japan in November 2012 for benchmarking. The NRN TFT started regular weekly online teleconferences in December 2012, which has been continued as an official KNN teleconference with all KNN executive board members, conducted every 1 or 2 weeks, for a total of more than 70 meetings thus far. By holding public hearings for the members of the KSN twice, in November and December 2012, opinions on the neonatal network were systemically gathered. Then, the NRN TFT submitted the final report for the project planning to the Korea CDC in December 2012 (21).
Fig. 1. Diagram of the launching and establishment of the Korean Neonatal Network (KNN). For the launching of the KNN, various activities, including planning, preparation, the actual process, structuring, and organization of the KNN were conducted since August 2012. Finally, KNN was officially started on April 15, 2013. TFT, task force team; CDC, Centers for Disease Control and Prevention; KSN, Korean Society of Neonatology; NRN, Neonatal Research Network.
With its official name "Korean Neonatal Network (KNN)," the KNN was started in January 2013, officially awarding in February 2013 the project called "Construction and operation of national registry for the development of management index of very-low-birth-weight infants in Korea," funded by the Korea CDC. The electronic case report form (e-CRF) for the KNN registry was designed based on the internet-based clinical research and trial (iCReaT) system, a Web-based clinical trial management system established by the Korea CDC in Osong. Before granting login right to the iCReaT system, KNN participants underwent an educational seminar for the use of the iCReaT system. Meanwhile, the KNN official homepage (http://knn.or.kr) (23) and a real-time data display system within the homepage linked with e-CRF data were developed. In March 2013, an official KNN logo was introduced, and members of the executive committee and extended advisory committee were also determined. In a formal ceremony for the establishment of the KNN was sponsored by the KSN and supported by the Korea CDC, held at Samsung Medical Center on April 15, 2013, during which the KNN commenced its official operation (7).
THE STRUCTURE OF THE KNN
Organization
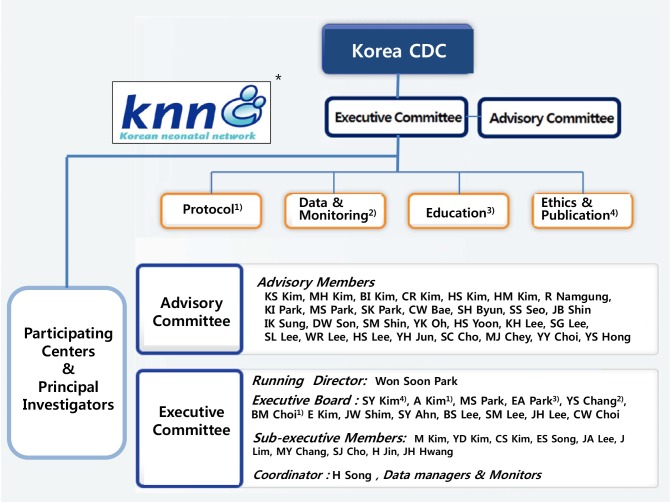
As shown in Fig. 2, KNN organizations are composed of mainly two parts, namely the executive and advisory committees under the supervision and support of the Korea CDC.
Fig. 2. Organizational structure and committee members of the Korean Neonatal Network (KNN) at present (April 2015). Under the supervision and support from the Korea CDC, KNN is composed of the executive and advisory committees. The executive committee comprises an executive director (Dr. Won Soon Park), an executive secretary general (Dr. Yun Sil Chang), 12 executive board members, and 10 sub-executive board members, along with a central coordinator, and data managers and monitors. The following are the four subcommittees in the KNN: 1) the protocol subcommittee comprised of short-term (Dr. Byung Min Choi as a sub-director) and long-term divisions (Dr. Ellen Ai-Rhan Kim as a sub-director), 2) data and monitoring subcommittee (Dr. Yun Sil Chang as a sub-director), 3) education subcommittee (Dr. Eun Ae Park as a sub-director), and 4) ethics and publication subcommittee (Dr. So Young Kim as a sub-director). The advisory committee comprises mainly the steering members of the Korean Society of Neonatology (KSN), including Dr. Chong Woo Bae (the former president of KSN), Dr. Rhan Namgung (the president of KSN), Dr. Beyong Il Kim (the vice president of KSN), Dr. Ki Soo Kim (the vice president of KSN), Dr. Soo Chul Cho (the vice president of KSN), and others. *Symbol of Korean Neonatal Network. KNN, Korean Neonatal Network; CDC, Centers for Disease Control and Prevention; PI, principal investigator.
The executive committee comprises an executive director, an executive secretary general, other executive board members, and sub-executive board members, along with a central coordinator, data managers, and monitoring clinical research assistants (CRA). It has four subcommittees, namely the protocol, data and monitoring, education, and ethics and publication committees, led by the executive members. The protocol subcommittee comprises short- and long-term e-CRF subcommittees that lead to develop and revise the short- and long-term e-CRF items and manual of operation (MOP), respectively. The data and monitoring subcommittee operates to conduct data management and monitoring and to provide the KNN Annual Report. The education subcommittee conducts various educational activities, including educational and principal investigator (PI) seminars, and maintains the KNN homepage, including a public announcement and the Q&A section on the Web site. The ethics and publication subcommittee mainly conducts various activities related to study proposals, publications, and newsletters, including education for publication ethics.
The executive board, spearheaded by the executive board members, conducts the actual management and final decision making regarding KNN organizations, registries, associated activities, and studies, as well as all the requests or suggestions related to the KNN.
The advisory committee comprises mainly the steering members of the KSN and some of the externally invited members, including one or more recommenders from the Korea CDC, epidemiologists, statisticians, and experts in relevant areas. They provide advice to the executive committee to maintain scientific rationality and effectiveness of the operation of the KNN at the highest level.
Aside from those, a PI committee, which is comprised of PIs from the KNN participating hospitals, was organized to preside actual KNN registration, data entry, study conduct, and writing of research articles. Once a year, the PI seminar is held for discussions and for educating members on various issues regarding registries, protocols, data quality, feedback for quality improvement, study proposals, and process changes in the KNN.
KNN member hospitals
Among about 100 hospitals with operational NICUs in Korea, 60 hospitals participate voluntarily at present (April 2015) and about 2,000 VLBWIs are registered per year in the KNN, covering about 70% of the overall admissions of VLBWIs born annually in Korea at present (8). The names of the KNN participating hospitals and corresponding principal investigators are as follows:
Ajou University Hospital (Dr. Jang Hoon Lee), Asan Medical Center (Dr. Byong Sop Lee), Busan ST Mary's Hospital (Dr. Sung Mi Kim), CHA Bundang Medical Center, CHA University (Dr. Heui Seung Jo), CHA Gangnam Medical Center, CHA University (Dr. Ji Hyun Jeon), Cheil General Hospital & Women's Healthcare Center (Dr. Sun Young Ko), Chonbuk National University Hospital (Dr. Jin Kyu Kim), Chonnam National University Hospital (Dr. Young Youn Choi), Chosun University Hospital (Dr. Sang Kee Park), Chung-Ang University Hospital (Dr. Na Mi Lee), Chungnam National University Hospital (Dr. Mea Young Chang), Daegu Catholic Univ. Medical Center (Dr. Woo Taek Kim), Dong-A University Hospital (Dr. Myo-Jing Kim), Eulji General Hospital (Dr. Hye Sun Yoon), Eulji University Hospital (Dr. Seung Yeon Kim), Ewha Womans University Medical Center (Dr. Eun Ae Park), Gachon University Gil Medical Center (Dr. Dong Woo Son), Gangnam Severance Hospital (Dr. Soon Min Lee), GangNeung Asan Hospital (Dr. Hyun-Seung Jin), Gyeongsang National University Hospital (Dr. Chan-Hoo Park), Hallym Univ. Medical Center (Dr. Yoon Ju Kim), Hanyang University Guri Hospital (Dr. Chang-Ryul Kim), Hanyang University Medical Center (Dr. Hyun Kyung Park), Inje University Busan Paik Hospital (Dr. Jong Beom Sin), Inje University Haeundae Paik Hospital (Dr. Mi Lim Chung), Inje University Ilsan Paik Hospital (Dr. Jong Hee Hwang), Inje University Sanggye Paik Hospital (Dr. Gyu Hong Shim), Jeju National University Hospital (Dr. Young Don Kim), Kangbuk Samsung Hospital (Dr. Jae Won Shim), Keimyung University Dongsan Medical Center (Dr. Chun Soo Kim), Konkuk University Medical Center (Dr. Min Hee Lee), Konyang University Hospital (Dr. Jae-Woo Lim), Korea University Anam Hospital (Dr. Eun Hee Lee), Korea University Ansan Hospital (Dr. Byung Min Choi), Korea University Guro Hospital (Dr. Young Sook Hong), Kosin University Gospel Hospital (Dr. Yoo Rha Hong), Kyung Hee University Hospital at Gangdong (Dr. Chong Woo Bae), Kyung Hee University Medical Center (Dr. Yong Sung Choi), Kyungpook National University Hospital (Dr. Heng Mi Kim), Pusan National University Children's Hospital (Dr. Shin-Yun Byun), Pusan National University Hospital (Dr. Kyung Hee Park), Samsung Changwon Medical Center (Dr. Seo Heui Choi), Samsung Medical Center (Dr. Won Soon Park), Seoul National University Bundang Hospital (Dr. Chang Won Choi), Seoul National University Hospital (Dr. Ee-Kyung Kim), Severance Hospital (Dr. Ran Namgung), SMG-SNU Boramae Medical Center (Dr. Jin A Lee), Soonchunhyang University Hospital Bucheon (Dr. Sung Shin Kim), Soonchunhyang University Hospital Cheonan (Dr. Jun Hwan Song), Soonchunhyang University Hospital Seoul (Dr. Woo Ryoung Lee), Sungae Hospital (Dr. Eun Ryoung Kim), The Catholic Univ of Korea Bucheon ST. Mary's Hospital (Dr. Ju Young Lee), The Catholic Univ of Korea Seoul ST. Mary's Hospital (Dr. In Kyung Sung), The Catholic Univ of Korea ST. Vincent's Hospital (Dr. Jung-Hyun Lee), The Catholic Univ of Korea Uijeongbu ST. Mary's Hospital (Dr. Hyun Seung Lee), The Catholic Univ of Korea Yeouido ST. Mary's Hospital (Dr. So Young Kim), Ulsan University Hospital (Dr. Ki Won Oh), Wonju Severance Christian Hospital (Dr. Baek Keun Lim), Wonkwang University School of Medicine & Hospital (Dr. Seung Hyun Lee), Yeungnam University Hospital (Dr. Eun Sil Lee).
UNIQUE SYSTEMS OF THE KNN
The KNN established unique systems (Fig. 3) for the operation of the multicenter registry and preparation for infrastructure for multicenter clinical studies.
Fig. 3. The unique systems specifically developed in the Korean Neonatal Network (KNN). KNN established unique systems, including not only organization structure and Web-based e-CRF registry but also a real-time data display system on the Website, a data-quality maintenance system using query generation and site-visit monitoring, and a feedback system using annual reports. The KNN consists of well-structured systems for education for registries and studies, as well as the online application of data use and study proposal. e-CRF, electronic case report form; iCReaT, internet-based clinical research and trial; CDC, Centers for Disease Control and Prevention; ID, identification; PI, principal investigator; Q&A, question and answer.
Web-based registry for VLBWIs
VLBWIs admitted to the NICU at birth or transferred from other hospitals within 28 days of life to the KNN participating hospitals are registered to the KNN registry. Any VLBWIs who died at delivery room or transferred to other hospitals within 28 days after birth are excluded. Moreover, each participating hospital should obtain institutional review board (IRB) approval for the KNN study. Case registration requires informed consent from parents for the use of patient's information.
A CRF covers 3 visits as follows: at discharge from the NICU (visit 1), long-term outpatient follow-up registry at 18-24 months of corrected age (visit 2), and at the age of 3 yr (visit 3). The KNN developed approximately 120 items for the short-term e-CRF based on about 80 items commonly used in other major international neonatal networks. The KNN also developed about 70 items each for visit 2 or 3 as a long-term outpatient follow-up. The protocol subcommittee spearheaded to develop these short- and long-term items and the MOP for each item thoroughly and to make revisions if necessary. Data entry is available at any time from all KNN member hospitals because the network is operating on a Web-based system with the iCReaT platform of the Korea CDC. Moreover, all the original data registered are stored safely, immediately, and confidentially in the server of the Korea CDC, with decoding of all the identification information of hospitals and patients.
Real-time data display
The KNN has a unique data display system on the authorized member's page on the KNN Web site, which requires a secure login information. The optimized graphs for each item registered are instantly showing with periodic syncing of updated data on iCReaT. Moreover, they present the most recent statuses of the demographics, treatment, and outcomes of the enrolled patients and allows for easy access and simultaneous, real-time comparisons between the overall accumulated data and data from each hospital. Each data display can be modified easily by a maximum of four conditions defined by the log-in user, which can include detailed and powerful analyses and feedback by providing real-time summaries and comparisons according to specific conditions.
Data quality management system
The KNN registry data should be entered by PIs or corresponding NICU staff members or by study coordinators with extensive data management experience at the participating hospitals. The PI at each hospital is responsible for precise data entry based on actual medical records according to the most recent version of the MOP of the KNN registry. Data entry staff should complete an official education on the iCReaT system and receive a certificate to qualify to enter data into the e-CRF of the KNN. Therefore, periodically circulating members (e.g., residents and fellows) in the hospital should be excluded from the data entry.
Moreover, each institution has an obligation to obtain IRB approval before the start of the KNN registry and obtain informed consent from the parents for the use of patient information.
Appropriate and continuous data management and monitoring are essential so that accurate data are obtained from the actual data for analysis. The KNN developed a unique data management system that comprises query generation and external site-visit monitoring. An auto-query is autogenerated primarily from the e-CRF system when incorrect data are entered. A manual query is generated secondarily through periodic inspection by the central data manager. The PI at each participating hospital has an obligation to solve the received queries by directly solving or supervising the process of solving the queries made by giving an appropriate answer to the person who enters the data. Moreover, the PI has an obligation to participate in the PI committee and to improve the quality of data collected by presenting and discussing the data quality issues, including the number of queries received and whether they are solved.
Site-visit monitoring
The KNN developed a unique site-visit monitoring system. The KNN member hospitals have an obligation to comply with the direct external site-visit monitoring for a more accurate data collection. Site-visit monitoring is conducted by two people per site-visit, one KNN member neonatologist and one professional monitor. Site-visit monitoring is conducted in each member hospital twice a year. During monitoring, the neonatologist monitor conducts source document verification (SDV) for 5%-10% of registered cases in the e-CRF from the institution by direct inspection of and comparison with the corresponding actual medical record of the cases on predetermined SDV items. Another monitor member conducts inspection for actual registration rate among the total VLBWIs admitted in the institution and the completeness of the IRB documents and informed consent forms. If necessary, they may determine the re-entry of data based on the result of the inspection. The PI has the obligation to comply with this decision. Site-visit monitoring contributes not only to assurance for data quality but also to providing relevant information by face-to-face education or gathering various opinions by direct discussion regarding issues related to the KNN registry. Site-visit monitoring is ongoing in 56 KNN participating hospitals in 2015, which requires 56 neonatologists and monitor members, which means that 244 monitor persons are required for annual visits to 56 participating hospitals.
Educational system
Since the first KNN educational seminar was held in Buyeo in May 2013, the KNN holds the education seminar once a year at the venue of the KSN Spring Symposium. The content of the educational seminar for staffs from the participating hospitals who are involved in the KNN regards short- and long-term e-CRF MOP, data management and monitoring, publication ethics, and implementation of study proposals. The PI seminar for PIs from the participating hospitals is held once a year to promote the collaborative network. The KNN education subcommittee also conducts the operation of the KNN homepage and various educational activities, including the operation of the Q&A section in the Web site.
Annual reports for total and individual institutions and feedback
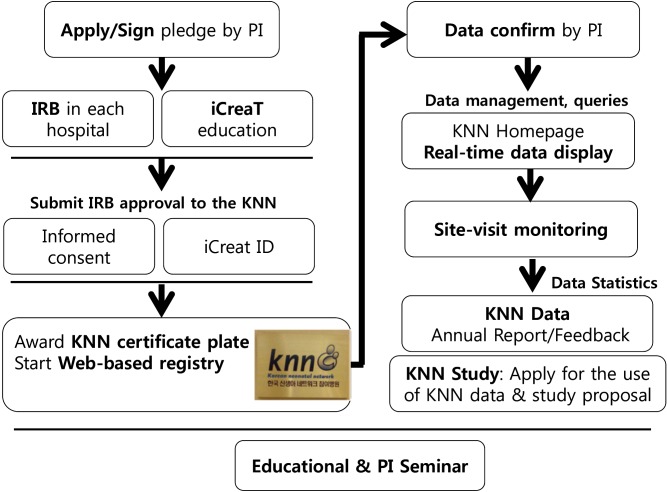
Following the application and participation to the KNN (Fig. 4), the KNN member institutions receive objective feedback with data-driven comparisons of the current statuses of their NICUs with that of the overall status of the KNN participating NICUs. They can do this by themselves by using the real-time display at the KNN Website at any time. Furthermore, the KNN executive committee publishes an annual report by using the data for the total registered cases born in the previous year, thus already locked and cleared through the data management process. Each annual report contains a summary of all of the e-CRF items analyzed according to 1-week gestational age and 100-g birth weight of the enrolled infants. Moreover, the KNN executive committee sends individual and confidential annual reports to each PI in each institution. The report contains a comparison of important items from e-CRF between those from all the participating hospitals and those from individual hospitals, along with a summary according to 1-week gestational age and 100-g birth weight of the enrolled infants. In addition, it contains the position of each institution among all the KNN participating hospitals for each category while showing the relative position in 25-75 percentile graphics or calculated odds ratio (OR) adjusted by gestational age of the enrolled VLBWIs (Fig. 5). These can provide objective evidence of the status of an NICU in relation to all the NICUs participating in the KNN, in terms of patient characteristics, treatment policies, care quality, and patient outcomes. Therefore, it provides powerful evidence-based feedback to promote quality improvement in each participating NICU eventually.
Fig. 4. Diagram of the participation process in the Korean Neonatal Network (KNN). When the principal investigator (PI) in a certain hospital applies to participate in the KNN, the PI has to sign the pledge first for fulfilling the obligations and commitments, keeping the information secure during KNN participation. Then, the PI has to obtain IRB approval for the KNN registry from his/her hospital and required official education for the use of the iCReaT. When the PI submit IRB approval document to the KNN, the iCReat ID that enables access to the KNN Web-based registry was provided. After receiving the KNN participation certificate plate, the institution can start the KNN registry. To view their own real-time data display on the secure KNN member Web site, PIs have to confirm the entered data to the KNN e-CRF and resolve the auto and manual queries sent from the KNN center periodically. In addition, the PI has to accept the KNN site-visit monitoring twice a year for quality surveillance of data and the KNN administrative process. Accumulated data are locked periodically, followed by cleaning and statistical analysis by central data managers. Then, the KNN executive committee publishes a total or individual annual report of the previous year. It provides powerful interactive data-driven evidence for quality improvement to the individual participating center. Academic studies that used the KNN data are promoted through the study proposal application system. Active collaborations among participants occur directly in the education or PI seminar once a year and indirectly but continuously through the KNN Web site. PI, principal investigator; IRB, institutional review board; iCReaT, internet-based clinical research and trial; ID, identification.
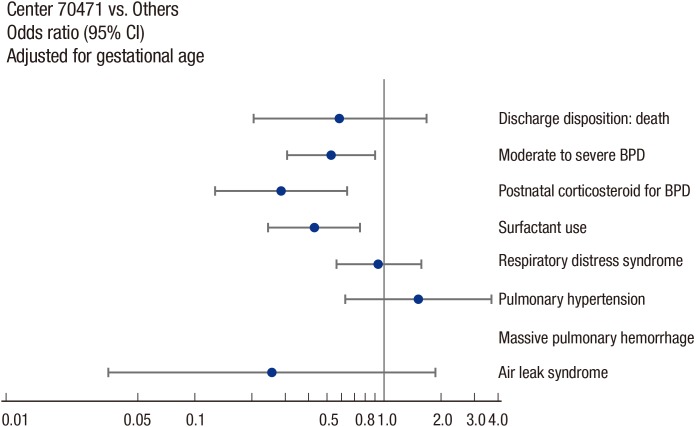
Fig. 5. A diagrams included in the individual and confidential annual report of the KNN, showing the odds ratios (ORs) and 95% confidence intervals (CIs) for various treatment policies and outcomes, with adjustment for the gestational age of the enrolled VLBWIs. This diagram shows that Center 70474 (virtual center, not real) has significantly lower incidences of moderate to severe BPD and postnatal corticosteroid use than all the other participating hospitals, with adjusted OR of 0.5 and 0.3, respectively. These data-driven analyses provide a valuable and powerful feedback for quality improvement to each institution, where the institution positions among all of the KNN participating institutions in terms of specific items indicating care qualities.
Application of the study proposal by using the KNN data
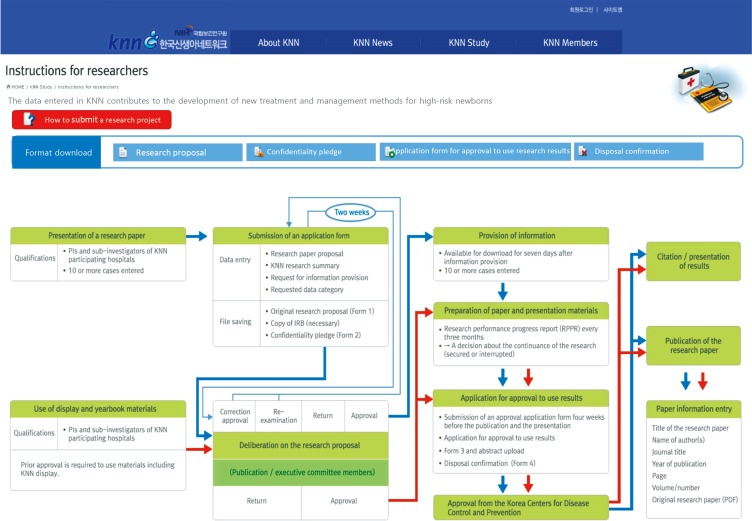
Any investigators from the participating hospitals, which registered more than 10 cases to the KNN, have the right to apply the study proposal by using the KNN data at any time. The KNN developed an electronic application system for study proposal and data use on the KNN homepage, which is managed by the ethics and publication subcommittee. The KNN encourages the motivated young investigator to submit the study proposal to use KNN data through this system. Once the applied proposal is approved by the KNN executive board, the requested data with decoding of the hospital and patient information are provided to the investigator. The investigator must report the progress of the study within a specified period and should acknowledge the use of KNN data in the article or presentation (Fig. 6).
Fig. 6. KNN Study site, an electronic application system for study proposal and data use on the Members site of KNN homepage (http://knn.or.kr). Any investigators of the participating hospitals, which registered more than 10 cases to the KNN, have the right to apply the study proposal by using this system at any time. KNN, Korean Neonatal Network.
Furthermore, to prevent excessive data use beyond the capability, a maximum of three studies can be conducted simultaneously per investigator, that is, until one of the applied studies is accepted for publication as a research article.
FUTURE PERSPECTIVES OF THE KNN
The KNN has been successfully established and run with unique systems, registering about 70% of VLBWI born in Korea annually, in a relatively short period, which took more than ten years in major developed countries. The KNN has to be maintained and developed further to generate appropriate, population-based, data-driven, health-care policies, and to facilitate active multicenter neonatal research, including quality improvement of neonatal care. We expect that these overall activities in KNN improve evidence-based neonatal medicine and ultimately lead to the improvement of the prognosis of high-risk newborns and subsequent reduction in health-care costs, nationally and internationally.
Important future perspectives of the KNN are as follows.
Generation of population-based healthcare data
Through the KNN, the Web-based national registry of VLBWIs, Korean large population-based data for VLBWI can be generated annually, which can facilitate the development of national health-care policies.
The KNN executive committee published the 2013 KNN Annual Report (22), in which 1,386 VLBWIs born in 2013 and registered from 49 hospitals were evaluated. Currently, 60 NICUs participate in the KNN, and more than 2,000 new VLBWIs are registered annually, which cover about 70% of VLBWIs born in Korea in a year (8). As the National Cancer Registry, the VLBWI registry has to be continued as a national health-care system project because one of the most important indexes in the global, national healthcare field is infant mortality rate. More than 50% of infant deaths are neonatal mortality, and most neonatal mortalities occur in small and weak VLBWIs. Therefore, the annual registry for these high-risk newborn infants will be an important basic database for formulating academic and health-care policies aimed at reducing infant mortality in the nation.
Implementation of quality improvement
By collecting large-scale KNN data, we can not only share, learn, and assess the variation in practices and outcomes of VLBWIs between NICUs but also obtain evidence-based quality improvement data. The benchmarking indexes for mortality and major complications can be established, such as bronchopulmonary dysplasia, intraventricular hemorrhage, sepsis, necrotizing enterocolitis, or retinopathy of prematurity, which are devastating complications of high-risk newborns. Improvement of neonatal intensive care quality can be achieved gradually through the development of potentially better practices and implementation of quality improvement activity in participating hospitals. These ultimately contribute to reducing health-care costs in the nation.
Infrastructure for neonatal multicenter clinical research
Moreover, the KNN is an infrastructure of multicenter randomized clinical trials to verify the effectiveness of a new treatment and care policies in high-risk newborn infants in Korea. Another high-risk newborn registry such as the national hypothermia or late preterm follow-up registry can be also established. Multicenter clinical trials can promote scientific development and qualitative improvement in the actual treatment of these high-risk newborn infants, which facilitate the increase in survival rate and decrease in complication rate in high-risk neonates through the development of new neonatal intensive-care policies, which is appropriate in our health-care environment.
Global impact via internetwork comparisons
The KNN plans to join in the International Network for Evaluation of Outcomes of Neonates (iNeo) (24), which is the international neonatal network for evaluating outcomes of very-low-birth-weight and very preterm neonates from 9 countries or others such as International Neonatal Consortium (INC) (25)Collaborative comparisons of international health services for quality improvement in neonatal care or efforts to concentrate on those conditions most commonly encountered in NICUs can contribute to the global health impact. Eventually, through this, improvements in quality of life can be expected internationally by increasing the survival rate of high-risk neonates and decreasing risks of major complications, decreasing socioeconomic burden, and increasing the proportion of the productive economic population.
ACKNOWLEDGMENTS
We are indebted to all the members of the KNN executive and advisory committees for their active participation in the KNN organization and operation. We thank Hyeyoung Song for her dedication to the KNN as Network Coordinator and Seong-Ran Lim for the incredible help to the KNN organization. We also thank all the members working for data management and site-visit monitoring and their tremendous efforts dedicated to the KNN.
We also express profound gratitude to the principal investigators and related researchers of the KNN participating hospitals.
Footnotes
Funding: This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2013-E63008-01).
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design of the study: Chang YS, Park HY, Park WS. First draft and revision of the manuscript: Chang YS, Park WS.
References
- 1.Moon JY, Hahn WH, Shim KS, Chang JY, Bae CW. Changes of maternal age distribution in live births and incidence of low birth weight infants in advanced maternal age group in Korea. Korean J Perinatol. 2011;22:30–36. [Google Scholar]
- 2.Park YS, Choi SH, Shim KS, Chang JY, Hahn WH, Choi YS, Bae CW. Multiple births conceived by assisted reproductive technology in Korea. Korean J Pediatr. 2010;53:880–885. doi: 10.3345/kjp.2010.53.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astolfi P, De Pasquale A, Zonta L. Late childbearing and its impact on adverse pregnancy outcome: stillbirth, preterm delivery and low birth weight. Rev Epidemiol Sante Publique. 2005;53:2S97–2S105. [PubMed] [Google Scholar]
- 4.Mukhopadhaya N, Arulkumaran S. Reproductive outcomes after in-vitro fertilization. Curr Opin Obstet Gynecol. 2007;19:113–119. doi: 10.1097/GCO.0b013e32807fb199. [DOI] [PubMed] [Google Scholar]
- 5.Bartels DB, Wypij D, Wenzlaff P, Dammann O, Poets CF. Hospital volume and neonatal mortality among very low birth weight infants. Pediatrics. 2006;117:2206–2214. doi: 10.1542/peds.2005-1624. [DOI] [PubMed] [Google Scholar]
- 6.Chung JH, Phibbs CS, Boscardin WJ, Kominski GF, Ortega AN, Needleman J. The effect of neonatal intensive care level and hospital volume on mortality of very low birth weight infants. Med Care. 2010;48:635–644. doi: 10.1097/MLR.0b013e3181dbe887. [DOI] [PubMed] [Google Scholar]
- 7.Chang YS, Ahn SY, Park WS Committee on Program and Planning and Advisory Committee of Korean Neonatal Network. The Establishment of the Korean Neonatal Network (KNN) Neonatal Med. 2013;20:169–178. [Google Scholar]
- 8.Korean Statistical Information Service. Birth Statistics. [accessed on 25 April 2015]. Available at http://www.kosis.kr.
- 9.Cho JH, Choi SK, Chung SH, Choi YS, Bae CW. Changes in neonatal and perinatal vital statistics during last 3 decades in the Republic of Korea: compared with OECD nations. Neonatal Med. 2013;20:402–412. [Google Scholar]
- 10.Hahn WH, Chang JY, Chang YS, Shim KS, Bae CW. Recent trends in neonatal mortality in very low birth weight Korean infants: in comparison with Japan and the USA. J Korean Med Sci. 2011;26:467–473. doi: 10.3346/jkms.2011.26.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim JW, Kim MJ, Kim EK, Park HK, Song ES, Lee SM, Lee JH, Jin HS, Kim ES, Chang YS. The impact of neonatal care resources on regional variation in neonatal mortality among very low birthweight infants in Korea. Paediatr Perinat Epidemiol. 2013;27:216–225. doi: 10.1111/ppe.12033. [DOI] [PubMed] [Google Scholar]
- 12.Choi CW, Kim BI, Kim EK, Song ES, Lee JJ. Incidence of bronchopulmonary dysplasia in Korea. J Korean Med Sci. 2012;27:914–921. doi: 10.3346/jkms.2012.27.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakkar M, O'Shea M. The role of neonatal networks. Semin Fetal Neonatal Med. 2006;11:105–110. doi: 10.1016/j.siny.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 14.NICHD Neonatal Research Network. [accessed on 25 April 2015]. Available at https://neonatal.rti.org/
- 15.Vermont Oxford Neonatal Network (VON) [accessed on 25 April 2015]. Available at http://www.vtoxford.org.
- 16.Australian and New Zealand Neonatal Network. [accessed on 25 April 2015]. Available at https://npesu.unsw.edu.au/data-collection/australian-new-zealand-neonatal-network-anznn.
- 17.Canadian Neonatal Network. [accessed on 25 April 2015]. Available at http://www.canadianneonatalnetwork.org.
- 18.Neonatal Research Network Japan. [accessed on 25 April 2015]. Available at http://nrn.shiga-med.ac.jp/Englishdefault.htm.
- 19.National Neonatal Audit Programme (NNAP) [accessed on 25 April 2015]. Available at http://www.rcpch.ac.uk/improving-child-health/quality-improvement-and-clinical-audit/national-neonatal-audit-programme-nn-3.
- 20.Europe Neonatal Network. [accessed on 25 April 2015]. Available at http://www.euroneonet.eu.
- 21.Park WS Korean Neonatal Network TFT. Development of the follow-up protocol to generate indicators for the prevention of neonatal complications. 2012 Report of Korea Centers for Disease and Prevention. Seoul: Korea Centers for Disease and Prevention; 2012. [Google Scholar]
- 22.The Executive Committee of Korean Neonatal Network. 2013 Korean Neonatal Network annual report. Seoul: Korea Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 23.The Korea Neonatal Network. [accessed on 25 April 2015]. Available at http://knn.or.kr.
- 24.Shah PS, Lee SK, Lui K, Sjörs G, Mori R, Reichman B, Håkansson S, Feliciano LS, Modi N, Adams M, et al. International Network for Evaluating Outcomes of Neonates (iNeo) The International Network for Evaluating Outcomes of very low birth weight, very preterm neonates (iNeo): a protocol for collaborative comparisons of international health services for quality improvement in neonatal care. BMC Pediatr. 2014;14:110. doi: 10.1186/1471-2431-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis JM, Turner MA. Global collaboration to develop new and existing drugs for neonates. JAMA Pediatr. 2015 doi: 10.1001/jamapediatrics.2015.1640. [DOI] [PubMed] [Google Scholar]