Abstract
Background
The purpose of this study was to determine the test characteristics of formal ultrasound when used to diagnose upper extremity soft tissue abscess in the setting of suspected infection.
Methods
We completed a retrospective chart review of all patients who had formal ultrasounds at our institution for the indication of diagnosing upper extremity abscess between July 2010 and July 2013. Using presence of purulence as the gold standard for diagnosis of abscess, we calculated the test characteristics of ultrasound. We then performed a series of logistic regression models with ultrasound being the independent variable of interest.
Results
Using search criteria consistent with upper extremity abscess, we identified 512 patients who underwent ultrasound examinations during our study period. Of these, 178 met the enrollment criteria. Ultrasound reports revealed 110 negative findings, 37 definitively positive findings, and 31 ambiguous findings. Forty-four patients had a final diagnosis of abscess, and 15 of these patients had negative or ambiguous ultrasounds. The sensitivity of definitively positive ultrasound was 65.9 %. The specificity was 94.0 %. Positive predictive value (PPV) of a definitively positive ultrasound result was 78.4 %, and negative predictive value (NPV) of a definitively negative result was 90 %. Logistic regression demonstrated a statistically significant association between definitively positive ultrasound and abscess, but no association between ambiguous ultrasound and abscess after adjustment for significant covariates.
Conclusions
Ultrasound is not a sensitive method to detect the presence of abscess in the setting of upper extremity infection. However, in this population of patients with suspected abscess, the negative predictive value was high with and without the inclusion of ambiguous results, suggesting reasonable utility of ultrasound as a rule-out test.
Level of Evidence
Diagnostic study, Level II
Keywords: Ultrasound, Soft tissue infection, Upper extremity abscess
Introduction
Over the past 20 years, soft tissue infections have become an increasing problem. Between 1993 and 2005, the incidence of emergency department (ED) visits for soft tissue infections doubled, reaching 3.4 million visits in 2005 [12]. Infections of the upper extremity in particular lead to significant morbidity if diagnosis is delayed or treatment is inadequate [13]. In some cases, presentation may include obvious fluctuance or drainage, suggesting a clear diagnosis of abscess. However, in many cases of soft tissue infection, it can be difficult to clinically distinguish between non-surgical cellulitis and abscess requiring drainage. Studies report clinical exam sensitivity for abscess ranging from 76 to 96 % [2, 8]. In an effort to improve rapid and accurate diagnosis of abscess, physicians often use imaging, including computed tomography, magnetic resonance imaging, and ultrasound [1, 4, 6, 16].
Ultrasound has gained popularity as a fast and cost-effective tool for evaluating soft tissue and musculoskeletal structures [3, 9]. However, studies that examine the use of ultrasound in diagnosis of abscess typically consist of bedside exams performed in the emergency department by clinicians with limited training in ultrasound performance and interpretation. While these studies have found sensitivities up to 98 %, they have also reported low specificities (67 to 69 %) [2, 4, 5, 8, 14, 15]. These operator-dependent results lead to difficulty with interpretation of the data and application of their results. Furthermore, these studies do not specifically investigate the hand and upper extremity, whose small potential spaces for infection are more challenging to image and assess. To our knowledge, no data exist regarding the diagnostic value of formal ultrasounds, done by trained ultrasound technicians and read by radiologists, in the diagnosis of purulent infections. These formal ultrasounds may be less operator-dependent compared to ultrasounds performed and interpreted by physicians with limited training. Understanding the testing circumstances and characteristics of ultrasound exams may improve surgeons’ ability to interpret ultrasound results and make treatment decisions.
Given the potential reliance upon musculoskeletal ultrasound findings to guide treatment decisions in the setting of soft tissue infections, it is important to determine the reliability of this test. The purpose of this retrospective chart review was to assess the test characteristics of formal ultrasound when used to differentiate abscesses from non-purulent infections in the upper extremity when there is clinical suspicion for an abscess. We defined formal ultrasound as one performed by a trained technician and read by an attending radiologist. We hypothesized that formal ultrasound would have a modest sensitivity but a higher specificity than has been previously reported in studies of bedside ultrasound.
Materials and Methods
Study Population and Design
After institutional review board approval, we performed a search of our hospital’s (a tertiary referral center) radiology database for all formal ultrasounds performed between July 2010 and July 2013. The ultrasound machines used for all studies were Philips IU22 (Philips Healthcare Bothel, WA). Our search criteria were use of at least one word from each of the following groups: “infection, abscess, fluid, collection,” and “shoulder, arm, forearm, wrist, hand, upper.” Exclusion criteria included repeat ultrasound examinations, arteriovenous fistula examinations, or ultrasound studies that were not primarily performed to evaluate the upper extremity.
Two investigators then completed chart abstractions for all eligible patients and collected information on age, gender, ultrasound result, clinical examination, laboratory findings, comorbidities, and final diagnosis.
Data Collection
Ultrasound results were recorded for all patients. They were determined by both abstractors to be positive, negative, or ambiguous. We recorded a positive result when the radiology report confirmed either abscess or drainable collection. We recorded a negative result when the report stated definitively that there was no drainable collection, or that a different diagnosis was present. We recorded an ambiguous result when the radiologist questioned abscess versus another diagnosis, or stated that findings were insufficient to either confirm or rule out abscess.
We reviewed laboratory values collected on each patient at the time point most closely predating the ultrasound exam to approximate the patient lab values at the time when the treating physician ordered the ultrasound. Laboratory data included white blood cell (WBC) count, percentage of neutrophils in the differential, and C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) when available.
Comorbidities of interest were those that are known to have an association with abscess [10], including diabetes, current IV drug use, and HIV infection. We did not consider a remote history of IV drug use to be a current comorbid factor. We used presence of pus either at time of aspiration or at time of drainage as the gold standard for diagnosis of abscess. We did not require culture-positive results, as some patients had received antibiotics prior to cultures being obtained. Our institution’s institutional review board approved this retrospective chart review.
Statistical Analysis
Following chart abstraction, we calculated the sensitivity and specificity of ultrasound for the diagnosis of abscess. These numbers were calculated twice: once by including ambiguous results in the negative test group, and again using ambiguous results in the positive test group. We then performed a logistic regression analysis using abscess as the dependent variable, and ultrasound as the independent variable of interest. In testing our other independent variables in this logistic regression model, we used significant variables when constructing our adjusted model. We calculated the odds ratio for ultrasound in a bivariate regression for our unadjusted mode, then calculated the odds ratio for our adjusted model. All analyses had an alpha of <0.05 for statistical significance.
Results
Our search through the radiology database yielded 512 ultrasound examinations. We excluded 97 studies because they were not performed on the upper extremity, 42 because they were done for the shoulder or axilla, 111 because they were not done to evaluate infection, 48 because they were done to evaluate infection adjacent to a vascular graft site, 22 because they were performed as follow-up exams, and 14 due to inadequate information being available from the electronic medical record. We have summarized demographic data and risk factors for the remaining 178 patients in Table 1. Table 2 presents data regarding the location of each ultrasound exam.
Table 1.
Patient demographics and risk factors
| Final diagnosis abscess | Final diagnosis other | All diagnoses | |
|---|---|---|---|
| Average age in years (range) | 35.2 (5–88) | 52.4 (3–91) | 48.2 (3–91) |
| Male gender (%) | 30 (68.2) | 62 (46.3) | 92 (51.7) |
| Female gender (%) | 14 (31.8) | 72 (63.7) | 86 (48.3) |
| Diabetes (%) | 9 (6.7) | 30 (22.4) | 39 (29.1) |
| Current IV drug use (%) | 13 (9.7) | 22 (16.4) | 35 (26.1) |
| HIV positive (%) | 2 (1.5) | 8 (6.0) | 10 (7.5) |
Table 2.
Location of suspected infection
| Final diagnosis abscess | Final diagnosis other | All diagnoses | |
|---|---|---|---|
| Arm (%) | 9 (20.5) | 18 (13.4) | 27 (15.2) |
| Elbow (%) | 3 (6.8) | 4 (3.0) | 7 (3.9) |
| Antecubital fossa (%) | 15 (34.1) | 30 (22.3) | 45 (25.3) |
| Forearm (%) | 10 (22.7) | 40 (29.9) | 50 (28.1) |
| Wrist (%) | 2 (4.5) | 26 (19.4) | 28 (15.7) |
| Hand (%) | 5 (11.4) | 16 (11.9) | 21 (11.8) |
We found that 44 patients had a final diagnosis of abscess, based on objective finding of purulence at incision or aspiration. Of these patients, 29 had a positive ultrasound, 11 had a negative ultrasound, and 4 had an ambiguous result. Figures 1, 2, and 3 demonstrate examples of negative, positive, and ambiguous findings, respectively. There were 37 definitively positive ultrasound results. In eight of the 37 positive ultrasounds, the final diagnosis was not an abscess. Thirty-one ultrasound results were ambiguous. These ambiguous results were read and interpreted by an attending radiologist, and the interpretation was neither confirmatory nor definitively negative that an abscess was present. The most common cause for an ambiguous report was a finding of “abscess versus hematoma.” Test characteristics for ultrasound examinations are displayed in Table 3.
Fig. 1.
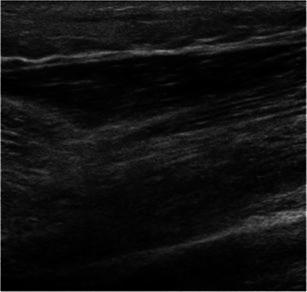
Ultrasound image demonstrating a negative finding. In this image, the tissue is generally hypoechoic, as in the case of cellulitis or tissue induration
Fig. 2.
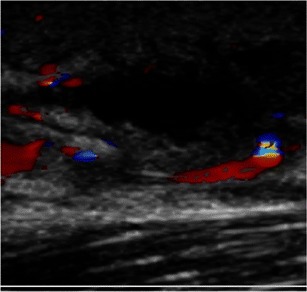
Ultrasound image demonstrating a positive finding. In this image, an anechoic area represents the presence of a discrete fluid collection within the tissue. Also noted is surrounding hyperemia/hyperemic rim
Fig. 3.
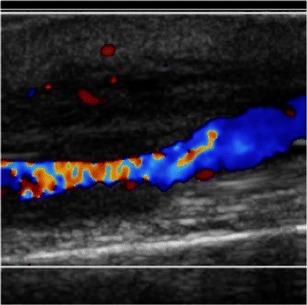
Ultrasound image demonstrating an ambiguous finding. Adjacent to a blood vessel is a relatively hypoechoic area of tissue. While this could represent an abscess, it may also be related to an inflamed vessel. The radiologist was not able to differentiate between these conditions
Table 3.
Test characteristics of ultrasound exam by result
| Definitively positive ultrasound (95 % confidence intervals) | Definitively positive or ambiguous ultrasound (95 % confidence intervals) | |
|---|---|---|
| Sensitivity | 65.9 % (50.1–79.5) | 75.0 % (59.7–86.8) |
| Specificity | 94.0 % (88.6–97.4) | 73.9 % (65.6–81.1) |
| Positive predictive value | 78.4 % (61.8–90.1) | 48.5 % (36.2–61.0) |
| Negative predictive value | 89.4 % (83.1–93.9) | 90.0 % (82.8–94.9) |
| Positive likelihood ratio | 11.0 (5.5–22.3) | 2.87 (2.1–4.0) |
| Negative likelihood ratio | 0.4 (0.2–0.5) | 0.3 (0.2–0.6) |
After completing a series of bivariate regressions, variables reaching significance included a definitive positive ultrasound result, age under 40 years, and white blood cell count over 10 × 1000/uL. We found that diabetes, HIV status, and current IV drug use, fever, and location of abscess were not significantly associated with a final diagnosis of abscess in the bivariate regression models. Table 4 displays the odds ratios for the finding of positive ultrasound in an unadjusted model and an adjusted model for age and WBC count.
Table 4.
Association of ultrasound results and abscess
| Ultrasound result | Unadjusted odds ratio | p value | Adjusted odds ratioa | p value |
|---|---|---|---|---|
| Definitively positive | 32.6 | <0.01* | 36.4 | <0.01* |
| Definitively positive and ambiguous | 8.5 | <0.01* | 10.8 | <0.01* |
| Ambiguous | 1.3 | 0.64 | 2.2 | 0.40 |
aAdjusted for age <40 and WBC >10 × 1000/uL
*p < 0.05
Discussion
The purpose of this study was to determine the diagnostic utility of formal ultrasound for patients with suspicion for hand and upper extremity abscesses. We found that ultrasound had only a modest sensitivity for detecting abscess. It was important to note that of the 44 patients with final diagnosis of abscess; only 29 of these patients had definitively positive ultrasound results. Ambiguous results were common (17.4 % of cases), and treating them as positive results increased sensitivity to only 75.0 % while reducing specificity from 94.0 to 73.9 %. Logistic regression analysis further showed that there was no association between ambiguous ultrasound results and abscess. This suggests that ambiguous results do not provide useful information for clinical decision-making. While the final radiology results were the interpretation of a single radiologist, we believe that the report of a trained radiologist may be less operator-dependent than a report by a physician with limited training, who is also guided by clinical patient characteristics. The finding of a high number of ambiguous results is a notable outcome. Other studies, which discuss operator-dependent bedside ultrasounds, do not comment on ambiguous findings. Given that there are a variety of conditions which may present similarly on ultrasound, we felt that the relatively high rate of ambiguous ultrasounds should be considered important by the clinician when ordering a formal ultrasound exam or acting on the results.
In this population of patients with suspected infection, the incidence of abscess was low. In this setting, ultrasound had a low positive predictive value, suggesting that ultrasound is a poor stand-alone test for diagnosing an abscess. Ultrasound did have a high negative predictive value that was not affected by inclusion of ambiguous results (90.0 and 89.4 %), which supports its use as a rule-out test in patients with suspected upper extremity abscess.
It is notable that the comorbidities commonly associated with a high risk of infection, including diabetes, HIV status, and current IV drug use, were not significantly associated with a final diagnosis of abscess in the bivariate regression models. Several factors may explain this. There may not have been enough patients in these subgroups to detect a significant association. Further, the patients in this group had intermediate concern for abscess, and the clinician desired further imaging prior to surgical decision-making. In this group, it is possible that high-risk patients, such as those with comorbidities, will have been included in this group despite other reassuring clinical characteristics. Our results suggest that in a group of patients with suspected abscess and moderate suspicion, comorbidities are not predictive of abscess.
Much of the existing literature regarding the ultrasound diagnosis of an abscess comes from the use of bedside ultrasound in the ED. One study, performed in the pediatric emergency setting, found a 97.5 % sensitivity of ultrasound, compared to only 78.7 % based on clinical exam. However, specificity of ultrasound in this study was only 69.2 % [8]. A prospective study performed in an adult emergency room examined ultrasound results done after a 30-min teaching course on the use of ultrasound. This study found a 98 % sensitivity when physicians combined ultrasound with clinical exam [14]. A similar study that enrolled 40 patients after emergency room clinicians underwent a 2-day course on the use of ultrasound demonstrated a sensitivity of 97 % and a specificity of 67 % [2]. These studies are limited by the nature of bedside ultrasound as an operator-dependent function. Compared to our findings, sensitivity was higher but specificity was lower. This may be due to the clinician training, which is potentially less able to differentiate between abscess and other abnormal findings. While ultrasound is noninvasive and rapid, it is a technical skill that requires training and experience to perform and interpret with accuracy. A study regarding the competence of radiology residents found that even after 200 cases, their competence at performing and interpreting ultrasound was low, with only a 16 % pass rate [7]. These studies draw into question whether physicians can perform bedside ultrasound reliably after a single, short training session. Further, available studies of bedside ultrasound do not focus specifically on upper extremity infection, which poses a unique set of challenges, given the complex anatomy and potential for closed-space infections.
The importance of ambiguous ultrasound results has not previously been highlighted in the literature. Ultrasound findings of abscess can include an anechoic mass, septations, gas, or fluid shift. However, in some cases, an abscess can appear sonographically identical to hematoma, solid mass, or cellulitis [11, 16]. Given the variety of pathology that can appear similar to an abscess, it is not surprising that a concrete diagnosis is not always achieved. In cases that are difficult or impossible for a radiologist to definitively interpret as cellulitis, hematoma, or abscess, clinical judgment is required for final decision-making. Clearly, in this series, 15 patients received some form of aspiration or debridement despite negative or ambiguous ultrasounds, indicating that clinical decision-making in some cases overrules ultrasound findings. However, when a clinician seeks to use imaging to guide clinical decisions, ultrasound provides a cost-effective and radiation-free modality compared to CT scan or MRI.
We found that treating ambiguous ultrasound results as positive leads to a positive predictive value of less than 50 %, indicating that ambiguous results are not clinically helpful, and in this setting should not be considered positive. Due to the low sensitivity of ultrasound for identifying an abscess and the poor utility of ambiguous results, we suggest that in situations of continued clinical suspicion after a negative ultrasound result, physicians consider other diagnostic modalities.
This study has several limitations. We performed a retrospective chart review, rather than a prospective assessment. In some cases, limited data was available regarding clinical exam or decision-making. We did analyze clinical data including labs, presence of fever, and risk factors in an effort to clarify the factors leading to decision to order ultrasound. Additionally, although we defined presence of purulence at aspiration or incision and debridement as the gold standard for abscess, many patients did not have these procedures, making it possible that some patients included in this study had a missed diagnosis. However, we did ensure that patients without diagnosis of abscess did not return for a second evaluation after initial ultrasound. Finally, the finding of ambiguous results was not able to be tested with inter-observer data, as a single attending radiologist provided the final read on all studies.
We found that the use of formal ultrasound in the workup of an upper extremity infection may not be useful in confirming the diagnosis of an abscess, but may be a useful test to rule out an abscess. We found that ambiguous results were common and were not associated with a final diagnosis of an abscess. Given the low sensitivity and the high incidence of ambiguous results, we suggest that if clinical suspicion remains high after a negative or ambiguous ultrasound, physicians should consider other diagnostic options in the workup of clinically suspected upper extremity infection.
Acknowledgments
Conflict of Interest
Andrea Haleim declares that she has no conflict of interest.
Yushane Shih declares that she has no conflict of interest.
Seth D. Dodds declares that he has no conflict of interest.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was not obtained for participants, as no identifying information is included in this article. IRB approval was obtained prior to data collection and analysis.
Contributor Information
Andrea Halim, Phone: (914)299-4096, Email: Andrea.Halim@gmail.com.
Yushane Shih, Email: Celestine.Shih@yale.edu.
Seth D. Dodds, Email: Seth.Dodds@yale.edu
References
- 1.Beauchamp NJ, Jr, Scott WW, Jr, Gottlieb LM, Fishman EK. CT evaluation of soft tissue and muscle infection and inflammation: a systematic compartmental approach. Skelet Radiol. 1995;24(5):317–24. doi: 10.1007/BF00197058. [DOI] [PubMed] [Google Scholar]
- 2.Berger T, Garrido F, Green J, Lema PC, Gupta J. Bedside ultrasound performed by novices for the detection of abscess in ED patients with soft tissue infections. Am J Emerg Med. 2012;30(8):1569–73. doi: 10.1016/j.ajem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: the orthopedic stethoscope. World J Orthod. 2011;2(2):13–24. doi: 10.5312/wjo.v2.i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau CL, Griffith JF. Musculoskeletal infections: ultrasound appearances. Clin Radiol. 2005;60(2):149–59. doi: 10.1016/j.crad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Gaspari R, Dayno M, Briones J, Blehar D. Comparison of computerized tomography and ultrasound for diagnosing soft tissue abscesses. Crit Ultrasound J. 2012;4(1):5. doi: 10.1186/2036-7902-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harish S, Chiavaras MM, Kotnis N, Rebello R. MR imaging of skeletal soft tissue infection: utility of diffusion-weighted imaging in detecting abscess formation. Skelet Radiol. 2011;40(3):285–94. doi: 10.1007/s00256-010-0986-1. [DOI] [PubMed] [Google Scholar]
- 7.Hertzberg BS, Kliewer MA, Bowie JD, et al. Physician training requirements in sonography: how many cases are needed for competence? AJR Am J Roentgenol. 2000;174(5):1221–7. doi: 10.2214/ajr.174.5.1741221. [DOI] [PubMed] [Google Scholar]
- 8.Iverson K, Haritos D, Thomas R, Kannikeswaran N. The effect of bedside ultrasound on diagnosis and management of soft tissue infections in a pediatric ED. Am J Emerg Med. 2012;30(8):1347–51. doi: 10.1016/j.ajem.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson JA. Musculoskeletal ultrasound: focused impact on MRI. AJR Am J Roentgenol. 2009;193(3):619–27. doi: 10.2214/AJR.09.2841. [DOI] [PubMed] [Google Scholar]
- 10.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341(25):1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 11.Loyer EM, DuBrow RA, David CL, Coan JD, Eftekhari F. Imaging of superficial soft-tissue infections: sonographic findings in cases of cellulitis and abscess. AJR Am J Roentgenol. 1996;166(1):149–52. doi: 10.2214/ajr.166.1.8571865. [DOI] [PubMed] [Google Scholar]
- 12.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA., Jr Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–8. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel JD, Szabo RM. A protocol for the treatment of severe infections of the hand. J Hand Surg Am. 1988;13(2):254–9. doi: 10.1016/S0363-5023(88)80060-1. [DOI] [PubMed] [Google Scholar]
- 14.Squire BT, Fox JC, Anderson C. ABSCESS: applied bedside sonography for convenient evaluation of superficial soft tissue infections. Acad Emerg Med Off J Soc Acad Emerg Med. 2005;12(7):601–6. doi: 10.1111/j.1553-2712.2005.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 15.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59(2):e10–52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 16.Struk DW, Munk PL, Lee MT, Ho SGF, Worsley DF. Imaging of soft tissue infections. Radiol Clin N Am. 2001;39(2):277–303. doi: 10.1016/S0033-8389(05)70278-5. [DOI] [PubMed] [Google Scholar]


