Abstract
A well-known tumor suppressor, p21, acts paradoxically by promoting tumor growth in some cellular conditions. These conflicting functions have been demonstrated in association with the HBx gene and in hepatocarcinogenesis. The molecular behavior of p21 depends on its subcellular localization. Nuclear p21 may inhibit cell proliferation and be proapoptotic, while cytoplasmic p21 may have oncogenic and anti-apoptotic functions. Because most typical tumor suppressive proteins also have different effects according to subcellular localization, elucidating the regulatory mechanisms underlying nucleo-cytoplasmic transport of these proteins would be significant and may lead to a new strategy for anti-hepatocellular carcinoma (HCC) therapy. Chromosome region maintenance 1 (CRM1) is a major nuclear export receptor involved in transport of tumor suppressors from nucleus to cytoplasm. Expression of CRM1 is enhanced in a variety of malignancies and in vitro studies have shown the efficacy of specific inhibition of CRM1 against cancer cell lines. Interestingly, interferon may keep p21 in the nucleus; this is one of the mechanisms of its anti-hepatocarcinogenic function. Here we review the oncogenic property of p21, which depends on its subcellular localization, and discuss the rationale underlying a new strategy for HCC treatment and prevention.
Keywords: p21, Tumor suppressors, Oncogene, Subcellular localization, Hepatocellular carcinoma, HBx, Nucleo-cytoplasmic export, Chromosome region maintenance 1, Selective inhibitors of nuclear export, Interferon
Core tip: A well-known tumor suppressor, p21, can act paradoxically by promoting tumor growth, depending on its subcellular localization. Nuclear p21 may inhibit cell proliferation while cytoplasmic p21 may be associated with anti-apoptotic and oncogenic functions. These conflicting roles are reviewed in the context of the HBx gene and hepatocarcinogenesis. Because most tumor suppressors act in a similar manner to p21, regulation of their nucleo-cytoplasmic export, which is mainly effected via chromosome region maintenance 1, may be a basis for developing a new strategy for anti-hepatocellular carcinoma therapy.
INTRODUCTION
Primary liver cancer is the 5th most common cancer in men and the 7th in women, with high mortality worldwide; therapeutic options for cure are urgently needed[1,2]. Hepatocellular carcinoma (HCC) accounts for most primary liver cancer. Although there is a geographic difference in incidence of HCC caused by some etiological variation, the major etiological agents are hepatitis B (HBV) and hepatitis C virus (HCV) infection. Once these viruses infect liver, they ingeniously evade host immune surveillance and induce chronic necroinflammation, leading to fibrosis and, ultimately, liver cirrhosis. Hepatocytes, via their innate regenerative capacity, continue to proliferate in order to compensate for the necrotic tissue. Genetic alterations continuously accumulate during these processes, resulting in pathogenic liver changes such as cirrhosis from which HCC frequently arises[3]. Once HBV-related cirrhosis is established, HCC develops at an annual rate of about 4% in Japan, for example[4].
Several lines of evidence support the direct involvement of HBV in the transformation processes. HBV is like a retrovirus in that it integrates into the host genome, causing chromosomal abnormalities. In addition, the HBx gene acts like an oncogene by trans-activating many genes involved in cellular transformation.
No common molecular mechanisms that account for the extremely complex process of hepatocarcinogenesis have yet been elucidated. Genetic alterations reported have been heterogenous, involving abnormalities of many signal transduction pathways[2,3]. However, a fundamental abnormality in hepatocarcinogenesis, like other malignancies, is deregulation of the cell cycle. The main regulators of the cell cycle are cyclin-dependent kinase inhibitors (CDKI), such as p21, p27, and p16, widely known as tumor suppressors. However, it is noteworthy that these tumor suppressors can function in an oncogenic manner depending on their precise intracellular localization. In this review, we explore the relevance of the intracellular localization of p21, in particular, and its function, to highlight the possibility that regulating the intracellular localization of tumor suppressors may be a potential future anti-HCC strategy in the context of both directly-killing tumor cells and preventive role.
P21 AS AN ONCOGENE
First identified in 1993[5,6], p21 is a universal CDKI that causes G1 growth arrest downstream of p53[7,8]. p21 binds to CDKs and inhibits the kinase activity, leading to growth arrest at specific stages in the cell cycle[9,10]. p21 also induces cellular differentiation and senescence.
Although p21 is one of the major tumor suppressors, it also can promotes oncogenesis. High expression of p21 is associated with poor prognosis of cancer[11-13]. Although mutation of the p21 gene has been reported in bladder cancer[14], most reported studies failed to show the loss-of-function mutations of p21[15-17]. These results suggest that p21 may not be a classical tumor suppressor.
Experimental results of using genetically-engineered mice also support conflicting functions of p21. Spontaneous tumors occurred in p21-deficient mice, providing evidence that p21 is a tumor suppressor[18]. p21 also causes genomic instability[19]. However, the timing of tumor formation in p21-deficient mice was later than p53-deficient mice[20]. Moreover, the occurrence of lymphoma was suppressed when p21-deficient mice were crossed with p53- or ATM-deficient mice[21,22]. This result indicates that p21 also acts in an oncogenic way in particular conditions, reflecting its versatile function[9]. In addition, mammary gland tumorigenesis was accelerated in mice in which p21 was overexpressed in cytoplasm[23], and the cyclin-binding motif of p21 has been reported to have a direct tumorigenic function[24].
HCC, HBX AND P21
There have been many reports regarding the expression of p21 in HCC tissues. p21 was found to be down-regulated in HCC tissues, demonstrating its tumor suppressive function[25-27]. Kao et al[28] also reported that p21 expression was observed in 37% of HCC tissues, regardless of p53 expression, and was an independent survival good prognosis factor. While most of the reports show that p21 acts as a tumor suppressor, expression levels of p21 in liver cirrhosis have been reported to be correlated with the cumulative incidence of the occurrence HCC[29] and to be dominant in cytoplasm when histology became more undifferentiated[30].
There are also some reported studies examining the relationship between HBV and p21. Some reports have shown that the HBx gene exerts oncogenic activity by suppressing p21 expression[31,32] and that HBx genes having core promoter mutations suppress p21 more effectively[33]. Inversely, HBx enhanced p21 in some reports[34,35]. Park et al[34] reported that when the cell cycle was prolonged by enhancement of p21 by HBx, cells had survival advantages and chances for gene mutations, eventually leading to preneoplastic hepatocytes. In addition, Yano et al[36] reported that HBx enhanced cytoplasmic p21 in protein kinase C (PKC)-dependent manner to induce cell proliferation. These conflicting results may partly come from differences in experimental conditions, but mostly reflect the conflictive function of p21.
ASSOCIATION BETWEEN MOLECULAR BEHAVIOR OF P21 AND ITS ONCOGENIC FUNCTION
What molecular behavior of p21 does correlate with its tumor-promoting function? It is well-known that p21 has not only inhibitory effects on cell cycle, but also has a promoting role. p21-associated CDKs exist in both active and inactive states[37]; p21 promotes the assembly of CDK4,6 and cyclin D and exerts oncogenic activity without inhibiting kinase activity[38]. Mantel et al[39] found a high level of induction of p21 in a myeloid cell line that was induced to proliferate by growth factors. p21 also induces nuclear retention of cyclin D1, inhibiting its cytoplasmic degradation[40]. p21 induces cell cycle progression in glioma[41] and in vascular smooth muscle cells[42] by promoting the formation of active cyclin-CDK complexes with PKC-alpha. Because the lymphoma observed in p21-deficient mice has high levels of apoptosis[21], the oncogenic activity of p21 may be closely associated with its anti-apoptotic function
P21 has a dual function with regard to apoptosis. p21 halts the cell cycle and prevents apoptosis induced by genotoxic agents. This anti-apoptotic function of p21 may be associated with its oncogenic property. However, p21 acts as a pro-apoptotic in some conditions. Forced expression of p21 induces the apoptotic response against cisplatin in glioma[43] and in ovarian cancer[44]. p21 is a modulator of apoptosis in a p53-dependent or -independent manner[10]. Masgras et al[45] reported that cell-specific sensitivity to oxidative stress determined whether the cell was fated to undergo p21-induced cell death.
ACTIONS OF P21 DEPEND ON SUBCELLULAR LOCALIZATION
The dual functions of p21, apoptotic or anti-apoptotic, depend on its subcellular localization[9,46]. Nuclear p21 is anti-proliferative and cytoplasmic p21, which is anti-apoptotic, may be associated with oncogenic function. Cytoplasmic p21 is associated with poor prognosis or the aggressiveness of human cancer[11,12,30,47]. Cytoplasmic localization of p21 is closely associated with the phosphorylation status. Phosphorylation at Thr57 and Ser130 by extracellular signal-regulated kinase (ERK) inhibits nuclear localization of p21 and causes its cytoplasmic accumulation, inducing cell cycle progression[48]. Phosphorylated p21 that locates in cytoplasm has anti-apoptotic action by inhibiting the apoptotic proteins. Koster et al[49] reported that cytoplasmic p21 conferred resistance against cisplatin-induced apoptosis, while it became pro-apoptotic when it entered in the nucleus by the inhibition of AKT. Involvement in signal transduction of phosphorylated p21 differs depending on the site of the phosphorylated amino acid; Thr145 by AKT[50,51] or Ser130 by p38 and JNK[52]. Cytoplasmic p21 prevents apoptosis by inhibiting procaspase 3[53], and apoptosis signal-regulating kinases (ASK) 1[54].
As a summary, p21 as a tumor suppressor may be associated with nuclear location that may be associated with inhibition of cell proliferation and pro-apoptotic function, while oncogenic, anti-apoptotic p21 may require a cytoplasmic location. Thus, the shifting subcellular localization of p21 may be the clearest way to explain its functional versatility.
It is well known that not only p21 but most tumor suppressive proteins have different effects in different subcellular compartment. Cancer cells respond to what are typically tumor suppressors, such as p21, Rb, p53, p27, breast cancer susceptibility gene (BRCA) 1 and FOXO (forkhead box-containing, O subfamily) by proliferating when these molecules relocate from nucleus to cytoplasm[55,56]. Thus, based on the discussion above regarding p21, it is possible to extend this view to tumor suppressors in general. Regulating the subcellular localization of these proteins may become a core rationale for anti-cancer strategy[55,56].
REGULATION OF THE SUBCELLULAR LOCALIZATION OF TUMOR SUPPRESSORS AND ITS POTENTIAL APPLICATION TO ANTI-HCC TREATMENT
Transport of macromolecules across the nuclear envelope occurs through nuclear pore complexes (NPC). Karyopherins, such as exportins and importins, are nuclear transport receptors that recognize nuclear export signal (NES) and nuclear localization signal (NLS) sequences, respectively, and transport cargo proteins at the NPC sites[55,57]. Subcellular localization of tumor suppressors is regulated by this nucleo-cytoplasmic transport system[55,56,58,59].
CRM1 (Exportin-1/chromosome region maintenance 1) is a major nuclear export receptor that forms NPC with nucleoporins, such as NUP214 and NUP88, transporting nuclear proteins with NES sequence to cytoplasm[55-59]. CRM1 is deeply involved in the mechanisms of cell proliferation by regulating the subcellular localization of tumor suppressors which have NES sequences, such as p53 and p21. For example, p53 accumulates in the nucleus when poly (ADP-ribosyl)ation blocks its interaction with CRM1[60], while interaction with SUMO (small ubiquitin-like modifier) promotes its export to cytoplasm by CRM1[61]. p21 inhibits CRM1 by binding phosphorylated cyclin D, which promotes its nuclear accumulation[40].
Cancer cells use nucleo-cytoplasmic transport system for their proliferation and inhibition of apoptosis. CRM1 expression is enhanced in many cancer tissues. High expression of CRM1 in gastric, ovarian, and pancreatic cancers show poor prognosis[62-64]. The increase in CRM1 leads to cytoplasmic abundance of tumor suppressors and cell cycle regulators, which in turn results in their aberrant activation. Knock-down of CRM1 expression prevents nuclear export of p27, resulting in cell cycle arrest[65]. Specific suppression of CRM1 caused nuclear retention of p21 and induced apoptosis[66]. The cellular apoptosis susceptibility (CAS)/importin pathway was found to be enhanced in HCC, confirming the importance of the transport machinery[67].
Orally available selective inhibitors of nuclear export (SINEs) which specifically inhibit CRM1, have been developed in recent years[58,59]. SINEs specifically bind Cys528, located in NES-binding groove of CRM1, to promote nuclear retention of p53, p21, p27, Rb, and BRCA 1[68]. The effects on hematologic malignancies of KPT-330, the most effective SINE, have been reported[69-73]. KPT-330 had anti-proliferative effects and induced apoptosis of an HCC cell line[74] in which p53-upregulated-modulator of apoptosis was markedly up-regulated; and this was shown to be one of the similar mechanisms by which sorafenib exerts anti-HCC effects[75].
Interestingly, interferon (IFN)-beta was reported to return cytoplasmic p21 to the nucleus and contributed to the prevention of hepatocarcinogenesis[36]. This was also true of p53 that was bound to cytoplasmic HBx and returned to the nucleus after IFN-treatment[76] (Figure 1). These observations suggest that natural substances such as IFN may be involved in an innate carcinogenesis prevention mechanisms, possibly by regulating CRM1. In fact, CRM1 is involved in the cytoplasmic localization of STAT2, which shifts to the nucleus by the action of IFN[77]. In addition, IFN inhibits beta-catenin signaling through the up-regulation of the nuclear RanBP3 which is a nuclear export factor[78].
Figure 1.
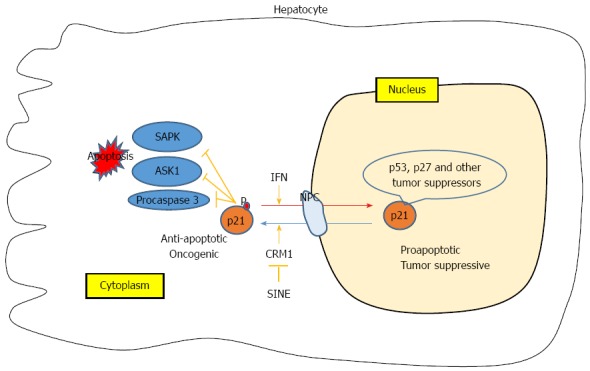
Outline of the overall aspects of this review. The subcellular localization of p21 and other tumor suppressors is regulated by CRM1. Inhibition of CRM1 by specific inhibitors and IFN may play a role in future anti-hepatocellular carcinoma strategies. CRM1: Chromosome region maintenance 1; IFN: Interferon; SINE: Selective inhibitors of nuclear export.
FUTURE PERSPECTIVE
Sorafenib is a tyrosine kinase inhibitor widely used for the treatment of advanced HCC, and many other molecular-targeted drugs are now in development[79]. However its effect is still limited in many patients and the appearance of drug-resistance is a significant problem. Regulation of CRM1 involves many genes and specific multiple pathways associated with nuclear-cytoplasmic export; a new therapeutic strategy could be based on these developing concepts. Because such regulation would normalize molecular changes caused by multiple genes, its use might not cause drug resistance, or even being suggested to reverse drug resistance[55]. Thus, combination of such regulation with specific inhibitor use might maximize the impact of treatment. While expecting some promising results of clinical trials, taking the molecular approach to explore innate mechanisms regulating nuclear-cytoplasmic distribution of tumor suppressors will become an intriguing theme for development of cancer prevention strategies. In particular, IFN might influence these mechanisms and play a role in anti-hepatocarcinogenesis. Future uses of this drug should be pursued in light of this functional biological aspect.
Footnotes
Supported by Grant-in-Aid for Scientific Research (C) (22590722 for Ohkoshi S) from the Japan Society for the promotion of Science (JSPS).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 31, 2015
First decision: May 18, 2015
Article in press: August 31, 2015
P- Reviewer: Huang SF, Wu ZJ S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 5.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 6.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 7.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 8.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 9.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- 11.Winters ZE, Hunt NC, Bradburn MJ, Royds JA, Turley H, Harris AL, Norbury CJ. Subcellular localisation of cyclin B, Cdc2 and p21(WAF1/CIP1) in breast cancer. association with prognosis. Eur J Cancer. 2001;37:2405–2412. doi: 10.1016/s0959-8049(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 12.Xia W, Chen JS, Zhou X, Sun PR, Lee DF, Liao Y, Zhou BP, Hung MC. Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin Cancer Res. 2004;10:3815–3824. doi: 10.1158/1078-0432.CCR-03-0527. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Kornblau SM, Kobayashi T, Gambel A, Claxton D, Deisseroth AB. High levels of constitutive WAF1/Cip1 protein are associated with chemoresistance in acute myelogenous leukemia. Clin Cancer Res. 1995;1:1051–1057. [PubMed] [Google Scholar]
- 14.Cazier JB, Rao SR, McLean CM, Walker AK, Wright BJ, Jaeger EE, Kartsonaki C, Marsden L, Yau C, Camps C, et al. Whole-genome sequencing of bladder cancers reveals somatic CDKN1A mutations and clinicopathological associations with mutation burden. Nat Commun. 2014;5:3756. doi: 10.1038/ncomms4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenzie KE, Siva A, Maier S, Runnebaum IB, Seshadri R, Sukumar S. Altered WAF1 genes do not play a role in abnormal cell cycle regulation in breast cancers lacking p53 mutations. Clin Cancer Res. 1997;3:1669–1673. [PubMed] [Google Scholar]
- 16.Patiño-García A, Sotillo-Piñeiro E, Sierrasesúmaga-Ariznabarreta L. p21WAF1 mutation is not a predominant alteration in pediatric bone tumors. Pediatr Res. 1998;43:393–395. doi: 10.1203/00006450-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Shiohara M, el-Deiry WS, Wada M, Nakamaki T, Takeuchi S, Yang R, Chen DL, Vogelstein B, Koeffler HP. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994;84:3781–3784. [PubMed] [Google Scholar]
- 18.Martín-Caballero J, Flores JM, García-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–6238. [PubMed] [Google Scholar]
- 19.Shen KC, Heng H, Wang Y, Lu S, Liu G, Deng CX, Brooks SC, Wang YA. ATM and p21 cooperate to suppress aneuploidy and subsequent tumor development. Cancer Res. 2005;65:8747–8753. doi: 10.1158/0008-5472.CAN-05-1471. [DOI] [PubMed] [Google Scholar]
- 20.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 21.De la Cueva E, García-Cao I, Herranz M, López P, García-Palencia P, Flores JM, Serrano M, Fernández-Piqueras J, Martín-Caballero J. Tumorigenic activity of p21Waf1/Cip1 in thymic lymphoma. Oncogene. 2006;25:4128–4132. doi: 10.1038/sj.onc.1209432. [DOI] [PubMed] [Google Scholar]
- 22.Wang YA, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA. 1997;94:14590–14595. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng X, Xia W, Yang JY, Hsu JL, Chou CK, Sun HL, Wyszomierski SL, Mills GB, Muller WJ, Yu D, et al. Activation of p21(CIP1/WAF1) in mammary epithelium accelerates mammary tumorigenesis and promotes lung metastasis. Biochem Biophys Res Commun. 2010;403:103–107. doi: 10.1016/j.bbrc.2010.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Yeh N, Zhu XH, Leversha M, Cordon-Cardo C, Ghossein R, Singh B, Holland E, Koff A. Somatic cell type specific gene transfer reveals a tumor-promoting function for p21(Waf1/Cip1) EMBO J. 2007;26:4683–4693. doi: 10.1038/sj.emboj.7601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi YZ, Hui AM, Takayama T, Li X, Cui X, Makuuchi M. Reduced p21(WAF1/CIP1) protein expression is predominantly related to altered p53 in hepatocellular carcinomas. Br J Cancer. 2000;83:50–55. doi: 10.1054/bjoc.2000.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui AM, Kanai Y, Sakamoto M, Tsuda H, Hirohashi S. Reduced p21(WAF1/CIP1) expression and p53 mutation in hepatocellular carcinomas. Hepatology. 1997;25:575–579. doi: 10.1002/hep.510250314. [DOI] [PubMed] [Google Scholar]
- 27.Furutani M, Arii S, Tanaka H, Mise M, Niwano M, Harada T, Higashitsuji H, Imamura M, Fujita J. Decreased expression and rare somatic mutation of the CIP1/WAF1 gene in human hepatocellular carcinoma. Cancer Lett. 1997;111:191–197. doi: 10.1016/s0304-3835(96)04509-0. [DOI] [PubMed] [Google Scholar]
- 28.Kao JT, Chuah SK, Huang CC, Chen CL, Wang CC, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM, et al. P21/WAF1 is an independent survival prognostic factor for patients with hepatocellular carcinoma after resection. Liver Int. 2007;27:772–781. doi: 10.1111/j.1478-3231.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 29.Wagayama H, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Yamanaka T, Takase K, Nakano T. High expression of p21WAF1/CIP1 is correlated with human hepatocellular carcinoma in patients with hepatitis C virus-associated chronic liver diseases. Hum Pathol. 2002;33:429–434. doi: 10.1053/hupa.2002.124724. [DOI] [PubMed] [Google Scholar]
- 30.Shiraki K, Wagayama H. Cytoplasmic p21(WAF1/CIP1) expression in human hepatocellular carcinomas. Liver Int. 2006;26:1018–1019. doi: 10.1111/j.1478-3231.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 31.Park SH, Jung JK, Lim JS, Tiwari I, Jang KL. Hepatitis B virus X protein overcomes all-trans retinoic acid-induced cellular senescence by downregulating levels of p16 and p21 via DNA methylation. J Gen Virol. 2011;92:1309–1317. doi: 10.1099/vir.0.029512-0. [DOI] [PubMed] [Google Scholar]
- 32.Ahn JY, Chung EY, Kwun HJ, Jang KL. Transcriptional repression of p21(waf1) promoter by hepatitis B virus X protein via a p53-independent pathway. Gene. 2001;275:163–168. doi: 10.1016/s0378-1119(01)00604-7. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Tong S, Tai AW, Hussain M, Lok AS. Hepatitis B virus core promoter mutations contribute to hepatocarcinogenesis by deregulating SKP2 and its target, p21. Gastroenterology. 2011;141:1412–1421, 1421.e1-5. doi: 10.1053/j.gastro.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park US, Park SK, Lee YI, Park JG, Lee YI. Hepatitis B virus-X protein upregulates the expression of p21waf1/cip1 and prolongs G1--& gt; S transition via a p53-independent pathway in human hepatoma cells. Oncogene. 2000;19:3384–3394. doi: 10.1038/sj.onc.1203674. [DOI] [PubMed] [Google Scholar]
- 35.Qiao L, Leach K, McKinstry R, Gilfor D, Yacoub A, Park JS, Grant S, Hylemon PB, Fisher PB, Dent P. Hepatitis B virus X protein increases expression of p21(Cip-1/WAF1/MDA6) and p27(Kip-1) in primary mouse hepatocytes, leading to reduced cell cycle progression. Hepatology. 2001;34:906–917. doi: 10.1053/jhep.2001.28886. [DOI] [PubMed] [Google Scholar]
- 36.Yano M, Ohkoshi S, Aoki YH, Takahashi H, Kurita S, Yamazaki K, Suzuki K, Yamagiwa S, Sanpei A, Fujimaki S, et al. Hepatitis B virus X induces cell proliferation in the hepatocarcinogenesis via up-regulation of cytoplasmic p21 expression. Liver Int. 2013;33:1218–1229. doi: 10.1111/liv.12176. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Hannon GJ, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 38.LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 39.Mantel C, Luo Z, Canfield J, Braun S, Deng C, Broxmeyer HE. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710–3719. [PubMed] [Google Scholar]
- 40.Alt JR, Gladden AB, Diehl JA. p21(Cip1) Promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J Biol Chem. 2002;277:8517–8523. doi: 10.1074/jbc.M108867200. [DOI] [PubMed] [Google Scholar]
- 41.Besson A, Yong VW. Involvement of p21(Waf1/Cip1) in protein kinase C alpha-induced cell cycle progression. Mol Cell Biol. 2000;20:4580–4590. doi: 10.1128/mcb.20.13.4580-4590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss RH, Joo A, Randour C. p21(Waf1/Cip1) is an assembly factor required for platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem. 2000;275:10285–10290. doi: 10.1074/jbc.275.14.10285. [DOI] [PubMed] [Google Scholar]
- 43.Kondo S, Barna BP, Kondo Y, Tanaka Y, Casey G, Liu J, Morimura T, Kaakaji R, Peterson JW, Werbel B, et al. WAF1/CIP1 increases the susceptibility of p53 non-functional malignant glioma cells to cisplatin-induced apoptosis. Oncogene. 1996;13:1279–1285. [PubMed] [Google Scholar]
- 44.Lincet H, Poulain L, Remy JS, Deslandes E, Duigou F, Gauduchon P, Staedel C. The p21(cip1/waf1) cyclin-dependent kinase inhibitor enhances the cytotoxic effect of cisplatin in human ovarian carcinoma cells. Cancer Lett. 2000;161:17–26. doi: 10.1016/s0304-3835(00)00586-3. [DOI] [PubMed] [Google Scholar]
- 45.Masgras I, Carrera S, de Verdier PJ, Brennan P, Majid A, Makhtar W, Tulchinsky E, Jones GD, Roninson IB, Macip S. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J Biol Chem. 2012;287:9845–9854. doi: 10.1074/jbc.M111.250357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cmielová J, Rezáčová M. p21Cip1/Waf1 protein and its function based on a subcellular localization [corrected] J Cell Biochem. 2011;112:3502–3506. doi: 10.1002/jcb.23296. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Wang W, Chen Y, Huang Y, Zhang J, He S, Tan Y, Qiang F, Li A, Røe OD, et al. The opposite prognostic significance of nuclear and cytoplasmic p21 expression in resectable gastric cancer patients. J Gastroenterol. 2014;49:1441–1452. doi: 10.1007/s00535-013-0900-4. [DOI] [PubMed] [Google Scholar]
- 48.Hwang CY, Lee C, Kwon KS. Extracellular signal-regulated kinase 2-dependent phosphorylation induces cytoplasmic localization and degradation of p21Cip1. Mol Cell Biol. 2009;29:3379–3389. doi: 10.1128/MCB.01758-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koster R, di Pietro A, Timmer-Bosscha H, Gibcus JH, van den Berg A, Suurmeijer AJ, Bischoff R, Gietema JA, de Jong S. Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J Clin Invest. 2010;120:3594–3605. doi: 10.1172/JCI41939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rössig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol. 2001;21:5644–5657. doi: 10.1128/MCB.21.16.5644-5657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 52.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277:29792–29802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki A, Tsutomi Y, Yamamoto N, Shibutani T, Akahane K. Mitochondrial regulation of cell death: mitochondria are essential for procaspase 3-p21 complex formation to resist Fas-mediated cell death. Mol Cell Biol. 1999;19:3842–3847. doi: 10.1128/mcb.19.5.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol. 2012;83:1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fabbro M, Henderson BR. Regulation of tumor suppressors by nuclear-cytoplasmic shuttling. Exp Cell Res. 2003;282:59–69. doi: 10.1016/s0014-4827(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 57.Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Gravina GL, Senapedis W, McCauley D, Baloglu E, Shacham S, Festuccia C. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7:85. doi: 10.1186/s13045-014-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao L, Yang Y. Targeting the nuclear transport machinery by rational drug design. Curr Pharm Des. 2013;19:2318–2325. doi: 10.2174/1381612811319120018. [DOI] [PubMed] [Google Scholar]
- 60.Kanai M, Hanashiro K, Kim SH, Hanai S, Boulares AH, Miwa M, Fukasawa K. Inhibition of Crm1-p53 interaction and nuclear export of p53 by poly(ADP-ribosyl)ation. Nat Cell Biol. 2007;9:1175–1183. doi: 10.1038/ncb1638. [DOI] [PubMed] [Google Scholar]
- 61.Santiago A, Li D, Zhao LY, Godsey A, Liao D. p53 SUMOylation promotes its nuclear export by facilitating its release from the nuclear export receptor CRM1. Mol Biol Cell. 2013;24:2739–2752. doi: 10.1091/mbc.E12-10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009;32:E315. [PubMed] [Google Scholar]
- 63.Walker CJ, Oaks JJ, Santhanam R, Neviani P, Harb JG, Ferenchak G, Ellis JJ, Landesman Y, Eisfeld AK, Gabrail NY, et al. Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood. 2013;122:3034–3044. doi: 10.1182/blood-2013-04-495374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou F, Qiu W, Yao R, Xiang J, Sun X, Liu S, Lv J, Yue L. CRM1 is a novel independent prognostic factor for the poor prognosis of gastric carcinomas. Med Oncol. 2013;30:726. doi: 10.1007/s12032-013-0726-1. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Wang Y, Xiang J, Ji F, Deng Y, Tang C, Yang S, Xi Q, Liu R, Di W. Knockdown of CRM1 inhibits the nuclear export of p27(Kip1) phosphorylated at serine 10 and plays a role in the pathogenesis of epithelial ovarian cancer. Cancer Lett. 2014;343:6–13. doi: 10.1016/j.canlet.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Wettersten HI, Landesman Y, Friedlander S, Shacham S, Kauffman M, Weiss RH. Specific inhibition of the nuclear exporter exportin-1 attenuates kidney cancer growth. PLoS One. 2014;9:e113867. doi: 10.1371/journal.pone.0113867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkler J, Ori A, Holzer K, Sticht C, Dauch D, Eiteneuer EM, Pinna F, Geffers R, Ehemann V, Andres-Pons A, et al. Prosurvival function of the cellular apoptosis susceptibility/importin-α1 transport cycle is repressed by p53 in liver cancer. Hepatology. 2014;60:884–895. doi: 10.1002/hep.27207. [DOI] [PubMed] [Google Scholar]
- 68.Monecke T, Dickmanns A, Ficner R. Allosteric control of the exportin CRM1 unraveled by crystal structure analysis. FEBS J. 2014;281:4179–4194. doi: 10.1111/febs.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azmi AS, Al-Katib A, Aboukameel A, McCauley D, Kauffman M, Shacham S, Mohammad RM. Selective inhibitors of nuclear export for the treatment of non-Hodgkin’s lymphomas. Haematologica. 2013;98:1098–1106. doi: 10.3324/haematol.2012.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, Christie AL, McCauley D, Rodig SJ, Kauffman M, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol. 2013;161:117–127. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y, Goettl V, Mahoney E, Berglund C, Gupta S, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–4634. doi: 10.1182/blood-2012-05-429506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M, Walker A, Klisovic R, Blum W, Caligiuri M, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012;120:1765–1773. doi: 10.1182/blood-2012-04-423160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tai YT, Landesman Y, Acharya C, Calle Y, Zhong MY, Cea M, Tannenbaum D, Cagnetta A, Reagan M, Munshi AA, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28:155–165. doi: 10.1038/leu.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng Y, Gery S, Sun H, Shacham S, Kauffman M, Koeffler HP. KPT-330 inhibitor of XPO1-mediated nuclear export has anti-proliferative activity in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2014;74:487–495. doi: 10.1007/s00280-014-2495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dudgeon C, Peng R, Wang P, Sebastiani A, Yu J, Zhang L. Inhibiting oncogenic signaling by sorafenib activates PUMA via GSK3β and NF-κB to suppress tumor cell growth. Oncogene. 2012;31:4848–4858. doi: 10.1038/onc.2011.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ueda H, Ullrich SJ, Gangemi JD, Kappel CA, Ngo L, Feitelson MA, Jay G. Functional inactivation but not structural mutation of p53 causes liver cancer. Nat Genet. 1995;9:41–47. doi: 10.1038/ng0195-41. [DOI] [PubMed] [Google Scholar]
- 77.Frahm T, Hauser H, Köster M. IFN-type-I-mediated signaling is regulated by modulation of STAT2 nuclear export. J Cell Sci. 2006;119:1092–1104. doi: 10.1242/jcs.02822. [DOI] [PubMed] [Google Scholar]
- 78.Thompson MD, Dar MJ, Monga SP. Pegylated interferon alpha targets Wnt signaling by inducing nuclear export of β-catenin. J Hepatol. 2011;54:506–512. doi: 10.1016/j.jhep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chuma M, Terashita K, Sakamoto N. New molecularly targeted therapies against advanced hepatocellular carcinoma: From molecular pathogenesis to clinical trials and future directions. Hepatol Res. 2014:Epub ahead of print. doi: 10.1111/hepr.12459. [DOI] [PubMed] [Google Scholar]


