Abstract
Background
Osteoarthritis of the trapeziometacarpal joint (TMJ) is a common condition causing significant disability. The aim of this study is to ascertain whether an intra-articular corticosteroid injection leads to pain relief and increased function and what is the duration and magnitude of this effect.
Methods
A systematic review with a critical appraisal of identified studies that met the inclusion criteria was performed. Two authors performed the literature review by independently searching the Cochrane, PubMed and Google Scholar databases.
Results
A total of 118 relevant articles were found, but only nine studies met the inclusion criteria which included 4 double-blinded randomised control trials (RCTs) and 5 prospective case series. There is some evidence in the literature to support the efficacy of steroid injections into the TMJ. Most studies do suggest a good short-term benefit. However, one identifies no benefit over placebo but two studies found a benefit lasting at least 6 months.
Conclusions
This study demonstrates that there are potentially significant although short-term benefits to be gained from steroid injections into the TMJ. They can lead to pain relief and improved function, certainly in the first 1 to 3 months post-injection. Steroid injections are a low-risk procedure and are helpful in delaying or avoiding the need for surgery.
Keywords: Intra-articular injection, Trapeziometacarpal joint, Corticosteroid
Background
Osteoarthritis of the basal thumb joint or TMJ is a common condition causing significant disability [9]. The prevalence of TMJ osteoarthritis increases with age and reaches 91 % in patients older than 80 years [29]. The condition is more common in women, particularly in those who are post-menopausal [9, 29]. A quarter of post-menopausal women have radiological evidence of TMJ osteoarthritis although most are asymptomatic [1].
The main symptoms of TMJ osteoarthritis are pain and stiffness at the base of the thumb. The thumb performs a very important role in the hand, enabling grip and pinch movements.
The TMJ is a saddle shaped joint that allows the extensive movements thumb function requires. There is very little inherent bony stability, and the joint relies on a series of ligaments to provide a constrained range of motion.
The aetiology of TMJ osteoarthritis is complex. It stems from weakness or laxity of the basal thumb joint ligaments causing instability and subluxation. In time, this leads to degeneration of articular cartilage. Joint laxity in the first 30 to 40 years of life leads to osteoarthritis in later years.
A range of non-operative and operative management options can be used to treat TMJ osteoarthritis [34, 35]. A range of surgical techniques have been employed over the years, but the mainstay is trapeziectomy [6]. This can be performed with or without ligament interposition, although this may confer no additional benefit [34]. Other surgical options include joint replacement [24] or fusion [14].
A Cochrane review to examine conservative management of TMJ osteoarthritis is currently in progress [4]. First-line treatments include activity modification, analgesic and anti-inflammatory tablets, physiotherapy, acupuncture and splints to support the thumb [35].
When these first-line treatments cease to control symptoms, intra-articular injections can be considered. Corticosteroid is the most frequently injected substance, and this technique has been employed with some success for at least 40 years [3, 19]. The aim is to ease pain by reducing inflammation. It is a commonly used treatment, even though its effectiveness is not well audited and the currently available literature has significant limitations [2, 5, 11, 13, 15, 17, 20, 30, 32]. Alternatively, hyaluronic acid may be injected into the TMJ [2, 11].
A corticosteroid injection such as 40 mg of triamcinolone acetonide can be used with or without a local anaesthetic, for example 0.5 ml 1 % lidocaine. The injections may be performed with fluoroscopic X-ray guidance using a mini C-arm image intensifier (Fig. 1). A significant proportion (17 to 42 %) of needle tips may be extra-articular in situations where fluoroscopy is not employed [10, 25]. In spite of this, some practitioners do not advocate using radiological guidance to deliver the injection. Even with accurate intra-articular placement, significant extravasation of injectate (25 %) can occur into the soft tissues demonstrated by the use of radiopaque dye in cadaveric specimens [25]. Success with correct placement may depend on experience [18]. Recent advances in imaging availability have seen an increase in the use of ultrasound for guided injections. The technique of ultrasound-guided CMCJ injections has been demonstrated in cadaveric models with a high intra-articular accuracy rate (94 %) and avoids exposure of the patient and operator to ionising radiation [33].
Fig. 1.

X-ray image of needle within TMJ
The injections can be painful, and the amount and duration of pain relief are unpredictable [2, 5, 11, 13, 15, 17, 20, 30, 32]. Response can vary with the severity of osteoarthritis which can be graded using the Eaton classification [8]. There is some evidence that patients with minimal arthritic changes on plain radiographs may benefit more and for a longer duration compared to those with more severe arthritic changes [30]. Complications are rare but potentially include local depigmentation, fat atrophy or infection of the joint [16, 22, 27, 31].
The aim of this study was to assess and critique the available evidence in the literature behind the use of intra-articular corticosteroid injections for the management of trapeziometacarpal joint osteoarthritis.
Methods
The research question was
Does an intra-articular corticosteroid injection for trapeziometacarpal joint osteoarthritis in adults lead to pain relief and increased function, and what is the duration and magnitude of this effect?
A systematic review of the literature is presented relevant to this topic.
Two authors (AF and MGS) independently searched the Cochrane, PubMed, and Google Scholar databases. The search strategy involved the combination of key words (Osteoarthritis, Trapeziometacarpal joint or carpometacarpal joint or basal thumb joint, steroid or corticosteroid injection, pain or hand function). The search keywords were derived from the clinical question and the two databases. The dates of the search were limited to the end of June 2010.
The Cochrane database was searched using all terms in the Title, Abstract or Keywords. PubMed was searched using the clinical queries tool for a broad search in the therapy category. The bibliographies of the eligible articles were also reviewed to discover any other relevant papers.
The following inclusion criteria were applied to the identified studies:
Injection of corticosteroid with or without local anaesthetic into the TMJ for osteoarthritis
Assessment of outcome as either pain relief and/or functional improvement
The following exclusion criteria were applied:
Non-human studies
Not published in English
Studies including injection into other joints
Descriptive studies or reviews
Studies not directly assessing response to steroid injections
Studies involving surgical interventions
The results were then combined to identify the most relevant studies. Independent analysis by the two authors was performed for each study using a Critical Appraisal Skills Programme (CASP) tool from the NHS Public Health Resource Unit, UK [26]. Randomised control trials (RCTs) were appraised using the CONSORT statement checklist [21, 28]. Both the CASP tool and the CONSORT checklist consist of a number of criteria that can be used to assess any study. These help ascertain the study’s precision when answering the research question. The checklists are a guide to assess a study’s thoroughness, validity and applicability.
Results
The PubMed database identified 14 clinical studies and one systematic review. The review was an RCT [11] and was therefore included. From the 14 clinical studies, seven were included—three RCTs and four case series. The reasons for excluding the other seven studies are as follows:
Studies including injection into other joints (n = 2)
Descriptive studies or reviews (n = 2)
Studies not directly assessing response to steroid (n = 2)
Studies involving surgical interventions (n = 1)
The Cochrane database found four clinical trials, all previously identified by the PubMed database search.
Google Scholar identified 121 studies. Of these, five studies could be included. Four of these studies had been previously identified, and one further case series was unique. The reasons for exclusion of the other 116 studies are as follows:
Descriptive studies or reviews (n = 33)
Studies involving surgical interventions (n = 54)
Studies not directly assessing response to steroid (n = 15)
Not relevant (n = 14)
In total, the literature search revealed nine relevant studies (Fig. 2). Four studies were double-blind RCTs [2, 11, 20, 30], and five were prospective case series [5, 13, 15, 17, 32].
Fig. 2.
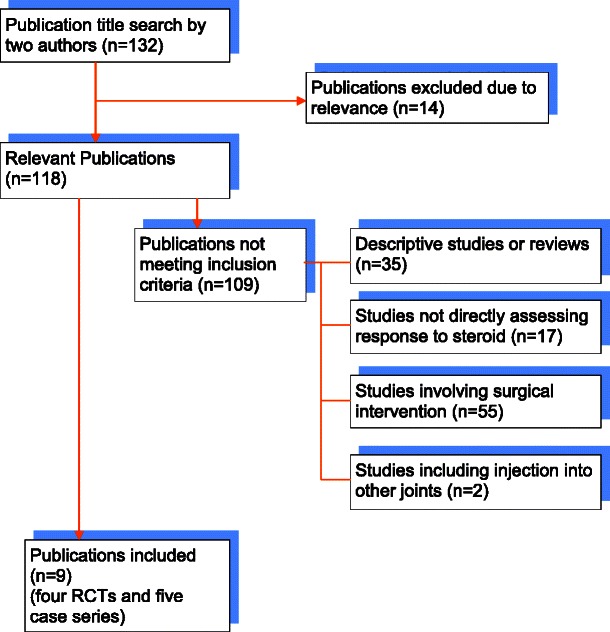
Flow diagram of search results and study inclusion
It was not possible to perform a formal meta-analysis of this topic from the identified papers because of the variation in practice and reporting. In addition to this, four of the studies were retrospective case series. A meta-analysis is not usually performed on this type of study due to their uncontrolled observational nature. A summary of the findings from the identified papers is displayed in Table 1.
Table 1.
Summary of the findings from identified papers
| Size | Patient demographics | Adequate power calculation | Radiologically guided | Outcome measures | Results | Conclusion | ||
|---|---|---|---|---|---|---|---|---|
| Mean age | M:F | |||||||
| [2] | 20 | 62.9 (52 to 84) | 0:20 | No | No | VAS, pinch + grip strength, hand function | VAS decrease to 12 months, grip improved to 3 months, no change in pinch, function improved to 6 months | Significant benefit to at least 6 months |
| [11] | 22 | 63 (48 to 85) | 2:20 | Yes | No | VAS, DASH, ROM, pinch + grip strength | VAS decrease only at 4 weeks, lower DASH to 26 weeks, ROM no change, slight pinch decrease and grip increase | Small, short-term improvement |
| [20] | 20 | 60.6 (41 to 71) | 1:19 | No | Yes | VAS, tenderness, stiffness, global assessment | No change in VAS, stiffness or tenderness. Global assessment improved to 12 weeks but not 24 | Steroid has no benefit over placebo in moderate to severe OA |
| [30] | 25 | 62 (50 to 91) | 4:21 | No | No | Pain, pinch + grip strength, hand function | Pain relief for 1 month, grip strength improvement to 6 months | Short-term pain relief |
| [5] | 30 | 61 (41 to 80) | 3:27 | No | No | VAS, DASH, pinch + grip strength | 17/30 had no improvement, 12/30 had sustained improvement to 18 months | Sustained pain relief in Eaton 1, may help 2 and 3, but unlikely for 4 |
| [13] | 25 | Unknown | 3:22 | No | No | VAS, Health Assessment Questionnaire | VAS decreased at 1 month but not beyond | Limited though well tolerated and safe, effective in short term |
| [15] | 40 | 65.1 (53 to 81) | 11:29 | No | No | VAS, DASH, subjective reporting | VAS, DASH and subjective all improved short-term. Sustained benefit up to 6 months only in early OA | Beneficial 4–6 months in early OA, only 4 weeks if advanced |
| [17] | 41 | 60 (39 to 83) | 10:31 | No | Yes | VAS, hand function | Short-term benefit in three quarters, longer-term benefit in one quarter | Short-term pain relief, not effective long-term |
| [32] | 83 | 62 (41 to 93) | 23:60 | No | Yes | VAS, subjective reporting | Two-thirds reported improvement at 2 months, one sixth had benefit at 6 months. VAS lower at 1 month | Benefit over short-term, irrespective of severity of OA |
Critical Appraisal
The four RCTs [2, 11, 20, 30] are considered first because they provide more powerful level 1b evidence [21]. The other five studies [5, 13, 15, 17, 32] are prospective case series, providing only level 4 evidence [23]. The case series are subject to selection bias with the consequence that the results may not be applicable to all patients. A critical appraisal of the nine studies follows.
Bahadir et al. [2]
This study is an evaluator-blinded randomised controlled trial, although this is not stated in the title. The abstract does clearly summarise the study, with the methods, findings and conclusion all briefly detailed. There is sufficient background information in the paper although the hypothesis is a little vague, stating the aim was ‘to provide more data on the clinical efficacy’ (of the injections).
The eligibility and exclusion criteria for the study are apparent, with 40 female patients identified with radiological and clinical criteria of TMJ osteoarthritis. Only Eaton [8] grades 2 or 3 were included, and previously injected patients were excluded. The study site is not presented nor the time period.
The patients were randomised to receive an intra-articular injection of either 20 mg triamcinolone acetonide or 5 mg sodium hyaluronate, delivered by the same investigator throughout, to minimise variation in injection technique. Post-intervention procedures are well described. The study did not allow patients to use any form of analgesia tablets or non-steroidal anti-inflammatory drugs or to wear a splint during the follow-up period. This is commendable for the purposes of the study to truly understand the effect of the injection alone, removing possible confounding factors.
Whilst the authors describe the outcome measures with careful explanation of the methods and timings of assessment, there is no mention of primary or secondary outcome measures. The clinician performing the assessment was blinded to the intervention given to the patient. However, there is no mention that the patient was blinded and it is possible that the patient was aware which group they were in. This has implications for the reliability of the study. Significantly, there was no sample size calculation. There is no justification for the particular number of patients included. Brief mention is given to statistical analysis, although the exact methods used are omitted. Randomisation was described as ‘simple’, although no details are given. The only demographic detail described was age, with no difference between the two groups. There was no significant difference between severities of osteoarthritis or initial pain level (measured by visual analogue scale (VAS)) between the two groups.
Heyworth et al. [11]
The title of this study clearly describes it as a double-blind randomised trial, giving the interventions and the patient group. The abstract is also informative and detailed. Three different interventions are compared for the treatment of thumb base osteoarthritis—hylan, corticosteroid and placebo. The background and objectives are apparent. The study was carried out over a 2-year period at one tertiary referral centre. Approval was sought and granted from the appropriate bodies. Funding was obtained, from the manufacturer of the Hylan used in the study. This may bias the conclusion of the study. Inclusion criteria are well described. All radiographic degrees of osteoarthritis were included. Patients were not included if they had had an injection previously that did not benefit or if they had had two previous injections or if the injection had been in the previous 6 months. By including patients that have benefitted before but not including patients who have not been helped, a selection bias may have been present for patients responsive to steroid.
The primary outcome measures were the VAS and the Disabilities of the Arm, Shoulder and Hand (DASH) functional assessment [12]. The DASH is a commonly used, validated questionnaire that is useful for assessing function of the upper limb. The secondary outcome measures were range of motion, as well as grip and pinch strength. A lot of detail is included regarding the various assessments and measurement techniques. Patients were allowed to wear a splint or take analgesia following the injection at their own discretion, and if they did, it was recorded in their diary.
A power calculation was performed based on the DASH. The power calculation was carried out using data from a previous paper assessing patients following surgery on the TMJ [7]. Randomisation methods are briefly described. The type of intervention was unknown to the patient and the evaluator (double blinding). Patients were allocated to receive either 1 ml normal saline (placebo), 1 ml hylan or 1 ml corticosteroid. Details of the methods of statistical analysis are given. Baseline demographics and measurements are tabulated and show minimal differences between each intervention group, although no statistical analysis was performed. A total of 60 patients were studied (18 controls, 22 steroid, 20 hylan). There were no adverse events.
Meenagh et al. [20]
This study is another RCT investigating intra-articular steroid injection for TMJ osteoarthritis. The title is less descriptive, not mentioning patient group or other interventions, but does state that it is an RCT. The abstract summarises the important details and the study rationale, and the objectives are clear. The study was carried out over a three-and-a-half-year period within one named hospital. Inclusion and exclusion criteria are explicit but brief. Patients who had previously had a steroid injection into either TMJ were excluded. Ethical approval was obtained prior to the study commencing, and consent was taken from each patient. Outcome measures are stated, with the primary measure being VAS. Secondary measures are joint stiffness, joint line tenderness and two global assessments (patient/physician). It is not clear what these global assessments included.
The study was a double-blind RCT, with both the patients and the assessor blind to the treatment (either steroid or placebo). The intra-articular injection was carried out under radiographic guidance to ensure correct placement. The randomisation method is clear, with tables being used relating to numbered opaque syringes. Either 5 mg (0.25 ml) triamcinolone or 0.25 ml sterile 0.9 % saline was injected into the TMJ. Following injection, the thumb was splinted for 48 h. A power calculation was performed prior to study commencement. It was deemed that 45 patients would be required in each intervention group to detect a significant difference. Unfortunately, recruitment proved difficult in this study and insufficient patients were recruited to meet the number required by the power calculation. Only 46 patients were recruited instead of the 90 needed. This rendered the power calculation redundant. Of the 46 eligible patients, only 40 agreed to take part in the study.
Stahl et al. [30]
The title does not mention that this is a randomised study, although the abstract is reasonably detailed. The research question of the study aims to ascertain whether intra-articular injection of hyaluronic acid into the TMJ could relieve symptoms but without the side effects of steroid injection. The precise side effects being assessed are not described.
Fifty-two patients were included, all of them suffering with grade 2 TMJ osteoarthritis. They were randomised to receive either methylprednisolone (steroid) or hyaluronate injection into the TMJ. Patients were randomised using a computer-generated identification number from their admission into the clinic. The injection was delivered without radiological guidance by a dorsal approach after palpation of the joint line. Each subject received an injection of 1 ml. In total, 25 patients received 40 mg methylprednisolone acetate and 27 received 15 mg sodium hyaluronate. There is no mention of whether patients or assessors were blinded to the intervention.
Outcomes are listed as pain, grip and pinch strength and also functional assessment using the Purdue Pegboard Test (PPT). This test involves the patient moving pegs from one area of a board to another. It specifically tests both gross and fine dexterity within a defined time period. Pain was assessed prior to injection using the VAS at rest and after activity. Evaluation was at 1, 3 and 6 months post-injection, and statistical methods were used for analysis (paired t test) with p values less than 0.05 taken as being significant. The initial mean level of pain on VAS was comparable between the two groups.
Recruitment details and timing of the study are not mentioned and neither are the demographics or flow of the participants.
Summarising the results for the four RCTs reveals similar sample sizes of between 20 to 25 patients receiving a steroid injection and a female preponderance with an average age of 62 years. Only one study delivered and met an adequate power calculation [11] which is one of the major limitations to these studies. Meenagh et al. performed a power calculation but were unable to fulfil it due to poor recruitment.
Meenagh et al. classified the degree of OA in their group of patients as moderate to severe compared to Bahadir et al. and Stahl et al. who graded their patients with an Eaton score of 2 or 3. Heyworth et al. did not specify the severity of OA. This makes direct comparisons between the studies difficult.
Outcome measures for all RCTs included a VAS assessment, and all studies except for Meenagh et al. included an assessment of pinch and grip strength. Three out of the four studies noted a significant decrease in VAS scores lasting for between 1 and 12 months [2, 11, 30], but Meenagh et al. did not find any significant decrease in VAS scores at any point post-injection of corticosteroid.
The use of additional analgesics in the post-operative period is not specified in two of the RCTs, but Bahadir et al. did not allow the patients to use such medications although the actual compliance would be unknown. Heyworth et al. specified that additional analgesics were permitted. This has implications again when comparing scores between studies.
Bahadir et al. and Stahl et al. reported a significant improvement in grip strength at 3 and 6 months (p = 0.008), respectively. In terms of hand function, Bahadir et al. noted an improvement lasting up to 6 months post-injection but Stahl et al. found no significant difference in PPT scores with corticosteroid injection when compared to the placebo group. None of the studies showed any improvement in pinch following the injection.
Overall, the RCTs except for Meenagh et al. concluded that there were significant short- to medium-term gains from corticosteroid injections lasting up to 1 year post-injection. This was possibly due to the higher grade of OA presenting in the Meenagh et al. study.
The five case series are critiqued below:
-
Day et al. [5]
This study prospectively observed a consecutive series of 37 patients. It evaluates the effectiveness of a single corticosteroid injection and 3 weeks splinting for patients with TMJ osteoarthritis. Patients were assessed using the DASH questionnaire before the injection and then 6 weeks later. The final review was completed at an average of 25 months. At this appointment, the patient was asked to remember whether they had pain relief at 3, 12 and 18 months after the injection. This is an unreliable method because it relies on the patient being able to accurately remember how long the pain relief lasted at over 2 years post-injection.
-
Joshi [13]
This study evaluates 25 patients who underwent 32 injections of 10 mg methylprednisolone into the TMJ. Patients were monitored for a maximum of 1 year. The primary outcome measure was the VAS, with secondary measures including clinical review and questions from the Health Assessment Questionnaire (HAQ). Variations in management limit the conclusions to be drawn from the study arising from inconsistent enrolment. Follow-up was arranged for 1, 3, 6 and 12 months post-injection and carried out by the same clinician who initially diagnosed the patient and provided treatment.
-
Khan et al. [15]
This case series studies 40 consecutive patients injected with 10 mg Kenalog (triamcinolone) and a local anaesthetic (no description of type or quantity) into the TMJ. The severity of osteoarthritis was graded using Dell’s radiological criteria [6], not one that is used commonly (compared to the Eaton grading). Some patients were using splints before enrolment in the study, whilst others were not. DASH and VAS were used to assess patients before injection and to follow them up at 6 weeks and between 3 and 6 months post-injection. Patients were asked to remember the duration of benefit after the injection.
-
Maarse et al. [17]
This is a combined retrospective and prospective review of patients who received an intra-articular steroid injection for symptomatic osteoarthritis during a 10-year period. This study method has limitations. The duration of the interval between injection and questionnaire evaluation is not specified. Patients may not be able to remember the duration of injection efficacy very accurately. Radiographic grading of pre-injection X-rays using the Eaton classification was carried out by an independent radiologist [8]. Radiological guidance was used throughout to check that the steroid was delivered into the joint. Pain was assessed after (but not before) the injection by using the VAS. Sixty-six patients were recruited to the study, although 15 of these were either lost to follow-up or were not reviewed for the 1 year minimum and were excluded. Ten patients did not respond to the questionnaire, leaving 43 thumbs from 41 patients included in the study. Most (49 %) of the patients had Eaton grade 1 (early) osteoarthritis. Confounding factors were not controlled, but some are described, including use of splints and anti-inflammatory medications. The duration of long-term follow-up was very varied, ranging from 1 year to nearly 10 years. The median was 39 months. Patient recollection of the short-term effects of a steroid injection up to 10 years after injection could not be considered reliable and dramatically reduces the impact of the results.
-
Swindells et al. [32]
This prospective observational study aimed to assess the duration of benefit gained from a single corticosteroid injection under fluoroscopic guidance. It was performed at a single centre but does not specify how many clinicians were involved in the selection and treatment of the patients which may cause variation.
The inclusion criteria were explained, suggesting that all patients presenting to the out-patient department with TMJ osteoarthritis were eligible. However, the decision to perform the injection was completely independent of the audit study.
A sample size of eighty-three patients each received a single injection of 40 mg triamcinolone with local anaesthetic under image guidance. Twenty-seven of these patients had previously received one or more steroid injections although the study does not state when the previous injection was given. All patients were scored via a VAS pre-injection and plain X-rays allowed an Eaton score [8] to be calculated.
The primary outcome of the study was subjective improvement in pain following injection. The secondary outcome was a semi-quantitative objective measurement of improvement using the VAS scoring. Monthly questionnaires were sent out to the patients via the post after treatment. Except from the initial 12 patients lost to follow-up after the first week, all patients were followed-up until the pain had returned to pre-injection levels, which was the end-point of the study.
This study was much larger than previous ones; however, it lost a significant proportion to follow-up. There was no blinding or randomisation which would allow selection bias even though the study specifies that the decision to perform the injection was independent of the audit. There is no information regarding the window between previous injections for the 27 patients and the injection given as part of this study which may confound the results. There was also no mention of adjuvant therapies such as oral analgesics, another potential confounding factor. VAS scores improved post-injection, but there is no statistical analysis of this.
The five case series provided a similar demographic of patients when compared to the RCTs in terms of age and sex. Similar scoring systems including VAS and DASH were used. Overall, the consensus was that short-term benefit was gained between 1 and 6 months post-injection, although Day et al. found that just under half the patients in the study had a sustained benefit for up to 18 months. Unfortunately, their method of follow-up was flawed as it required the patient to recall their symptoms at set time points post-injection.
Maarse et al. had similar design flaws in their study, and although specifying a quarter of the group had long-term pain relief, they do not mention the time frame. They documented that 14 out of the 41 patients had pain relief lasting at least 6 weeks.
In keeping with the RCTs, Day et al. and Khan et al. suggested that patients with less severe OA would benefit more than those with a more advanced stage. Khan et al. reported significant reductions in VAS and DASH scores at the 6-week stage but continuing relief was only noted for patients with grade 1 and 2 OA changes (based on Dell’s radiological criteria) thereafter. The largest study by Swindells et al. found no correlation between grade of OA and outcome.
Joshi et al. reported a statistical reduction in VAS scores at 1 month (p < 0.001), but this improvement was not maintained after this time point. Swindells et al. also noted a median VAS score reduction for the whole group from 68 to 16 at 1 month; however, there was no statistical analysis for this. They also found that over half of the patients who continued with follow-up (42/71) had a benefit for at least 2 months with ten patients reporting an improvement lasting over 6 months.
There was very little evidence from any of the studies to suggest an improvement in hand function post-injection.
The results of the case series are limited by the study designs with many confounding factors (such as the use of adjuvant analgesics) not being controlled. However, they do reinforce the results of the RCTs with improvements (in particular pain relief) noted over the shorter-term period. Whether or not the severity of OA plays a major role in the longer-term outcomes is undecided.
Discussion
There is some evidence in the literature to support the efficacy of steroid injections into the TMJ. Studies show a range of responses, from no benefit over placebo in moderate to severe osteoarthritis [20], to reported useful benefit with steroid injection and splinting, particularly in early osteoarthritis [13, 30]. Many studies have reported short-term effectiveness [5, 11, 15, 17, 32], although one showed longer-term effectiveness [2] of steroid injection into the TMJ. Two of these studies include power calculations although only one was able to recruit sufficient numbers to satisfy the calculation [11] (Table 1).
The information obtained from previous studies has to be used carefully. Each study performed the injection using a slightly different technique; different types of steroid were used for injection, and post-injection treatment varied. In some studies, a splint was used to immobilise the thumb; in others, no splint was supplied. Some studies allowed use of analgesic medications, whilst others did not, and several did not record this information. Studies not published in English were excluded which could result in some degree of bias.
The optimum method of assessing benefits arising from steroid injection into the basal thumb joint would be a meta-analysis of well-designed RCTs. However, this does not currently exist. There are also hazards with meta-analyses. The studies that are included must match each other closely so there is little variation in practice. Often, the RCTs are so diverse that they cannot be brought together and compared usefully. A systematic review of the literature as was carried out in this study is a useful alternative and often delivers a more accurate assessment of existing studies. A well-designed RCT could answer the research question, something that four previous groups have attempted [2, 11, 20, 30]. RCTs are challenging to set up, requiring a lot of time and money to accomplish. To deliver a meaningful RCT, it is necessary to perform a power calculation prior to its commencement. Without a power calculation, the study will not be able to answer the question posed. The study also has to be able to recruit the required number of patients to satisfy the power calculation. If it does not, or too many patients are lost to follow-up, the study has not achieved its objective. Only one of the four evaluated RCTs had a satisfied power calculation [11].
Many of the identified studies were case series [5, 13, 15, 17, 32]. These provide comparatively weak evidence. They are subject to many sources of error, most notably selection bias. Patients are usually selected for the intervention first and then included in the study. This can reduce the external validity of the study. One major limitation with all currently published studies is the timing of follow-up. When the patient is seen for assessment at a period of time post-injection, the clinician or assessor will only see a ‘snapshot’ at that moment in time. The patient may deteriorate immediately following that or only just before the next follow-up. Seeing patients at, for example, 3-month intervals, does not lead to a particularly accurate estimation of the duration of action of the steroid. It could be more helpful for a series of patients to record their pain and function in a diary on a daily basis.
There are other issues with regard to assessing response to steroid injections into the TMJ. Inappropriate needle positioning has been found in a significant proportion of patients (17 to 42 %) where fluoroscopy was employed after needle placement but prior to injection [10, 25]. Only three previous studies describe the injections being carried out under radiological guidance [17, 20, 32]. Consequently, the various outcomes in other previous studies [2, 5, 11, 13, 15] may in part indicate inappropriate deposition of steroids outside the affected joints. In the authors’ unit, radiological guidance is usually used to confirm correct steroid placement into the TMJ. However other, albeit very experienced, authors do not promote the need for radiological guidance [18].
The Eaton grading system [8] is a well-known but coarse instrument for recording the degree of trapeziometacarpal osteoarthritis. It is known that ordinal scales (i.e., 4-point classification) for a continuous variable (i.e., degree of osteoarthritis) are very prone to inter- and intra-observer error.
The visual analogue scale (VAS) is a semi-quantitative subjective outcome measurement. It was used in all the identified studies to measure pain levels. Reported effectiveness does not always correlate with VAS, which makes it hard to fully interpret the patients’ assessments. The VAS provides a snapshot of pain at one particular time point. The patient’s perception of whether the steroid is still working is more reliable since it will guide future intervention.
No adverse events were recorded in any of the papers included in the systematic review. There were no known side effects caused by any of the steroid injections. A total of approximately 307 patients were treated in the nine analysed studies. This may confirm the general belief that complications are rare [16, 22, 27, 31], although the sample presented here is still relatively small.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conclusion
This study demonstrates that there are potentially significant although short-term benefits to be gained from steroid injections into the TMJ. They can lead to pain relief and improved function, certainly in the first 1 to 3 months post-injection. However, the currently available evidence is somewhat limited. Severity of the osteoarthritis may or may not affect the patient’s response to steroid injection. The outcome of TMJ injections may be improved by using fluoroscopic assistance (Fig. 1). Steroid injections are a low-risk procedure and can be a helpful form of treatment prior to consideration of more invasive treatments.
Acknowledgments
Conflict of Interest
The authors Andrew Fowler, Mark Swindells and Frank Burke confirm that they have no relationships or interests that could influence or bias this work.
Statement of Human and Animal Rights
The above work is a systematic review of available studies and we did not carry out experiments on human or animal subjects.
Statement of Informed Consent
We confirm there to be no identifying information about participants in the article.
References
- 1.Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg (Br) 1994;19(3):340–1. doi: 10.1016/0266-7681(94)90085-X. [DOI] [PubMed] [Google Scholar]
- 2.Bahadir C, Onal B, Dayan VY, Gürer N. Comparison of therapeutic effects of sodium hyaluronate and corticosteroid injections on trapeziometacarpal joint osteoarthritis. Clin Rheumatol. 2009;28(5):529–33. doi: 10.1007/s10067-008-1079-6. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell JR. Intra-articular corticosteroids. Guide to selection and indications for use. Drugs. 1996;52(4):507–14. doi: 10.2165/00003495-199652040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane review: conservative therapy for thumb (trapeziometacarpal joint) osteoarthritis. Stage: registered title; date of creation: 10. 2009; last updated: 2. March 2010; Entity: musculoskeletal group.
- 5.Day CS, Gelberman R, Patel AA, Vogt MT, Ditsios K, Boyer MI. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg [Am] 2004;29(2):247–51. doi: 10.1016/j.jhsa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Dell PC, Brushart TM, Smith RJ. Treatment of trapeziometacarpal arthritis: results of resection arthroplasty. J Hand Surg [Am] 1978;3(3):243–9. doi: 10.1016/S0363-5023(78)80088-4. [DOI] [PubMed] [Google Scholar]
- 7.De Smet L. Responsiveness of the DASH score in surgically treated basal joint arthritis of the thumb: preliminary results. Clin Rheumatol. 2004;23:223–4. doi: 10.1007/s10067-004-0866-y. [DOI] [PubMed] [Google Scholar]
- 8.Eaton RG, Lane LB, Littler JW, Keyser JJ. Ligament reconstruction for the painful thumb carpometacarpal joint: a long-term assessment. J Hand Surg [Am] 1984;9(5):692–9. doi: 10.1016/S0363-5023(84)80015-5. [DOI] [PubMed] [Google Scholar]
- 9.Harrison J, Fahmy N. (ii) Management of peri-trapezial osteoarthritis. Curr Orthop. 2005;19(3):190–5. doi: 10.1016/j.cuor.2005.02.015. [DOI] [Google Scholar]
- 10.Helm AT, Higgins G, Rajkumar P, Redfern DR. Accuracy of intra-articular injections for osteoarthritis of the trapeziometacarpal joint. Int J Clin Pract. 2003;57(4):265–6. [PubMed] [Google Scholar]
- 11.Heyworth BE, Lee JH, Kim PD, Lipton CB, Strauch RJ, Rosenwasser MP. Hylan versus corticosteroid versus placebo for treatment of basal joint arthritis: a prospective, randomized, double-blinded clinical trial. J Hand Surg [Am] 2008;33(1):40–8. doi: 10.1016/j.jhsa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand). The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29(6):602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Joshi R. Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J Rheumatol. 2005;32(7):1305–6. [PubMed] [Google Scholar]
- 14.Kenniston JA, Bozentka DJ. Treatment of advanced carpometacarpal joint disease: arthrodesis. Hand Clin. 2008;24(3):285–94. doi: 10.1016/j.hcl.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Khan M, Waseem M, Raza A, Derham D. Quantitative assessment of improvement with single corticosteroid injection in thumb CMC joint osteoarthritis? Open Orthop J. 2009;19(3):48–51. doi: 10.2174/1874325000903010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar N, Newman RJ. Complications of intra- and peri-articular steroid injections. Br J Gen Pract. 1999;49(443):465–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Maarse W, Watts AC, Bain GI. Medium-term outcome following intra-articular corticosteroid injection in first cmc joint arthritis using fluoroscopy. Hand Surg. 2009;14(2–3):99–104. doi: 10.1142/S0218810409004311. [DOI] [PubMed] [Google Scholar]
- 18.Mandl LA, Hotchkiss RN, Adler RS, Ariola LA, Katz JN. Can the carpometacarpal joint be injected accurately in the office setting? Implications for therapy. J Rheumatol. 2006;33(6):1137–9. [PubMed] [Google Scholar]
- 19.Mayer JH. Carpometacarpal osteoarthritis of the thumb. Lancet. 1970;2(7666):270. doi: 10.1016/S0140-6736(70)92628-0. [DOI] [PubMed] [Google Scholar]
- 20.Meenagh GK, Patton J, Kynes C, Wright GD. A randomised controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63(10):1260–3. doi: 10.1136/ard.2003.015438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, For the CONSORT group et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trial. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman RJ. Local skin depigmentation due to corticosteroid injection. Br Med J (Clin Red Ed) 1984;288(6432):1725–6. doi: 10.1136/bmj.288.6432.1725-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oxford Centre for Evidence Based Medicine—Levels of Evidence (2009). Produced by Bob Phillips, Chris Ball, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin Dawes since November 1998. Updated by Jeremy Howick,.
- 24.Pendse A, Nisar A, Shah SZ, Bhosale A, Freeman JV, Chakrabarti I. Surface replacement trapeziometacarpal joint arthroplasty—early results. J Hand Surg Eur. 2009;34(6):748–57. doi: 10.1177/1753193409343750. [DOI] [PubMed] [Google Scholar]
- 25.Pollard MA, Cermak MB, Buck WR, Williams DP. Accuracy of injection into the basal joint of the thumb. Am J Orthop (Belle Mead NJ) 2007;36(4):204–6. [PubMed] [Google Scholar]
- 26.Public Health Resource Unit, Institute of Health Science, Oxford. Critical Appraisal Skills Programme (CASP), www.phru.nhs.uk/Pages/PHD/resources.htm. Accessed 20/07/2010.
- 27.Rogojan C, Hetland ML. Depigmentation—a rare side effect to intra-articular glucocorticoid treatment. Clin Rheumatol. 2004;23(4):373–5. doi: 10.1007/s10067-004-0905-8. [DOI] [PubMed] [Google Scholar]
- 28.Schulz KF, Altman DG, Moher D, for the CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sodha S, Ring D, Zurakowski D, Jupiter JB. Prevalence of osteoarthrosis of the trapeziometacarpal joint. J Bone Joint Surg Am. 2005;87(12):2614–8. doi: 10.2106/JBJS.E.00104. [DOI] [PubMed] [Google Scholar]
- 30.Stahl S, Karsh-Zafrir I, Ratzon N, Rosenberg N. Comparison of intraarticular injection of depot corticosteroid and hyaluronic acid for treatment of degenerative trapeziometacarpal joints. J Clin Rheumatol. 2005;11(6):299–302. doi: 10.1097/01.rhu.0000191194.39926.c9. [DOI] [PubMed] [Google Scholar]
- 31.Stefanich RJ. Intraarticular corticosteroids in treatment of osteoarthritis. Orthop Rev. 1986;15(2):65–71. [PubMed] [Google Scholar]
- 32.Swindells MG, Logan AJ, Armstrong DJ, Chan P, Burke FD, Lindau TR. The benefit of radiologically-guided steroid injections for trapeziometacarpal osteoarthritis. Ann R Coll Surg Engl. 2010;92(8):680–4. doi: 10.1308/003588410X12699663905078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umphrey GL, Brault JS, Hurdle MB, Smith J. Ultrasound-guided intra-articular injection of the trapeziometacarpal joint: description of technique. Arch Phys Med Rehabil. 2008;89:153–6. doi: 10.1016/j.apmr.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 34.Wajon A, Carr E, Edmunds I, Ada L. Surgery for thumb (trapeziometacarpal joint) osteoarthritis. Cochrane Database Syst Rev. 2009;4:CD004631. doi: 10.1002/14651858.CD004631.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Yao J, Park MJ. Early treatment of degenerative arthritis of the thumb carpometacarpal joint. Hand Clin. 2008;24(3):251–61. doi: 10.1016/j.hcl.2008.03.001. [DOI] [PubMed] [Google Scholar]


