Abstract
Acute-on-chronic liver failure (ACLF) is increasingly recognized as a complex syndrome that is reversible in many cases. It is characterized by an acute deterioration of liver function in the background of a pre-existing chronic liver disease often associated with a high short-term mortality rate. Organ failure (OF) is always associated, and plays a key role in determining the course, and the outcome of the disease. The definition of ACLF remains controversial due to its overall ambiguity, with several disparate criteria among various associations dedicated to the study of liver diseases. Although the precise pathogenesis needs to be clarified, it appears that an altered host response to injury might be a contributing factor caused by immune dysfunction, ultimately leading to a pro-inflammatory status, and eventually to OF. The PIRO concept (Predisposition, Insult, Response and Organ Failure) has been proposed to better approach the underlying mechanisms. It is accepted that ACLF is a different and specific form of liver failure, where a precipitating event is always involved, even though it cannot always be ascertained. According to several studies, infections and active alcoholism often trigger ACLF. Viral hepatitis, gastrointestinal haemorrhage, or drug induced liver injury, which can also provoke the syndrome. This review mainly focuses on the physiopathology and prognostic aspects. We believe these features are essential to further understanding and providing the rationale for improveddisease management strategies.
Keywords: Acute on-chronic liver failure, Immune dysfunction, Systemic inflammatory response, Hepatic encephalopathy, Hepatorenal syndrome, Acute decompensation of cirrhosis, Liver failure, Organ failure, Severity score, Chronic liver failure-sequential organ failure assessment
Core tip: Acute on-chronic liver failure is a newly recognized syndrome characterized by acute deterioration of a compensated or decompensated chronic liver disease, leading to organ failure, and a mortality rate ≥ 15% at 28-d. Pathogenesis involves an exaggerated systemic inflammatory response in the setting of immune dysregulation and oxidative stress. Alcohol is a frequent precipitating factor seen most commonly in the West, and untreated hepatitis B virus infection is more prevalently seen in the East. However, it must be noted, that specific precipitant factors cannot be established in up to the 40% of cases. Recent prospective work has generated data on definition, prevalence, precipitating factors and scoring systems. Treatment of precipitant factors, complications, organ failure support, and liver transplantation are the current therapeutic options.
INTRODUCTION
In recent years, a new clinical form of liver failure has been recognised. Traditionally there were two types of liver failure: Acute liver failure (ALF), a rapid deterioration of the liver function in the absence of pre-existing liver disease, in the setting of an acute hepatic insult and chronic liver failure (CLF), a progressive and slow deterioration over the course of pre-existing end-stage liver disease[1-4]. In 1995, a third type of liver failure was first described[5]: Acute-on-chronic liver failure (ACLF). This new entity is characterised by acute complications of compensated or even decompensated cirrhosis and is characterised by a high rate of organ/system failure(s), and a high short-term mortality rate (> 15% at 28-d). Over the last decade, many definitions have been proposed, based on expert’s opinion rather than on evidence-based data. The heterogeneity of definitions illustrates the differences in underlying aetiologies of liver disease between Eastern and Western countries[6-9]. The Asian Pacific Association for the Study of the Liver (APASL) defines ACLF as an “Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 wk by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease”[10]. Whereas, the American Association for the Study of Liver Disease (AASLD)/as well as the European Association for the Study of the Liver (EASL) consensus defines it as: “Acute deterioration of pre-existing, chronic liver disease (CLD), usually related to a precipitating event and associated with increased mortality at 3 mo due to multi-system organ failure”[6,11]. Given the lack of consensus among researchers, a group of investigators from the EASL-Chronic Liver Failure (CLIF) Consortium, undertook a prospective multicenter study in patients with cirrhosis suffering from acute decompensation (AD). The study identified patients with cirrhosis at a high risk of short term mortality. The study also aimed to develop a definition of ACLF. This large study was called EASL-CLIF Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC)[12]. Based on data analysis obtained from 1343 hospitalized patients with cirrhosis and AD, at 29 liver units in 8 European countries this study established diagnostic criteria for ACLF. This study also permitted to know prevalence, precipitating factors, pathogenic mechanism and the phenotypic features of patients with ACLF.
DIAGNOSTIC CRITERIA OF ACLF
In the CANONIC study, the overall prevalence of ACLF was 30.9%. The definition of the ACLF diagnostic criteria was based on the presence of the 3 key characteristics of the syndrome: (1) AD: defined by acute development of large volume ascites, hepatic encephalopathy (HE), gastrointestinal haemorrhage, bacterial infections, or a combination of any of these[4,13-16]. In other words, the acute development of at least one of these major complications of liver disease must be present; (2) organ Failure: defined by a modified SOFA scale (Sequential Organ Failure Assessment) the CLIF-SOFA scale that takes into account some specificities of cirrhosis (Table 1)[17-21]; and (3) short-term mortality (28-d) at least 15%[12].
Table 1.
Chronic liver failure-sequential organ failure assessment score
| Organ failure | 0 | 1 | 2 | 3 | 4 |
| Liver (Tbil, mg/L) | < 1.2 | ≥ 1.2 to < 2.0 | ≥ 2.0 to < 6.0 | ≥ 6.0 to < 12 | ≥ 12.0 |
| Kidney (cr, mg/dL) | < 1.2 | ≥ 1.2 to < 2.0 | ≥ 2.0 to < 3.5 | ≥ 6.0 to < 12 | ≥ 5.0 |
| Or use of renal replacement therapy | |||||
| Cerebral (HE grade) | No HE | I | II | III | IV |
| Coagulation (INR) | < 1.1 | ≥ 1.1 to < 1.25 | ≥ 1.25 to < 1.5 | ≥ 1.5 to < 2.5 | ≥ 2.5 or PLT ≤ 20 × 109/L |
| Circulation (MAP, mmHg) | ≥ 70 | < 70 | DA ≤ 5 or DOB or Terlipressin | DA > 5 or E ≤ 0.1 or NE ≤ 0.1 | DA > 15 or E 0.1 or NE > 0.1 |
| Lung PaO2/FiO2 | > 400 | > 300 to ≤ 400 | > 200 to ≤ 300 | > 100 to ≤ 200 | ≤ 100 |
| Or SpO2/FiO2 | > 512 | > 357 to ≤ 512 | > 214 to ≤ 357 | > 89 to ≤ 214 | ≤ 89 |
This score is used to categorize patients into grades of ACLF. ACLF: Acute-on-chronic liver failure; CLIF: Chronic liver failure; SOFA: Sequential organ failure assessment; Tbil: Total bilirubin; cr: Serum creatinine; HE: Encephalopathy; INR: International normalized ratio; PLT: Plateletes; DA: Dopamine; DOB: Dobutamine; E: Epinephrine; NE: Norepinephrine; PaO2: Partial pressure of arterial oxygen; FiO2: Fraction of inspired oxygen; SpO2: Pulse oximetry saturation. Data from Moreau et al[12].
According to these characteristics, patients admitted to the hospital for an AD can be classified into 4 groups (Table 2). However, the majority of the patients did not have ACLF (77.5%). The Figure 1 summarizes the mortality rate according to the ACLF subtype.
Table 2.
Grades of acute-on-chronic liver failure according to the number of organ failure and the type of organ
| No. ACLF | |
| ACLF grade 1 | Single- organ failure (coagulation, liver, circulation, lungs) in patients with sCr 1.5-1.9 mg/dL and/or grades 1-2 HE or braine failure with sCr range from 1.5-1.9 mg/dL |
| ACLF grade 2 | Two organ failures |
| ACLF grade 3 | Three or more organ failures |
Data from the CANONIC study[12]. ACLF: Acute-on-chronic liver failure; OF: Organ failure.
Figure 1.
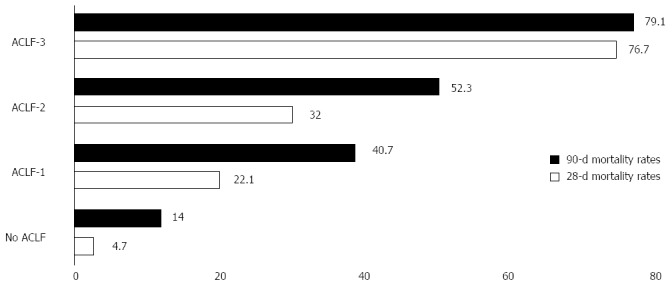
Mortality rates according to the grade of acute-on-chronic liver failure at 28 d and 90 d according to the grade of acute-on-chronic liver failure. Data from the CANONIC study[12]. Mortality increases with the grade of ACLF, directly related to the type and number of organ failure. ACLF: Acute-on-chronic liver failure.
PATHOPHYSIOLOGY
PIRO concept (predisposition, injury, response and organ failure)
The PIRO model is a useful approach in understanding the clinical sequence of the ACLF. It also consists of a scoring system that classifies severity, estimates risk, stratification, and prognosis in critically ill patients.
Initially postulated in 1900, and later modeled by Marshall et al[22] and Levy et al[23] the PIRO score was designed originally to measure the clinical features and outcomes in sepsis. The PIRO concept arises from the comprehensive examination of ACLF as a severe liver dysfunction, linked to other organs failure, as a strong and characteristic response to an insult that might be identified as an aggression within an underlying CLD that predisposes the whole situation[22]. It is proposed that organ dysfunction is the most predictive item among the four PIRO factors as it predicts 28-d mortality and multiple organ dysfunction[24]. Taking into account the great capacity of this concept to summarize and breakdown the physiopathology of ACLF, it has been proposed to also explain the cascade of facts in this entity.
Predisposition
Almost any kind of CLD can be a main predisposing factor on its own. In the Western countries, alcoholic cirrhosis is the cause of 50%-70% of all predisposing liver diseases of ACLF, comparing to the 10%-30% caused by chronic viral infection. In the Eastern countries hepatitis B virus (HBV) accounts for 70%, and only 15% is related to alcohol[6,10]. Nevertheless, some widespread infections like simple steatosis are not included as an underlying factor, whereas non-alcoholic steatohepatitis is. Also, metabolic and cholestatic liver diseases constitute part of susceptibility of the ACLF. This status of chronic liver impairment predisposes not only to an altered pro-inflammatory situation based on elevated serum cytokines, but also to a dysfunction in cellular immune system, reticulo-endothelial, and impairment in the bacterial translocation defense system[25].
Insult
Similarly to sepsis syndrome, infection may play a major role in triggering the whole inflammatory response. In the Asian continent HBV reactivation is one of the principal causes of ACLF. Other hepatotropic viruses like virus C reactivation might also provoke this failure[26]. In India, superimposed hepatitis E has been described as a major precipitant of ACLF[27,28].
Bacterial, fungal or viral primary infection can lead to systemic inflammatory response syndrome (SIRS) that has the potential to cause acute liver failure. In the CANONIC study, the principal infections related to ACLF were spontaneous bacterial peritonitis (SBP) and pneumonia[12].
Among the non-infective precipitating events, alcoholic hepatitis is one of the most common causes[12]. In the CANONIC study, one of the main predisposing events of ACLF was active alcoholism during the previous 3 mo (about 25%). Other situations described as precipitating events were less frequent (about 8%), and included acute toxic hepatitis, major surgery, or TIPS insertion. Paracentesis without adequate albumin replacement, has been reported as well[12,25]. However, in 40% of cases an obvious precipitating event could not be identified[12].
Host response
Host response is probably the leading factor in determining the severity of the ACLF and its prognostic outcome. The extension and range of inflammatory activation may result in the development of SIRS, characterized by a strong pro-inflammatory status (despite of an impairment of immune response) that can lead to ALF, and dysfunction in other organs.
Role of inflamatory response: The host immune response and the inflammatory cascade take especially high importance in this syndrome. The similarity between the SIRS produced by sepsis, and ACLF suggests that both entities share common pathogenic mechanisms. In SIRS there is an activation of the immune system relating leukocytes, endothelial cells, monocyte/macrophages, cytokines, enzymes, chemotactic mediators, and adhesion molecules overproduction. In this state, hepatocytes are believed to result in sensitized tumor necrosis factor (TNF)-induced apoptosis[29].
Comparing septic patients to ACLF patients, Wasmuth et al[30] formulated the concept of “sepsis- like immune paralysis” based on a profoundly decreased production of TNF-α and low monocyte HLA-DR expression in both groups. He postulated that this cellular immune impairment could contribute to increased mortality.
Endotoxins have also been proposed to play a role in mediating the full activation of neutrophils, which paradoxically would render them unable to act against the insult. An enhanced pro inflammatory cytokine environment was proved present in ACLF, as compared with cirrhosis alone. As ACLF is a pro-inflammatory state, it could result in chronically primed neutrophils, but in a deleterious form that might cause functional failure in phagocytosis due to a continuous energy depletion, which would prevent them from fighting against further infections[31].
The role that cytokines play in ACLF remains a key point in the pathogenesis of the inflammatory response. Elevated serum levels of many cytokines including TNF-α, sTNF-αR1, sTNF-αR2, interleukin (IL)-2, IL-2R, IL-4, IL-6, IL-8, IL-10, and interferon-α has been described. In particular IL-6 and TNF-α had been proposed to have a dual action, producing hepatocyte death and also enhancing hepatocyte proliferation through a complex interplay with Kupffer cells (KCs) and hepatocytes[10,32].
The mechanism in the rise of cytokines can be related to necrotic liver cells, decreased hepatic clearance or, probably the most important, activation of toll-like receptors (TLRs). These receptors activate KCs[33,34]. Causing KCs to change into M1 pro-inflammatory macrophages[35]. TLRs have the capacity to interact with many different agents, recognizing multiple molecular patterns in pathogen or damage- associated pathways[36]. KCs play a key role in liver injury, as they internalize ligands and activate the signaling cascades, transcription of pro-inflammatorycytokines, and superoxide agents. This promotes oxidative stress and releases proteolytic enzymes, vasoactive substances such as endothelin-1 (ET-1), thromboxane A2, nitric oxide (NO), and prostaglandins, thereby contributing to microcirculatory dysfunction[37].
This entire cascade eventually leads to hepatocyte death and liver dysfunction. Hepatocyte apoptosis rather than necrosis seems to be the predominant mode of cell death in ACLF, as high levels of the apoptosis marker cytokeratin M30 occurs in ACLF patients[38]. Nevertheless, both paths are not mutually exclusive and the concept of “necroapoptosis” is only as of late been proposed. Also, the same patient can present both forms of cell damage dynamically[38].
Role of bacterial infection
Though the precise mechanisms involved in ACLF have yet to be clarified, the immune system seems to be play a predominant role in the setting of cirrhosis, which paradoxically is one of the most common forms of immunodeficiency[39,40].
The homeostatic role of the liver in the systemic immune response is well known[41-43]. This role is defines as “cirrhosis-associated immune dysfunction” which includes the main syndromic abnormalities of immune function, immunodeficiency, and systemic inflammation[44]. This is a dynamic condition which leads to oscillation from predominantly pro-inflammatory to predominantly immunodeficient situations[44,45].
Immune dysfunction in cirrhosis is multifactorial and reflects a complex interaction between many systems, predisposing these patients to infections. It is thought that this susceptibility is not due to a sole responsible factor, but rather to the concomitant presence of various facilitating mechanisms such as: portal hypertension with porto-systemic shunting (thus impairing detoxification and reticuloendothelial system phagocytic activity), increased gut permeability and bacterial overgrowth (all of them increases the risk of bacteremia and the occurrence of endotoxemia), albumin and lipoprotein dysfunction, or aberrant toll-like receptor expression in KCs[33,46-49].
The presence of innate immune dysfunction in ACLF can be inferred from susceptibility to infections: 30% to 50% of cirrhotic patients presented bacterial infections upon their admission or during hospitalization[50-53]. The most common bacterial infections are SBP (25%), urinary tract infections (20%), pneumonia (15%) and spontaneous bacteremia (12%)[54]. Clinical and biochemical parameters in bacterial infection were generally correlated with the severity of liver disease. Child-Pugh score (CPs) showed a predominance of class C in infected cirrhotic patients compared to non-infected ones[55].
PATHOPHYSIOLOGY OF ORGANS FAILURE
Hepato-adrenal axis failure
Adrenal dysfunction is frequently reported in patients with CLD (compensated or decompensated), and severe sepsis (51%-68%), especially in patients with high CPs, model of end stage liver disease (MELD) scores, and hemodynamic instability, thereby reflecting a more advanced liver disease[56-58]. Some hypotheses to explain adrenal dysfunction pathophysiology have been proposed, such as: decreased cholesterol levels, overstimulation of the hypothalamus-pituitary-adrenal axis by cytokines, and endotoxemia[56,59]. However, the mechanism leading to adrenal insufficiency remains unclear[60].
This dysfunction called “hepato-adrenal syndrome”, is associated with renal failure, hemodynamic instability, and increased mortality. Hydrocortisone administration can have initial favourable effects on hemodynamic parameters, but it has not been confirmed to improve the outcome[57,61].
Test to assess adrenal function and its interpretation in cirrhosis and ACLF is difficult due to the absence of consensus, and normal values of this test, therefore recommendations cannot be made[62].
Pulmonary failure
Respiratory failure can be classified in two types of complications. First, complications typically related to cirrhosis, like hepatic hydrothorax (that can become infected), portopulmonary hypertension, hepatopulmonary syndrome, and transfusion-related acute lung injury (among others)[63,64]. Secondly, infectious complications (which are the most common), like aspiration pneumonia. Bacterial respiratory tract infections in cirrhotic patients represent 14% to 48% of all bacterial infections[65]. These patients are at increased risk of pneumonia due to unprotected airway from altered consciousness, increased intra-abdominal pressure from ascites, endoscopic procedures for gastrointestinal bleeding and increased risk of bacterial translocation because of excessive use of proton pump inhibitors[66-68].
The relevance of this OF, and its impact on mortality in ACLF, can be emphasized by its incorporation into the CLIF-SOFA score. Respiratory failure is defined in CLIF-SOFA by gasometric parameters, as a partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio of 200 or less, or a pulse oximetry saturation/FiO2 ratio of 200 or less[12,69].
Haematological failure
Failure in the coagulation system is defined in CLIF-SOFA as an international normalized ratio (INR) of 2.5 or more, or a platelet count < 20000/μL[12].
Patients with liver disease are in a state of “rebalanced haemostasis” which results in an increase of both pro-thrombotic and anti-thrombotic factors[70,71]. As explained above, in ACLF the inflammatory process may trigger the “unstable balance” to any of these two states and may be manifested by either bleeding or thrombotic complications. Anti-thrombotic alterations are thrombocytopenia, abnormal platelet functions, deficiency in the coagulation factors (except for Factor VIII), and increased fibrinolysis. On the other side of the balance, the pro-thrombotic state, which is manifested by a decrease of anti-coagulation and plasminogen, associated with an increase in the plasminogen activator inhibitor (PAI), Von Willebrand factor and in factor VIII[72-76]. Summarizing, the most significant haematological abnormalities described in ACLF are defective platelet function and increased fibrinolysis[77,78].
Coagulopathy is worsened in sepsis by the presence of endogenous low-molecular weight heparinoids which disappear with resolution of infection. In addition, there is an increased risk of bleeding complications due to further increased portal pressure secondary to infections and may explain the beneficial role of antibiotics administration in reducing early variceal rebleeding[79-81]. Standard laboratory values, such as the determination of the INR or the activated partial thromboplastin time, poorly reflect the pathophysiological changes in ACLF, therefore a deeper comprehension of underlying mechanisms is needed to guide correction of coagulation abnormalities on these patients[71,82].
Neurological failure
HE is a common manifestation of ACLF. Neurological failure is defined by CLIF-SOFA by the development of encephalopathy grade III or IV[83]. Local and systemic disturbances have been implicated in the development of this syndrome. Patients with HE show a functional derangement in the blood brain barrier leading to increased transport of neutral amino acids and reduced transport of basic amino acids[84]. Elevated brain ammonia level and cerebral hemodynamic dysfunction are known to be the major etiological factors. Recent data suggest that in light of functional immunoparesis of patients with liver dysfunction, a poorly understood relationship between ammonia, inflammation and oxidative stress may underlie the HE pathogenesis[85-87]. These alterations include abnormalities in neurotransmission [i.e., disturbances in aminobutyric acid (GABA) ergic systems], energy impairment (i.e., decrease in cerebral blood flow, inhibition of cerebral energy metabolism by ammonia), brain oedema (i.e., elevated ammonia levels, hyponatremia) and neuro-inflammation (generation on nitric oxide, prostanoids, astrocytic swelling)[88,89].
EH in the setting of ACLF have a different course from cirrhotic patients with AD but without ACLF[90]. Isolated EH usually develops in the context of long-term diuretic use, and is not associated to an impairment of liver function. The absence of significant inflammatory reaction and the low prevalence of organ’s failure relatively preserve good prognosis. By contrast, patients with HE associated with ACLF has an extremely poor survival rate, as a consequence of a generalized inflammatory reaction that may play a role in brain and other organs dysfunction. In addition to liver dysfunction, HE in the setting of ACLF is frequently associated with bacterial infections, active alcoholism or dilutional hyponatremia[91,92].
Circulatory failure
According to the CLIF-SOFA, patients requiring inotropic drugs are considered to present circulatory failure[6,12].
Mechanisms underlying haemodynamic and cardiac dysfunction in ACLF resembles closely to those in severe sepsis, as TNF and NO are increased and cortisol is decreased[57,58]. This circulatory dysfunction is typically characterised by an intense hyperdynamic state with the inability to obtain adequate perfusion pressure despite volume expansion and requirement of large doses of inotropic agents, with subsequent development of lactic acidosis[93-95]. The increased infections risk is often coupled to cardiac dysfunction. This situation may be aggravated by sepsis related to increased susceptibility to infections, by impairment in cardiac systolic and/or diastolic function or by the presence of hepatoadrenal syndrome[96,97]. It has been speculated that any acute inflammatory insult in patients with underlying cirrhotic cardiomyopathy may precipitate cardiovascular collapse[97]. In ACLF there is often incapacity to appropriately increase the cardiac output in response to the insult[98]. This finding is in contrast to decompensated cirrhosis, where cardiac output remains elevated, until advanced stages of liver disease, secondary to splanchnic vasodilatation. This cardiovascular abnormality is associated with an increased risk of death, particularly in those patients who present with renal dysfunction[99]. Inotropic support is often needed, however the best therapeutic approach remains unclear[100].
Kidney dysfunction
Renal failure is defined by the CLIF-SOFA as a creatinine ≥ 2 mg/d and the use of renal replacement therapy[12]. In the CANONIC study kidney failure was the most frequent OF for ACLF grades (55.8%), followed by liver, cerebral, and coagulation failures (43.6%, 27.7% and 24.1%, respectively)[12].. As shown, acute kidney injury (AKI) is a frequent and an important component of ACLF, as it is associated with poor prognosis[101-105]. Mortality is associated to the type and number of organs failure, and was higher in the subgroup of patients with single kidney failure than in those with involvement of other organs. A study including 562 hospitalised patients with cirrhosis, suggested that the most frequent causes of renal failure were related to bacterial infections (46%), hypovolaemia (32%), hepato-renal syndrome (HRS) (13%) and intrinsic renal failure (9%)[106]. Other studies support these results[107-109]. Renal failure may be categorized into four types: HRS, parenchymal disease, hypovolemia-induced and drug-induced renal failure[106,107,110]. Attributing the renal failure to a single mechanism in patients with multiorgan failure is usually difficult. There are many aetiologies of renal failure in patients with ACLF[111,112]. Prerenal factors are generally associated with renal hypoperfusion, which may be associated with intravascular volume depletion (haemorrhage, renal and gastro-intestinal fluid loss) or marked deterioration of effective arterial blood volume, leading to HRS[99]. Most intrarenal causes are related to ischemic acute tubular necrosis, also due to renal hypoperfusion[109,113].
Undoubtedly, systemic haemodynamics and cardiac dysfunction play an important role in the development of renal failure. Thus, in some patients the circulatory changes may predominate, whilst in other patients there may be increased synthesis of pro-inflammatory mediators (or both). SIRS has also been suggested to be involved, accompanying and aggravating the above mentioned mechanisms[114-116]. The benefit of the anti-inflammatory or immunomodulatory agents such as corticosteroids or pentoxifylline in the prevention of renal failure in patients with acute alcoholic hepatitis might support this observation[106,109,117,118].
Liver failure
Liver failure is defined by the CLIF-SOFA as a total bilirubin ≥ 12 mg/dL. The hallmark of the liver manifestation of ACLF is hyperbilirubinemia and coagulopathy[12].
Some lines of evidence suggest that the histopathological characteristics of the liver during ACLF will be determined by the underlying cause of cirrhosis and the nature of the precipitating event[6,25]. From the pathophysiological point of view, in ACLF there is a further exacerbation of haemodynamic derangements besides the already existing liver structural changes[6]. Liver inflammation has a capital importance on increased portal pressure[119]. Mechanisms proposed are changes in vascular smooth muscle cells, activation of hepatic stellate cells, reduced nitric oxide activity secondary to endothelial dysfunction and upregulation of sympathetic tone[113,120-123]. Another key component is angiogenesis, which plays an important role in increasing intrahepatic resistance, and therefore in ACLF pathogenesis[124-127]. It should be noted that according to definition on diagnostic criteria, differences in portal haemodynamics have been described. When ACLF was defined according to the APASL criteria no differences were observed in portal haemodynamics between decompensated cirrhosis and ACLF[128]. However, using the EASL-AASLD definition, the portal pressure was markedly higher in those with ACLF portal pressure, in comparison to those with decompensated cirrhosis when ACLF was defined according to the AASLD/EASL definition[129]. These results point to the need for cautious definition of the population studied.
SIRS and bilirubinostasis had been associated with an increased risk of subsequent infection[130-132]. These infections begets a greater inflammatory response with aggravation in portal hypertension and further worsening of an already poor prognosis[80,133].This concept is supported by the reduction of portal pressure by antibiotics administration that modulates gut-derived endotoxemia and bacterial translocation[79,80,134].
Another characteristic feature of liver dysfunction is coagulopathy. Coagulation tests are usually abnormal in cirrhotic patients due to impaired synthesis and increased consumption of coagulation factors (see haematological failure). Bleeding abnormalities and hyper-coagulability may coexist[70,81,82,107].
PROGNOSIS, PREDICTORS OF MORTALITY
ACLF is associated with a high mortality rate of 50%-90% (which means it is 15 times higher of a rate in patients with ACLF), as compared to patients with an AD without ACLF[12]. Unfortunately, there are no well-established prognostic indicators available for predicting ACLF progression. The discrepancies and unevenness in the definition of ACLF, and therefore the different characteristics of the population under study, has limited research into the identification of clear indicators of severity and outcome predictors[9,135-138]. As previously mentioned, ACLF is a serious illness, in which reversibility is sometimes suggested in about half of the patients, or in other cases can progress to a life-threatening situation. It is, therefore, of fundamental importance to have accurate prognostic indicators in place, to be able to identify patients at high risk of ACLF that may require intensive care treatment, concise clinical decision making to improve management and minimize futile and expensive care. Due to a lack of universally accepted prognostic model for ACLF, many already widely used prognostic models for cirrhosis have been applied for the evaluation of this syndrome. In this regard prognosis scores can be categorized in two: the former that evaluates the severity of liver dysfunction (CPs, MELD) and the latter, global prognostic scores [Acute Physiology and Chronic Health Evaluation (APACHE II) and SOFA]. Several lines of evidence demonstrate that global prognostic scores are superior to specific liver scores for estimation of prognosis in these patients[103,105,139-141]. These findings emphasize the importance of OF in defining the prognosis of ACLF, because once extrahepatic failure has begun, outcome is mainly determined by the degree of end-organ dysfunction and less by the severity of the liver disease[101,104,142-144].
Some studies suggested that APACHE-II is the best predictive scoring system, owing to the fact that in ACLF once liver failure is established the prognosis is determined by the degree of other organ dysfunction and not by the severity of liver failure[10,98,142,145]. In some studies, MELD has been found to be a discrimination factor similar to SOFA and APACHE II[146]. The CLIF-SOFA also proved to be a strong predictor of short-term mortality but does not significantly improve the prediction accuracy of MELD and MELD-Na[18,19]. Recently, based on data from the CANONIC study, a specific prognostic score for ACLF has been developed named the “CLIF-CONSORTIUM score for ACLF” (CLIF-C ACLF score). This score is the result of combining “CLIF-Consortium Organ Failure (CLIF-C OF)” score (designed for the diagnosis of ACLF), and two other independent predictors of mortality (age and white-cell count)[7,135,147]. This new score at ACLF diagnosis showed a significantly higher predictive accuracy than MELDs, MELD-Na and CPs[7,135]. CLIF-C ACLF score has also been shown to be an independent predictor of course severity[45,148].
Furthermore, ACLF has been shown to be dynamic process. In this connection, scoring taking into account dynamic changes, or improvement/impairment in the same score, have shown to predict outcomes[149,150]. In this line, Kumar et al[151] has demonstrated that any improvement in the MELD score over 2 wk suggests a good outcome.
A large number of studies have indicated that the greater the number of organ dysfunction or OF at diagnosis, the lower the ACLF patient survival[12,98,152,153]. The basic mechanism is the importance of systemic inflammation on OF, and its impact on prognosis[132]. Along these lines, ACLF mortality has been associated with loss of organ function (Higher CLIF-SOFA score), high leukocyte counts, and high C-reactive protein (CRP). ACLF is especially severe in patients with no prior history of AD, characterized by higher numbers of OF, higher levels of inflammatory mediators, leukocyte count and higher rates of mortality[12,154]. Patients with ACLF are younger than those without, and age is associated with more vigorous immune response[154]. These data sets do not coincide with the findings from Shi et al[155] suggesting that ACLF patients with or without prior decompensation had comparable short-term prognosis, but the former group was characterized by increased delayed mortality.
Many studies suggest that HE is associated with higher mortality, especially in those with grade III-IV encephalopathy[83,90,151,156]. This association is highlighted by the incorporation of HE to modified scores [i.e., integrated-MELD (iMELD) score] with the aim of improving its predictive value[157,158]. In a recent study from Shi et al[159] when compared ACLF precipitated by hepatic insults to those precipitated by extrahepatic ones, the latter group had significantly higher 90-d and 1-year mortality; however, both groups had comparably high short-term mortality. This study also, demonstrates that the iMELD score may be a better predictor for hepatic-ACLF short-term prognosis, whereas CLIF-C-ACLF might be more beneficial for extrahepatic-ACLF patients. This novel score incorporates age and HE into MELD score, both, strong predictors of prognosis in hepatic-ACLF patients. The iMELD score has better predictive value of 3-mo mortality than the original MELD, SOFA, CLIF-SOFA and CPs in HBV-ACLF patients[158].
Recently, Wu et al[160] established and validated a new score to predict mortality risk in patients with HBV-ACLF. This score named “ALPH-Q score”, integrates electrocardiography parameters, age, liver cirrhosis, prothrombin time and HE greater performance than CPs, MELD, and Logistic regression model (LRM) for predicting short-term mortality of patients with HBV-ACLF.
Many other factors summarized at Table 3 had been described.
Table 3.
Summary of predicting factors
| High levels of bilirubin | Gustot et al[148], López-Velázquez et al[206], Cordoba et al[90] |
| Age and high INR | Shi et al[155], Cordoba et al[90], Garg et al[146], Kumar et al[151], Moreau et al[152] |
| Decreased serum thyroid-stimulation hormone (TSH) levels | Wu et al[207] |
| Low free T3 levels | Agiasotelli et al[208] |
| Systemic haemodynamic changes | Garg et al[146] |
| Iron metabolism and transport | Maras et al[209] |
| Neutrophil-lymphocyte ratio (NRL) | Lin et al[210], Liu et al[211] |
| Presence of SIRS | Katoonizadeh et al[177], Thabut et al[108] |
| Infection and sepsis | Sargenti et al[212], Bruns et al[164], Linderoth et al[213] |
No well-established prognostic indicators are available for predicting ACLF outcome. Ambiguity in the diagnostic criteria has limited researches into the identification of clear indicators of severity. According to diverse population under study and definition of the syndrome, different indicators have been proposed. ACLF: Acute-on-chronic liver failure; INR: International normalized ratio.
MANAGEMENT
General management
At present, there is no ACLF-specific treatment. Current treatment consists of supportive measures, and therefore it should rely on enhanced care or intensive care units where the management of patients with multiorgan failure is protocolised, and patients can be closely monitored[107,140,161]. The aim of the general management should be focused on early recognition of any condition or precipitating factor which can cause ACLF, or, even more importantly, on avoiding exposure to those factors known to trigger multiple OFs. Although not proven, it is thought that the greatest impact on patient’s outcome will be achieved by preventing or slowing a further progression of ACLF. Patients with ACLF present some unique features that may differentiate them from the non-cirrhotic patients and thus, a multidisciplinary approach is essential.
The main principle of treatment should therefore be to support organ function and treat precipitating factors while the liver recovers[6,12,25,45,162]. Treatment should be directed at addressing each specific dysfunction. For example, plasma expansion with albumin or crystalloids to improve the circulatory system or kidney function; administration of prednisolone in the setting of acute alcoholic hepatitis; renal replacement therapy to treat fluid, electrolyte, and acid-base abnormalities; vasoactive amines to improve ventricular function when circulatory failure or sepsis occurs; endotracheal intubation for airway control in patients with severe encephalopathy, in the presence of active upper gastrointestinal bleeding and/or lung failure. It should be noted, the great importance of both, prophylaxis and treatment of infections, given their crucial role of in the development of ACLF[66,69,144,163,164].
Liver support devices and liver transplantation
Liver support devices: When medical treatment fails, artificial liver support can be considered as a bridge therapy to liver transplantation or while the precipitating event is reversed. Yet organ shortage, cost, complications and side effects associated with immunosuppression, strongly limit this option. Furthermore, unstable clinical conditions of patients with ACLF are often contraindications for liver transplant.
Two types of devices can be distinguished; acellular devices such as albumin dialysis and plasma exchange [mainly molecular adsorbents recirculating system (MARS), and Prometheus devices], and cell-based devices, which incorporate cells from human, animal sources, or immortalized cells. The use of liver-assisting devices is based on their ability to remove toxic substances, inflammatory molecules, reduce NO, improve systemic hemodynamics and severe HE[165,166]. However, two prospective randomised studies, the RELIEF Study Group and the HELIOS Study Group compared treatment with conventional therapy to MARS or to Prometheus, respectively, failed to show any survival benefit, despite improvement on biochemical parameters[167,168]. In contrast, Xu et al[169] and Ling et al[170] found that downgrading MELD in ACLF using these systems therapies improved the outcomes after liver transplantation.
Therefore, although these systems have some beneficial effects in patients with ACLF, their overall usefulness in this setting is uncertain. Until today, given the lack of acquiring a strict definition of ACLF, has undoubtedly made prospective studies in this field more difficult. Consensus on definition is needed to perform clinical trials able to translate liver assist devices application to a survival benefit in patients with ACLF[168,171-173].
Liver transplantation: Available information on liver transplantation for ACLF patients’ is scarce, even though this represents the only definitive therapeutic option for the vast majority of patients with ACLF[174,175]. Nonetheless, as mentioned above, numerous reasons, including advanced age, active alcoholism, uncontrolled infections, concomitant diseases, and the presence of associated OFs, make patients with ACLF often unsuitable to undergo transplantation.
ACLF is associated with high short-term mortality rates of 50% to 90% and may evolve rapidly into a fatal clinical situation, thus the timeframe for evaluating patients and assessing them for LT is short[12,176,177]. More than 50% of the listed ACLF patients died on the waiting list which further demonstrates that the time period to transplantation is crucial, and that the window of opportunity is small[178]. Where the time of transplantation is a critical element in the patient’s prognosis, living donor transplantation is an attractive alternative, since there are no waiting list constraints, and long-term survival has been shown to be comparable to living donor transplants[179-182]. There are limited evidences regarding the long-term outcome of patients transplanted for ACLF. Some studies showed similar survival rates of patients with ACLF to patients with chronic liver disease who underwent transplantation for other indications[174,178,183]. When interpreting these data sets, differences between western and eastern transplant centers must be taken into consideration.
Further studies are still necessary to determine timing of liver transplantation, optimal selection, and whether ACLF patients should be prioritized on a high-urgency list.
Antiviral therapy in ACLF
Antiviral therapy deserves particular mention due to the relevance of reactivation of HBV among aetiologies of ACLF in the Asia-Pacific region, where hepatitis-B-related cirrhosis constitutes around 70% of the underlying chronic liver diseases[30,105]. Furthermore, a large number of HBV-ACLF cases do not have underlying cirrhosis, as evidenced APASL ACLF Research Consortium (AARC) data based on the liver biopsy studies[184].
The aim of antiviral treatment for HBV-ACLF is to reduce viral DNA, so that reduction in hepatocyte cell death, helps prevent decompensation related multiorgan complications, and thereby improves survival outcomes[185-188]. Early treatment with nucleos(t)ide analogues such as lamivudine, tenofovir, entecavir or telbuvidine should be started[188-190]. Low pretreatment HBV DNA load and a rapid decrement in viral load improves outcomes in ACLF[191-194]. Some studies suggest that initial combination antiviral therapy is more effective than monotherapy[195-197].
New therapeutic targets
A few recent studies have tested the possibility of liver regeneration in a small group of patients with ACLF using granulocyte-colony stimulating factor therapy[192,198-201]. This cytokine mobilises bone marrow-derived stem cells and then restores neutrophil function, promotes hepatic regeneration, and thereby reducing the risk of developing kidney, or brain failure, and sepsis and thus improving survival of patients with ACLF. More studies are needed to provide clearer evidence.
Other proposed therapy for patients with ACLF, has been cell transplantation, either using hepatocytes or stem cells, to improve liver function thought cell repopulation of the liver and their potential anti-inflammatory effects, but again, these results await confirmation[202-205].
CONCLUSION
ACLF is a devastating syndrome since it remains a highly prevalent, life-threatening disease, which is clinically, pathophysiologically and prognostically a distinct entity from a mere decompensation of cirrhosis. In ACLF, altered host response to precipitating injury plays a pivotal pathophysiological role, such as SIRS. The degree of background immune paralysis and severity of OF determine the outcome of this syndrome. Ambiguity and variability among researcher groups on definitions criteria hampers precise characterization of this entity. Considerable efforts have been made to delve into the knowledge of this syndrome. Despite the progress, especially in pathophysiology, several questions remain: Fist, treatment strategies are currently limited to organ support; thereby a better understanding of underlying mechanisms will allow the development of new drugs and devices. Second, the absence of consensus on diagnostic criteria hampers the recognition of biomarkers and factors determining the outcome. Third, the most ambitious goal is, probably, the early recognition of this syndrome, in order to implement strategies to avoid the development of OF owing to the reversibility of this profile of liver failure. Finally, a universally accepted definition is urgently needed.
ACKNOWLEDGMENTS
We thank Mrs. Eulalia Grifol for facilitating information searching and providing bibliographic support and Lady Suzanne Edwards for writing and editing assistance.
Footnotes
Conflict-of-interest statement: The authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 1, 2015
First decision: July 20, 2015
Article in press: September 30, 2015
P- Reviewer: Tai DI S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 4.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi H, Sugihara J, Moriwaki H, Muto Y. [Acute-on-chronic liver failure] Ryoikibetsu Shokogun Shirizu. 1995;(7):217–219. [PubMed] [Google Scholar]
- 6.Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, Gines P, Kim WR, Kamath PS. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4–10. doi: 10.1053/j.gastro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Wlodzimirow K, Abu-Hanna A, Chamuleau RA. Acute-on-chronic liver failure - its definition remains unclear. J Hepatol. 2013;59:190–191. doi: 10.1016/j.jhep.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS. Defining acute-on-chronic liver failure: will East and West ever meet? Gastroenterology. 2013;144:1337–1339. doi: 10.1053/j.gastro.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165–169. doi: 10.1097/MCC.0b013e328344b42d. [DOI] [PubMed] [Google Scholar]
- 12.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1-9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 13.Blei DA, Cordoba J. [Hepatic encephalopathy] Rom J Gastroenterol. 2002;11:163–165. [PubMed] [Google Scholar]
- 14.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256.e1-5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Herrera JL. Management of acute variceal bleeding. Clin Liver Dis. 2014;18:347–357. doi: 10.1016/j.cld.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680–1704. doi: 10.1136/gutjnl-2015-309262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516–1523. doi: 10.1111/liv.12597. [DOI] [PubMed] [Google Scholar]
- 19.Dhiman RK, Agrawal S, Gupta T, Duseja A, Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014;20:14934–14941. doi: 10.3748/wjg.v20.i40.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont B, Delvincourt M, Koné M, du Cheyron D, Ollivier-Hourmand I, Piquet MA, Terzi N, Dao T. Retrospective evaluation of prognostic score performances in cirrhotic patients admitted to an intermediate care unit. Dig Liver Dis. 2015;47:675–681. doi: 10.1016/j.dld.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 21.McPhail MJ, Shawcross DL, Abeles RD, Chang A, Patel V, Lee GH, Abdulla M, Sizer E, Willars C, Auzinger G, et al. Increased Survival for Patients With Cirrhosis and Organ Failure in Liver Intensive Care and Validation of the Chronic Liver Failure-Sequential Organ Failure Scoring System. Clin Gastroenterol Hepatol. 2015;13:1353–1360.e8. doi: 10.1016/j.cgh.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Marshall J, Sweeney D. Microbial infection and the septic response in critical surgical illness. Sepsis, not infection, determines outcome. Arch Surg. 1990;125:17–22; discussion 22-23. doi: 10.1001/archsurg.1990.01410130019002. [DOI] [PubMed] [Google Scholar]
- 23.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 24.Chen YX, Li CS. Risk stratification and prognostic performance of the predisposition, infection, response, and organ dysfunction (PIRO) scoring system in septic patients in the emergency department: a cohort study. Crit Care. 2014;18:R74. doi: 10.1186/cc13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol. 2013;19:349–359. doi: 10.3350/cmh.2013.19.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WM, Squires RH, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Saraswat VA. Hepatitis E and Acute-on-Chronic Liver Failure. J Clin Exp Hepatol. 2013;3:225–230. doi: 10.1016/j.jceh.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, Kumar Panda S. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387–394. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 30.Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A, Purucker EA, Gressner AM, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol. 2005;42:195–201. doi: 10.1016/j.jhep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, Jalan R. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosino G, Naso A, Feltracco P, Carraro P, Basso SM, Varotto S, Cillo U, Zanus G, Boccagni P, Brolese A, et al. Cytokines and liver failure: modification of TNF- and IL-6 in patients with acute on chronic liver decompensation treated with Molecular Adsorbent Recycling System (MARS) Acta Biomed. 2003;74 Suppl 2:7–9. [PubMed] [Google Scholar]
- 33.Verbeke L, Nevens F, Laleman W. Bench-to-beside review: acute-on-chronic liver failure - linking the gut, liver and systemic circulation. Crit Care. 2011;15:233. doi: 10.1186/cc10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steib CJ, Bilzer M, Härtl JM, Beitinger F, Gülberg V, Göke B, Gerbes AL. Kupffer cell activation by hydrogen peroxide: a new mechanism of portal pressure increase. Shock. 2010;33:412–418. doi: 10.1097/SHK.0b013e3181b85934. [DOI] [PubMed] [Google Scholar]
- 35.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 36.Holland-Fischer P, Grønbæk H, Sandahl TD, Moestrup SK, Riggio O, Ridola L, Aagaard NK, Møller HJ, Vilstrup H. Kupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPS. Gut. 2011;60:1389–1393. doi: 10.1136/gut.2010.234542. [DOI] [PubMed] [Google Scholar]
- 37.Rockey DC, Fouassier L, Chung JJ, Carayon A, Vallee P, Rey C, Housset C. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472–480. doi: 10.1002/hep.510270222. [DOI] [PubMed] [Google Scholar]
- 38.Adebayo D, Morabito V, Andreola F, Pieri G, Luong TV, Dhillon A, Mookerjee R, Jalan R. Mechanism of cell death in acute-on-chronic liver failure: a clinico-pathologic-biomarker study. Liver Int. 2015:Epub ahead of print. doi: 10.1111/liv.12850. [DOI] [PubMed] [Google Scholar]
- 39.Brann OS. Infectious complications of cirrhosis. Curr Gastroenterol Rep. 2001;3:285–292. doi: 10.1007/s11894-001-0051-2. [DOI] [PubMed] [Google Scholar]
- 40.Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M. Immune dysfunction in cirrhosis. World J Gastroenterol. 2014;20:2564–2577. doi: 10.3748/wjg.v20.i10.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 42.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. [Google Scholar]
- 43.Runyon BA, Morrissey RL, Hoefs JC, Wyle FA. Opsonic activity of human ascitic fluid: a potentially important protective mechanism against spontaneous bacterial peritonitis. Hepatology. 1985;5:634–637. doi: 10.1002/hep.1840050419. [DOI] [PubMed] [Google Scholar]
- 44.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Arroyo V, Moreau R, Jalan R, Ginès P. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62:S131–S143. doi: 10.1016/j.jhep.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 46.Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Semin Respir Crit Care Med. 2012;33:80–95. doi: 10.1055/s-0032-1301737. [DOI] [PubMed] [Google Scholar]
- 47.Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–262. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 48.Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53–58. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]
- 49.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1–12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 50.Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, Boccia S, Colloredo-Mels G, Corigliano P, Fornaciari G, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Dig Liver Dis. 2001;33:41–48. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 51.Navasa M, Fernández J, Rodés J. Bacterial infections in liver cirrhosis. Ital J Gastroenterol Hepatol. 1999;31:616–625. [PubMed] [Google Scholar]
- 52.Yang YY, Lin HC. Bacterial infections in patients with cirrhosis. J Chin Med Assoc. 2005;68:447–451. doi: 10.1016/S1726-4901(09)70072-3. [DOI] [PubMed] [Google Scholar]
- 53.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 54.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 55.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 56.Anastasiadis SN, Giouleme OI, Germanidis GS, Vasiliadis TG. Relative adrenal insufficiency in cirrhotic patients. Clin Med Insights Gastroenterol. 2015;8:13–17. doi: 10.4137/CGast.S18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández J, Escorsell A, Zabalza M, Felipe V, Navasa M, Mas A, Lacy AM, Ginès P, Arroyo V. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288–1295. doi: 10.1002/hep.21352. [DOI] [PubMed] [Google Scholar]
- 58.Tsai MH, Peng YS, Chen YC, Liu NJ, Ho YP, Fang JT, Lien JM, Yang C, Chen PC, Wu CS. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43:673–681. doi: 10.1002/hep.21101. [DOI] [PubMed] [Google Scholar]
- 59.Karagiannis AK, Nakouti T, Pipili C, Cholongitas E. Adrenal insufficiency in patients with decompensated cirrhosis. World J Hepatol. 2015;7:1112–1124. doi: 10.4254/wjh.v7.i8.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trifan A, Chiriac S, Stanciu C. Update on adrenal insufficiency in patients with liver cirrhosis. World J Gastroenterol. 2013;19:445–456. doi: 10.3748/wjg.v19.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182:1971–1977. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fede G, Spadaro L, Tomaselli T, Privitera G, Germani G, Tsochatzis E, Thomas M, Bouloux PM, Burroughs AK, Purrello F. Adrenocortical dysfunction in liver disease: a systematic review. Hepatology. 2012;55:1282–1291. doi: 10.1002/hep.25573. [DOI] [PubMed] [Google Scholar]
- 63.Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: a clinical review. Lancet. 2013;382:984–994. doi: 10.1016/S0140-6736(12)62197-7. [DOI] [PubMed] [Google Scholar]
- 64.Vlaar AP. Transfusion-related acute lung injury: Current understanding and preventive strategies. Transfus Clin Biol. 2012;19:117–124. doi: 10.1016/j.tracli.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Christou L, Pappas G, Falagas ME. Bacterial infection-related morbidity and mortality in cirrhosis. Am J Gastroenterol. 2007;102:1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 66.Sargenti K, Prytz H, Nilsson E, Bertilsson S, Kalaitzakis E. Bacterial infections in alcoholic and nonalcoholic liver cirrhosis. Eur J Gastroenterol Hepatol. 2015;27:1080–1086. doi: 10.1097/MEG.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 67.O’Leary JG, Reddy KR, Wong F, Kamath PS, Patton HM, Biggins SW, Fallon MB, Garcia-Tsao G, Subramanian RM, Malik R, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:753–9.e1-753-9.e2. doi: 10.1016/j.cgh.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mackavey CL, Hanks R. Hemostasis, coagulation abnormalities, and liver disease. Crit Care Nurs Clin North Am. 2013;25:435–46, v. doi: 10.1016/j.ccell.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, Tripodi A, Sanyal AJ. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–1046. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 72.Mannucci PM, Tripodi A. Liver disease, coagulopathies and transfusion therapy. Blood Transfus. 2013;11:32–36. doi: 10.2450/2012.0151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tripodi A, Primignani M, Lemma L, Chantarangkul V, Mannucci PM. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J Hepatol. 2013;59:265–270. doi: 10.1016/j.jhep.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 74.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 75.Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9:1713–1723. doi: 10.1111/j.1538-7836.2011.04429.x. [DOI] [PubMed] [Google Scholar]
- 76.Mannucci PM, Tripodi A. Hemostatic defects in liver and renal dysfunction. Hematology Am Soc Hematol Educ Program. 2012;2012:168–173. doi: 10.1182/asheducation-2012.1.168. [DOI] [PubMed] [Google Scholar]
- 77.Lisman T, Stravitz RT. Rebalanced Hemostasis in Patients with Acute Liver Failure. Semin Thromb Hemost. 2015;41:468–473. doi: 10.1055/s-0035-1550430. [DOI] [PubMed] [Google Scholar]
- 78.Lisman T, Porte RJ. Platelet function in patients with cirrhosis. J Hepatol. 2012;56:993–994; author reply 994-995. doi: 10.1016/j.jhep.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, Tsianos EV. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815–818. doi: 10.1016/j.cgh.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 80.Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992–999. doi: 10.1111/j.1365-2036.2009.03958.x. [DOI] [PubMed] [Google Scholar]
- 81.Schaden E, Saner FH, Goerlinger K. Coagulation pattern in critical liver dysfunction. Curr Opin Crit Care. 2013;19:142–148. doi: 10.1097/MCC.0b013e32835ebb52. [DOI] [PubMed] [Google Scholar]
- 82.Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11:1064–1074. doi: 10.1016/j.cgh.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 83.Lee GH. Hepatic encephalopathy in acute-on-chronic liver failure. Hepatol Int. 2015;9:520–526. doi: 10.1007/s12072-015-9626-0. [DOI] [PubMed] [Google Scholar]
- 84.Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252–261. doi: 10.1159/000047017. [DOI] [PubMed] [Google Scholar]
- 85.Atluri DK, Prakash R, Mullen KD. Pathogenesis, diagnosis, and treatment of hepatic encephalopathy. J Clin Exp Hepatol. 2011;1:77–86. doi: 10.1016/S0973-6883(11)60126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- 87.Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7–S20. doi: 10.1016/j.jceh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5:S21–S28. doi: 10.1016/j.jceh.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22 Suppl 2:5–13. doi: 10.1034/j.1600-0676.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 90.Cordoba J, Ventura-Cots M, Simón-Talero M, Amorós À, Pavesi M, Vilstrup H, Angeli P, Domenicali M, Ginés P, Bernardi M, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF) J Hepatol. 2014;60:275–281. doi: 10.1016/j.jhep.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 91.Córdoba J, García-Martinez R, Simón-Talero M. Hyponatremic and hepatic encephalopathies: similarities, differences and coexistence. Metab Brain Dis. 2010;25:73–80. doi: 10.1007/s11011-010-9172-3. [DOI] [PubMed] [Google Scholar]
- 92.Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011;54:640–649. doi: 10.1016/j.jhep.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 93.Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, Verslype C, Fevery J, Nevens F. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. doi: 10.1186/cc4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt LE, Sørensen VR, Svendsen LB, Hansen BA, Larsen FS. Hemodynamic changes during a single treatment with the molecular adsorbents recirculating system in patients with acute-on-chronic liver failure. Liver Transpl. 2001;7:1034–1039. doi: 10.1053/jlts.2001.29108. [DOI] [PubMed] [Google Scholar]
- 95.Prin M, Bakker J, Wagener G. Hepatosplanchnic circulation in cirrhosis and sepsis. World J Gastroenterol. 2015;21:2582–2592. doi: 10.3748/wjg.v21.i9.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Theocharidou E, Krag A, Bendtsen F, Møller S, Burroughs AK. Cardiac dysfunction in cirrhosis - does adrenal function play a role? A hypothesis. Liver Int. 2012;32:1327–1332. doi: 10.1111/j.1478-3231.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 97.Liu H, Lee SS. Acute-on-chronic liver failure: the heart and systemic hemodynamics. Curr Opin Crit Care. 2011;17:190–194. doi: 10.1097/MCC.0b013e328344b397. [DOI] [PubMed] [Google Scholar]
- 98.Laleman W, Verbeke L, Meersseman P, Wauters J, van Pelt J, Cassiman D, Wilmer A, Verslype C, Nevens F. Acute-on-chronic liver failure: current concepts on definition, pathogenesis, clinical manifestations and potential therapeutic interventions. Expert Rev Gastroenterol Hepatol. 2011;5:523–537; quiz 537. doi: 10.1586/egh.11.47. [DOI] [PubMed] [Google Scholar]
- 99.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 100.Møller S, Hansen EF, Becker U, Brinch K, Henriksen JH, Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver. 2000;20:51–59. doi: 10.1034/j.1600-0676.2000.020001051.x. [DOI] [PubMed] [Google Scholar]
- 101.Aggarwal A, Ong JP, Younossi ZM, Nelson DR, Hoffman-Hogg L, Arroliga AC. Predictors of mortality and resource utilization in cirrhotic patients admitted to the medical ICU. Chest. 2001;119:1489–1497. doi: 10.1378/chest.119.5.1489. [DOI] [PubMed] [Google Scholar]
- 102.Fang JT, Tsai MH, Tian YC, Jenq CC, Lin CY, Chen YC, Lien JM, Chen PC, Yang CW. Outcome predictors and new score of critically ill cirrhotic patients with acute renal failure. Nephrol Dial Transplant. 2008;23:1961–1969. doi: 10.1093/ndt/gfm914. [DOI] [PubMed] [Google Scholar]
- 103.Karvellas CJ, Bagshaw SM. Advances in management and prognostication in critically ill cirrhotic patients. Curr Opin Crit Care. 2014;20:210–217. doi: 10.1097/MCC.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 104.Zimmerman JE, Wagner DP, Seneff MG, Becker RB, Sun X, Knaus WA. Intensive care unit admissions with cirrhosis: risk-stratifying patient groups and predicting individual survival. Hepatology. 1996;23:1393–1401. doi: 10.1002/hep.510230615. [DOI] [PubMed] [Google Scholar]
- 105.Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G, Shaw S, Burroughs AK. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883–893. doi: 10.1111/j.1365-2036.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 106.Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488–496.e4. doi: 10.1053/j.gastro.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 107.Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, Garcia-Tsao G, Kamath PS. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864–1872. doi: 10.1002/hep.24622. [DOI] [PubMed] [Google Scholar]
- 108.Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–1882. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- 109.Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–1290. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 110.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 111.Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 112.Angeli P, Rodríguez E, Piano S, Ariza X, Morando F, Solà E, Romano A, García E, Pavesi M, Risso A, et al. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64:1616–1622. doi: 10.1136/gutjnl-2014-307526. [DOI] [PubMed] [Google Scholar]
- 113.Stadlbauer V, Wright GA, Banaji M, Mukhopadhya A, Mookerjee RP, Moore K, Jalan R. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111–119. doi: 10.1053/j.gastro.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 114.Nazar A, Pereira GH, Guevara M, Martín-Llahi M, Pepin MN, Marinelli M, Solá E, Baccaro ME, Terra C, Arroyo V, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219–226. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- 115.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 116.Ortega R, Ginès P, Uriz J, Cárdenas A, Calahorra B, De Las Heras D, Guevara M, Bataller R, Jiménez W, Arroyo V, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–948. doi: 10.1053/jhep.2002.35819. [DOI] [PubMed] [Google Scholar]
- 117.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 118.Cárdenas A, Ginès P. Acute-on-chronic liver failure: the kidneys. Curr Opin Crit Care. 2011;17:184–189. doi: 10.1097/MCC.0b013e328344b3da. [DOI] [PubMed] [Google Scholar]
- 119.Triantos C, Samonakis D, Thalheimer U, Patch D, Burroughs A. The relationship between liver function and portal pressure: what comes first, the chicken or the egg? J Hepatol. 2005;42:146–147; author reply 147-148. doi: 10.1016/j.jhep.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 120.Mookerjee RP. Acute-on-chronic liver failure: the liver and portal haemodynamics. Curr Opin Crit Care. 2011;17:170–176. doi: 10.1097/MCC.0b013e328344a076. [DOI] [PubMed] [Google Scholar]
- 121.Bauer M, Press AT, Trauner M. The liver in sepsis: patterns of response and injury. Curr Opin Crit Care. 2013;19:123–127. doi: 10.1097/MCC.0b013e32835eba6d. [DOI] [PubMed] [Google Scholar]
- 122.Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015;35:724–734. doi: 10.1111/liv.12559. [DOI] [PubMed] [Google Scholar]
- 123.Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776–778. doi: 10.1016/0140-6736(91)91384-7. [DOI] [PubMed] [Google Scholar]
- 124.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, Kodama Y, Miura K, Ikai I, Uemoto S, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135:1729–1738. doi: 10.1053/j.gastro.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 126.Medina J, Arroyo AG, Sánchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–1195. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 127.Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558–567. doi: 10.1016/j.jhep.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 128.Kumar A, Das K, Sharma P, Mehta V, Sharma BC, Sarin SK. Hemodynamic studies in acute-on-chronic liver failure. Dig Dis Sci. 2009;54:869–878. doi: 10.1007/s10620-008-0421-9. [DOI] [PubMed] [Google Scholar]
- 129.Jalan R, Mookerjee RP. Systemic hemodynamics, hepatic blood flow and portal pressure in patients with cirrhosis and multiorgan failure: The role of systemic inflammatory response and sympathetic activation [Abstract] Hepatology. 2008;48(Suppl 1):1078A. [Google Scholar]
- 130.Benten D, Wiest R. Gut microbiome and intestinal barrier failure--the “Achilles heel” in hepatology? J Hepatol. 2012;56:1221–1223. doi: 10.1016/j.jhep.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y, Guo J, Qian G, Fang D, Shi D, Guo L, Li L. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol. 2015;30:1429–1437. doi: 10.1111/jgh.12932. [DOI] [PubMed] [Google Scholar]
- 132.Jalan R, Stadlbauer V, Sen S, Cheshire L, Chang YM, Mookerjee RP. Role of predisposition, injury, response and organ failure in the prognosis of patients with acute-on-chronic liver failure: a prospective cohort study. Crit Care. 2012;16:R227. doi: 10.1186/cc11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jalan R, Mookerjee RP. Acute-on-chronic liver failure: an early biopsy is essential? Gut. 2010;59:1455–1456. doi: 10.1136/gut.2010.214627. [DOI] [PubMed] [Google Scholar]
- 134.Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003;139:186–193. doi: 10.7326/0003-4819-139-3-200308050-00008. [DOI] [PubMed] [Google Scholar]
- 135.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Q, Li Y, Han T, Nie C, Cai J, Liu H, Liu Y. Comparison of current diagnostic criteria for acute-on-chronic liver failure. PLoS One. 2015;10:e0122158. doi: 10.1371/journal.pone.0122158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stravitz RT. Liver: Acute-on-chronic liver failure--no longer an entity without definition. Nat Rev Gastroenterol Hepatol. 2014;11:580–581. doi: 10.1038/nrgastro.2014.161. [DOI] [PubMed] [Google Scholar]
- 138.Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int. 2013;33:40–52. doi: 10.1111/j.1478-3231.2012.02790.x. [DOI] [PubMed] [Google Scholar]
- 139.Warrillow SJ. Predictions and outcomes for the critically ill patient with cirrhosis: is it time to settle on the SOFA and let jaundiced views on outcome MELD away? Crit Care Med. 2010;38:2259–2260. doi: 10.1097/CCM.0b013e3181f84a23. [DOI] [PubMed] [Google Scholar]
- 140.Das V, Boelle PY, Galbois A, Guidet B, Maury E, Carbonell N, Moreau R, Offenstadt G. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;38:2108–2116. doi: 10.1097/CCM.0b013e3181f3dea9. [DOI] [PubMed] [Google Scholar]
- 141.Karvellas CJ, Pink F, McPhail M, Austin M, Auzinger G, Bernal W, Sizer E, Kutsogiannis DJ, Eltringham I, Wendon JA. Bacteremia, acute physiology and chronic health evaluation II and modified end stage liver disease are independent predictors of mortality in critically ill nontransplanted patients with acute on chronic liver failure. Crit Care Med. 2010;38:121–126. doi: 10.1097/CCM.0b013e3181b42a1c. [DOI] [PubMed] [Google Scholar]
- 142.Wehler M, Kokoska J, Reulbach U, Hahn EG, Strauss R. Short-term prognosis in critically ill patients with cirrhosis assessed by prognostic scoring systems. Hepatology. 2001;34:255–261. doi: 10.1053/jhep.2001.26522. [DOI] [PubMed] [Google Scholar]
- 143.Stauber R, Stadlbauer V, Struber G, Kaufmann R. Evaluation of four prognostic scores in patients with acute-on-chronic liver failure. J Hepatol. 2006;44(Suppl 2):S69–70. [Google Scholar]
- 144.Amarapurkar D, Dharod MV, Chandnani M, Baijal R, Kumar P, Jain M, Patel N, Kamani P, Issar S, Shah N, et al. Acute-on-chronic liver failure: a prospective study to determine the clinical profile, outcome, and factors predicting mortality. Indian J Gastroenterol. 2015;34:216–224. doi: 10.1007/s12664-015-0574-3. [DOI] [PubMed] [Google Scholar]
- 145.Mikolasevic I, Milic S, Radic M, Orlic L, Bagic Z, Stimac D. Clinical profile, natural history, and predictors of mortality in patients with acute-on-chronic liver failure (ACLF) Wien Klin Wochenschr. 2015;127:283–289. doi: 10.1007/s00508-015-0707-9. [DOI] [PubMed] [Google Scholar]
- 146.Garg H, Kumar A, Garg V, Sharma P, Sharma BC, Sarin SK. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis. 2012;44:166–171. doi: 10.1016/j.dld.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 147.Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, Sawhney R, Mookerjee R, Caraceni P, Moreau R, et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 148.Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, Trebicka J, Elkrief L, Hopf C, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 149.Zheng YB, Huang ZL, Wu ZB, Zhang M, Gu YR, Su YJ, Lin CS, Zhu RH, Lin BL, Gao ZL. Dynamic changes of clinical features that predict the prognosis of acute-on-chronic hepatitis B liver failure: a retrospective cohort study. Int J Med Sci. 2013;10:1658–1664. doi: 10.7150/ijms.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yi ZQ, Lu MH, Xu XW, Fu XY, Tan DM. A novel prognostic score for acute-on-chronic hepatitis B liver failure. J Huazhong Univ Sci Technolog Med Sci. 2015;35:87–92. doi: 10.1007/s11596-015-1394-5. [DOI] [PubMed] [Google Scholar]
- 151.Kumar R, Krishnamoorthy TL, Tan HK, Lui HF, Chow WC. Change in model for end-stage liver disease score at two weeks, as an indicator of mortality or liver transplantation at 60 days in acute-on-chronic liver failure. Gastroenterol Rep (Oxf) 2015;3:122–127. doi: 10.1093/gastro/gou075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moreau R, Jalan R, Arroyo V. Acute-on-Chronic Liver Failure: Recent Concepts. J Clin Exp Hepatol. 2015;5:81–85. doi: 10.1016/j.jceh.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsai MH, Peng YS, Lien JM, Weng HH, Ho YP, Yang C, Chu YY, Chen YC, Fang JT, Chiu CT, et al. Multiple organ system failure in critically ill cirrhotic patients. A comparison of two multiple organ dysfunction/failure scoring systems. Digestion. 2004;69:190–200. doi: 10.1159/000078789. [DOI] [PubMed] [Google Scholar]
- 154.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi Y, Zheng MH, Yang Y, Wei W, Yang Q, Hu A, Hu Y, Wu Y, Yan H. Increased delayed mortality in patients with acute-on-chronic liver failure who have prior decompensation. J Gastroenterol Hepatol. 2015;30:712–718. doi: 10.1111/jgh.12787. [DOI] [PubMed] [Google Scholar]
- 156.Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890–895. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- 157.Xia Q, Dai X, Zhang Y, Guo Y, Xu X, Yang Q, Du W, Liu X, Chen Y, Huang J, et al. A modified MELD model for Chinese pre-ACLF and ACLF patients and it reveals poor prognosis in pre-ACLF patients. PLoS One. 2013;8:e64379. doi: 10.1371/journal.pone.0064379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yan H, Wu W, Yang Y, Wu Y, Yang Q, Shi Y. A novel integrated Model for End-Stage Liver Disease model predicts short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure patients. Hepatol Res. 2015;45:405–414. doi: 10.1111/hepr.12362. [DOI] [PubMed] [Google Scholar]
- 159.Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, Xu Z, Wu Y, Yan H, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232–242. doi: 10.1002/hep.27795. [DOI] [PubMed] [Google Scholar]
- 160.Wu SJ, Yan HD, Zheng ZX, Shi KQ, Wu FL, Xie YY, Fan YC, Ye BZ, Huang WJ, Chen YP, et al. Establishment and validation of ALPH-Q score to predict mortality risk in patients with acute-on-chronic hepatitis B liver failure: a prospective cohort study. Medicine (Baltimore) 2015;94:e403. doi: 10.1097/MD.0000000000000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Siddiqui MS, Stravitz RT. Intensive care unit management of patients with liver failure. Clin Liver Dis. 2014;18:957–978. doi: 10.1016/j.cld.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 162.Zamora Nava LE, Aguirre Valadez J, Chávez-Tapia NC, Torre A. Acute-on-chronic liver failure: a review. Ther Clin Risk Manag. 2014;10:295–303. doi: 10.2147/TCRM.S59723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sargenti K, Prytz H, Strand A, Nilsson E, Kalaitzakis E. Healthcare-associated and nosocomial bacterial infections in cirrhosis: predictors and impact on outcome. Liver Int. 2015;35:391–400. doi: 10.1111/liv.12625. [DOI] [PubMed] [Google Scholar]
- 164.Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sen S, Davies NA, Mookerjee RP, Cheshire LM, Hodges SJ, Williams R, Jalan R. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004;10:1109–1119. doi: 10.1002/lt.20236. [DOI] [PubMed] [Google Scholar]
- 166.Hassanein TI, Schade RR, Hepburn IS. Acute-on-chronic liver failure: extracorporeal liver assist devices. Curr Opin Crit Care. 2011;17:195–203. doi: 10.1097/MCC.0b013e328344b3aa. [DOI] [PubMed] [Google Scholar]
- 167.Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, Hoste E, Van Vlierberghe H, Escorsell A, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782–789.e3. doi: 10.1053/j.gastro.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 168.Bañares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, Saliba F, Sauerbruch T, Klammt S, Ockenga J, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153–1162. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 169.Xu X, Liu X, Ling Q, Wei Q, Liu Z, Xu X, Zhou L, Zhang M, Wu J, Huang J, et al. Artificial liver support system combined with liver transplantation in the treatment of patients with acute-on-chronic liver failure. PLoS One. 2013;8:e58738. doi: 10.1371/journal.pone.0058738. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Ling Q, Xu X, Wei Q, Liu X, Guo H, Zhuang L, Chen J, Xia Q, Xie H, Wu J, et al. Downgrading MELD improves the outcomes after liver transplantation in patients with acute-on-chronic hepatitis B liver failure. PLoS One. 2012;7:e30322. doi: 10.1371/journal.pone.0030322. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Stadlbauer V, Davies NA, Sen S, Jalan R. Artificial liver support systems in the management of complications of cirrhosis. Semin Liver Dis. 2008;28:96–109. doi: 10.1055/s-2008-1040324. [DOI] [PubMed] [Google Scholar]
- 172.Maiwall R, Maras JS, Nayak SL, Sarin SK. Liver dialysis in acute-on-chronic liver failure: current and future perspectives. Hepatol Int. 2014;8 Suppl 2:505–513. doi: 10.1007/s12072-014-9534-8. [DOI] [PubMed] [Google Scholar]
- 173.Khuroo MS, Khuroo MS, Farahat KL. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl. 2004;10:1099–1106. doi: 10.1002/lt.20139. [DOI] [PubMed] [Google Scholar]
- 174.Chan AC, Fan ST. Criteria for liver transplantation in ACLF and outcome. Hepatol Int. 2015;9:355–359. doi: 10.1007/s12072-014-9585-x. [DOI] [PubMed] [Google Scholar]
- 175.Reddy MS, Rajalingam R, Rela M. Liver transplantation in acute-on-chronic liver failure: lessons learnt from acute liver failure setting. Hepatol Int. 2015;9:508–513. doi: 10.1007/s12072-014-9603-z. [DOI] [PubMed] [Google Scholar]
- 176.Duseja A, Chawla YK, Dhiman RK, Kumar A, Choudhary N, Taneja S. Non-hepatic insults are common acute precipitants in patients with acute on chronic liver failure (ACLF) Dig Dis Sci. 2010;55:3188–3192. doi: 10.1007/s10620-010-1377-0. [DOI] [PubMed] [Google Scholar]
- 177.Katoonizadeh A, Laleman W, Verslype C, Wilmer A, Maleux G, Roskams T, Nevens F. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut. 2010;59:1561–1569. doi: 10.1136/gut.2009.189639. [DOI] [PubMed] [Google Scholar]
- 178.Finkenstedt A, Nachbaur K, Zoller H, Joannidis M, Pratschke J, Graziadei IW, Vogel W. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879–886. doi: 10.1002/lt.23678. [DOI] [PubMed] [Google Scholar]
- 179.Liu CL, Fan ST, Lo CM, Wei WI, Yong BH, Lai CL, Wong J. Live-donor liver transplantation for acute-on-chronic hepatitis B liver failure. Transplantation. 2003;76:1174–1179. doi: 10.1097/01.TP.0000087341.88471.E5. [DOI] [PubMed] [Google Scholar]
- 180.Lin KH, Liu JW, Chen CL, Wang SH, Lin CC, Liu YW, Yong CC, Lin TL, Li WF, Hu TH, et al. Impacts of pretransplant infections on clinical outcomes of patients with acute-on-chronic liver failure who received living-donor liver transplantation. PLoS One. 2013;8:e72893. doi: 10.1371/journal.pone.0072893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen Z, Wen T, Zeng Y, Wang L, Lu JJ, Gong S, Tan H, Feng P, Li B, Zhao J, et al. A single institution experience with living donor liver transplantation for acute-on-chronic hepatitis B liver failure. Hepatogastroenterology. 2011;58:1267–1273. doi: 10.5754/hge10148. [DOI] [PubMed] [Google Scholar]
- 182.Chan AC, Fan ST, Lo CM, Liu CL, Chan SC, Ng KK, Yong BH, Chiu A, Lam BK. Liver transplantation for acute-on-chronic liver failure. Hepatol Int. 2009;3:571–581. doi: 10.1007/s12072-009-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bahirwani R, Shaked O, Bewtra M, Forde K, Reddy KR. Acute-on-chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation. 2011;92:952–957. doi: 10.1097/TP.0b013e31822e6eda. [DOI] [PubMed] [Google Scholar]
- 184.Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 185.Chen T, He Y, Liu X, Yan Z, Wang K, Liu H, Zhang S, Zhao Y. Nucleoside analogues improve the short-term and long-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Clin Exp Med. 2012;12:159–164. doi: 10.1007/s10238-011-0160-7. [DOI] [PubMed] [Google Scholar]
- 186.Liu XY, Wang HF, Hu JH, He WP, Wang HQ, Liu N. [The short-term efficacy of nucleoside analogue on the treatment of acute-on-chronic liver failure] Zhonghua Gan Zang Bing Za Zhi. 2010;18:845–848. doi: 10.3760/cma.j.issn.1007-3418.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 187.Cui YL, Yan F, Wang YB, Song XQ, Liu L, Lei XZ, Zheng MH, Tang H, Feng P. Nucleoside analogue can improve the long-term prognosis of patients with hepatitis B virus infection-associated acute on chronic liver failure. Dig Dis Sci. 2010;55:2373–2380. doi: 10.1007/s10620-010-1257-7. [DOI] [PubMed] [Google Scholar]
- 188.Xie F, Yan L, Lu J, Zheng T, Shi C, Ying J, Shen R, Yang J. Effects of nucleoside analogue on patients with chronic hepatitis B-associated liver failure: meta-analysis. PLoS One. 2013;8:e54773. doi: 10.1371/journal.pone.0054773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Philips CA, Sarin SK. Potent antiviral therapy improves survival in acute on chronic liver failure due to hepatitis B virus reactivation. World J Gastroenterol. 2014;20:16037–16052. doi: 10.3748/wjg.v20.i43.16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu S, Jianqin H, Wei W, Jianrong H, Yida Y, Jifang S, Liang Y, Zhi C, Hongyu J. The efficacy and safety of nucleos(t)ide analogues in the treatment of HBV-related acute-on-chronic liver failure: a meta-analysis. Ann Hepatol. 2013;12:364–372. [PubMed] [Google Scholar]
- 191.Huang K, Hu JH, Wang HF, He WP, Chen J, Duan XZ, Zhang AM, Liu XY. Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol. 2011;17:3448–3452. doi: 10.3748/wjg.v17.i29.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Piscaglia AC, Arena V, Passalacqua S, Gasbarrini A. A case of granulocyte-colony stimulating factor/plasmapheresis-induced activation of granulocyte-colony stimulating factor-positive hepatic progenitors in acute-on-chronic liver failure. Hepatology. 2015;62:649–652. doi: 10.1002/hep.27708. [DOI] [PubMed] [Google Scholar]
- 193.Garg H, Sarin SK, Kumar M, Garg V, Sharma BC, Kumar A. Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology. 2011;53:774–780. doi: 10.1002/hep.24109. [DOI] [PubMed] [Google Scholar]
- 194.Sun LJ, Yu JW, Zhao YH, Kang P, Li SC. Influential factors of prognosis in lamivudine treatment for patients with acute-on-chronic hepatitis B liver failure. J Gastroenterol Hepatol. 2010;25:583–590. doi: 10.1111/j.1440-1746.2009.06089.x. [DOI] [PubMed] [Google Scholar]
- 195.Yang J, Chen G, Chen X, Zhang H, Jiang D, Yang G. Initial combination anti-viral therapy with lamivudine and adefovir dipivoxil decreases short-term fatality rate of hepatitis-B-virus-related acute-on-chronic liver failure. Virol J. 2015;12:97. doi: 10.1186/s12985-015-0323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu F, Wang X, Wei F, Hu H, Zhang D, Hu P, Ren H. Efficacy and resistance in de novo combination lamivudine and adefovir dipivoxil therapy versus entecavir monotherapy for the treatment-naive patients with chronic hepatitis B: a meta-analysis. Virol J. 2014;11:59. doi: 10.1186/1743-422X-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sheng YJ, Liu JY, Tong SW, Hu HD, Zhang DZ, Hu P, Ren H. Lamivudine plus adefovir combination therapy versus entecavir monotherapy for lamivudine-resistant chronic hepatitis B: a systematic review and meta-analysis. Virol J. 2011;8:393. doi: 10.1186/1743-422X-8-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gustot T. Beneficial role of G-CSF in acute-on-chronic liver failure: effects on liver regeneration, inflammation/immunoparalysis or both? Liver Int. 2014;34:484–486. doi: 10.1111/liv.12356. [DOI] [PubMed] [Google Scholar]
- 199.Khanam A, Trehanpati N, Garg V, Kumar C, Garg H, Sharma BC, Sarin SK. Altered frequencies of dendritic cells and IFN-gamma-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on- chronic liver failure. Liver Int. 2014;34:505–513. doi: 10.1111/liv.12415. [DOI] [PubMed] [Google Scholar]
- 200.Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505–512.e1. doi: 10.1053/j.gastro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 201.Duan XZ, Liu FF, Tong JJ, Yang HZ, Chen J, Liu XY, Mao YL, Xin SJ, Hu JH. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2013;19:1104–1110. doi: 10.3748/wjg.v19.i7.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ma XR, Tang YL, Xuan M, Chang Z, Wang XY, Liang XH. Transplantation of autologous mesenchymal stem cells for end-stage liver cirrhosis: a meta-analysis based on seven controlled trials. Gastroenterol Res Pract. 2015;2015:908275. doi: 10.1155/2015/908275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun K, Xie X, Xie J, Jiao S, Chen X, Zhao X, Wang X, Wei L. Cell-based therapy for acute and chronic liver failures: distinct diseases, different choices. Sci Rep. 2014;4:6494. doi: 10.1038/srep06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang F, Zhou L, Ma X, Ma W, Wang C, Lu Y, Chen Y, An L, An W, Yang Y. Monitoring of intrasplenic hepatocyte transplantation for acute-on-chronic liver failure: a prospective five-year follow-up study. Transplant Proc. 2014;46:192–198. doi: 10.1016/j.transproceed.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 206.López-Velázquez JA, Chávez-Tapia NC, Ponciano-Rodríguez G, Sánchez-Valle V, Caldwell SH, Uribe M, Méndez-Sánchez N. Bilirubin alone as a biomarker for short-term mortality in acute-on-chronic liver failure: an important prognostic indicator. Ann Hepatol. 2013;13:98–104. [PubMed] [Google Scholar]
- 207.Wu Y, You S, Zang H, Liu H, Mao Y, Mao P, Zhu B, Xu J, Xie G, Guo J, et al. Usefulness of serum thyroid-stimulation hormone (TSH) as a prognostic indicator for acute-on-chronic liver failure. Ann Hepatol. 2015;14:218–224. [PubMed] [Google Scholar]
- 208.Agiasotelli D, Alexopoulou A, Vasilieva L, Dourakis SP. Low free T3 levels are related to early mortality in patients with decompensated cirrhosis and acute-on chronic liver failure. J Hepatol. 2014;61:1446–1447. doi: 10.1016/j.jhep.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 209.Maras JS, Maiwall R, Harsha HC, Das S, Hussain MS, Kumar C, Bihari C, Rastogi A, Kumar M, Trehanpati N, et al. Dysregulated iron homeostasis is strongly associated with multiorgan failure and early mortality in acute-on-chronic liver failure. Hepatology. 2015;61:1306–1320. doi: 10.1002/hep.27636. [DOI] [PubMed] [Google Scholar]
- 210.Lin BY, Zhou L, Geng L, Zheng ZY, Jia JJ, Zhang J, Yao J, Zheng SS. High neutrophil-lymphocyte ratio indicates poor prognosis for acute-on-chronic liver failure after liver transplantation. World J Gastroenterol. 2015;21:3317–3324. doi: 10.3748/wjg.v21.i11.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu H, Zhang H, Wan G, Sang Y, Chang Y, Wang X, Zeng H. Neutrophil-lymphocyte ratio: a novel predictor for short-term prognosis in acute-on-chronic hepatitis B liver failure. J Viral Hepat. 2014;21:499–507. doi: 10.1111/jvh.12160. [DOI] [PubMed] [Google Scholar]
- 212.Sargenti K, Prytz H, Nilsson E, Kalaitzakis E. Predictors of mortality among patients with compensated and decompensated liver cirrhosis: the role of bacterial infections and infection-related acute-on-chronic liver failure. Scand J Gastroenterol. 2015;50:875–883. doi: 10.3109/00365521.2015.1017834. [DOI] [PubMed] [Google Scholar]
- 213.Linderoth G, Jepsen P, Schønheyder HC, Johnsen SP, Sørensen HT. Short-term prognosis of community-acquired bacteremia in patients with liver cirrhosis or alcoholism: A population-based cohort study. Alcohol Clin Exp Res. 2006;30:636–641. doi: 10.1111/j.1530-0277.2006.00074.x. [DOI] [PubMed] [Google Scholar]


