Abstract
This study aimed to investigate current therapeutic strategies for patent ductus arteriosus (PDA) in very-low-birth-weight (VLBW) infants in Korea. A total of 2,254 VLBW infants among 2,386 from Korean Neonatal Network cohort born from January 2013 to June 2014 were included. No PDA was seen for 1,206 infants (53.5%) and the infants diagnosed or treated for PDA were 1,048 infants (46.5%). The proportion of infants with PDA was decreased according to the increase in gestational age (GA) and birthweight. Infants with PDA were divided into groups according to the therapeutic strategies of PDA: prophylactic treatment (PT, n = 69, 3.1%), pre-symptomatic treatment (PST, n = 212, 9.4%), symptomatic treatment (ST, n = 596, 26.4%), and conservative treatment (CT, n = 171, 7.6%). ST was the most preferred treatment modality for preterm PDA and the proportion of the patients was decreased in the order of PST, CT, and PT. Although ST was still the most favored treatment in GA < 24 weeks group, CT was more preferred than PST or ST when compared with GA ≥ 32 weeks group [CT vs. PST, OR 5.3, 95% CI 1.56-18.18; CT vs. ST, OR 2.9, 95% CI 1.03-8.13]. A total of 877 infants (38.9%) received pharmacological or surgical treatment about PDA, and 35.5% (801 infants) received pharmacological treatment, mostly with ibuprofen. Seventy-six infants (3.4%) received primary ligation and 8.9% (201 infants) received secondary ligation. Diverse treatment strategies are currently used for preterm PDA in Korea. Further analyses of neonatal outcomes according to the treatment strategies are necessary to obtain a standardized treatment guideline for preterm PDA.
Keywords: Very-Low-Birth-Weight Infant, Patent Ductus Arteriosus, Treatment, Korea
Graphical Abstract
INTRODUCTION
Failure of patent ductus arteriosus (PDA) constriction is common in extremely premature infants, affecting 60-70% of infants with less than 28 weeks of gestation (1). Failure to close PDA in the early postnatal days is associated with increased mortality (2) and serious morbidities including bronchopulmonary dysplasia (BPD) (3), periventricular hemorrhage (4), and necrotizing enterocolitis (NEC) (5). However, causality between these morbidities and failure to close PDA has not yet been proven. Because there are potential risks associated with pharmacologic treatment and surgical ligation, we have to carefully weigh the potential benefits of the pharmacological and/or surgical intervention against its significant risks when we decide on PDA management (6).
There are conflicting or inconclusive results on optimal treatment strategies for preterm PDA. Results from the few randomized controlled clinical trials and recent observational studies have not provided a consensus on PDA treatment (1,7,8,9,10). Many reports support the pharmacologic closure of symptomatic PDA and PDA ligation for cases of symptomatic PDA refractory to pharmacologic treatment (11). However, a few reports support conservative treatment due to a spontaneous closure rate of 73% without any specific treatment in infants born before 28 weeks of gestation (12). Diverse treatment strategies are still used, so the current management practice of preterm PDA in Korea has to be evaluated using a national cohort in order to obtain a standardized treatment guideline.
Korean Neonatal Network (KNN) database is the first nationwide prospective cohort of infants discharged from neonatal intensive care units (NICUs) whose birthweight is less than 1,500 g. We aimed to investigate current status of the therapeutic strategies for PDA in very-low-birth-weight (VLBW) infants in Korea using the KNN database. We assessed whether demographic and baseline characteristics are different between PDA and no PDA groups, and also between diverse therapeutic strategies for preterm PDA groups. We also assessed whether the proportion of infants diagnosed or treated as PDA and the proportion of PDA treatment strategies were different according to the gestational age (GA) and birthweight. Finally, we searched for a pattern of pharmacological and surgical treatment of preterm PDA in Korea.
MATERIALS AND METHODS
Study population
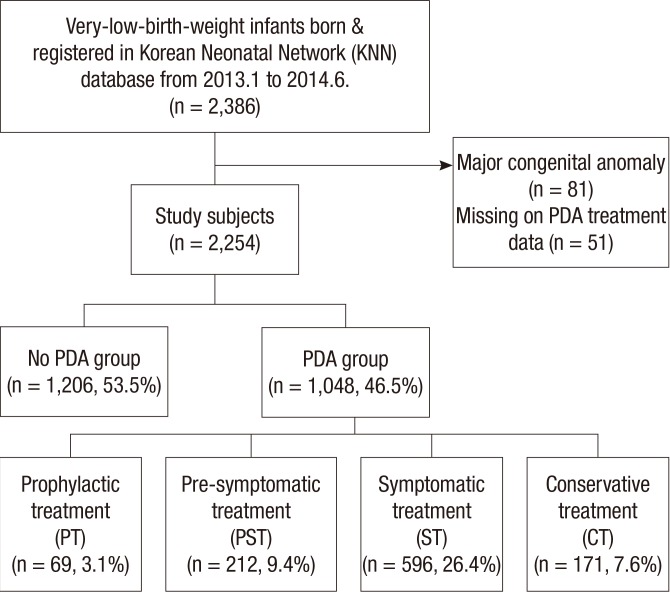
We identified a cohort of total 2,386 VLBW infants born between January 2013 and June 2014 that were registered with the KNN. Clinical data were prospectively recorded in the KNN database from 55 NICUs of the KNN participating hospitals as previously described (13) and analyzed retrospectively for the purposes of this manuscript. We excluded 81 infants due to any major congenital or chromosomal anomaly from analysis. Fifty-one infants were also excluded because of missing or mismatched information about the PDA treatment strategy and details of pharmacological or surgical treatment of PDA. A total of 2,254 VLBW infants were finally included in the analysis. Among these infants, we identified cases whose PDA closed spontaneously or had minimal ductal shunting before any signs and symptoms were attributable to PDA (defined as "no PDA group"). Subsequently, the remaining patients (we defined as "PDA group") were classified into 4 groups according to the therapeutic treatment strategy: prophylactic treatment group (PT), pre-symptomatic treatment group (PST), symptomatic treatment group (ST), and conservative treatment without medication or surgical intervention group (CT) (Fig. 1).
Fig. 1. Study population. A total of 2,254 very-low-birth-weight (VLBW) infants registered in the Korean Neonatal Network (KNN) from 2013.1. to 2014.6. were included. "No PDA group" was 1,206 patients (53.5%) and "PDA group" was 1,048 patients (46.5%).
First, we compared the baseline characteristics between the PDA group and no PDA group. We also compared the baseline characteristics in patients between 5 groups (no PDA, PT, PST, ST, and CT). After that, we examined the differences in the proportion of each PDA treatment strategy according to the GA and birthweight group. GA group was divided into 4 groups (<24 weeks, 24-27 weeks, 28-31 weeks, and ≥32 weeks) and birth-weight group was divided into 4 groups (<750 g, 750-999 g, 1,000-1,249 g, and 1,250-1,499 g). Finally, we searched for the treatment pattern of pharmacological and surgical treatment i.e., kinds of medicine, route of pharmacological treatment, postnatal days for the first day of pharmacological or surgical treatment.
Definitions
We defined "no PDA group" as the group of patients whose PDA had closed spontaneously or had minimal ductal shunting before any signs and symptoms were attributable to PDA.
PDA treatment strategies were defined according to the KNN manual of operation as follows: 1) PT, namely treatment of PDA was done without any clinical symptoms or diagnostic abnormalities in echocardiography or increased B-natriuretic peptide (BNP) suggestive of PDA; 2) PST, where PDA was confirmed by echocardiography or BNP and treatment of PDA was done without any clinical symptoms or signs attributable to PDA; 3) ST, as treatment of PDA was done because there were clinical symptoms by hemodynamically significant left to right shunt through PDA; and 4) CT, applied only conservative and supportive treatment without any pharmacological or surgical intervention for PDA although there were clinical symptoms of PDA.
Presence of clinical symptoms attributable to PDA was defined when there were 2 out of 5 symptoms caused by PDA as follows: 1) systolic or continuous murmur; 2) bounding pulse or hyperactive precordial pulsation; 3) hypotension; 4) respiratory difficulty; and 5) pulmonary edema or cardiomegaly (cardiothoracic ratio >60%) on chest radiograph.
Small for gestational age (SGA) was defined according to the definitions published by Olsen et al. (14). Pregnancy induced hypertension (PIH) was defined if there was any maternal diagnosis of eclampsia or preeclampsia. Delivery room (DR) resuscitation was defined when cardiac compression was done or medication was administered in the DR. Early sepsis was defined as a positive blood culture less than 7 days from birth. Primary ligation was defined when the patient received PDA ligation without any pharmacological treatment about preterm PDA. Secondary ligation was defined when the patient received PDA ligation after failing to close a PDA with pharmacological treatment. Postnatal days (PND) of first day of treatment about preterm PDA was defined as the difference between the date of first day of treatment and the date of birth. 0 day meant less than 24 hr from birth.
Statistical analysis
All the continuous variables were expressed as the mean±standard deviation (SD) and categorical variables were expressed as numbers and proportions. For analysis, we first compared the demographic and baseline characteristics between PDA group and no PDA group. Chi-square test and t-test were done to compare variables between PDA group and no PDA group. We also compared the baseline characteristics among 5 different treatment groups (no PDA, PT, PST, ST, and CT). The one-way analysis of variance (ANOVA) with Tukey test for multiple comparisons was used for continuous variables and chi-square test with Bonferroni correction was used for the analysis of categorical variables. We examined the proportion of different PDA treatment strategies according to the GA and birthweight groups by chi-square test. Multinomial logit model analysis was conducted to assess the preference of certain treatment strategies according to the GA and birthweight group. The statistical analysis was conducted using IBM SPSS Statistics version 20 (IBM Corp., Amarok NY, USA) and R version 3.1.2. (http://www.r-project.org). P values less than 0.05 were considered statistically significant.
Ethics statement
The KNN registry was approved by the institutional review board at each participating hospital and informed consent was obtained from the parents at enrollment in NICUs participating in the KNN.
RESULTS
Study groups
A total of 2,254 infants with birthweight <1,500 g born from January 2013 to June 2014 were included in our analysis. PDA that closed spontaneously or with minimal ductal shunting before any signs and symptoms were attributable to PDA (no PDA group) was observed in 1,206 (53.5%) infants. The 1,048 (46.5%) infants in whom PDA was diagnosed or who received PDA treatment (PDA group) were divided into study groups according to the therapeutic strategies of PDA: PT (69 infants, 3.1%), PST (212 infants, 9.4%), ST (596 infants, 26.4%), and CT (171 infants, 7.6%) (Fig. 1). ST was the most preferred treatment modality of preterm PDA, and infants received other treatment modalities in the order of PST, CT, and PT. A total of 877 infants (38.9%) of patients received pharmacological or surgical treatment about preterm PDA.
Demographic and baseline characteristics of groups according to the PDA treatment strategies
When compared with no PDA group, PDA group was younger and lighter at birth. Fewer infants were delivered by cesarean section in the PDA group. Maternal histologic chorioamnionitis was more prevalent; however, maternal PIH was less prevalent in PDA group when compared with no PDA group. One minute Apgar score and five minute Apgar score of at least 3 or less were more frequent in PDA group. More infants received surfactant and early sepsis was more prevalent in PDA group when compared with no PDA group.
All 4 subgroups were younger at birth when compared with no PDA group. PST and ST were older at birth when compared with CT. There was no difference in sex ratio between the 5 groups. When compared with no PDA group, histologic chorioamnionitis was more prevalent in ST and PIH was less prevalent in CT. SGA was less prevalent in all PDA subgroups when compared with no PDA group. Five minute Apgar score of at least 3 or less were more frequent in PT and ST. All PDA subgroups received more surfactants. Early sepsis was more prevalent in CT group. When compared with CT, fewer infants received cesarean section in PT. SGA was less prevalent in PT and five minute Apgar score of at least 3 or less were more frequent in PT. Surfactant was less frequently used in PST (Table 1).
Table 1. Patient characteristics according to the patent ductus arteriosus (PDA) treatment strategies in very-low-birth-weight infants in Korea.
| Characteristics | No PDA (n = 1,206) | PDA (n = 1,048) | PDA treatment strategies | |||
|---|---|---|---|---|---|---|
| PT (n = 69) | PST (n = 212) | ST (n = 596) | CT (n = 171) | |||
| % Total | 53.5 | 46.5 | 3.1 | 9.4 | 26.4 | 7.6 |
| % PDA | NA | 100.0 | 6.6 | 20.2 | 56.9 | 16.3 |
| GA (week)* | 29.6 ± 2.9 | 26.9 ± 2.6∥ | 26.5 ± 2.9† | 27.3 ± 2.5†,‡ | 27.1 ± 2.4†,‡ | 26.0 ± 2.7† |
| Birthweight (g)* | 1,177 ± 258 | 944 ± 270∥ | 943 ± 280†,‡ | 984 ± 260†,‡ | 966 ± 265†,‡ | 819 ± 265† |
| Male | 622 (51.6%) | 508 (48.5%) | 32 (46.4%) | 104 (49.1%) | 277 (46.5%) | 95 (55.6%) |
| Cesarean section* | 921 (76.4%) | 759 (72.4%)∥ | 42 (60.9%)†,‡ | 153 (72.2%) | 430 (72.1%) | 134 (78.4%) |
| Multiple gestation* | 435 (36.1%) | 383 (36.5%) | 23 (33.3%) | 61 (28.8%)‡ | 217 (36.4%)‡ | 82 (48.0%)† |
| HCA* | 309 (30.7%) | 338 (37.9%)∥ | 20 (31.3%) | 66 (33.3%) | 191 (40.1%)† | 61 (39.6%) |
| PIH* | 246 (20.4%) | 163 (15.6%)∥ | 6 (8.7%) | 35 (16.5%) | 104 (17.4%) | 18 (10.5%)† |
| Antenatal steroid, complete | 573 (48.6%) | 515 (50.0%) | 33 (48.5%) | 101 (48.1%) | 284 (48.6%) | 97 (57.4%) |
| SGA* | 404 (33.8%) | 189 (18.3%)∥ | 4 (6.3%)†,‡ | 39 (18.6%)† | 111 (18.6%)† | 35 (21.5%)† |
| DR resuscitation* | 53 (4.4%) | 52 (5.0%) | 2 (2.9%) | 2 (1.0%)‡,§ | 35 (6.0%) | 13 (7.6%) |
| 1 min AS ≤ 3* | 297 (24.8%) | 405 (38.9%)∥ | 35 (51.5%)† | 64 (30.3%)§ | 247 (41.8%)† | 59 (34.5%)† |
| 5 min AS ≤ 3* | 52 (4.3%) | 98 (9.4%)∥ | 18 (26.5%)†,‡,§ | 12 (5.7%) | 54 (9.1%)† | 14 (8.2%) |
| Surfactant use* | 798 (66.2%) | 996 (95.0%)∥ | 69 (100.0%)† | 195 (92.0%)†,‡ | 563 (94.5%)† | 169 (95.0%)† |
| Early sepsis* | 33 (2.7%) | 47 (4.5%)∥ | 2 (2.9%) | 6 (2.8%) | 26 (4.4%) | 13 (7.6%)† |
*P<0.05 in one-way ANOVA with Tukey or chi-square test with Bonferroni correction between no PDA, PT, PST, ST, and CT groups; †P<0.05 when compared with no PDA group; ‡P<0.05 when compared with CT group; §P<0.05 when compared with ST group; ∥P<0.05 in chi-square test or t-test between no PDA group and PDA group. PT, prophylactic treatment; PST, pre-symptomatic treatment; ST, symptomatic treatment; CT, conservative treatment without any intervention; PDA, patent ductus arteriosus; GA, gestational age at birth; HCA, histologic chorioamnionitis; PIH, pregnancy induced hypertension; SGA, small for gestational age; DR, delivery room; AS, apgar score, NA: not appropriate.
Proportion of patients according to the GA
Infants with PDA were 75.2%, 70.9%, 36.3%, and 12.3% of GA groups <24 weeks, 24-27 weeks, 28-31 weeks, and ≥32 weeks, respectively. The proportion of PDA group was decreased as the GA was increased (Table 2).
Table 2. Patent ductus arteriosus (PDA) treatment strategies according to the gestational age group.
| Treatment | Gestational age at birth (weeks) | Total (n = 2,254) |
|||
|---|---|---|---|---|---|
| <24 (n=113) |
24-27 (n=766) |
28-31 (n=1,043) |
≥32 (n=332) |
||
| No PDA group | 28 (24.8%) |
223 (29.1%) |
664 (63.7%) |
291 (87.7%) |
1,206 (53.5%) |
| PDA group | 85 (75.2%) |
543 (70.9%) |
379 (36.3%) |
41 (12.3%) |
1,048 (46.5%) |
| PT group | 11 (9.7%) |
31 (4.0%) |
24 (2.3%) |
3 (0.9%) |
69 (3.1%) |
| PST group | 10 (8.8%) |
107 (14.0%) |
84 (8.1%) |
11 (3.3%) |
212 (9.4%) |
| ST group | 35 (31.0%) |
307 (40.1%) |
233 (22.3%) |
21 (6.3%) |
596 (26.4%) |
| CT group | 29 (25.7%) |
98 (12.8%) |
38 (3.6%) |
6 (1.8%) |
171 (7.6%) |
*In multinomial logit model, the odds ratio (OR) of the ratio of CT vs. PST was 5.3 [95% confidence interval (CI) 1.56-18.18] and OR of the ratio of CT vs. ST was 2.9 (95% CI 1.03-8.13) in GA <24 weeks group when compared with GA≥32 weeks group. P<0.001 in 4 PDA treatment subgroups according to GA by chi-square test. PT, prophylactic treatment; PST, pre-symptomatic treatment; ST, symptomatic treatment; CT, conservative treatment; PDA, patent ductus arteriosus; GA, gestational age; OR, odds ratio.
The proportion of PDA treatment strategy was different between subgroups of GA in PDA group (Table 2, P<0.001). ST was the most favored treatment strategies in all GA subgroups. Although ST was still the most favored treatment in GA<24 weeks group, when compared with infants of GA≥32 weeks in multinomial logit model, the chance of choosing CT rather than PST or ST was higher [CT vs. PST, odds ratio (OR) 5.3, 95% confidence interval (CI) 1.56-18.18; CT vs. ST, OR 2.9, 95% CI 1.03-8.13]. When compared with infants with GA 28-32 weeks in multinomial logit model, the chance of choosing CT rather than PST or ST was also higher in GA<24 weeks group [CT vs. PST, odds ratio (OR) 6.4, 95% confidence interval (CI) 2.84-14.48; CT vs. ST, OR 5.1, 95% CI 2.79-9.26].
Proportion of patients according to the birthweight
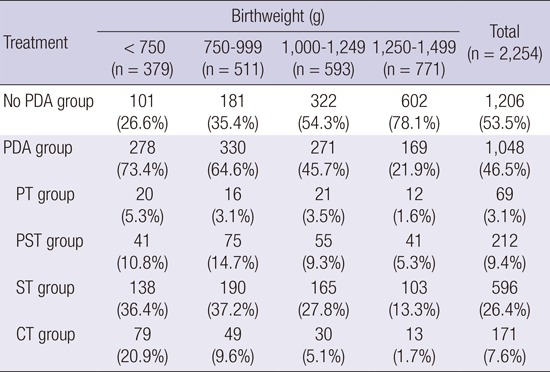
PDA group was 73.4%, 64.6%, 45.7%, and 21.9% of infants with birthweight <750 g, 750-999 g, 1,000-1,249 g, and ≥1,250 g, respectively. The proportion of PDA group was decreased according to the increase in birthweight (Table 3).
Table 3. Patent ductus arteriosus (PDA) treatment strategies according to the birthweight group.
| Treatment | Birthweight (g) | Total (n = 2,254) |
|||
|---|---|---|---|---|---|
| <750 (n=379) |
750-999 (n=511) |
1,000-1,249 (n=593) |
1,250-1,499 (n=771) |
||
| No PDA group | 101 (26.6%) |
181 (35.4%) |
322 (54.3%) |
602 (78.1%) |
1,206 (53.5%) |
| PDA group | 278 (73.4%) |
330 (64.6%) |
271 (45.7%) |
169 (21.9%) |
1,048 (46.5%) |
| PT group | 20 (5.3%) |
16 (3.1%) |
21 (3.5%) |
12 (1.6%) |
69 (3.1%) |
| PST group | 41 (10.8%) |
75 (14.7%) |
55 (9.3%) |
41 (5.3%) |
212 (9.4%) |
| ST group | 138 (36.4%) |
190 (37.2%) |
165 (27.8%) |
103 (13.3%) |
596 (26.4%) |
| CT group | 79 (20.9%) |
49 (9.6%) |
30 (5.1%) |
13 (1.7%) |
171 (7.6%) |
*In multinomial logit model, the odds ratio (OR) of the ratio of CT vs. PT was 3.6 (95% CI 1.45-9.17), OR of the ratio of CT vs. PST was 9.2 (95% CI 2.93-12.66), and the OR of the ratio of CT vs. ST was 4.5 (95% CI 2.39-8.62) in birthweight <750 g group when compared with birthweight 1,250-1,499 g group. P<0.001 in 4 PDA treatment subgroups according to the birthweight by chi-square test. PT, prophylactic treatment; PST, pre-symptomatic treatment; ST, symptomatic treatment; CT, conservative treatment without any intervention; PDA, patent ductus arteriosus; OR, odds ratio.
The proportion of PDA treatment strategy was different among subgroups of birthweight in PDA group (Table 3, P<0.001). ST was the most favored treatment strategy in all birthweight subgroups; however, the proportion of CT among 4 different PDA treatment strategy groups increased according to the decrease in birthweight. Although ST was still the most favored treatment in infants with birthweight <750 g group when compared with infants with birthweight 1,250-1,499 g group, CT was more preferred than PT, PST, and ST (CT vs. PT, OR 3.6, 95% CI 1.45-9.17; CT vs. PST, OR 9.2, 95% CI 2.93-12.66; CT vs. ST, OR 4.5, 95% CI 2.39-8.62). CT was also preferred in infants with birthweight 750-999 g group when compared with birthweight 1,250-1,499 g group (CT vs. PT, OR 2.8, 95% CI 1.08-7.41; CT vs. PST, OR 2.1, 95% CI 1.00-4.24; CT vs. ST, OR 2.0, 95% CI 1.06-3.94).
Pharmacological treatment of preterm PDA
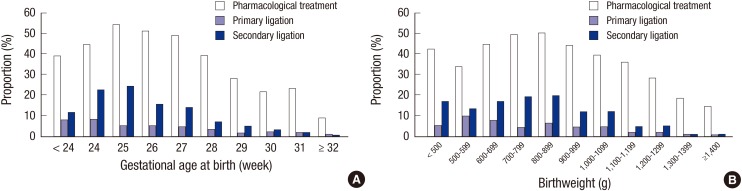
Eight hundred and one infants (35.5%) among the total VLBW infants in the KNN received pharmacological treatment. The proportion of infants with pharmacological treatment was different according to the GA (P<0.001, Fig. 2A) and birthweight (P<0.001, Fig. 2B).
Fig. 2. Preterm patent ductus arteriosus (PDA) treatment. Proportion of patients who received pharmacological (with or without surgery) and surgical treatment (both primary and secondary ligation) was decreased with the increase in gestational age and birthweight. (A) According to the gestational age, (B) According to the birthweight.
Most of the patients received ibuprofen as pharmacological treatment of PDA (787 infants, 98.2% of total patients with pharmacological treatment) and only 13 infants received indomethacin. In infants treated with ibuprofen, intravenous ibuprofen was used in 503 patients (62.8%), oral ibuprofen was used in 275 patients (34.3%) and both oral and intravenous ibuprofen was used in 9 infants (1.1%, Table 4).
Table 4. Pharmacological treatment of preterm PDA according to the PDA treatment strategies.
| PT group (n = 67) |
PST group (n = 209) |
ST roup (n = 525) |
Total (n = 801) |
||
|---|---|---|---|---|---|
| Ibuprofen | Total | 64 (95.5%) | 208 (99.5%) | 515 (98.1%) | 787 (98.2%) |
| Oral | 2 (3.0%) | 121 (57.9%) | 152 (29.0%) | 275 (34.3%) | |
| IV | 62 (92.5%) | 85 (40.7%) | 356 (67.8%) | 503 (62.8%) | |
| Oral+IV | 0 (0.0%) | 2 (0.9%) | 7 (1.3%) | 9 (1.1%) | |
| Indomethacin | 2 (3.0%) | 1 (0.5%) | 10 (1.9%) | 13 (1.7%) | |
| Others | 1 (1.5%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) |
PT, prophylactic treatment; PST, pre-symptomatic treatment; ST, symptomatic treatment; IV, intravenous; others, other medications used for treatment of preterm patent ductus arteriosus such as acetaminophen.
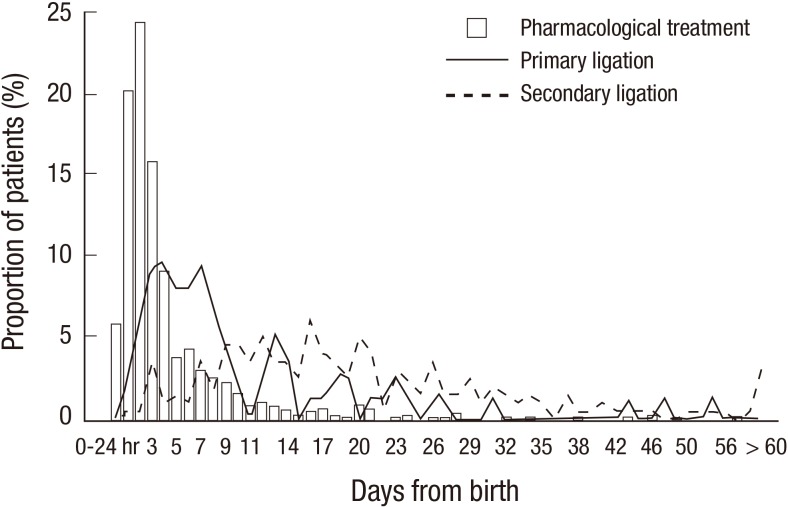
When we divided patients with pharmacological treatment according to the PDA treatment strategies, pharmacological treatment was done in 67 infants (97.1%) in PT, 209 infants (98.6%) in PST, and 525 infants (88.1%) in ST. Intravenous ibuprofen was used in 92.5% of PT, 40.7% of PST, and 67.8% of ST in infants with pharmacological treatment (Table 4). Mean postnatal days (PND) at the first dose of cyclooxygenase inhibitors treatment was 4.6±6.4 days in total patients (Fig. 3), and 0.7±1.3 days in PT, 3.2±4.3 days in PST, and 5.6±7.1 days in ST (P<0.001, Table 5). Secondary ligation was done in 6 infants (8.7%) in PT, 38 infants (17.9%) in PST, and 157 infants (26.3%) in ST, with significant differences among the 3 groups in univariate analysis (P=0.001).
Fig. 3. Proportion of patients according to the postnatal days of the first day of patent ductus arteriosus (PDA) treatment (Proportion of patients in each treatment modality: pharmacological treatment, primary ligation, and secondary ligation). Mean postnatal days at the first dose of pharmacological treatment was 4.6±6.4 days. Mean postnatal days of surgery was 10.9±10.4 days in primary ligation group and 21.3±16.2 days in secondary ligation group.
Table 5. Postnatal days of the first days of pharmacological treatment according to the different strategies of patent ductus arteriosus (PDA) treatment (days).
| PT group | PST group | ST group | Total | ||
|---|---|---|---|---|---|
| Ibuprofen* | Total | 0.7±1.3 | 3.2±4.3 | 5.6±7.2 | 4.6±6.4 |
| Oral | 0.0±0.0 | 3.7±5.1 | 7.4±9.0 | 5.7±7.7 | |
| IV | 0.7±1.3 | 2.6±2.8 | 4.8±6.1 | 4.0±5.5 | |
| Oral+IV | NA | 3.5±0.7 | 3.9±4.2 | 3.8±3.6 | |
| Indomethacin | 0.5±0.7 | 6.0 | 7.5±6.7 | 6.3±6.3 | |
| Others | 0.0 | NA | NA | 0.0 | |
| Total | 0.7±1.3 | 3.2±4.3 | 5.6±7.1 | 4.6±6.4 |
P<0.05 compared between oral, intravenous, and both oral and intravenous treatment of ibuprofen groups by one-way ANOVA test. PT, prophylactic treatment; PST, pre-symptomatic treatment; ST, symptomatic treatment; IV, intravenous; NA, not applicable; others, other medications used for treatment of preterm patent ductus arteriosus such as acetaminophen.
When we compared the baseline characteristic between intravenous ibuprofen group and oral ibuprofen group, intravenous ibuprofen was used in infants with younger GA (26.9±2.4 weeks vs. 27.8±2.5 weeks, P<0.001) and lower birthweight (943±259 g vs. 1,041±263 g, P<0.001) as compared with oral ibuprofen group. Intravenous ibuprofen was used in earlier PND (4.0±5.5 days vs. 5.7±7.7 days, P<0.001), when compared with oral ibuprofen group. There was no difference in the frequency of secondary ligation between intravenous and oral ibuprofen groups [128 infants (25.4%) vs. 62 infants (22.5%), P=0.368].
Surgical treatment of preterm PDA
A total of 277 infants (12.3%) received PDA ligation. Of which, 76 infants (3.4%) received primary ligation and 201 infants (8.9%) received secondary ligation. The proportion of infants with surgical treatment was different according to the GA (P<0.001, Fig. 2A). Mean PND was 10.9±10.4 days in infants with primary ligation and 21.3±16.2 days in infants with secondary ligation (P=0.024, Fig. 3).
DISCUSSION
Based on the KNN database, no PDA group was 53.5% and PDA group was 46.5% of the VLBW infants. Infants with pharmacological or surgical treatment of preterm PDA were 38.9% of the infants. Pharmacological treatment was done in 35.5% of VLBW infants and surgical treatment was done in 12.3% of VLBW infants. ST was the most preferred treatment modality for preterm PDA; however, more infants received CT rather than PST or ST in infants with lower GA and birthweight.
Ductus arteriosus in normal term infants usually constricts within 72 hr of birth. Most preterm infants >29 weeks GA achieve ductal contraction by day 4; however, failure rate of constriction was up to 70% of infants with GA <28 weeks and 80% in 24-25 weeks (6). Clyman et al. (15) reported that PDA was closed in 90% of infants ≥30 weeks GA on the day 4 and 98% at discharge. However, in infants with GA 24 weeks, 8% had PDA closed on day 4 and 13% on day 7 in his report. According to the birthweight in his report, PDA was closed in 35% of infants with birthweight 1,000-1,500 g on day 4, 67% on day 7, and 94% at discharge. In infants with birthweight <1,000 g, PDA was closed in 21% on day 4 and 34% on day 7. Recently, contrary to the previous reports, Rolland et al. (12) performed a retrospective cohort analysis of 103 infants with GA 24-27 weeks, and they reported that spontaneous closure was observed in 73% of infants without any specific treatment to close PDA. According to such concepts, quite a number of centers in Korea started to favor conservative treatment strategies.
There are several reports on the epidemiologic data of treatment of preterm PDA using large national cohort data. According to the National Institute of Children Health and Human Development and Neonatal Research Network (NICHD-NRN) data in infants with GA 22-28 weeks and birthweight <1,000 g between 2003 and 2007 (16), indomethacin therapy for PDA was done in 71% and surgical treatment of PDA was performed in 27% of the infants, which was a higher treatment rate than from the KNN data (49.0% infants with GA ≤28 weeks received pharmacological treatment and 20.0% infants received surgical treatment, data not shown in the results). According to the Canadian Neonatal Network database (17), of the infants with GA ≤32 weeks between 2004 and 2008, 3,673 (25%) infants were diagnosed with PDA. Laughon et al. (9) reported in a cohort study of infants born between 23 and 30 weeks gestation managed by Pediatrix Medical Group from 1997 and 2004 that 52% had no PDA, which was higher than the KNN data (44.6% of infants with GA ≤30 weeks had no PDA in our cohort).
Established treatments for PDA are prostaglandin H2 synthetase inhibitors (indomethacin and ibuprofen) and surgical ligation. Recently, the effects of paracetamol (acetaminophen) have been studied (18). PDA treatment can be classified according to the timing of treatment as prophylactic, pre-symptomatic, and symptomatic treatment. Currently, many neonatologists prefer supportive therapy alone in infants who appear to tolerate PDA awaiting spontaneous closure because of no clear evidence of benefits in neonatal outcomes and potential side effects. Also in our results with the KNN, CT was more preferred than PST or ST in infants with lower GA and birthweight, which suggests that quite a number of centers in Korea now favor CT.
For prophylactic treatment, most demonstrable benefit is a reduction in rates of intra-ventricular hemorrhage (IVH) in prophylactic indomethacin treatment. However, lack of improved long-term developmental outcome and mortality makes prophylactic indomethacin treatment less compelling (19). Prophylactic treatment of ibuprofen decreased the need for rescue treatment with cyclo-oxygenase inhibitors and decreased the need for surgical closure. However, prophylactic ibuprofen treatment exposes many infants to a drug that has potential renal and gastrointestinal side effects without any short-term benefits (20). Pre-symptomatic treatment with indomethacin showed a trend towards a reduction in chronic lung disease (CLD) without statistical significance on meta-analysis (21).
Laughon et al. (9) reported that in infants with GA ≤30 weeks managed by Pediatrix, 18% patients received prophylactic indomethacin, 16% received the indicated treatment after day 1 of birth, and 11% had a PDA without treatment. Our data indicates that PT was 3.7%, PST was 11.1%, ST was 31.1% and CT was 9.5% in infants with GA ≤30 weeks (data are not shown in results). In our cohort, fewer infants received PT and more infants received ST when compared with infants in Pediatrix group. According to the Canadian Neonatal Network data, for infants with GA ≤32 weeks between 2004 and 2008, 16% received conservative management. Hoellering et al. (22) performed a survey on the management of patent ductus arteriosus in Australia and New Zealand, and they reported that in infants with GA ≤28 weeks or birthweight ≤1,000 g, prophylactic treatment was done in 17%, pre-symptomatic treatment in 16% and echocardiographic targeted prophylaxis was done in 32% of the entire patient cohort reflecting a variation in management approach across Australia and New Zealand. Because the evidence that prophylactic or pre-symptomatic treatment of PDA improve neonatal outcomes in preterm infants is not sufficient, most Korean neonatologists hesitate to perform prophylactic treatment than neonatologists in other countries do.
Contrary to data from other countries, the most frequently used drug for PDA treatment was ibuprofen, because indomethacin is not supplied in Korea. Intravenous ibuprofen group was younger and lighter at birth when compared with oral ibuprofen group. Although there is some evidence that oral ibuprofen is more effective in closure of preterm PDA when compared with intravenous ibuprofen (23), as enteral feeding can be started in later days after birth and advanced slower in infants with younger GA, intravenous ibuprofen may be preferred in younger infants.
Proportion of patients who received primary ligation was 9% of infants with GA ≤32 weeks in the Canadian Neonatal Network (17). In a telephone interview of preferences and practices to the 136 Canadian neonatologist (24), 9% of neonatologists chose surgery as first-line treatment for infants, 4% choose it for infants 23-26 weeks' gestation, 2% for infants older than 21 days of age, and 2% for infants weighing less than 800 g. No neonatologist used surgery as a prophylactic treatment. Our data indicated that more infants received secondary ligation in ST when compared with PT and PST; however, multivariate analysis should be done to adjust for the risk factors of secondary ligation to assess the association between PDA treatment strategies and the frequency of secondary ligation.
According to our data, the mean PND of the first day of preterm PDA treatment was 4.6±6.4 days for pharmacological treatment. Mean PND at the first dose of cyclooxygenase inhibitor treatment was 0.7±1.3 days in PT, 3.2±4.3 days in PST, and 5.6±7.1 days in ST. For the association between the timing of pharmacological treatment for PDA and the risk of secondary surgery, death or bronchopulmonary dysplasia (BPD), Gudmundsdottir et al. (25) reported that intermediate start (postnatal days 3-6 days after birth) was not associated with any risk of BPD, while late PDA treatment (≥7 days) was associated with a lower BPD risk when compared with early start (0 to 2 days). So, further analysis about the relationship between the timing of PDA treatment and the neonatal outcomes are needed using the KNN database.
This study had several limitations. First, although it was the first epidemiologic study representing the current therapeutic strategies of preterm PDA in Korea, the number of patients included in this study were still small due to short inclusion period of the KNN cohort. More infants should be included for both epidemiologic and outcome analysis to obtain a standardized protocol on preterm PDA treatment. Second, when we checked whether the classification of PDA treatment strategy matched the other information such as pharmacological treatment and PDA ligation, there were several cases with discordant information. For example, some cases without any pharmacological or surgical treatment were coded as PT, PST or ST. We excluded such cases (2.3%) from the analysis, which was not a big portion of the total patient set. Third, because our cohort was based on the birthweight, and not on the GA especially for infants with GA >30 weeks, not all infants at each GA could be included in the analysis. Fourth, we could not obtain the information about the centers where a given treatment was performed, hence we could not assess the variation of treatment strategies according to the centers. Fifth, we defined prophylactic PDA treatment as treatment without any test for PDA regardless of the start time of the medication. Some reports defined prophylactic treatment only when treatment was done within 24 hr after birth (9,17), so the proportion of patients who received prophylactic treatment could differ according to the definition for prophylactic treatment.
This study was the first epidemiologic study on the current treatment practices of preterm PDA in VLBW infants in Korea. For VLBW infants, 46.5% were confirmed as preterm PDA or received treatment for PDA and although ST was the most preferred method, more infants received CT in lower GA and lower birthweight group. For a standardized treatment guideline for PDA, outcome analysis including in-hospital outcome and long-term neurodevelopmental outcome are needed for a more complete analysis. In addition, a multi-center, randomized controlled trial should be planned in order to more effectively and thoroughly assess the effect of treatment for PDA in preterm infants.
Footnotes
Funding: This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2013-E63008-01).
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design of the study: Lee JA, Choi BM. Acquisition of data: Lee JA. Statistical analysis: Oh S. First draft of the manuscript: Lee JA, Choi BM. Revision and critical review of the manuscript: Lee JA, Kim MJ, Choi BM. Manuscript approval: all authors.
References
- 1.Madan JC, Kendrick D, Hagadorn JI, Frantz ID, 3rd National Institute of Child Health and Human Development Neonatal Research Network. Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics. 2009;123:674–681. doi: 10.1542/peds.2007-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, Sekar K. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123:e138–e144. doi: 10.1542/peds.2008-2418. [DOI] [PubMed] [Google Scholar]
- 3.Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O'Shea TM. Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics. 1999;104:1345–1350. doi: 10.1542/peds.104.6.1345. [DOI] [PubMed] [Google Scholar]
- 4.Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F183–F186. doi: 10.1136/fn.75.3.f183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005;40:184–188. doi: 10.1097/00005176-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Heuchan AM, Clyman RI. Managing the patent ductus arteriosus: current treatment options. Arch Dis Child Fetal Neonatal Ed. 2014;99:F431–F436. doi: 10.1136/archdischild-2014-306176. [DOI] [PubMed] [Google Scholar]
- 7.Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. 1983;102:895–906. doi: 10.1016/s0022-3476(83)80022-5. [DOI] [PubMed] [Google Scholar]
- 8.Benitz WE. Patent ductus arteriosus: to treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012;97:F80–F82. doi: 10.1136/archdischild-2011-300381. [DOI] [PubMed] [Google Scholar]
- 9.Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. 2007;27:164–170. doi: 10.1038/sj.jp.7211662. [DOI] [PubMed] [Google Scholar]
- 10.Chorne N, Leonard C, Piecuch R, Clyman RI. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. 2007;119:1165–1174. doi: 10.1542/peds.2006-3124. [DOI] [PubMed] [Google Scholar]
- 11.Alan S, Karadeniz C, Okulu E, Kilic A, Erdeve O, Ucar T, Atasay B, Atalay S, Arsan S. Management of patent ductus arteriosus in preterm infants: clinical judgment might be a fair option. J Matern Fetal Neonatal Med. 2013;26:1850–1854. doi: 10.3109/14767058.2013.801956. [DOI] [PubMed] [Google Scholar]
- 12.Rolland A, Shankar-Aguilera S, Diomande D, Zupan-Simunek V, Boileau P. Natural evolution of patent ductus arteriosus in the extremely preterm infant. Arch Dis Child Fetal Neonatal Ed. 2015;100:F55–F58. doi: 10.1136/archdischild-2014-306339. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Ahn SY, Park WS. The establishment of the Korean Neonatal Network (KNN) Neonatal Med. 2013;20:169–178. [Google Scholar]
- 14.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 15.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36:123–129. doi: 10.1053/j.semperi.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirea L, Sankaran K, Seshia M, Ohlsson A, Allen AC, Aziz K, Lee SK, Shah PS Canadian Neonatal Network. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. 2012;161:689–694.e1. doi: 10.1016/j.jpeds.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, Canpolat FE, Dilmen U. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164:510–514.e1. doi: 10.1016/j.jpeds.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010:CD000174. doi: 10.1002/14651858.CD000174.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2011:CD004213. doi: 10.1002/14651858.CD004213.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003:CD003745. doi: 10.1002/14651858.CD003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoellering AB, Cooke L. The management of patent ductus arteriosus in Australia and New Zealand. J Paediatr Child Health. 2009;45:204–209. doi: 10.1111/j.1440-1754.2008.01461.x. [DOI] [PubMed] [Google Scholar]
- 23.Cherif A, Jabnoun S, Khrouf N. Oral ibuprofen in early curative closure of patent ductus arteriosus in very premature infants. Am J Perinatol. 2007;24:339–345. doi: 10.1055/s-2007-981853. [DOI] [PubMed] [Google Scholar]
- 24.Lai LS, McCrindle BW. Variation in the diagnosis and management of patent ductus arteriosus in premature infants. Paediatr Child Health. 1998;3:405–410. doi: 10.1093/pch/3.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudmundsdottir A, Johansson S, Håkansson S, Norman M, Källen K, Bonamy AK. Timing of pharmacological treatment for patent ductus arteriosus and risk of secondary surgery, death or bronchopulmonary dysplasia: a population-based cohort study of extremely preterm infants. Neonatology. 2015;107:87–92. doi: 10.1159/000367887. [DOI] [PubMed] [Google Scholar]