Abstract
Here, we aimed to evaluate the incidence and mortality of intraventricular hemorrhage (IVH) and post-hemorrhagic hydrocephalus (PHH) among very-low-birth-weight (VLBW) infants in Korea and assess the associated factors of PHH. This cohort study used prospectively collected data from the Korean Neonatal Network (KNN). Among 2,386 VLBW infants in the KNN database born between January 2013 and June 2014, 63 infants who died without brain ultrasonography results were excluded. Maternal demographics and neonatal clinical characteristics were assessed. The overall incidence of IVH in all the VLBW infants was 42.2% (987 of 2,323), while those of IVH grade 1, 2, 3, and 4 were 25.1%, 7.0%, 4.8%, and 5.5%, respectively. The incidence and severity of IVH showed a negatively correlating trend with gestational age and birth weight. PHH developed in 0%, 3.5%, 36.1%, and 63.8% of the surviving infants with IVH grades 1, 2, 3, and 4, respectively. Overall, in the VLBW infants, the IVH-associated mortality rate was 1.0% (24/2,323). Only IVH grade severity was proven to be an associated with PHH development in infants with IVH grades 3-4. This is the first Korean national report of IVH and PHH incidences in VLBW infants. Further risk factor analyses or quality improvement studies to reduce IVH are warranted.
Keywords: Infant, Newborn; Infant, Premature; Infant, Very-Low-Birth-Weight; Intracranial Hemorrhage; Hydrocephalus; Registries; Incidence; Epidemiology
INTRODUCTION
Intraventricular hemorrhage (IVH) is a cause of neonatal morbidity and mortality and is strongly associated with adverse neurological outcomes (1,2). The incidence of severe IVH (grade 3 or 4) has persisted despite the overall decline in IVH because the survival rate of very-low-birth-weight (VLBW) infants has consistently increased (3,4). According to multicenter studies, the incidence of IVH varies inversely with gestational age with an overall incidence of 15%-42%, while severe IVH develops in 7%-16% of VLBW infants (5,6,7,8,9). Approximately 35% of VLBW infants with IVH develop post-hemorrhagic hydrocephalus (PHH), and 15% of VLBW infants with IVH require intervention (2,5,8,10). In Korea, however, no nationwide data for the incidences of IVH and PHH have been reported to date. IVH is graded based on the Papile classification system (11), but the grading of PHH is not yet standardized. Although some studies have suggested using the criteria for ventricular dilatation (12,13), there is currently no commonly accepted grading system. The decision to treat PHH has typically been made in clinical situations of increasing ventricular width from serial brain imaging. To date, only a few studies have addressed the risk factors for or outcomes of PHH in VLBW infants with IVH (14,15,16). Identifying the associated risk factors for PHH would assist the decision-making process for serial brain imaging or procedures to remove cerebral spinal fluid (CSF) from VLBW infants who develop PHH after IVH.
The objective of the present study was to report the distribution of IVH and the associated risk factors for PHH among VLBW infants in Korea. For this objective, we first investigated the incidence of IVH in VLBW infants as well as the IVH induced mortality rate according to gestational age at birth and birth weight. Second, we evaluated the incidence of clinically significant PHH after IVH and the associated risk factors for procedures used to remove CSF in VLBW infants. Finally, we compared the Korean nationwide data to those in other national databases. The present study is to describe nationwide data of the incidence and mortality of IVH and PHH among VLBW infants in Korea.
MATERIALS AND METHODS
Study population
We performed a cohort study using prospectively collected data from 55 Korean Neonatal Network (KNN) centers. A total of 2,386 VLBW infants born between January 2013 and June 2014 and registered in the KNN were reviewed. We excluded 63 VLBW infants who did not have documented brain ultrasonography (BUS) results, and among them, 60 infants were expired within 7 days after birth. Finally, the remaining 2,323 were included in the present study. The infants were grouped according to the most severe IVH grade reported on BUS documents during neonatal intensive care unit hospitalization.
Data collection and analysis
Informed consent was obtained to access the KNN database. Neonatology staff members or trained research coordinators prospectively gathered maternal, delivery, and neonatal data until the first patient discharge according to the KNN manual of operational procedure (17). For infants with persistent hospitalization, limited data were collected to postnatal day 365. Gestational age was determined by the best obstetric estimate using the date of the last menstrual period and/or ultrasonography. Antenatal corticosteroid exposure was defined as the maternal receipt of one or more doses of any corticosteroid. Pulmonary hemorrhage was defined as massive and significant pulmonary hemorrhage that destabilized vital signs. Hypotension referred to the hypotension that developed within the first week of life that required medication including cardiotonics or steroids. Infants who received medication or surgery to treat patent ductus arteriosus (PDA) for therapeutic and/or prophylactic purposes were identified as PDA medication or PDA ligation. The most severe stage of IVH of all BUS findings until first discharge was recorded irrespective of the time of first observation or occurrence as this information was not collected. IVH staging was made according to Papile's classification (11). PHH was defined as IVH-induced hydrocephalus that required spinal tapping, external ventricular drainage, and/or a shunt operation. Mortality that occurred before the first discharge was termed in-hospital death, while mortality that was primarily related to IVH was defined as death of which primary cause was severe grade IVH and its sequelae in the KNN dataset. Bronchopulmonary dysplasia (BPD) was defined by the use of the National Institutes of Health Workshop severity-based diagnostic criteria (18). We analyzed the gestational age- and birth weight-dependent incidence of IVH and compared the IVH incidences of the KNN to other national registry datasets including the Canadian Neonatal Network (CNN) (19), Australian and New Zealand Neonatal Network (ANZNN) (20), European Neonatal Network (EuroNeoNet) (21), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (4). IVH grade-dependent incidences of PHH in surviving infants and mortality with time of death were assessed. Among surviving infants with IVH grades 3-4, we grouped them according to PHH occurrence (PHH and non-PHH groups) and compared the maternal demographics and neonatal characteristics between groups.
Statistical analysis
Unadjusted comparisons of maternal demographics and neonatal characteristics between the non-PHH and PHH groups among IVH grade 3-4 infants were performed using the chi-square or Fisher exact test for categorical data and Student's t-test for continuous variables. A binary logistic regression was performed for the adjusted analysis. P values<0.05 were considered statistically significant. SPSS version 18 (IBM Corp., Chicago, IL, USA) was used for all of the statistical analyses.
Ethics statement
The data registration was approved by the institutional review board of every hospital participating in the KNN. Informed consent was obtained prospectively from the parents of the infants registered in the KNN.
RESULTS
Incidence of IVH
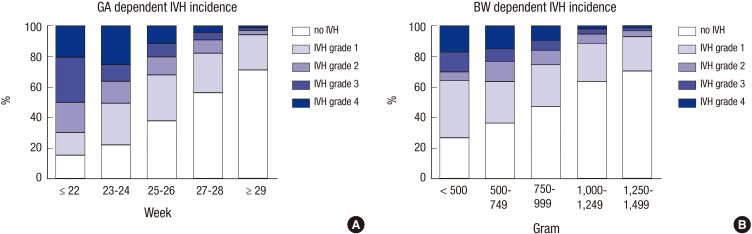
A total of 2,386 VLBW infants were enrolled in the KNN registry during the study period. Of these infants, 2,323 with documented BUS were included in the analysis. The overall incidence of IVH in the VLBW infants registered in the KNN was 42.5% (987 of 2,323) (Table 1). Severe (grade 3-4) IVH occurred in 10.3% (240 of 2,323) of VLBW infants (Table 1). Among infants with any grade IVH, the incidence of grade 1, 2, 3, and 4 disease was 59.2% (584), 16.5% (163), 11.3% (112), and 13.0% (128), respectively. Fig. 1 shows the gestational age- and birth weight-dependent incidence of IVH by grade. Overall and severe (grade 3-4) IVH were more common in smaller and more immature infants (Table 2).
Table 1. Incidence of intraventricular hemorrhage in very-low-birth-weight infants.
| IVH grade | Incidence (n = 2,323) | Cumulative incidence |
|---|---|---|
| 4 | 128 (5.5%) | 128 (5.5%) |
| 3 | 112 (4.8%) | 240 (10.3%) |
| 2 | 163 (7.0%) | 403 (17.3%) |
| 1 | 584 (25.1%) | 987 (42.5%) |
| 0 | 1,336 (57.5%) | 2,323 (100%) |
IVH, intraventricular hemorrhage.
Fig. 1. Incidence of intraventricular hemorrhage (IVH) according to gestational age (GA) (A) and birth weight (BW) (B). The proportions of infants with grades 1, 2, 3, and 4 IVH and without IVH are presented according to GA and BW.
Table 2. Gestational age- and birth weight-dependent incidence of significant intraventricular hemorrhage.
| IVH grade | Incidence of IVH | ||||
|---|---|---|---|---|---|
| Gestational age (week) | ≤ 22 | 23-24 | 25-26 | 27-28 | ≥ 29 |
| Grade ≥ 2 IVH | 14/20 (70.0%) |
105/207 (50.7%) |
126/391 (32.2%) |
96/551 (17.4%) |
62/1,154 (5.4%) |
| Grade ≥ 3 IVH | 10/20 (50.0%) |
76/207 (36.7%) |
78/391 (19.9%) |
47/551 (8.5%) |
29/1,154 (2.5%) |
| Birth weight (gram) | < 500 | 500-749 | 750-999 | 1,000-1,249 | 1,250-1,499 |
| Grade ≥ 2 IVH | 19/53 (35.8%) |
112/304 (36.8%) |
135/528 (25.6%) |
75/622 (12.1%) |
62/816 (7.6%) |
| Grade ≥ 3 IVH | 16/53 (30.2%) |
72/304 (23.7%) |
86/528 (16.3%) |
34/622 (5.5%) |
32/816 (3.9%) |
IVH, intraventricular hemorrhage.
Incidence of PHH
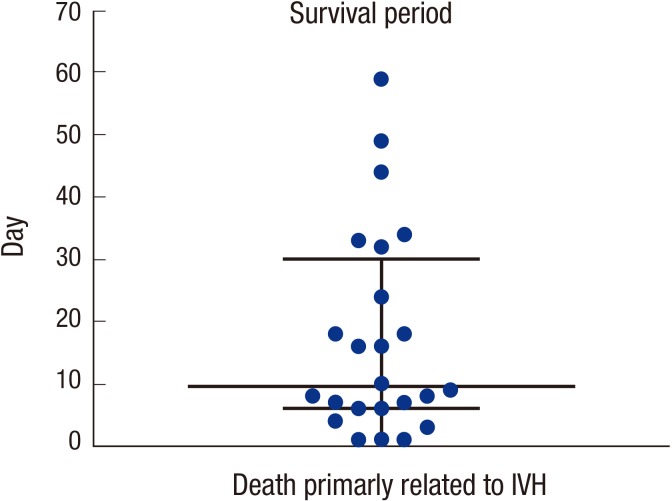
IVH grade-dependent incidence of PHH is described in Table 3. In the IVH grade 3-4 infants who expired in the early period (the median survival time of IVH grade 3-4 infants was postnatal day 12; Fig. 2), there is a limitation to assessing accurate occurrence of PHH because it usually requires therapeutic intervention in the later period. Thus, incidences of PHH or death in all registered infants or PHH in only survived infants were displayed. PHH did not develop in survived infants with IVH grade 1, and it occurred only in 3.5% of survived infants with grade 2 IVH. However, the incidence significantly increased in infants with grades 3-4 IVH. Moreover, incidence of PHH or death also showed IVH grade dependent pattern and it occurs in the 52.7% and 86.7% of all infants with grade 3 and grade 4 IVH, respectively (Table 3).
Table 3. Incidence of post-hemorrhagic hydrocephalus in very-low-birth-weight infants.
| Variables | no IVH (n=1,336) | Incidence of IVH and PHH by IVH grade | ||||
|---|---|---|---|---|---|---|
| Grade 1 (n=584) | Grade 2 (n=163) | Grade 3 (n=112) | Grade 4 (n=128) | Total (n=987) | ||
| Gestational age | 29.4±2.8 | 28.2±2.9 | 26.6±2.5 | 26.1±2.3 | 25.3±2.3 | 27.2±2.9 |
| Birth weight | 1,147±258 | 1,045±284 | 949±273 | 943±289 | 807±252 | 978±293 |
| Total mortality | 64 (4.8%) | 30 (5.1%) | 19 (11.7%) | 29 (25.9%) | 81 (63.3%) | 223 (9.6%) |
| IVH directly induced mortality | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (3.6%) | 20 (15.6%) | 24 (1.0%) |
| PHH or death | 64 (4.8%) | 30 (5.1%) | 25 (15.3%) | 59 (52.7%) | 111 (86.7%) | 225 (22.8%) |
| PHH in survived infants | 0/1,272 (0.0%) | 0/554 (0.0%) | 5/144 (3.5%) | 30/83 (36.1%) | 30/47 (63.8%) | 65/764 (8.5%) |
IVH, intraventricular hemorrhage; PHH, post-hemorrhagic hydrocephalus.
Fig. 2. Survival period of infants who expired primarily of intraventricular hemorrhage (IVH). The time of death is shown in terms of postnatal age (day). The median and interquartile range (25%-75%) are indicated.
Mortality primarily related to IVH
Total and primarily IVH-related mortality rates are displayed in Table 3. None of the infants with grade 1-2 IVH expired because of IVH. However, 3.6% and 15.6% of infants with grade 3-4 IVH expired, respectively, due primarily to IVH (Table 3). As per the KNN dataset, of the 110 infants who had died, 24 (22%) had a primary cause of death as severe IVH and its sequelae. The median time of death in infants who expired primarily because of IVH was on the 10th postnatal day and ranged from the 3rd to the 124th postnatal day (25-75 percentile, 6th-32nd postnatal day) (Fig. 2).
Factors related to PHH development
To assess the factors associated with PHH development in the surviving infants with grade 3-4 IVH, the maternal demographic findings and neonatal characteristics were compared between the PHH and non-PHH groups (Table 4). Factors found to be significantly higher in the PHH group than in the non-PHH group included the chest compressions at initial resuscitation, significant pulmonary hemorrhage destabilized vital signs, significant hypotension requiring medication within the 1st week of life, PDA ligation, and IVH grade 4. After logistic regression adjustment for above factors, only the incidence of IVH grade 4 was significantly higher in the PHH group than in the non-PHH group with an adjusted odds ratio (OR) of 3.1 and a 95% confidence interval (CI) of 1.35-6.91 (Table 5). Although the use or non-use of chest compressions at initial resuscitation did not reach statistical significance (P=0.05), it showed a high adjusted OR of 5.1 (95% CI, 0.97-27.1).
Table 4. Maternal demographics and neonatal clinical characteristics of surviving infants with grade 3-4 intraventricular hemorrhage according to the development of post-hemorrhagic hydrocephalus.
| Characteristics | non-PHH (n = 70) | PHH (n = 60) | P value |
|---|---|---|---|
| Gestational age (week) | 26.4 ± 2.3 | 26.1 ± 2.4 | 0.56 |
| Birth weight (g) | 956 ± 286 | 954 ± 273 | 0.52 |
| Apgar score (1 min) | 3.4 ± 1.4 | 3.2 ± 1.6 | 0.85 |
| Apgar score (5 min) | 5.8 ± 1.7 | 5.7 ± 1.9 | 0.39 |
| Female sex, number (%) | 26/70 (37) | 24/60 (43) | 0.55 |
| Cesarean section, number (%) | 45/70 (64) | 44/60 (73) | 0.27 |
| Antenatal corticosteroids, number (%) | 50/65 (77) | 41/57 (72) | 0.72 |
| Pathologic chorioamnionitis, number (%) | 27/59 (46) | 27/49 (55) | 0.58 |
| Maternal hypertension, number (%) | 8/68 (16) | 5/59 (8.4) | 0.75 |
| Gestational diabetes, number (%) | 4/70 (6) | 7/60 (12) | 0.32 |
| Intubation at initial resuscitation | 63/68 (93) | 54/56 (97) | 0.36 |
| Chest compression at initial resuscitation | 2/68 (3) | 8/56 (14) | 0.02 |
| Body temperature at admission (℃) | 36.0 ± 0.7 | 36.1 ± 0.7 | 0.94 |
| Base excess at admission (mM/L) | -6.4 ± 4.2 | -6.7 ± 4.0 | 0.68 |
| RDS, number (%) | 67/70 (96) | 56/60 (93) | 0.55 |
| Pneumothorax, number (%) | 2/70 (2) | 4/60 (1) | 0.30 |
| Pulmonary hemorrhage, number (%) | 10/70 (14) | 19/60 (32) | 0.02 |
| Hypotension, number (%) | 31/70 (44) | 40/60 (67) | 0.01 |
| PDA medication, number (%) | 31/70 (44) | 35/60 (58) | 0.11 |
| PDA operation, number (%) | 16/70 (23) | 24/60 (40) | 0.04 |
| Intubation duration (day) | 38.2 ± 35.2 | 42.1 ± 30.4 | 0.50 |
| TPN duration (day) | 49.3 ± 30.8 | 47.5 ± 28.8 | 0.73 |
| Steroid usage for BPD, number (%) | 41/70 (59) | 28/60 (47) | 0.18 |
| BPD ( ≥ moderate), number (%) | 44/70 (63) | 45/59 (76) | 0.20 |
| Seizure, number (%) | 30/70 (43) | 33/60 (55) | 0.17 |
| Sepsis, number (%) | 90/70 (43) | 27/60 (45) | 0.81 |
| NEC ( ≥ stage 2), number (%) | 7/70 (10) | 6/60 (10) | 1.00 |
| IVH (grade 4), number (%) | 17/70 (24) | 30/60 (50) | < 0.01 |
Data are presented as mean±standard deviation or as number and percentage of total. RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; TPN, total parenteral nutrition; BPD, bronchopulmonary dysplasia; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage.
Table 5. Adjusted odds ratios for neonatal characteristics in the development of post-hemorrhagic hydrocephalus in the survived infants with grade 3-4 intraventricular hemorrhage.
| Characteristics | P value | Odds ratio (95% confidence interval) |
|---|---|---|
| Chest compression at initial resuscitation | 0.05 | 5.1 (0.97-27.1) |
| Hypotension | 0.09 | 2.0 (0.89-4.58) |
| Pulmonary hemorrhage | 0.18 | 2.0 (0.74-5.18) |
| PDA ligation | 0.26 | 1.6 (0.69-3.91) |
| Grade 4 IVH | 0.01 | 3.1 (1.35-6.91) |
Odds ratio for post-hemorrhagic hydrocephalus development was adjusted for the chest compressions at initial resuscitation, significant pulmonary hemorrhage destabilized vital signs, significant hypotension requiring medication within the 1st week of life, PDA ligation, and IVH grade 4. PDA, patent ductus arteriosus; IVH, intraventricular hemorrhage.
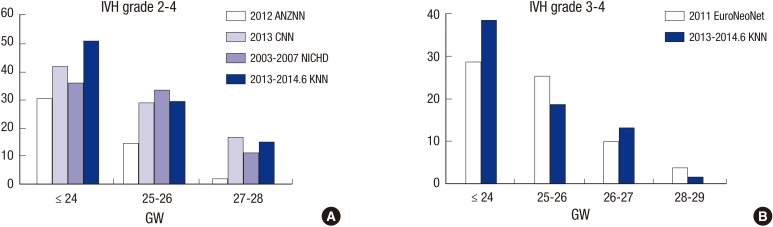
National comparisons of IVH incidence
The incidences of IVH grades 2-4 in the KNN (January 2013-June 2014) were compared with those in the ANZNN (2012), CNN (2013), and NICHD (2003-2007), and the incidence of IVH grades 3-4 in KNN was compared with that in EURONET (2011) based on the annual report of each registry or published papers (Fig. 3). In VLBW infants born at ≤24 gestational weeks, KNN appears the trend of having a higher incidence of IVH grades 2-4 or 3-4 than the ANZNN, CNN, NICHD, and EuroNeoNet networks. Among infants born at 25-28 gestational weeks, the incidence of IVH grade 2-4 or 3-4 in the KNN seemed similar to those in the other networks.
Fig. 3. Cross-national comparisons of intraventricular hemorrhage incidences. Incidences of grade 2-4 (A) or grade 3-4 IVH (B) in the KNN were compared with those of the ANZNN, CNN, NICHD, and EuroNeoNet registries. IVH, intraventricular hemorrhage; KNN, Korean Neonatal Network; ANZNN, Australian and New Zealand Neonatal Network; CNN, Canadian Neonatal Network; NICHD, Eunice Kennedy Shriver National Institute of Child Health and Human Development; EuroNeoNet, European Neonatal Network; GW, gestational weeks.
DISCUSSION
This study reported the Korean nationwide incidences of IVH and PHH requiring a procedure to remove CSF after IVH. This population-based study suggests that IVH is a common problem in VLBW infants in Korea, with 42.5% of VLBW infants developing IVH of any grade and 10.3% developing IVH grade 3 or 4. The more immature infants were at greater risk of severe IVH and subsequent PHH. The finding in the present study is consistent with previous reports derived from CNN (19), NICHD (4), and EuroNeoNet (21) data (Fig. 3). A comparison of the KNN and other national registry datasets revealed that the incidence of IVH in infants who were ≤24 gestational weeks at birth in the KNN seemed to be higher than those in the other national registries, while ANZNN appeared to show a trend of a lower incidence of IVH compared to other registries. One possible explanation might be that the KNN registry contains population-based data whereas the ANZNN registry includes data from only tertiary centers.
In the present study, infants born at ≤22 gestational weeks had a strikingly high incidence of IVH (70%), while those with a birth weight ≤500 g had a similar incidence of IVH as those with a birth weight of 500-999 g. In the development of IVH, gestational age seems to be a more significant factor than birth weight, although both gestational age and birth weight influence IVH development. Consistent with previous studies, the present study also demonstrated that IVH incidence and severity in VLBW infants decreases gradually as gestational age or birth weight increases (16,22) and that infants born after 29 gestational weeks or weighing>1,250 g at birth are at a low risk of IVH.
PHH is a major consequence of IVH (23). PHH is thought to cause white matter injury by multiple mechanisms, such as disturbing periventricular fibers, increasing intracranial pressure, introducing iron-induced hydroxyl free radicals, and eliciting cytokine responses (23,24,25). Although the precise mechanism has not been completely delineated, the pathogenesis of IVH-induced PHH has been explained to be related with multiple small blood clots partially occlude the channels of CSF circulation or blood induced inflammation within subarachnoid spaces and ensuing obliterative arachnoditis which reduces CSF resorption (26,27,28,29). Previous studies suggested that PHH induces ischemia, oligodendrocyte and axonal injury in the white matter, and eventually motor or cognitive dysfunction (15,30,31). In the present study, 8.5% of survived infants with IVH of any grade showed progression of PHH and underwent spinal tapping, external ventricular drainage, and/or a shunt operation to remove the CSF. In the infants with severe IVH (grade 3-4), PHH or death developed in the 52.7% and 86.7% of infants with IVH grade 3 and 4, respectively. The incidence of PHH requiring a procedural intervention among survived VLBW infants with grade 3 or 4 IVH was similar to those of previous studies (2,14). One previous study suggested that the incidences of PHH and shunt operations have been increasing over time (15), a finding that might be related to an increased survival rate of VLBW infants with severe IVH and, thereby, an increased risk of PHH.
The optimal treatment strategy for PHH is not yet known, and the clinical management for PHH varies considerably among centers (28). There have been only a few arguments for early intervention to remove the CSF prior to the development of clinically significant PHH (32,33,34,35,36,37). We noted inconsistency in the interpretations among radiologists as well as variability in the PHH procedure rates among medical centers, indicating the need to establish a standardized guideline for the diagnosis and treatment of PHH (16). To date, only a few studies have reported the risk factors of PHH development after IVH (14,15). Most suggested that IVH severity would be the most important predictive factor for a shunt operation. Consistent with above findings, the present study also showed that about 3 fold higher development of PHH in infants with IVH grade 3 than infants with IVH grade 4. We also investigated the presence of other clinical factors affecting PHH development in VLBW infants with IVH grade 3 or 4. On univariate analysis, variables related with circulation, such as chest compressions at initial resuscitation, pulmonary hemorrhage, hypotension, and PDA operation differed significantly between the PHH and non-PHH groups. Although all factors except IVH grade 4 were no longer significant after adjustment, it is interesting that chest compressions at initial resuscitation occurred more frequently in the PHH group (P=0.05) and showed a high OR of 5.1 even after adjustment.
Mortality is a major consequence of severe IVH or PHH. Recent studies have reported that the mortality rate is approximately 30% in infants with IVH grade 3 and 60% in infants with IVH grade 4 (15,38), and the results of the present study support these findings. Furthermore, we analyzed the mortality rate primarily related to IVH and found that it was 3.6% in infants with grade 3 IVH and 15.6% in infants with grade 4 IVH. Because the KNN registry collected data about the primary cause of death, the present study could show the mortality rate that was primarily related to IVH. However, a possible limitation of the study could be that the combined diseases such as disseminated intravascular coagulopathy, BPD, PDA, and other mortality-associated variables could not be considered. The present study showed that the median time of death caused primarily by IVH was the 10th postnatal day, indicating that infants with severe IVH are in a generally deteriorated state. Another issue requiring consideration is that the withdrawal of life-sustaining treatment is often discussed with the parents of infants with severe IVH, although it is not clear whether this affected the survival time recorded in the KNN registry.
A major limitation of the present study is that the KNN registry has various criteria for the diagnosis of PHH as well as different guidelines for its treatment. However, this is a global problem that needs to be addressed. Because of the absence of a definite criteria, the KNN registry collected data for only PHH cases in which procedures to remove CSF were needed; therefore, we could not determine the overall incidence of PHH including non-treated cases.
In this context, early expired VLBW infants with severe IVH were excluded from the analysis for associated factors with PHH development. In addition, infants with IVH grade 2 were also excluded from this analysis because of the potential bias stems from infrequency of PHH development in survived infants with IVH grade 2.
In conclusion, the present study shows that IVH is a common problem in VLBW infants and that infants with severe IVH tend to develop PHH that requires procedures to remove the CSF. The present study is the first to report the incidence and mortality rates of IVH and PHH based on a Korean nationwide dataset. A subsequent study of the long-term neurodevelopmental outcomes of the infants in our study should be conducted.
Footnotes
Funding: This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2013-E63008-01).
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: All authors participated in designing the study as well as writing and revising the manuscript. All authors agree to this final version of the manuscript for publication.
References
- 1.Bassan H. Intracranial hemorrhage in the preterm infant: understanding it, preventing it. Clin Perinatol. 2009;36:737–762. v. doi: 10.1016/j.clp.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–F41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35:777–792. vii. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radic JA, Vincer M, McNeely PD. Temporal trends of intraventricular hemorrhage of prematurity in Nova Scotia from 1993 to 2012. J Neurosurg Pediatr. 2015;15:573–579. doi: 10.3171/2014.11.PEDS14363. [DOI] [PubMed] [Google Scholar]
- 6.Batton DG, DeWitte DB, Boal DK, Nardis EE, Maisels MJ. Incidence and severity of intraventricular hemorrhage: 1981-1984. Am J Perinatol. 1986;3:353–356. doi: 10.1055/s-2007-999896. [DOI] [PubMed] [Google Scholar]
- 7.Philip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics. 1989;84:797–801. [PubMed] [Google Scholar]
- 8.Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 2012;9:242–258. doi: 10.3171/2011.12.PEDS11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergani P, Patanè L, Doria P, Borroni C, Cappellini A, Pezzullo JC, Ghidini A. Risk factors for neonatal intraventricular haemorrhage in spontaneous prematurity at 32 weeks gestation or less. Placenta. 2000;21:402–407. doi: 10.1053/plac.1999.0499. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy CR, Ayers S, Campbell MJ, Elbourne D, Hope P, Johnson A. Randomized, controlled trial of acetazolamide and furosemide in posthemorrhagic ventricular dilation in infancy: follow-up at 1 year. Pediatrics. 2001;108:597–607. doi: 10.1542/peds.108.3.597. [DOI] [PubMed] [Google Scholar]
- 11.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer MJ, de Vries LS, Pistorius L, Rademaker KJ, Groenendaal F, Benders MJ. Ultrasound measurements of the lateral ventricles in neonates: why, how and when? A systematic review. Acta Paediatr. 2010;99:1298–1306. doi: 10.1111/j.1651-2227.2010.01830.x. [DOI] [PubMed] [Google Scholar]
- 13.Whitelaw A, Aquilina K. Management of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2012;97:F229–F233. doi: 10.1136/adc.2010.190173. [DOI] [PubMed] [Google Scholar]
- 14.Behjati S, Emami-Naeini P, Nejat F, El Khashab M. Incidence of hydrocephalus and the need to ventriculoperitoneal shunting in premature infants with intraventricular hemorrhage: risk factors and outcome. Childs Nerv Syst. 2011;27:985–989. doi: 10.1007/s00381-010-1387-4. [DOI] [PubMed] [Google Scholar]
- 15.Kazan S, Güra A, Uçar T, Korkmaz E, Ongun H, Akyuz M. Hydrocephalus after intraventricular hemorrhage in preterm and low-birth weight infants: analysis of associated risk factors for ventriculoperitoneal shunting. Surg Neurol. 2005;64:S77–S81. doi: 10.1016/j.surneu.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R Nichd Research Network. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:e1167–e1177. doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YS, Ahn SY, Park WS. The Establishment of the Korean Neonatal Network (KNN) Neonatal Med. 2013;20:169–178. [Google Scholar]
- 18.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 19.Canadian Neonatal Network. CNN 2013 Annual Report. [accessed on 1 April 2015]. Available at http://www.canadianneonatalnetwork.org/portal/
- 20.Australian & New Zealand Neonatal Network. Report of the Australian and New Zealand Neonatal Network 2012. [accessed on 1 April 2015]. Available at https://npesu.unsw.edu.au/data-collection/australian-new-zealand-neonatal-network-anznn.
- 21.EuroNeoNet. EuroNeoNet Annual Report for VLGAI and Individual Report for Each Unit Practicipating in the EuroNeoNet Project, YEAR 2011. [accessed on 1 April 2015]. Available at http://www.euroneonet.eu/Paginas/Publicas/euroNeo/euroNeoNet/ennet_documents.htm.
- 22.Singh R, Gorstein SV, Bednarek F, Chou JH, McGowan EC, Visintainer PF. A predictive model for SIVH risk in preterm infants and targeted indomethacin therapy for prevention. Sci Rep. 2013;3:2539. doi: 10.1038/srep02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallevi H, Walker KC, Kasam M, Bornstein N, Grotta JC, Savitz SI. Inflammatory response to intraventricular hemorrhage: time course, magnitude and effect of t-PA. J Neurol Sci. 2012;315:93–95. doi: 10.1016/j.jns.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin N Am. 2001;12:639–649. vii. [PubMed] [Google Scholar]
- 25.Whitelaw A, Thoresen M, Pople I. Posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2002;86:F72–F74. doi: 10.1136/fn.86.2.F72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 2012;3:25–38. doi: 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, Oh WI, Park WS. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44:497–504. doi: 10.1161/STROKEAHA.112.679092. [DOI] [PubMed] [Google Scholar]
- 28.Larroche JC. Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biol Neonate. 1972;20:287–299. doi: 10.1159/000240472. [DOI] [PubMed] [Google Scholar]
- 29.Hudgins RJ, Boydston WR, Hudgins PA, Morris R, Adler SM, Gilreath CL. Intrathecal urokinase as a treatment for intraventricular hemorrhage in the preterm infant. Pediatr Neurosurg. 1997;26:281–287. doi: 10.1159/000121207. [DOI] [PubMed] [Google Scholar]
- 30.Boillat CA, Jones HC, Kaiser GL, Harris NG. Ultrastructural changes in the deep cortical pyramidal cells of infant rats with inherited hydrocephalus and the effect of shunt treatment. Exp Neurol. 1997;147:377–388. doi: 10.1006/exnr.1997.6617. [DOI] [PubMed] [Google Scholar]
- 31.Wright LC, McAllister JP, 2nd, Katz SD, Miller DW, Lovely TJ, Salotto AG, Wolfson BJ. Cytological and cytoarchitectural changes in the feline cerebral cortex during experimental infantile hydrocephalus. Pediatr Neurosurg. 1990;16:139–155. doi: 10.1159/000120516. [DOI] [PubMed] [Google Scholar]
- 32.Limbrick DD, Jr, Mathur A, Johnston JM, Munro R, Sagar J, Inder T, Park TS, Leonard JL, Smyth MD. Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr. 2010;6:224–230. doi: 10.3171/2010.5.PEDS1010. [DOI] [PubMed] [Google Scholar]
- 33.de Vries LS, Liem KD, van Dijk K, Smit BJ, Sie L, Rademaker KJ, Gavilanes AW Dutch Working Group of Neonatal Neurology. Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in The Netherlands. Acta Paediatr. 2002;91:212–217. doi: 10.1080/080352502317285234. [DOI] [PubMed] [Google Scholar]
- 34.Lin JP, Goh W, Brown JK, Steers AJ. Neurological outcome following neonatal post-haemorrhagic hydrocephalus: the effects of maximum raised intracranial pressure and ventriculo-peritoneal shunting. Childs Nerv Syst. 1992;8:190–197. doi: 10.1007/BF00262843. [DOI] [PubMed] [Google Scholar]
- 35.Bruinsma N, Stobberingh EE, Herpers MJ, Vles JS, Weber BJ, Gavilanes DA. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin Microbiol Infect. 2000;6:202–206. doi: 10.1046/j.1469-0691.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 36.Johnson A, Wincott E, Grant A, Elbourne D. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation: results at 30 months. Arch Dis Child. 1994;71:F147. doi: 10.1136/fn.71.2.f147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anwar M, Kadam S, Hiatt IM, Hegyi T. Serial lumbar punctures in prevention of post-hemorrhagic hydrocephalus in preterm infants. J Pediatr. 1985;107:446–450. doi: 10.1016/s0022-3476(85)80532-1. [DOI] [PubMed] [Google Scholar]
- 38.Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K New South Wales and Australian Capital Territory Intensive Care Units' Data Collection. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133:55–62. doi: 10.1542/peds.2013-0372. [DOI] [PubMed] [Google Scholar]