Abstract
Immunotherapeutic approaches for treating cancer overall have been receiving a considerable amount of interest due to the recent approval of several clinical formulations. Among the different modalities, anticancer vaccination acts by training the body to endogenously generate a response against tumor cells. However, despite the large amount of work that has gone into the development of such vaccines, the near absence of clinically approved formulations highlights the many challenges facing those working in the field. The generation of potent endogenous anticancer responses poses unique challenges due to the similarity between cancer cells and normal, healthy cells. As researchers continue to tackle the limited efficacy of vaccine formulations, fresh and novel approaches are being sought after to address many of the underlying problems. In this review, we discuss the application of nanoparticle technology towards the development of anticancer vaccines. Specifically, we focus on the benefits of using such strategies to manipulate antigen presenting cells (APCs), which are essential to the vaccination process, and how nanoparticle-based platforms can be rationally engineered to elicit the appropriate downstream immune responses.
Keywords: nanomedicine, cancer immunotherapy, cancer vaccine, antigen presentation, immunoengineering
Graphical Abstract
Immunotherapeutic approaches to cancer treatment have been gaining significant traction in the clinic Of the different modalities, the goal of anticancer vaccination is to train the body to detect and eliminate tumors. This review details the application of nanoparticle technology towards the development of more effective vaccine formulations that address the limitations of current approaches.
1. Introduction
As the incidence of deaths worldwide due to more preventable causes continues to decline thanks to advancing technology, wider accessibility to treatments, and better education, diseases such as cancer continue their rise to the forefront as major threats to public health.[1] Despite the great amount of progress made in the past century with regards to cancer treatment using traditional methods such as surgical resection, radiotherapy, and chemotherapy, many forms of the disease remain fundamentally difficult to manage.[2] Per capita fatalities from certain types of cancer, such as lung cancer, have seen dramatic declines as a result of increased awareness or early screening, but these encouraging trends are not universal.[3] The incredible heterogeneity of cancer is largely responsible for these discrepancies, and it has become clear that there is no cure-all magic bullet to be discovered.[4] In the post-genomic age, more personalized treatments with the aid of targeted therapies and combinatorial approaches are slowly changing the way that physicians attack malignancies.[5–6] Within this shifting landscape, immunotherapy has been garnering a great deal of attention and has emerged as a promising strategy with the potential to significantly alter the cancer treatment paradigm.[7]
Cancer immunotherapies operate on the notion that the body is capable of recognizing and destroying its own aberrant cells. Indeed, under normal functioning, the immune system is constantly detecting and destroying abnormal growth.[8] All cancers must find ways to evade or overcome these natural defenses in order to succeed, and as such disease progression can be viewed as a failure in immunological containment.[9] Immunotherapeutic approaches seek to tip the balance back into the immune system’s favor, and by leveraging biological machinery that has evolved over millions of years, can theoretically achieve levels of specificity that far surpasses other types of cancer therapy.[10] They exist in many forms, ranging from administration of immune stimulatory compounds[11] to adoptive T cell transfer,[12] with the goal of augmenting different aspects of the immune process.
Among these immunotherapeutic strategies is anticancer vaccination, which will be the main focus of this review. The goal in developing an anticancer vaccine is to better train the body itself to detect and eliminate tumors.[13–14] Much like with traditional vaccination against pathogens, anticancer vaccines attempt to redirect and focus immune specificity to targets of interest. Unfortunately, despite intense interest in the subject and a great deal of effort dedicated to anticancer vaccine research, results thus far have been underwhelming. This is highlighted by the fact that there is only one U.S. FDA approved formulation in the clinic.[15] This exists in stark contrast to traditional vaccines, which represent one of the biggest success stories of the 20th century, helping to all but eliminate some diseases.[16] Unlike vaccines targeted against foreign agents, however, the development of anticancer vaccines poses unique challenges that will require tremendous ingenuity to solve.[17–18]
Central to the success of the body’s ability to generate a response against tumors is training the immune system to recognize the correct antigen specificities. This involves a class of cells known as professional antigen presenting cells (APCs), of which dendritic cells (DCs) are perhaps the most important[19] (Figure 1). These cells process antigens and, in the correct context, present them for downstream immune activation. Effective anticancer vaccine design revolves around correct manipulation of these cells, namely: (i) selection of the correct antigenic material for a tumor-specific or tumor-biased response, (ii) inclusion of immune-boosting adjuvants that force downstream immune activation, and (iii) delivery of both antigen an adjuvant to APCs. It has proven difficult to address all of these considerations simultaneously, and promising strategies oftentimes are labor-intensive and not cost effective. This review will focus on emerging strategies using nanotechnology for more effective anticancer vaccine design. First, the role of APCs in cancer immunity and traditional anticancer vaccination strategies will be covered. This will be followed by discussion on leveraging nanoparticle technology for better vaccine design to address issues with traditional formulations. We conclude with the most recent and promising works concerning the use of nanoparticles for anticancer vaccination as well as our views on the future of such efforts.
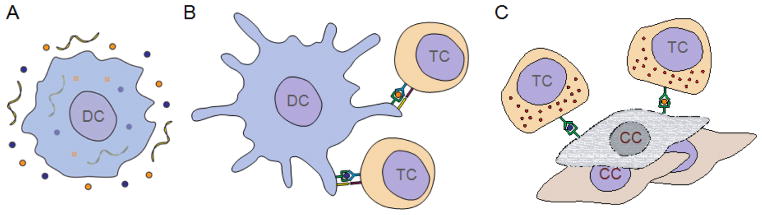
Figure 1.
Schematic illustration showing the induction of anticancer immunity via dendritic cells (DCs). (A) An immature DC takes up tumor antigens (orange and blue circles) along with an immune stimulating adjuvant (yellow ribbons). (B) A mature DC presents processed antigens along with co-stimulatory signals to tumor antigen-specific cytotoxic T cells (TCs). (C) Cytotoxic TCs upregulate effector functions and are capable of destroying cancer cells (CCs) presenting the original tumor antigens.
2. Manipulation of Dendritic Cells for Anticancer Vaccination
Over time, cancer has increasingly become understood as having a significant immunological component.[20–21] In order for aberrant cells to grow, they must successfully evade detection and attack by the body’s own defenses.[22] This is accomplished through a variety of means, with tumors becoming increasingly better at overcoming the immune system throughout disease progression. Armed with this knowledge, immunotherapeutic strategies such as anticancer vaccination have emerged that empower the body to fight back against tumors.
2.1. Approaching Cancer Treatment from an Immunological Perspective
The past several decades have brought about an incredible increase in our understanding about how cancer develops. The concept of immune surveillance suggests that the body is constantly monitoring for neoplastic growth, and the relative rarity of malignant tumors indicates that the immune system is fairly adept at this task.[8] This is especially impressive given the amount of cell divisions and potentially harmful mutations that may occur in an average individual’s lifespan.[23] Indeed, tumors must slowly evolve creative ways to escape detection.[22] Over time, they can shed immunogenic antigens while also downregulating major histocompatibility complexes (MHCs) or stress markers, which helps them to avoid the cell-based effectors that seek their destruction.[24–25] Further, tumors can secrete immune deactivating molecules or actively recruit T-regulatory cells that promote a tolerogenic microenvironment.[26] The biological interactions between a nascent tumor and the immune system can operate in a delicate balance for decades in a game of cat and mouse until malignant disease finally manifests itself.
Immunotherapy purposefully manipulates the immune system in order to turn the fight back in the body’s favor. By encouraging immune activation over tolerance, the goal is to promote antitumor immunity and overcome the different tumor escape mechanisms. Cytokine therapies such as interferon alpha (IFNα) and interleukin 2 (IL-2) enhance immune activation and have long been investigated in the clinic with mixed results.[11] These therapies attempt to overcome immune suppression by hyperactivating targeted immune subsets. More recently, anti-blockade therapies such as anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4) and anti-programmed cell death protein 1 (anti-PD1) seek to directly address immunosuppression, nonspecifically unleashing effector T cells to freely seek out and destroy their targets.[27] These treatments, having both gained U.S. FDA approval within the last several years, have provided strikingly good clinical outcomes in large patient populations, with autoimmunity being a potential but manageable side effect.[28] Adoptive T cell therapies have similarly garnered a great deal of interest due to their ability to produce impressive clinical responses.[12] In such treatments, cancer-specific T cells are generated and are then infused back into the patient, largely bypassing endogenous immune processes. This specificity can prevent unwanted side effects seen from other therapies, but the treatment is complex to execute and the long-term safety is unknown.[29] Anticancer vaccines in theory occupy a balance between specificity, efficacy, and ease of use. In their most ideal form, they can be directly injected into a patient, activating the necessary T cell specificities. However, their reliance on endogenous machinery serves as a double-edged sword, adding layers of complexity that often compromise efficacy and has proven difficult to navigate.[17]
2.2. Design Considerations for Anticancer Vaccines
Because cancer cells are derived from one’s own cells, anticancer vaccination presents a sort of paradox: how do you eliminate something that largely looks like “self”? It is known that most successfully growing tumors are poorly immunogenic, having found ways to make themselves either undetectable or unassailable by the immune system.[9] To address this, researchers have identified a class of immune cells known as professional APCs that must be correctly manipulated in order to maximize vaccine efficacy.[30] These cells are responsible for taking up antigenic material, processing it, and then presenting epitopes via MHCs on their surface in order to train downstream immune effectors such as cytotoxic T cells. Success of anticancer vaccines is centered on how to most effectively modulate these APCs to promote the desired responses.
Perhaps the most critical consideration when designing an anticancer vaccine is the choice of antigenic material that is delivered to APCs, which helps the immune system to correctly identify and generate a response against the desired targets. There are two basic approaches: using either identified tumor antigens[31] or lysate-based formulations.[32] Regarding the first approach, researchers have identified a number of tumor-associated or tumor-specific antigens that are commonly upregulated or mutated in cancers.[33] When using such previously identified antigens, population heterogeneity represents a major hurdle, as a patient’s tumor must be explicitly analyzed for appropriate expression of the target of interest. Further, in many instances a tumor may simply not have a suitable phenotype at all, leaving no viable options for formulating a vaccine. In the future, a different approach may involve personalized identification of less common candidate epitopes and custom synthesis of vaccines.[34–35] Such an approach would also have the benefit of potential multiplexing via the identification of multiple targets for vaccination, but widespread use currently remains infeasible given practical constraints of current technology and the associated costs and labor. In stark contrast to a single antigen approach, lysate-based formulations source their antigenic material from a patient’s own tumor, relying on endogenous immune machinery to identify the epitopic targets against which to generate a response.[36] Indeed, many neoantigens present in tumors, even those resulting from passenger mutations, have potential to serve as targets for the immune system. This enables facile personalization and thus broad applicability across large patient populations, but comes at the cost of specificity and efficacy due to the presence of unwanted antigens that dilute immune focus.[37] As a result of this major hurdle, lysate-based formulations have had limited success in the clinic.[38]
Because cancer cells originate from endogenous cells and share most of the same antigens, it is hard for the immune system to distinguish between the two. This makes the generation of a potent antitumor response extremely difficult, and a significant impediment lies in the fact that high affinity T cell clones against potential tumor antigens are usually eliminated by the body, leaving only low affinity clones to work with.[39] It is widely accepted now that any anticancer vaccine formulation must be co-delivered with an immune boosting adjuvant in order to help break tolerance.[40] Such a concept is not new, as it was observed as far back as the late 19th century that infections could actually boost antitumor immunity.[41] Today, adjuvants are generally based on pathogen-associated molecular patterns (PAMPs) that serve as danger signals to the body.[42] These PAMPs, which generally compose some integral part of pathogen structure or function, have specific receptors on APCs known as toll-like receptors (TLRs). Upon TLR engagement, PAMPs will facilitate maturation of DCs, inducing upregulation of co-stimulatory markers such as CD80 and CD86 and the production of pro-inflammatory cytokines. This prevents T cell anergy upon co-engagement with the relevant MHC-peptide complexes, enabling the immune cells to carry out their effector functions on tumors.[43]
The final major consideration when designing an anticancer vaccine is correct delivery of both antigen and adjuvant to APCs. Cell biology dictates that correct immune modulation requires individual APCs to take up both of them in sufficient amounts.[44] Co-localization can be a challenging task given the varying transport kinetics of antigens, which are generally proteins or peptides, and adjuvants, which can be lipid-like, nucleic acid-based, or small molecules. Strategies to address this challenge include fusion or conjugation of the two components, enabling unification of pharmacokinetics after administration.[45] Beyond co- localization, efficient transport to the correct organs and immune cell subsets can also pose a significant challenge.[46] Given all of these challenges with delivery, it is perhaps no coincidence that the first and only U.S. FDA-approved formulation is based on the ex vivo pulsing of DCs, a strategy that, while laborious and expensive, has the benefit of finely controlled cell manipulation.
2.3. Current Therapeutic Anticancer Vaccination Strategies in the Clinic
Thus far, single antigen anticancer vaccines have demonstrated the most promise, and there are many formulations that are under clinical investigation. A peptide form of glycoprotein 100 (gp100), an upregulated protein in melanomas, is currently being used for two vaccine formulations. One formulation using IL-2 as an immune boosting agent is in clinical Phase III trials, and another using a tetanus toxoid peptide as an adjuvant is currently in a Phase I trial.[47–48] GlaxoSmithKline’s melanoma-associated antigen A3 (MAGE A3) vaccine combines MAGE-A3 protein and the multi-adjuvant immunostimulant AS02B for treatment of non-small-cell lung cancer.[49] After a randomized Phase II trial showed potential, a Phase III trial (MAGRIT) was conducted but failed to meet primary endpoints.[50] However the company will continue to evaluate a second Phase III trial, DERMA, to determine if a sub-population of patients is more likely to respond to the MAGE A3 vaccine.[51] Similarly, Stimuvax, a liposomal formulation containing the mucin 1 (MUC1) antigen and monophosphoryl lipid A (MPLA) adjuvant in the membrane layers, has been successful through Phase II clinical trials for non-small-cell lung cancer, but only resulted in a statistically insignificant 3.3 month survival extension in Phase III trials. It was identified that a subset of patients who had previously received chemotherapy and radiation concurrently showed a 10.2 month increase in survival time, and these findings have become the basis for another Phase III trial.[52] There are also Phase III trials ongoing for NeuVax, a breast cancer peptide vaccine comprising of a human epidermal growth factor receptor 2 (HER2) peptide and granulocyte macrophage colony- stimulating factor (GM-CSF) adjuvant, after a successful Phase I/II trial showing significant efficacy.[53–54]
Provenge (Sipuleucel-T), currently the only FDA approved anticancer vaccine, consists of autologous APCs pulsed ex vivo by a fusion of a single prostate cancer antigen, prostatic acid phosphatase, and an adjuvant, GM-CSF. These APCs are then collected and adoptively transferred back into the patient.[55] In a randomized, placebo-controlled Phase III trial, men with metastatic castration-resistant prostate cancer who were vaccinated with Provenge had a 4.5 month improvement in survival.[56] This result was confirmed during a large Phase III trial called Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT), in which treatment with Provenge resulted in a 4.1 month median improvement in survival, and a 38% increase in survival at the 3 year follow up.[15] The IMPACT trial led to FDA approval in April 2010, marking a milestone for cancer immunotherapy.[57] Other promising APC pulsing vaccines are currently being tested using a wide variety of adjuvants and antigens, such as the cancer-testis antigen NY-ESO-1 protein or combinations of p53, survivin, and telomerase-derived peptides.[58–62]
Whole tumor cell vaccines have been investigated in human clinical trials for more than twenty years. Resected patient tumors, made nonviable through irradiation or lysing, are an obvious source of whole tumor cells due to their unique profile of antigens. For example, Oncovax uses processed and irradiated autologous tumor cells in conjunction with the bacillus Calmette-Guerin adjuvant to vaccinate against colon cancer. This method was first tested in a small pilot study in 1980, resulting in statistically significant reduction in tumor recurrence and improved survival rates. After much development in the intermediate years, the vaccine is currently about to begin a Phase IIIb trial.[63–67] Patient derived tumors can also be genetically modified to increase immunogenicity, exemplified by a chronic lymphocytic leukemia cancer vaccine which manipulates autologous tumor cells to transgenically express CD40L and IL-2 for increased T cell response.[68] Allogenic cancer cell lines can be another antigen source for whole tumor cell vaccines. Lucanix, a vaccine for non-small-cell lung cancer, uses tumor cell lines genetically modified to have a downregulated expression of tumor growth factor β (TGFβ), an immunosuppressor, which demonstrated a survival advantage in an international Phase III trial.[69] The GVAX vaccine similarly uses select cell lines, such as a HER2-positive breast cancer cell line and two pancreatic cancer cell lines, which have been genetically modified to secrete adjuvants like GM-CSF.[70–71] Phase I/II studies demonstrated a dose dependent increase in median survival time, and justified two Phase III trials, VITAL-1 and VITAL-2. These latter trials, however, were terminated early due to a lack of efficacy.[72–73]
2.4. Current Challenges
Despite the tremendous amount of research geared towards the development and translation of anticancer vaccines, the scarcity of approved treatments in the clinic highlights the many challenges that remain. It has become apparent that current strategies have difficulty addressing all of the necessary requirements for eliciting potent and specific anticancer immune responses. Single antigen formulations, which have been getting the most attention recently due to their ability to generate efficacy, are only applicable to specific patient populations. As is the case with most monotherapies, they are susceptible to the development of resistance, which can happen via in vivo immune selection.[74–75] Lysate-based formulations derived from patient tumors would represent the ideal source of multi-antigenic material, but have failed to display efficacy due to the presence of large amounts of interfering antigens.[37] Further, delivery of both antigenic and adjuvant material to the same target can be challenging, often necessitating ex vivo manipulation of DCs. Such autologous cell manipulation strategies, unfortunately, are extremely costly and are arguably hard to justify given the modest therapeutic benefits.[76] Ultimately, an ideal platform for anticancer vaccination would be: (i) formulated with an enriched set of multi-antigenic tumor material, (ii) co-encapsulated with large amounts of adjuvant for simultaneous immune stimulation, (iii) targeted to the correct cell subsets, and (iv) personalized yet cost-effective. Formulating a vaccine with all of the above qualities represents a difficult task; to aid in the process, some researchers have turned towards new technologies in hopes of engineering a viable solution.
3. Nanoparticle-Based Strategies for Anticancer Vaccination
From computer chips to green energy, nanotechnology has become ubiquitous in everyday human life. Likewise, the prevalence of nanomedicine, or the application of nanotechnology to medical problems, has steadily risen in the clinic for the past several decades.[77–78] This has largely been driven by the approval of clinical chemotherapeutic nanoformulations. At their size scale, nanoparticle drug carriers offer advantages over free drug formulations that can lead to higher specificity and lower systemic toxicity.[79–82] As the drug delivery applications of nanomedicine platforms have been maturing over time, attention has turned towards leveraging the technology to benefit other therapeutic modalities, including immunotherapy and specifically vaccine design.
3.1. Background on Nanoparticle-Based Cancer Therapy
The first clinical approval of a chemotherapeutic nanoformulation was in 1995 for Doxil, a stealth liposomal formulation of doxorubicin for the treatment of Kaposi’s sarcoma.[83] Several other liposome-based formulations have since been approved for cancer therapy.[84] Besides liposomal systems, Abraxane and Genexol-PM represent albumin-bound and polymeric micellar formulations of paclitaxel, respectively.[85–86] More recently, targeted nanoformulations are being explored in clinical trials, including Accurins, a prostate-specific membrane antigen (PSMA)-targeted polymeric nanoparticle formulation of docetaxel.[87] The continued development of nanoparticle-based formulations highlights the advantages that they hold over traditional free drug formulations, which ultimately enables increased specificity, fewer systemic side effects, and better patient compliance. For example, the size scale of the particles leads to passive targeting via the enhanced permeation and retention (EPR) effect, which results in passive accumulation of nanoparticles within tumors.[88] Further, tumors can be actively targeted using a variety of ligands, including small molecules, peptides, aptamers, and antibodies.[89] Carriers can be designed to encapsulate drugs of varying properties, including potent hydrophobic drugs that would otherwise perform poorly in their free form.[90] Additionally, properties such as particle size, drug release, and in vivo circulation can be finely tuned, enabling the engineering of nanoparticle-based drug delivery platforms that can be tailored to specific applications.
Beyond chemotherapy, nanoparticle technology has also been explored for use in immunotherapeutic applications. The earliest examples of such were liposomal carriers for the delivery and release of cytokines such as IL-2 and interferon gamma (IFNγ).[91–92] In such cases, the nanocarrier acts as a depot that helps to modulate pharmacokinetics with the hopes of increasing therapeutic benefit while minimizing the harsh side effects associated with high-dose cytokine therapy.[93–94] Using these delivery systems, improved therapeutic efficacy has been demonstrated in a variety of murine tumor models.[92, 95–96] Multiple agents have been encapsulated within the same liposome for concurrent delivery.[97] Liposomal cytokines have also been used in combinatorial approaches where they are co-delivered with chemotherapeutics or used as a supplement for adoptive T cell therapy.[98–99] In more recent examples, sophisticated strategies involving adsorption of the payload onto magnetic particles for guided delivery and remote release have been reported.[100–101] Polymeric and hydrogel-based systems with extended release capabilities have also been explored for cytokine release.[102–103] Other than cytokine delivery, nanoparticles have been employed in artificial antigen presentation.[104–105] Peptide-loaded MHCs and co-stimulatory signals are conjugated together onto the same particle to promote clonal expansion of cancer specific T cells. Traditionally done using micron-sized particles, nanoparticles offer some unique advantages, including the ability to facilitate cell clustering upon application of a magnetic field when fabricated using iron oxide nanoparticles.[106] The shape of the nanoparticles also has significant implications on the efficiency of the antigen presentation and has been well studied.[107] Overall, as an increasing number of these platforms are translated from the lab bench to the clinic, the influence of nanotechnology will continue to grow and will have an increasing impact on the landscape of cancer therapy.
3.2. Advantages of Nanoparticles for Vaccine Design
Among the different immunotherapeutic approaches, perhaps one of the strongest cases for the application of nanotechnology is in vaccine design. Many novel platforms have been developed in recent years, including those based on liposome-like,[108] inorganic,[109] and polymeric[110] nanoparticles. These nanoscale particles have many properties that make them well suited for eliciting immune responses via delivery of payloads to APCs (Figure 2). One of the key advantages of nanoformulations is that they enable control over transport kinetics upon administration. This maximizes the chance that APCs will have sufficient amounts of both antigen and adjuvant to further promote downstream immune responses. There are many different ways by which this can be accomplished, including co-encapsulation of antigen and adjuvant into a single particle or single encapsulation into separate particles with similar properties. Such strategies will be discussed in-depth later in the review.
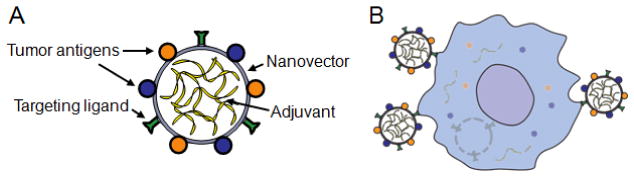
Figure 2.
Designing nanoparticles for anticancer vaccination. (A) Nanoparticles can be engineered with numerous properties that make them well suited for vaccination applications. This includes the ability to display multiple tumor antigens, load large amounts of antigens, target DCs, and respond to environmental stimuli. (B) Engineered nanoparticles taken up by a DC can release their antigen and adjuvant cargos intracellularly.
Beyond formulation parameters, the inherently small size of nanoparticles offers advantages in terms of payload localization within the body. Much has been studied about the transport kinetics of particles upon administration via common vaccination routes. The subcutaneous space consists of an extracellular meshwork, and it has been shown that, below a certain cutoff, particulate material is subject to efficient lymphatic drainage.[111–112] This size dependent transport of nanoparticles and their associated cargoes is an important design consideration given that these organs are extremely rich in immune cells, which can help to maximize utilization of antigen and adjuvant. Further, it has also been established that nanoparticle-mediated delivery of immune modulatory compounds to the lymph nodes has implications across a wide range of cell subsets, including APCs and T-regulatory cells.[113]
An additional advantage of nanoparticulates is the fact that they are prone to cellular uptake, especially when lacking stealth moieties such as polyethylene glycol (PEG), which is commonly used for drug delivery applications.[114] This enhanced uptake facilitates intracellular localization of antigen to the endosomal compartments, which can improve the ability of cells to process and present epitopic material.[115] It has been shown that antigenic material loaded into poly(lactic-co-glycolic acid) (PLGA) nanoparticles can even improve the cross-presentation of antigenic material via MHC class I molecules by DCs,[116] which can be leveraged to promote the cell-based immunity that is essential for anticancer immunotherapy. The delivery of nanoparticles to APCs and their subsequent internalization can further be promoted via facile targeting functionalization strategies.[117–118] Examples of ligands that can be employed include mannose and Lewis blood group antigens, which target endocytic receptors such as DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN.[119–121] To improve the availability of cargoes upon uptake by APCs, pH responsive nanoparticles can be used to trigger release within the endosomal compartment.[122–123] It has been shown that attaching antigen onto nanoparticles via reduction-sensitive linkages can significantly increase performance both in vitro and in vivo.[124] Further, encapsulation into environmentally sensitive hydrogels can result in a similar boost.[125]
3.3. Nonspecific Modulation of APCs using Nanoparticles
There exists a large body of work centering on nanoparticle-mediated delivery of immune modulators, supporting the notion that nanoparticles can be used to effectively manipulate APCs. Many have focused on the use of unmethylated cytosine-phosphate-guanine oligonucleotides (CpG ODN), which has been previously shown to be a potent vaccine adjuvant that binds to the endosomal toll-like receptor 9 (TLR9).[126] One of the first studies involving adjuvant delivery investigated the use of cationic gelatin nanoparticles to encapsulate and deliver CpG ODN to DCs.[127] The results indicated that the CpG gelatin nanoparticles were efficiently taken up into murine myeloid DCs, primarily through phagocytosis. It was also shown that the particles were able to induce DC maturation both in vitro and in vivo, as represented by increases in IL-12p70 production and tumor necrosis factor α (TNFα) production. In addition, liposomal nanoparticles have been used for adjuvant encapsulation such as liposome-protamine-DNA (LPD) nanoparticles with similar results.[128–130] In particular, a cationic PEGylated liposome encapsulating G3139, a DNA strand containing two CpG motifs, was able to increase the production of a variety of cytokines as well as increase the mean survival rate of L1210 tumor bearing mice compared to empty lipid nanoparticles or an equivalent amount of free G3139.[131] Adjuvant delivery particles can also target APCs to increase efficiency of delivery, such as a CpG loaded Pluronic F127-stabilized poly(propylene) sulfide nanoparticles for tumor-draining lymph node delivery, which boosted CD8+ T cell production and showed efficacy during in vivo tumor challenges.[113]
Immobilizing adjuvant onto nanoparticle surface is another strategy for loading. To make the CpG strands more accessible for receptor binding, CpG was bound to the surface of small gold nanoparticles (AuNPs) using spacers to increase the degrees of freedom for CpG movement.[132] The optimal formulation was found to be a 15 nanometer AuNP with both poly-thymidine and triethylene glycol spacers to reduce the steric crowding of the CpG strands and allow rotation for maximum binding ability. The CpG AuNPs elicited significantly higher TNFα, IL-6, and GM-CSF production, as well as significant tumor inhibition and prolonged survival compared to equivalent free CpG treatment. In addition to increasing CpG bioavailability via configurational geometry, it is possible to increase efficacy through sustained release. To deliver CpG with a slower release profile, magnetic mesoporous silica (MMS) nanoparticles have been explored as CpG delivery vehicles.[133] Through both encapsulation in the pores and electrostatic binding on the surface, the MMS nanoparticles enable a rapid delivery for CpG on the surface, with a depot effect as the CpG is also released from the pores.
The material and shape of the nanoparticles themselves have also been shown to elicit immunomodulatory effects. Polyhydroxylated fullerenes alone were able to activate DCs and macrophage cells both in vitro and in vivo, even reducing tumor volume in animals challenged with cancer cells.[134–135] Immunomodulation was also achieved using RNA complexes as nanovehicles for CpG delivery.[136] RNA conjugated to CpG strands was designed to self-assemble into different shapes, with the smaller triangular complexes showing the highest amount of DC activation per CpG input, through the high production of IL-6 and TNFα. TLR agonists and antagonists themselves can be assembled into spherical carriers around either gold or liposomal cores to form spherical nucleic acids, which have been successful in showing immunomodulatory effects and antitumor efficacy in a lymphoma model (Figure 3).[137] These studies have all shown that adjuvant delivery to DCs in a nanosized package, in whatever form, can have powerful immunomodulatory effects that can stimulate the immune system into action against malignancies.
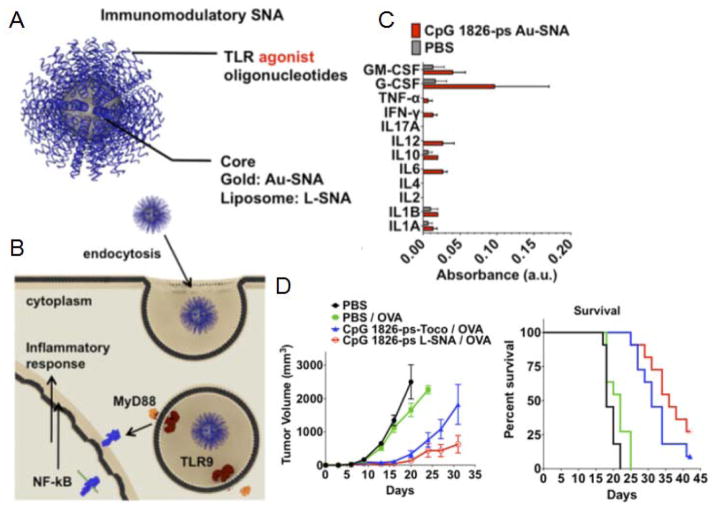
Figure 3.
A spherical nucleic acid (SNA) platform for immune modulation. (A) Design of SNA platform, which contains a TLR agonist oligonucleotide shell around a liposome or gold core. (B) Schematic of proposed mechanism for uptake and TLR interaction between APCs and SNAs. (C) Cytokine profiling of draining popliteal lymph node collected 4 hours after footpad administration. (D) Tumor growth curves (left) and corresponding survival (right) for mice injected with E.G7-OVA cells on day 0, then treated on days 3, 5, and 7. Reproduced with permission.[137] Copyright 2015, National Academy of Sciences.
3.4. Nanoparticle-Based Anticancer Vaccines
In much the same manner as with adjuvant material, nanoparticles have proven effective at loading antigenic material, and many antigen-only nanoparticle vaccines have been reported in scientific literature. PLGA nanoparticles have been used to encapsulate either tumor lysates or defined tumor antigens such as gp100 for use as a prophylactic against B16-F10 melanoma challenge in a murine model.[138] Tumor lysate from head and neck squamous carcinoma cells derived from human patients have been incorporated into nanoparticles and pulsed into patient derived DCs.[139] Delivery of multiple tumor peptides has also been achieved using lipid-coated polymeric nanoparticles.[140] Unsurprisingly, such antigen-only systems generally demonstrate limited efficacy due to the problem with low immunogenicity. Many studies have corroborated the need for the inclusion of immune stimulatory compounds in nanoparticle-based vaccine platforms.[141–145] As such, the focus in nanoparticle vaccine engineering has been on designing strategies for the co-delivery of antigen and adjuvant.
One such strategy is the simultaneous delivery of antigen and adjuvant in two separate particles, which enables unification of transport kinetics and encourages delivery to the same cells. Co-administration of two types of PLGA nanoparticles, one loaded with ovalbumin (OVA) and another loaded with the immunomodulatory components STAT3 siRNA and R837 (imiquimod), was effective in increasing cytokine levels and decreasing tumor volume in a prophylactic setting (Figure 4).[146] In the same vein, it has been shown that a lipid-calcium-phosphate nanoparticle delivering both TRP2 peptide antigen and CpG adjuvant had efficacy for early stage tumors, but less efficacy for more progressed tumors. Administering a second liposome-protamine-hyaluronic acid nanoparticle loaded with TGFβ siRNA for these late stage tumors resulted in a 52% tumor growth inhibition compared to the vaccine alone.[147] Two different nanoparticles of similar size, which experience similar pharmacokinetics, such as ultra-small CpG nanoparticles injected alongside ultra-small OVA conjugated nanoparticles, can also greatly enhance efficacy compared to only antigen-loaded particles.[148] A similar strategy has also been reported using co-administered AuNPs conjugated with either CpG or OVA.[149]
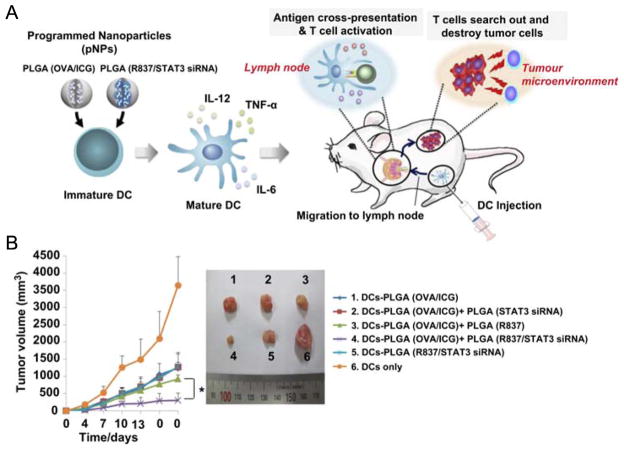
Figure 4.
Antigen and adjuvant loaded into separate nanoparticles for programmed immune responses. (A) Schematic illustrating ex vivo DC pulsing with an antigen carrying programmed nanoparticles (pNP) (PLGA OVA/ICG) and adjuvant encapsulating pNP (PLGA R837/STAT3 siRNA), and the antitumor immunity effects once pulsed DCs are administered. (B) Antitumor activity of DCs pulsed with pNPs. One week after immunization with pulsed DCs, mice were challenged with E.G7-OVA tumor cells, and tumor volume was measured every 3 days. Representative photographs are shown for each group. Reproduced with permission.[146] Copyright 2013, Elsevier.
A streamlined method for introducing adjuvant properties to a nanoparticle is to employ a material that is inherently immunostimulatory. Poly(γ-glutamic acid) (γ-PGA) is a popular polymeric material for antigen encapsulation due to having such properties. γ-PGA nanoparticles loaded with OVA have been shown to be taken up into APCs efficiently, and slowly release payload during endosomal breakdown. After introduction into APCs, the particles were able to efficiently promote secretion of pro-inflammatory cytokines and DC maturation.[150–152] In some cases, γ-PGA loaded with OVA was sufficient to have cytotoxic effects against OVA expressing cells, moderately retard tumor growth when challenged with E.G7-OVA, and significantly reduce metastatic growth in a B16-OVA metastatic tumor model.[150, 153–155] Another platform, which involved conjugation of OVA onto α-Al2O3, demonstrated the ability to induce antigen-specific CD8+ T cell proliferation and showed impressive control of tumor growth in a B16-OVA mouse melanoma model (Figure 5).[109] Additionally, DOTAP liposomes have been shown to be immunostimulatory and have been used to entrap antigens for vaccine formulation.[156]
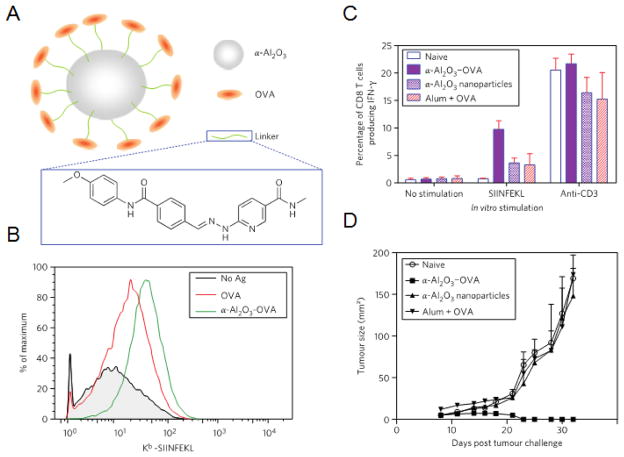
Figure 5.
Alpha-alumina (α-Al2O3) nanoparticles as an inherent adjuvant. (A) Illustration depicting α-Al2O3 nanoparticle design including an α-Al2O3 core connected to OVA antigen through a linker. (B) Surface expression of MHC I-peptide complexes is higher for DCs pulsed with α-Al2O3-OVA nanoparticles. (C) Vaccination with α-Al2O3-OVA nanoparticles elicited a high frequency of OVA-specific IFNγ producing CD8+ T cells from mice spleens as determined by flow cytometry. (D) Seven days after B16-OVA injection, mice were vaccinated with α-Al2O3-OVA nanoparticles and controls and tumors were measured. Reproduced with permission.[109] Copyright 2011, Macmillan Publishers Limited.
Perhaps the most appealing method of delivering antigen and adjuvant together is co-encapsulation in the same particle. The benefit of this strategy is that a single particle can theoretically provide the requisite material to an APC for generating specific downstream antitumor responses. Improved DC maturation and cytokine production have been achieved using polymeric particles or liposomes encapsulating combinations of TLR agonists and peptide antigens.[157–159] Additional functionality can also be built into these nanoparticle systems to improve vaccine performance. For example, targeting functionalization via CD40 antibody or mannose can enable improved localization and antigen delivery to APCs, leading to enhanced antitumor activity in vivo.[160–161] Conjugating both antigen and adjuvant onto AuNPs generates a theranostic platform that is capable of being concurrently imaged.[162] Also promising are vaccine formulations that introduce additional cargoes that combat immune suppression, as was demonstrated with micelles encapsulating OVA with TLR agonist poly I:C and STAT3 siRNA that were able to increase DC maturation and IL-12 production in vivo.[163]
Recent nanoparticle-based vaccine platforms have incorporated naturally inspired design cues that involve compartmentalization of the two vaccine components. One example is a multilamellar crosslinked vesicle structure that incorporates MPLA, an adjuvant derived from an integral bacterial membrane component, into its lipid bilayers while encapsulating antigen in the center (Figure 6).[108] These particles were able to induce efficient DC maturation and further promoted prolonged antigen specific CD8+ T cell responses, suggesting utility for anticancer vaccination purposes. A different example is based off of a recently developed cancer cell membrane-coating approach where naturally derived tumor cell membrane is fused onto the surface of a polymeric nanoparticle core (Figure 7).[110] The benefit of such an approach is that it presents a facile method for the incorporation of personalized cancer antigens, which can potentially be sourced from a patient’s own tumor. Further, the polymeric core can be loaded with a plethora of different cargoes, allowing for the fine-tuning of antitumor immune responses. In the study, it was demonstrated that the particles retained membrane-bound tumor antigens while removing many intracellular, housekeeping proteins that can serve to dilute immune responses. The particles, when incorporated with adjuvant, were able to induce efficient DC maturation and promoted tumor-specific T cell responses. Such bioinspired platforms are still in their infancy, and their ability to elicit long-term, durable antitumor responses in vivo requires validation. Nonetheless, their rational design can serve as a model for future anticancer vaccine development.
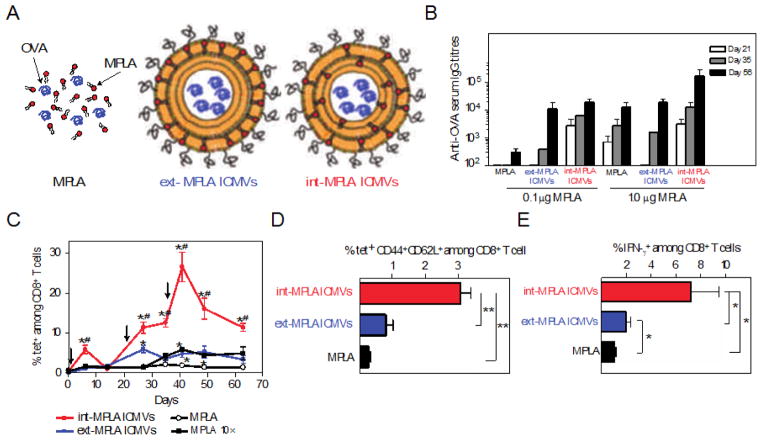
Figure 6.
Interbilayer-crosslinked multilamellar vesicles (ICMV). (A) Illustration of free antigen and adjuvant, and two ICMV formulations containing liposome encapsulated OVA antigen with MPLA embedded into the lipid layers. (B) Anti-OVA serum IgG titers analyzed from mice serum collected on days 21, 35, and 56 after immunization. (C) Frequency of OVA-specific T cells in peripheral blood determined by flow cytometry analysis of tetramer+ CD8+ T cells over time after vaccination. (D) Percentage of central memory T cells in peripheral blood collected on day 41 after vaccination determined by flow cytometry analysis of stained tetramer+ CD44+CD62L+ T cells among CD8+ T cells. Peripheral blood mononuclear cells were collected day 49 after immunization and restimulated ex vivo with OVA peptide. (E) Functionality of antigen-specific CD8+ T cells against the presentation of OVA antigen was then assayed using flow cytometry histograms of IFNγ+CD8+ T cells. Reproduced with permission.[108] Copyright 2011, Macmillan Publishers Limited.
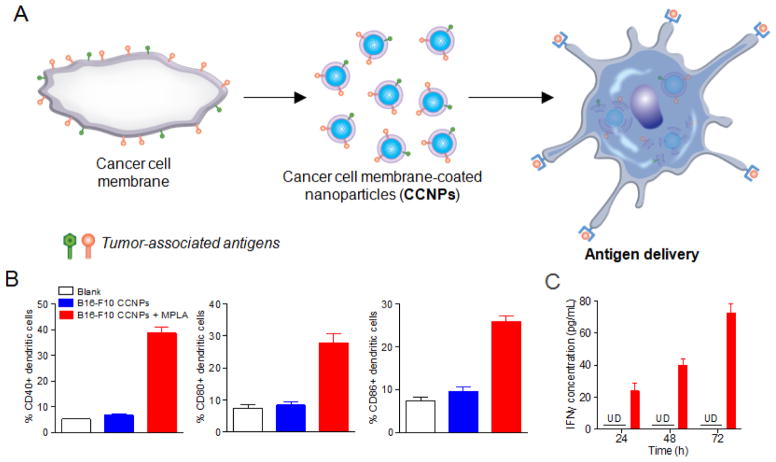
Figure 7.
Cancer cell membrane-coated nanoparticles (CCNPs). (A) Schematic representation of CCNP fabrication and application. (B) Maturation of DCs after pulsing with blank solution, CCNPs from B16-F10 cell membrane, or CCNPs with MPLA adjuvant for 48 hours. Cells were immunostained with antibodies against CD40 (left), CD80 (middle), or CD86 (right) as maturation markers and analyzed by flow cytometry. (C) Pulsed DCs were co-cultured with T cells derived from pmel-1 transgenic mice. Response against the presentation of gp100 antigen was assayed using an ELISA for IFNγ at 24, 48, and 72 hours after co-culturing. Reproduced with permission.[110] Copyright 2014, American Chemical Society.
4. Conclusion
Vaccination represents a promising approach for the treatment of cancer, but successful clinical translation has been hampered by challenges in designing specific, potent, and cost-effective formulations. Much like it has done for other areas of cancer therapy, nanotechnology has the ability to solve many of these problems and propel anticancer vaccination forward. Specifically, nanoparticle platforms can aid in the effective manipulation of APCs, which are essential to the endogenous induction of anticancer immunity, and development along these lines has accelerated over the past decade. Looking forward, as an increasing number of nanoparticle-based platforms are reported, researchers will need to move beyond the use of model antigens such as OVA and to evaluate their formulations using systems that more accurately recapitulate tumor immunology, which will help to better facilitate clinical translation. Ultimately, the flexibility to engineer carriers that can incorporate virtually endless combinations of antigenic and adjuvant materials at controllable ratios while also targeting the correct immune subsets offers the exciting prospect of allowing anticancer vaccines to realize their full potential.
Recent developments have also suggested that combinatorial immunotherapy treatments will eventually be necessary to achieve maximal efficacy.[164] This is a natural conclusion given the complexity associated with generating antitumor immunity and the fact that most treatments only focus on one aspect of the immune process. For example, anti-blockade therapies may be extremely effective at unlocking existing immune responses, but may not be optimal for generating new specificities. On the other hand, while they can do very little to directly overcome immune suppression, vaccines excel at expanding the immune repertoire, increasing the breadth to include subdominant epitopic targets. This compartmentalized view of immunotherapies has important implications in the pre-clinical evaluation of new vaccine formulations, as lack of treatment efficacy does not necessarily correlate to a lack of utility. The discrepancy can be addressed by readjusting views on what it means for a vaccine to be successful. As development progresses, it is not difficult to envision that, in the future, personalized vaccine nanoformulations will be routinely combined with other treatment modalities to generate therapies that can make a significant and lasting impact on the patient population.
Acknowledgments
This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK095168.
Biographies

Dr. Ronnie H. Fang is a postdoctoral researcher in the Department of NanoEngineering at the University of California, San Diego. He received his Ph.D. in NanoEngineering at the University of California, San Diego. His thesis work was on the synthesis and application of cell membrane-coated nanoparticles for novel therapeutic applications. He is currently interested in leveraging biomimetic nanoparticles for manipulating immune responses for disease treatment.

Ashley V. Kroll is a graduate student researcher in the Department of NanoEngineering at the University of California, San Diego. She received her B.S. in NanoEngineering at the University of California, San Diego in 2014. Her research interests lie in the development of biomimetic nanoparticles for improved treatments of human diseases. She is currently working on cell membrane-coated nanoparticles for cancer immunotherapy.

Dr. Liangfang Zhang is a Professor in the Department of NanoEngineering and Moores Cancer Center at the University of California, San Diego. He received his Ph.D. in Chemical Engineering at the University of Illinois at Urbana-Champaign. His research aims to create cutting-edge biomimetic nanotechnologies and exploit them for various biomedical applications with a particular focus on drug delivery, biodetoxification and vaccination.
References
- 1.Siegel RL, Miller KD, Jemal A. CA-Cancer J Clin. 2015;65(1):5. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. CA-Cancer J Clin. 2014;64(4):252. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. CA-Cancer J Clin. 2014;64(1):9. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Strebhardt K, Ullrich A. Nat Rev Cancer. 2008;8(6):473. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 5.van't Veer LJ, Bernards R. Nature. 2008;452(7187):564. doi: 10.1038/nature06915. [DOI] [PubMed] [Google Scholar]
- 6.Al-Lazikani B, Banerji U, Workman P. Nat Biotechnol. 2012;30(7):679. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 7.Mellman I, Coukos G, Dranoff G. Nature. 2011;480(7378):480. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swann JB, Smyth MJ. J Clin Invest. 2007;117(5):1137. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim R, Emi M, Tanabe K. Immunology. 2007;121(1):1. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Angelis C. Curr Oncol. 2008;15(4):198. doi: 10.3747/co.v15i4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dranoff G. Nat Rev Cancer. 2004;4(1):11. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 12.Restifo NP, Dudley ME, Rosenberg SA. Nat Rev Immunol. 2012;12(4):269. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H. Nat Rev Clin Oncol. 2014;11(9):509. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 14.Vergati M, Intrivici C, Huen NY, Schlom J, Tsang KY. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/596432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS. N Engl J Med. 2010;363(5):411. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, Santosham M, Schmitt HJ. Bull World Health Organ. 2008;86(2):140. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, Restifo NP. Nat Med. 2004;10(9):909. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabi Z, Man S. Adv Drug Deliv Rev. 2006;58(8):902. doi: 10.1016/j.addr.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Banchereau J, Steinman RM. Nature. 1998;392(6673):245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 20.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Annu Rev Immunol. 2011;29:235. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 21.Chen DS, Mellman I. Immunity. 2013;39(1):1. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Drake CG, Jaffee E, Pardoll DM. Adv Immunol. 2006;90:51. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 23.Lynch M. Proc Natl Acad Sci USA. 2010;107(3):961. doi: 10.1073/pnas.0912629107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubenik J. Int J Oncol. 2004;25(2):487. [PubMed] [Google Scholar]
- 25.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S, Pistoia V. Neoplasia. 2004;6(5):558. doi: 10.1593/neo.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM, Huang RP. Biochim Biophys Acta. 2014;1845(2):182. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Pardoll DM. Nat Rev Cancer. 2012;12(4):252. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, Kleiner D, Mavroukakis SA, Yellin M, Rosenberg SA. Ann Surg Oncol. 2005;12(12):1005. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.June CH. J Clin Invest. 2007;117(6):1466. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinman RM, Pope M. J Clin Invest. 2002;109(12):1519. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buonaguro L, Petrizzo A, Tornesello ML, Buonaguro FM. Clin Vaccine Immunol. 2011;18(1):23. doi: 10.1128/CVI.00286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Cancer Res. 2004;64(14):4973. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 33.Vigneron N, Stroobant V, Van den Eynde BJ, van der Bruggen P. Cancer Immun. 2013;13:15. [PMC free article] [PubMed] [Google Scholar]
- 34.Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, de Graaf J, Selmi A, Diken M, Boegel S, Paret C, Koslowski M, Kuhn AN, Britten CM, Huber C, Tureci O, Sahin U. Cancer Res. 2012;72(5):1081. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 35.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, Linette GP. Science. 2015 doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu LN, Shivakumar R, Allen C, Fratantoni JC. Methods Mol Biol. 2008;423:139. doi: 10.1007/978-1-59745-194-9_9. [DOI] [PubMed] [Google Scholar]
- 37.Lokhov PG, Balashova EE. J Cancer. 2010;1:230. doi: 10.7150/jca.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen BM, Ohlfest JR. Cancer Lett. 2012;325(2):155. doi: 10.1016/j.canlet.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obenaus M, Leitao C, Leisegang M, Chen X, Gavvovidis I, van der Bruggen P, Uckert W, Schendel DJ, Blankenstein T. Nat Biotechnol. 2015;33(4):402. doi: 10.1038/nbt.3147. [DOI] [PubMed] [Google Scholar]
- 40.Dubensky TW, Jr, Reed SG. Semin Immunol. 2010;22(3):155. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Hoption Cann SA, van Netten JP, van Netten C. Postgrad Med J. 2003;79(938):672. [PMC free article] [PubMed] [Google Scholar]
- 42.Akira S, Hemmi H. Immunol Lett. 2003;85(2):85. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 43.Appleman LJ, Boussiotis VA. Immunol Rev. 2003;192:161. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 44.Blander JM, Medzhitov R. Nature. 2006;440(7085):808. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 45.Kreutz M, Giquel B, Hu Q, Abuknesha R, Uematsu S, Akira S, Nestle FO, Diebold SS. PLoS ONE. 2012;7(7):e40208. doi: 10.1371/journal.pone.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palucka K, Banchereau J. Nat Rev Cancer. 2012;12(4):265. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. N Engl J Med. 2011;364(22):2119. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slingluff CL, Jr, Yamshchikov G, Neese P, Galavotti H, Eastham S, Engelhard VH, Kittlesen D, Deacon D, Hibbitts S, Grosh WW, Petroni G, Cohen R, Wiernasz C, Patterson JW, Conway BP, Ross WG. Clin Cancer Res. 2001;7(10):3012. [PubMed] [Google Scholar]
- 49.Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, Lopez-Brea M, Vanakesa T, Jassem J, Kalofonos H, Perdeus J, Bonnet R, Basko J, Janilionis R, Passlick B, Treasure T, Gillet M, Lehmann FF, Brichard VG. J Clin Oncol. 2013;31(19):2396. doi: 10.1200/JCO.2012.43.7103. [DOI] [PubMed] [Google Scholar]
- 50.Tyagi P, Mirakhur B. Clin Lung Cancer. 2009;10(5):371. doi: 10.3816/CLC.2009.n.052. [DOI] [PubMed] [Google Scholar]
- 51.Cuppens K, Vansteenkiste J. Curr Opin Oncol. 2014;26(2):165. doi: 10.1097/CCO.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 52.Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquee L, Trigo JM, Spira A, Tremblay L, Nyman J, Ramlau R, Wickart-Johansson G, Ellis P, Gladkov O, Pereira JR, Eberhardt WE, Helwig C, Schroder A, Shepherd FA S. t. team. Lancet Oncol. 2014;15(1):59. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 53.Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, Peoples GE. Ann Oncol. 2014;25(9):1735. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneble EJ, Berry JS, Trappey FA, Clifton GT, Ponniah S, Mittendorf E, Peoples GE. Immunotherapy. 2014;6(5):519. doi: 10.2217/imt.14.22. [DOI] [PubMed] [Google Scholar]
- 55.Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson RL, Smits BJ, Sopapan P, Strang G, Valone FH, Vuk-Pavlovic S. Clin Cancer Res. 2000;6(6):2175. [PubMed] [Google Scholar]
- 56.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. J Clin Oncol. 2006;24(19):3089. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 57.Cheever MA, Higano CS. Clin Cancer Res. 2011;17(11):3520. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 58.Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, Kluger H, Tejwani S, Green J, Ramakrishna V, Crocker A, Vitale L, Yellin M, Davis T, Keler T. Sci Transl Med. 2014;6(232):232. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kandalaft LE, Chiang CL, Tanyi J, Motz G, Balint K, Mick R, Coukos G. J Transl Med. 2013;11:149. doi: 10.1186/1479-5876-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karbach J, Neumann A, Atmaca A, Wahle C, Brand K, von Boehmer L, Knuth A, Bender A, Ritter G, Old LJ, Jager E. Clin Cancer Res. 2011;17(4):861. doi: 10.1158/1078-0432.CCR-10-1811. [DOI] [PubMed] [Google Scholar]
- 61.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS. J Clin Oncol. 2011;29(3):330. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trepiakas R, Berntsen A, Hadrup SR, Bjorn J, Geertsen PF, Straten PT, Andersen MH, Pedersen AE, Soleimani A, Lorentzen T, Johansen JS, Svane IM. Cytotherapy. 2010;12(6):721. doi: 10.3109/14653241003774045. [DOI] [PubMed] [Google Scholar]
- 63.Hanna MG, Jr, Hoover HC, Jr, Vermorken JB, Harris JE, Pinedo HM. Vaccine. 2001;19(17–19):2576. doi: 10.1016/s0264-410x(00)00485-0. [DOI] [PubMed] [Google Scholar]
- 64.Hoover HC, Jr, Brandhorst JS, Peters LC, Surdyke MG, Takeshita Y, Madariaga J, Muenz LR, Hanna MG., Jr J Clin Oncol. 1993;11(3):390. doi: 10.1200/JCO.1993.11.3.390. [DOI] [PubMed] [Google Scholar]
- 65.Hoover HC, Jr, Surdyke MG, Dangel RB, Peters LC, Hanna MG., Jr Cancer. 1985;55(6):1236. doi: 10.1002/1097-0142(19850315)55:6<1236::aid-cncr2820550616>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 66.Uyl-de Groot CA, Vermorken JB, Hanna MG, Jr, Verboom P, Groot MT, Bonsel GJ, Meijer CJ, Pinedo HM. Vaccine. 2005;23(17–18):2379. doi: 10.1016/j.vaccine.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 67.Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, Hanna MG, Jr, Pinedo HM. Lancet. 1999;353(9150):345. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 68.Okur FV, Yvon E, Biagi E, Dotti G, Carrum G, Heslop H, Mims MP, Fratantoni JC, Peshwa MV, Li LH, Brenner MK. Cytotherapy. 2011;13(9):1128. doi: 10.3109/14653249.2011.592523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, DeVol E, Maples PB, Liu L, Chamberlin T, Shawler DL, Fakhrai H. Mol Ther. 2006;13:S424. [Google Scholar]
- 70.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, Jaffee EM. J Clin Oncol. 2009;27(35):5911. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Clin Cancer Res. 2008;14(5):1455. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drake CG. J Clin Oncol. 2009;27(25):4035. doi: 10.1200/JCO.2009.22.2299. [DOI] [PubMed] [Google Scholar]
- 73.Higano CS, Corman JM, Smith DC, Centeno AS, Steidle CP, Gittleman M, Simons JW, Sacks N, Aimi J, Small EJ. Cancer. 2008;113(5):975. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 74.Riker A, Cormier J, Panelli M, Kammula U, Wang E, Abati A, Fetsch P, Lee KH, Steinberg S, Rosenberg S, Marincola F. Surgery. 1999;126(2):112. [PubMed] [Google Scholar]
- 75.Law SK. Clin Exp Immunol. 1991;85(1):1. doi: 10.1111/j.1365-2249.1991.tb05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tacken PJ, Figdor CG. Semin Immunol. 2011;23(1):12. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. Nanomedicine. 2013;9(1):1. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parveen S, Misra R, Sahoo SK. Nanomedicine. 2012;8(2):147. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 79.Chow EK, Ho D. Sci Transl Med. 2013;5(216):216rv4. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- 80.Wang AZ, Langer R, Farokhzad OC. Annu Rev Med. 2012;63:185. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 81.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Clin Cancer Res. 2008;14(5):1310. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 82.Davis ME, Chen ZG, Shin DM. Nat Rev Drug Discov. 2008;7(9):771. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 83.Barenholz Y. J Control Release. 2012;160(2):117. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 84.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nat Nanotechnol. 2007;2(12):751. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 85.Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, Hawkins MJ. Ann Oncol. 2006;17(8):1263. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 86.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, Kim NK, Bang YJ. Clin Cancer Res. 2004;10(11):3708. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 87.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Sci Transl Med. 2012;4(128):128. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 88.Iyer AK, Khaled G, Fang J, Maeda H. Drug Discov Today. 2006;11(17–18):812. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 89.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Nat Nanotechnol. 2010;5(1):42. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fonseca C, Simoes S, Gaspar R. J Control Release. 2002;83(2):273. doi: 10.1016/s0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 91.Saiki I, Fidler IJ. J Immunol. 1985;135(1):684. [PubMed] [Google Scholar]
- 92.Anderson PM, Katsanis E, Leonard AS, Schow D, Loeffler CM, Goldstein MB, Ochoa AC. Cancer Res. 1990;50(6):1853. [PubMed] [Google Scholar]
- 93.Christian DA, Hunter CA. Immunotherapy. 2012;4(4):425. doi: 10.2217/imt.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson PM, Katsanis E, Sencer SF, Hasz D, Ochoa AC, Bostrom B. J Immunother (1991) 1992;12(1):19. [PubMed] [Google Scholar]
- 95.Oya M. Keio J Med. 1994;43(1):37. doi: 10.2302/kjm.43.37. [DOI] [PubMed] [Google Scholar]
- 96.Kanaoka E, Takahashi K, Yoshikawa T, Jizomoto H, Nishihara Y, Uchida N, Maekawa R, Hirano K. J Control Release. 2002;82(2–3):183. doi: 10.1016/s0168-3659(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 97.Anderson PM, Hanson DC, Hasz DE, Halet MR, Blazar BR, Ochoa AC. Cytokine. 1994;6(1):92. doi: 10.1016/1043-4666(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 98.Saxton ML, Longo DL, Wetzel HE, Tribble H, Alvord WG, Kwak LW, Leonard AS, Ullmann CD, Curti BD, Ochoa AC. Blood. 1997;89(7):2529. [PubMed] [Google Scholar]
- 99.Cabanes A, Even-Chen S, Zimberoff J, Barenholz Y, Kedar E, Gabizon A. Clin Cancer Res. 1999;5(3):687. [PubMed] [Google Scholar]
- 100.Mejias R, Costo R, Roca AG, Arias CF, Veintemillas-Verdaguer S, Gonzalez-Carreno T, del Puerto Morales M, Serna CJ, Manes S, Barber DF. J Control Release. 2008;130(2):168. doi: 10.1016/j.jconrel.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 101.Mejias R, Perez-Yague S, Gutierrez L, Cabrera LI, Spada R, Acedo P, Serna CJ, Lazaro FJ, Villanueva A, del Morales MP, Barber DF. Biomaterials. 2011;32(11):2938. doi: 10.1016/j.biomaterials.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 102.Yao H, Ng SS, Huo LF, Chow BK, Shen Z, Yang M, Sze J, Ko O, Li M, Yue A, Lu LW, Bian XW, Kung HF, Lin MC. Mol Cancer Ther. 2011;10(6):1082. doi: 10.1158/1535-7163.MCT-10-0717. [DOI] [PubMed] [Google Scholar]
- 103.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Nat Mater. 2012;11(10):895. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sunshine JC, Green JJ. Nanomedicine (Lond) 2013;8(7):1173. doi: 10.2217/nnm.13.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Weijden J, Paulis LE, Verdoes M, van Hest JCM, Figdor CG. Chem Sci. 2014;5(9):3355. [Google Scholar]
- 106.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. ACS Nano. 2014;8(3):2252. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ. Small. 2015;11(13):1519. doi: 10.1002/smll.201402369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, Goodwin JT, Ramos J, Chiu W, Irvine DJ. Nat Mater. 2011;10(3):243. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li H, Li Y, Jiao J, Hu HM. Nat Nanotechnol. 2011;6(10):645. doi: 10.1038/nnano.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O'Connor DE, Zhang L. Nano Lett. 2014;14(4):2181. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Nat Biotechnol. 2007;25(10):1159. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 112.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. J Control Release. 2006;112(1):26. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 113.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Biomaterials. 2014;35(2):814. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 114.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanomedicine (Lond) 2011;6(4):715. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kou L, Sun J, Zhai Y, He Z. Asian J Pharm Sci. 2013;8(1):1. [Google Scholar]
- 116.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Immunology. 2006;117(1):78. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sperling RA, Parak WJ. Philos Trans A Math Phys Eng Sci. 2010;368(1915):1333. doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- 118.Fang RH, Hu CM, Chen KN, Luk BT, Carpenter CW, Gao W, Li S, Zhang DE, Lu W, Zhang L. Nanoscale. 2013;5(19):8884. doi: 10.1039/c3nr03064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh SK, Stephani J, Schaefer M, Kalay H, Garcia-Vallejo JJ, den Haan J, Saeland E, Sparwasser T, van Kooyk Y. Mol Immunol. 2009;47(2–3):164. doi: 10.1016/j.molimm.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 120.Sheng KC, Kalkanidis M, Pouniotis DS, Esparon S, Tang CK, Apostolopoulos V, Pietersz GA. Eur J Immunol. 2008;38(2):424. doi: 10.1002/eji.200737578. [DOI] [PubMed] [Google Scholar]
- 121.Cui Z, Han SJ, Huang L. Pharm Res. 2004;21(6):1018. doi: 10.1023/b:pham.0000029292.66792.4f. [DOI] [PubMed] [Google Scholar]
- 122.Kwon YJ, Standley SM, Goh SL, Frechet JM. J Control Release. 2005;105(3):199. doi: 10.1016/j.jconrel.2005.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A, Stayton PS. ACS Nano. 2013;7(5):3912. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Vaccine. 2010;28(50):7897. doi: 10.1016/j.vaccine.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 125.Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, Liu L, Wang C, Cai L, Ma Y. J Control Release. 2013;168(3):271. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 126.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. Expert Rev Vaccines. 2011;10(4):499. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zwiorek K, Bourquin C, Battiany J, Winter G, Endres S, Hartmann G, Coester C. Pharm Res. 2008;25(3):551. doi: 10.1007/s11095-007-9410-5. [DOI] [PubMed] [Google Scholar]
- 128.de Jong S, Chikh G, Sekirov L, Raney S, Semple S, Klimuk S, Yuan N, Hope M, Cullis P, Tam Y. Cancer Immunol Immun. 2007;56(8):1251. doi: 10.1007/s00262-006-0276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vangasseri DP, Cui Z, Chen W, Hokey DA, Falo LD, Jr, Huang L. Mol Membr Biol. 2006;23(5):385. doi: 10.1080/09687860600790537. [DOI] [PubMed] [Google Scholar]
- 130.Cui Z, Han SJ, Vangasseri DP, Huang L. Mol Pharm. 2005;2(1):22. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 131.Pan X, Chen L, Liu S, Yang X, Gao JX, Lee RJ. Mol Pharm. 2009;6(1):211. doi: 10.1021/mp800146j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin AY, Almeida JPM, Bear A, Liu N, Luo L, Foster AE, Drezek RA. Plos One. 2013;8(5) doi: 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tao C, Zhu Y. Dalton Trans. 2014;43(41):15482. doi: 10.1039/c4dt01984a. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, Jiao F, Qiu Y, Li W, Qu Y, Tian C, Li Y, Bai R, Lao F, Zhao Y, Chai Z, Chen C. Nanotechnology. 2009;20(41):415102. doi: 10.1088/0957-4484/20/41/415102. [DOI] [PubMed] [Google Scholar]
- 135.Zhu J, Ji Z, Wang J, Sun R, Zhang X, Gao Y, Sun H, Liu Y, Wang Z, Li A, Ma J, Wang T, Jia G, Gu Y. Small. 2008;4(8):1168. doi: 10.1002/smll.200701219. [DOI] [PubMed] [Google Scholar]
- 136.Khisamutdinov EF, Li H, Jasinski DL, Chen J, Fu J, Guo P. Nucleic Acids Res. 2014;42(15):9996. doi: 10.1093/nar/gku516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Radovic-Moreno AF, Chernyak N, Mader CC, Nallagatla S, Kang RS, Hao L, Walker DA, Halo TL, Merkel TJ, Rische CH, Anantatmula S, Burkhart M, Mirkin CA, Gryaznov SM. Proc Natl Acad Sci USA. 2015;112(13):3892. doi: 10.1073/pnas.1502850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ. Mol Pharm. 2007;4(1):47. doi: 10.1021/mp060107e. [DOI] [PubMed] [Google Scholar]
- 139.Prasad S, Cody V, Saucier-Sawyer JK, Saltzman WM, Sasaki CT, Edelson RL, Birchall MA, Hanlon DJ. Nanomedicine. 2011;7(1):1. doi: 10.1016/j.nano.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tan S, Sasada T, Bershteyn A, Yang K, Ioji T, Zhang Z. Nanomedicine (Lond) 2014;9(5):635. doi: 10.2217/nnm.13.67. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, Reinhold B, Keskin DB, Reinherz EL, Sasada T. Biomaterials. 2011;32(14):3666. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 142.Schlosser E, Mueller M, Fischer S, Basta S, Busch DH, Gander B, Groettrup M. Vaccine. 2008;26(13):1626. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 143.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Vaccine. 2008;26(39):5046. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 144.Fischer NO, Rasley A, Corzett M, Hwang MH, Hoeprich PD, Blanchette CD. J Am Chem Soc. 2013;135(6):2044. doi: 10.1021/ja3063293. [DOI] [PubMed] [Google Scholar]
- 145.Bal SM, Hortensius S, Ding Z, Jiskoot W, Bouwstra JA. Vaccine. 2011;29(5):1045. doi: 10.1016/j.vaccine.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 146.Heo MB, Lim YT. Biomaterials. 2014;35(1):590. doi: 10.1016/j.biomaterials.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 147.Xu Z, Wang Y, Zhang L, Huang L. ACS Nano. 2014;8(4):3636. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA. Proc Natl Acad Sci USA. 2013;110(49):19902. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Almeida JP, Lin AY, Figueroa ER, Foster AE, Drezek RA. Small. 2015;11(12):1453. doi: 10.1002/smll.201402179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yoshikawa T, Okada N, Oda A, Matsuo K, Matsuo K, Mukai Y, Yoshioka Y, Akagi T, Akashi M, Nakagawa S. Biochem Biophys Res Commun. 2008;366(2):408. doi: 10.1016/j.bbrc.2007.11.153. [DOI] [PubMed] [Google Scholar]
- 151.Akagi T, Wang X, Uto T, Baba M, Akashi M. Biomaterials. 2007;28(23):3427. doi: 10.1016/j.biomaterials.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 152.Uto T, Wang X, Sato K, Haraguchi M, Akagi T, Akashi M, Baba M. J Immunol. 2007;178(5):2979. doi: 10.4049/jimmunol.178.5.2979. [DOI] [PubMed] [Google Scholar]
- 153.Yoshikawa T, Okada N, Oda A, Matsuo K, Matsuo K, Kayamuro H, Ishii Y, Yoshinaga T, Akagi T, Akashi M, Nakagawa S. Vaccine. 2008;26(10):1303. doi: 10.1016/j.vaccine.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 154.Kurosaki T, Kitahara T, Nakamura T, Nishida K, Fumoto S, Kodama Y, Nakagawa H, Higuchi N, Sasaki H. Pharm Res. 2012;29(2):483. doi: 10.1007/s11095-011-0571-x. [DOI] [PubMed] [Google Scholar]
- 155.Seth A, Heo MB, Sung MH, Lim YT. Int J Biol Macromol. 2015;75:495. doi: 10.1016/j.ijbiomac.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 156.Chen W, Yan W, Huang L. Cancer Immunol Immunother. 2008;57(4):517. doi: 10.1007/s00262-007-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Elamanchili P, Lutsiak CM, Hamdy S, Diwan M, Samuel J. J Immunother. 2007;30(4):378. doi: 10.1097/CJI.0b013e31802cf3e3. [DOI] [PubMed] [Google Scholar]
- 158.Ilyinskii PO, Roy CJ, O'Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, Radovic-Moreno AF, Langer RS, Farokhzad OC, von Andrian UH, Johnston LP, Kishimoto TK. Vaccine. 2014;32(24):2882. doi: 10.1016/j.vaccine.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, Gyobu H, Kawarada Y, Kondo S, Akira S, Katoh H, Ikeda H, Nishimura T. Cancer Res. 2004;64(23):8754. doi: 10.1158/0008-5472.CAN-04-1691. [DOI] [PubMed] [Google Scholar]
- 160.Rosalia RA, Cruz LJ, van Duikeren S, Tromp AT, Silva AL, Jiskoot W, de Gruijl T, Lowik C, Oostendorp J, van der Burg SH, Ossendorp F. Biomaterials. 2015;40:88. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 161.Xu Z, Ramishetti S, Tseng YC, Guo S, Wang Y, Huang L. J Control Release. 2013;172(1):259. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 162.Lee IH, Kwon HK, An S, Kim D, Kim S, Yu MK, Lee JH, Lee TS, Im SH, Jon S. Angew Chem Int Ed Engl. 2012;51(35):8800. doi: 10.1002/anie.201203193. [DOI] [PubMed] [Google Scholar]
- 163.Luo Z, Wang C, Yi H, Li P, Pan H, Liu L, Cai L, Ma Y. Biomaterials. 2015;38:50. doi: 10.1016/j.biomaterials.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 164.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Nature. 2015;520(7547):373. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]