Abstract
Objectives
The purpose of this study was to estimate the volumetric change of augmented autobone harvested from mandibular body cortical bone, using cone-beam computed tomography (CBCT) and three-dimensional reconstruction. In addition, the clinical success of dental implants placed 4 to 6 months after bone grafting was also evaluated.
Materials and Methods
Ninety-five patients (48 men and 47 women) aged 19 to 72 years were included in this study. A total of 128 graft sites were evaluated. The graft sites were divided into three parts: anterior and both posterior regions of one jaw. All patients included in the study were scheduled for an onlay graft and implantation using a two-stage procedure. The dental implants were inserted 4 to 6 months after the bone graft. Volumetric stability was evaluated by serial CBCT images.
Results
No major complications were observed for the donor sites. A total of 128 block bones were used to augment severely resorbed alveolar bone. Only 1 of the 128 bone grafts was resorbed by more than half, and that was due to infection. In total, the average amount of residual grafted bone after resorption at the recipient sites was 74.6%±8.4%.
Conclusion
Volumetric stability of mandibular body autogenous block grafts is predictable. The procedure is satisfactory for patients who want dental implants regardless of atrophic alveolar bone.
Keywords: Alveolar bone grafting, Augment bone graft, Dental implant
I. Introduction
If the quantity and quality of alveolar bone for a dental implant is appropriate, then the prosthesis using an osseointegrated dental implant will have a favorable long-term result 1,2. Implant-supported restorations can be successful, even in atrophic jaws, using bone augmentation that includes bone graft and tissue regeneration techniques3. Among the various techniques, autogenous bone is believed to be the most effective bone graft material and is still regarded as the "gold standard" for augmentation procedures because of its osteogenic potential4. In cases where large amounts of bone are required, autogenous bone is considered the best choice. Autogenous bone can be harvested from sites such as the iliac crest, tibia, skull, or mandible5,6. Moreover, block bone can be harvested from intraoral sites, including the retromolar region, zygoma, maxilla, and mandible7,8. Intraoral harvesting has several advantages when compared with extraoral donor sites, such as proximity of the donor site to the recipient site, convenient surgical access, shorter operation time, lower morbidity of the donor site, and ease of performing the procedure in an outpatient or office environment. The intraoral surgical operation can be performed in the office under local anesthesia or in the operating room under general anaesthesia9,10.
Several studies have shown that intramembranous bone grafts (skull or mandible), compared to endochondral bone grafts (iliac), have minimal resorption and better incorporation at the recipient sites11,12. These reports suggest that embryonic origins make a difference in terms of resorption patterns. Microarchitectural features, such as the cortical/cancellous ratio, may affect the volumetric stability of the bone grafts in the craniofacial skeleton13,14.
On computed tomography (CT) scans, the Hounsfield unit (HU) is proportional to the degree of X-ray attenuation by the tissue. On cone-beam CT (CBCT), the degree of X-ray attenuation is shown by greyscale or voxel values. We calculated the volumetric changes using the grayscale of the harvested cortical bone and volume of interest (VOI) using the computer program Ez3D2009 (Vatech, Yongin, Korea).
The aim of this study was to evaluate volumetric changes of bone reconstruction of severely atrophic jaws, using a block consisting of autogenous mandibular cortical bone prior to dental implant placement. In addition, the clinical success of dental implants placed at the grafted alveolar bone sites was also evaluated.
II. Materials and Methods
1. Patients and study design
A retrospective chart review of patients who underwent onlay graft surgery prior to dental implant placement was conducted. The patients included in this study were treated from March 2010 to April 2014 at the Pusan National University Dental Hospital (Yangsan, Korea). Ninety-five patients (48 men and 47 women) aged 19 to 72 years were included in the study. A total of 128 graft sites were used. The graft sites were divided into three parts; anterior and both posterior regions of one jaw. All patients included in the study were scheduled for onlay grafts and implantation using a two-stage procedure. The dental implants were inserted 4 to 6 months after the bone graft.
Volumetric stability was evaluated by serial CBCT images. Personal information, such as age, sex, and grafting direction (horizontal/vertical), location (anterior/posterior or maxilla/mandible) and whether or not sinus lift procedures were conducted, were collected from the patient records. All patients underwent an onlay bone grafting procedure using a mandibular bone block, consisting of buccal cortical bone from the posterior part of the mandibular body to the external oblique ridge. No patient had undergone any oncologic treatment, and there was no use of any allografts or xenograft materials. Allografts were only used in those patients who also required sinus lift procedures.
A CBCT was taken before the grafting surgery (T0) and after the grafting surgery (T1, within 3 days) and just before the implant surgery (T2). The authors calculated the volumetric change of the grafted bone between T1 and T2.
Dental implants were placed at recipient sites 5.5±0.8 months after grafting surgery in all cases. The authors used several dental implants, including a sand blasted with large grit and acid etched sand large acid (SLA) surface, USII (Osstem, Seoul, Korea), SOLAR (Shinhung, Seoul, Korea), and Straumann SLActive (Straumann AG, Basel, Switzerland). The authors observed the state of the dental implants and evaluated them clinically over 2.6±1.2 years. Occlusion function, marginal bone loss (by dental panorama), peri-implantitis, osseointegration of the fixture, and prosthetic problems were investigated at all dental implant sites. Only 11 patients could not be evaluated with respect to their implant-based prosthetics. Six patients were not seen again for personal reasons, and prosthetics were not yet completed for five patients. The authors used panoramic radiographs and standard dental films for long-term follow-up since CBCT was not beneficial for patients, and taking a CBCT solely for research purposes was not considered ethical.
2. Surgery
Preoperative CBCTs were used to evaluate the need for a bone graft with mandibular cortical bone in those patients who had a severe atrophic jaw. A total of 95 patients had grafting surgery. Of these, 85 surgeries were performed under local anesthesia. In order to improve patient comfort, conscious sedation with midazolam was used in these surgeries from March 2010 to October 2011 at the Pusan National University Dental Hospital. A total of 38 surgeries were conducted under midazolam sedation. Another sedative agent, dexmedetomidine (Precedex; Hospira, Lake Forest, IL, USA), was used for surgeries performed in November 2011. A total of 48 surgeries were performed using this new sedative. Another nine patients were treated under general anesthesia due to long operative times required for multiple recipient sites or patients' demands. Lidocaine with epinephrine (1:100,000 epinephrine) was administered locally to reduce pain and bleeding.
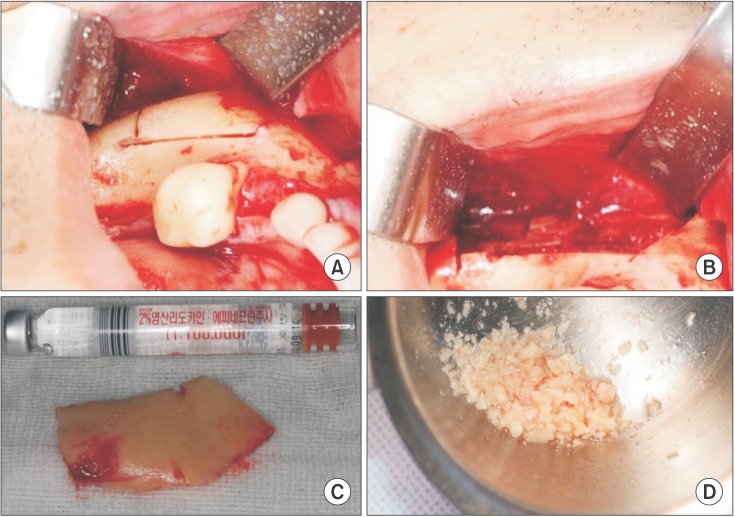
Harvesting the mandibular block bone was initiated with linear incision over the lower external oblique ridge. After reflection of the full-thickness flap and exposure of the mandibular body bone, the osteotomy was completed with copious irrigation. One part of the harvested bone was ground into small particles using a bone crusher.(Fig. 1)
Fig. 1. A. Cortical bone after sawing targeted bone. B. Donor site after harvesting cortical bone. C. Harvested cortical bone. D. Particulate bone from cortical bone.
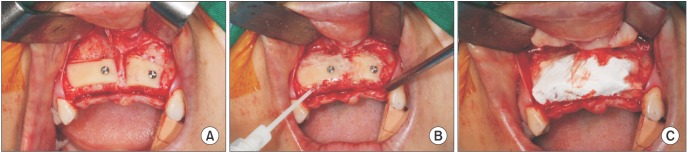
The harvested block bone was positioned as an onlay using the "lag screw" technique in order to stabilize the bone block15. The authors fixed the block bone and surrounded it with particulate cortical bone mixed with fibrin sealant (Tisseel; Baxter Healthcare GmbH, Wien, Austria). Then, a bioresorbable collagen membrane (OssGuide; Bioland, Cheonan, Korea) was used for covering the grafted bone. For increased bone volume and tension (full-coverage onlay), elongation of the buccal flap was achieved through a small incision and dissection of the periosteum.(Fig. 2. A, 2. B)
Fig. 2. A. Fixed block bone using the "lag screw" technique. B. Particulate bone surrounding block bone with Tisseel (Baxter Healthcare GmbH, Wien, Austria). C. Membrane materials covering block and particulate bone.
Antibiotics were given preoperatively 30 minutes before and postoperatively for approximately one week intravenously and by mouth. Patients were prescribed analgesics, acetaminophen, or steroidal anti-inflammatory drugs.
3. Calculation of volumetric change
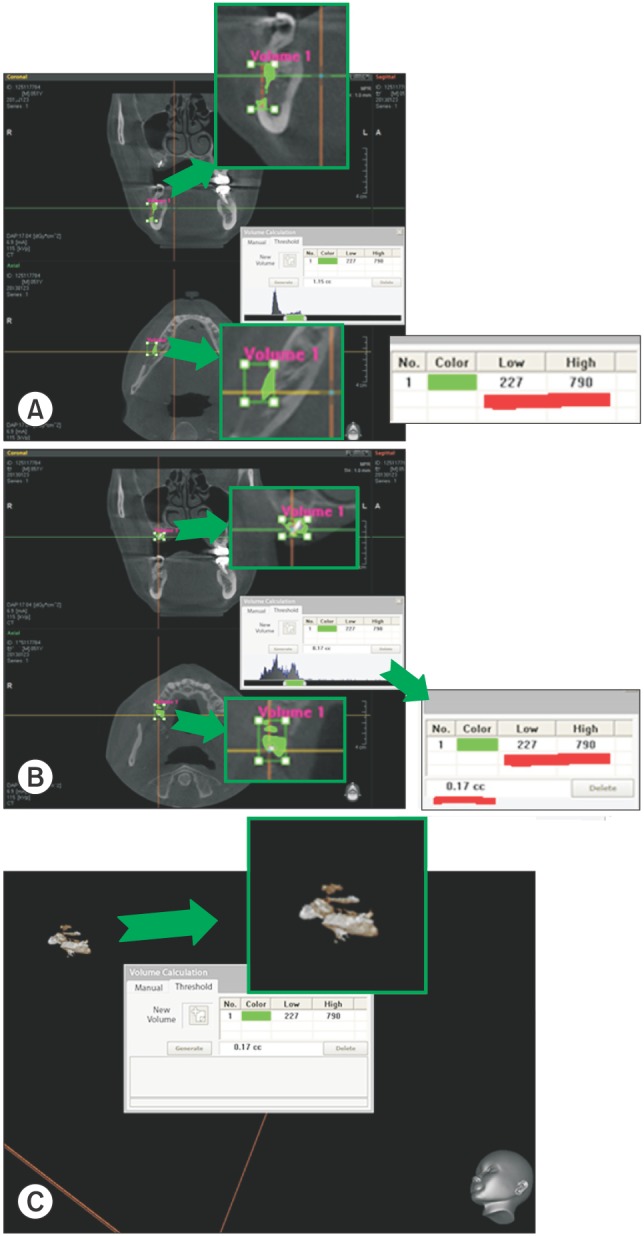
The donor site used was the mandibular buccal cortical bone from the body of the mandible. The authors assumed that the harvested cortical bone was the same as the adjacent non-harvested cortical bone on the grayscale range. Therefore, the authors established the grayscale range of the harvested cortical bone by measuring the grayscale of the adjacent cortical bone. The authors enclosed the grafted bone in a rectangular parallelepiped, namely, the VOI in the Ez3D2009 program setting. This program is able to calculate the volume of grafted bone by integrating the materials corresponding to the determined grayscale range. For the onlay graft, it is not difficult to draw lines for the VOI because the grafted bone is not surrounded by recipient bone.(Fig. 3)
Fig. 3. A. Evaluating adjacent cortical bone not harvested with the grayscale range. B. Enclosing grafted bone in a rectangular parallelepiped by using an evaluated grayscale range. C. Calculating grafted bone in the Ez3D2009 program (Vatech, Yongin, Korea) setting.
4. Statistics
The Mann-Whitney U test was used for statistical analysis in order to identify differences due to sex. The one-way ANOVA test was used for onlay direction in the same manner. Scheffe's test combined with one-way ANOVA was used to identify differences due to age. The Kruskal-Wallis test was used for the recipient location, and the Student's t-test was used for the sinus lift procedures. The normality of the data was evaluated with the Kolmogorov-Smirnov test. The level of statistical significance was set at P<0.05.
All statistical analyses were performed using IBM SPSS Statistics 21.0 (IBM Co., Armonk, NY, USA).
III. Results
The recipient sites were divided into three parts for each jaw (anterior, left posterior, and right posterior). There were 69 maxillary sites and 59 mandibular sites that were grafted with mandibular block bone in this retrospective study. Another categorization included 27 anterior sites and 101 posterior sites. A total of 128 recipient sites were placed in 95 patients. The patients consisted of 66 women and 62 men, and the average age was 49.1 years. Each patient had an average of 1.35 recipient sites.
With the exception of 11 patients who did not have final prosthetics placed on their implant fixtures, or had not come to our hospital for implantation, 84 patients had 267 implants placed in their onlay-grafted sites. The authors excluded implants placed on non-grafted sites. An average of 3.18 implants were placed on augmented alveolar bone in each patient.
Three infections occurred at the recipient sites less than a week after the grafting surgery. The authors reopened the recipient sites to allow for irrigation. The grafted bone was intact after 4.2 months. In one case, granulation tissue was found on the recipient site 2 months after surgery, and the tissue was curetted. For that case, a resorption rate of 0.38 was recorded.
The average residual rate for all of the recipient sites was 74.6%±8.4%. The authors compared the residual graft rate with age, sex, graft direction (horizontal/vertical), graft location (anterior/posterior, maxilla/mandible), and whether or not the sinus lift procedure was conducted. For the analysis of differences due to age, patients were categorized into 10-year units. As shown in Table 1, a significant difference was only observed between a and b, which corresponds to patients in their 30s and 50s, respectively. In other words, differences due to age and sex were not statistically significant according to this study.(Table 2) Statistically significant esdifferences were found for grafting direction. Horizontal onlay grafts were more stable than vertical or vertical plus horizontal grafts in graft volume.(Table 1) Graft location partially affected volumetric stability.(Table 1) For the maxilla, the residual rate was higher than the mandible, but the difference was not statistically significant. For the anterior jaw, statistical analyses showed that less grafted bone was lost as compared with the posterior jaw. For cases where onlay graft surgery was performed at the same time as the sinus lift procedure on the posterior maxilla, the volumetric stability of the bone was poorer than without the sinus lift, but the difference was not statistically significant.(Table 3)
Table 1. Differences due to grafting direction and location.
| Grafting direction | No. of recipient sites | Residual rate (%) | Results in Scheffe's test | P-value |
|---|---|---|---|---|
| Direction | ||||
| Horizontal | 63 | 78.4±6.9 | a | |
| Vertical | 30 | 71.0±5.6 | b | |
| Horizontal+vertical | 35 | 71.0±10.0 | b | |
| Site | ||||
| Maxilla | 69 | 75.6±7.1 | 0.154 | |
| Mandible | 59 | 73.4±9.6 | ||
| Anterior | 27 | 79.5±6.7 | 0.001 | |
| Posterior | 101 | 73.3±7.1 |
The difference between 'a' and 'b' was statistically significant in Scheffe's test.
Statistical significance level, P<0.05
Table 2. Differences due to age and sex.
| Variable | No. of recipient sites | Residual rate (%) | Results in Scheffe's test | P-value |
|---|---|---|---|---|
| Age (yr) | ||||
| ≤19 | 2 | - | - | |
| 20-29 | 8 | 77.6±8.9 | a, b | |
| 30-39 | 15 | 81.0±6.6 | b | |
| 40-49 | 28 | 76.4±7.3 | a, b | |
| 50-59 | 50 | 71.5±8.8 | a | |
| 60-69 | 23 | 73.6±6.7 | a, b | |
| 70-79 | 2 | - | - | |
| Total | 128 | |||
| Sex | 0.148 | |||
| Male | 62 | 73.3±8.9 | ||
| Female | 66 | 75.8±7.8 | ||
| Total | 128 |
In Scheffe's test, the difference between 'a' and 'a, b', and 'b' and 'a, b', were not statistically significant; however, the difference between 'a' and 'b' was statistically significant.
Statistical significance level, P<0.05
Table 3. Differences due to whether or not the sinus lift procedure was conducted.
| Sinus lift | Site (n) | Residual rate (%) | P-value |
|---|---|---|---|
| With sinus lift | 22 | 72.3±6.1 | 0.21 |
| Without sinus lift | 25 | 74.7±6.6 |
Statistical significance level, P<0.05
Two hundred sixty-seven implants were placed on 111 onlay-grafted sites. An average of 3.18 implants were placed on recipient bone per grafted site. All prosthetic restorations were completed and functioned in occlusion. Approximately 24.4±14.3 months after implant-placing surgery, only 3 of the 267 implants had undergone mild marginal bone loss (under 2 mm, vertically); however, these implants did not have peri-implantitis or mobility. Although the follow-up period was short, all implant-based prosthetics were clinically successful until November 2014.
IV. Discussion
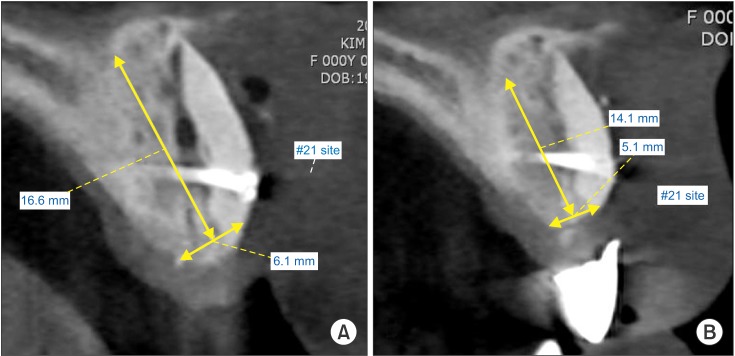
A previous volumetric study showed that a significant amount of resorption of bone grafts occurred during the first 6 months following surgery. Furthermore, these authors concluded that the initial loss of bone grafts subsides after 6 months and does not significantly continue after 12 months16. Therefore, in the current study, the authors placed implants 4.3±0.8 months after bone graft surgery. The present study demonstrated the volumetric stability of block bone grafts in 95 patients. The CBCT evaluation 4.2 months after block bone grafting showed very high volumetric stability.(Fig. 4)
Fig. 4. A. Cross-section of grafted bone 1 day after grafting surgery. B. Cross-section of grafted bone 4.2 months after grafting surgery. Hyeong-Geun Lee et al: Volumetric.
Several investigators who compared two-dimensional images from standard radiographs with data obtained from CT scans showed widely varying results; underestimations of 21% and overestimations of 18% were observed17. This result was attributed to a correlation between the bone height and the total volume of resorption and enlargement, as well as the distortion of standard radiographic imaging17. Therefore, the current authors calculated three-dimensional (3D) volumetric changes for 4.2 months.
Several clinical and radiological studies have used CT scans to gain sectional information; however, the methods used for obtaining the actual measurements are not Universal; they vary according to different software programs18. The precision of the maxillary sinus volume calculated by CT was reported to be more than 95%19. The accuracy of CTs in estimating the volume of grafted bone has proved to be almost 100%20. Although analysis of the volume of grafted bone by CT scans appears to be precise in the literature, the actual volumetric measurements of each grafted site can have some source of error, depending on the method used for calculation. It is impossible to detect and distinguish a particular patient's existing bone from the grafted areas18. Fortunately, we selected onlay-graft cases only, not inlay-grafts; therefore, the authors could distinguish grafted bone from recipient bone. However, the method for drawing the VOI rectangle was not always perfect. If the gap between the grafted bone and the recipient bone is narrow, it is difficult to draw lines for the VOI. Furthermore, even though the recipient bone is included in the VOI, unless it corresponds to the determined grayscale range, the program does not calculate it as a meaningful volume.
Therefore, we formed a hypothesis that the grayscale range the of grafted bone is almost the same as that of the adjacent bone not harvested. It may be accurate to apply this hypothesis to CT at T1, but the grayscale range of grafted bone may diminish over time. Further studies are required in order to understand the change in the pattern of the grayscale range for grafted cortical bone during the remodeling period.
HU were utilized in order to standardize the density in the CT, and air and water have been used to analyze lesions in other medical fields. CBCT, which was used in this study as an imaging system, has many advantages over CT, including lower levels of radiation exposure, shorter acquisition times, cost-effectiveness, and submillimeter resolution21. In CT scans, the HU is proportional to the degree of X-ray attenuation by the tissue. In CBCT, the degree of X-ray attenuation is shown by the grayscale (voxel value). Strong correlations between the grayscales of CBCT and HUs of CT scans have been shown22.
Several authors reported that membranous bone grafts maintain their volume to a greater extent compared with endochondral bone grafts23,24,25. One possible explanation for this result could be that bone grafts of membranous origin have higher cortical bone quality than those of endochondral origin. In addition, cortical bone grafts maintain their volume better than cancellous bone grafts, independent of embryogenic origin13,14. Cancellous bone grafts can revascularize much more quickly than cortical bone; however, cortical bone is much stronger26. Therefore, the authors utilized several operative techniques to maintain revascularizing ability and the microarchitecture of cancellous bone. Capillary ingrowth, perforation of existing cortical bone, and tamping particulate cortical bone around cortical bone are important factors to consider in this assessment. As shown in Fig. 4, these techniques could make some of the bone between the grafted cortical bone and existing bone similar to the materials surrounded by the sinus membrane and sinus floor, and thus, allowing for the formation of new bone.
von Arx and Buser27 performed horizontal ridge augmentation using autogenous block grafts covered with anorganic bovine bone mineral (ABBM) and a bioabsorbable collagen membrane in the atrophic jaw. Their study demonstrated successful horizontal ridge augmentation with high predictability. A mean initial crest width of 3.06 mm was measured. At re-entry, the width was 7.66 mm, with a calculated mean gain of horizontal bone thickness of 4.6 mm. After 5.8 months, only minor surface resorption of 0.36 mm was observed from augmentation to re-entry. We used a similar technique, but we selected a particulate block bone in place of ABBM. Thus, better biocompatibility was achieved at no additional cost. The block bone was used for the main volume to augment the alveolar ridge. Particulate bone was used as an auxiliary to fill the boundary of block bone.
Significant amounts of resorption of the bone graft take place during the first 6 months after the grafting surgery. Furthermore, initial loss of bone grafts subsides after 6 months and does not significantly continue after 12 months16. Thus, 4.2 months (average period from T1 to T2 in this study) is not too short to evaluate volumetric changes. Moreover, implants protect the graft from continuous resorption vertically in areas without implants, where resorption has been prolonged such that implant fixture threads are no longer engaged in bone28. Thus, it is not a good idea to wait for full remineralization and revascularization of the grafted bone. In this study, no grafted block bone had fallen out during the 4.2 ±0.8 months after the surgery when the implant fixture was inserted.
Following implant-placing surgery, volumetric calculation by the method used in this study is almost impossible, or very inaccurate, because the titanium fixture causes many artifacts on the CBCT scan image, and the borders that distinguish grafted bone from residual bone gradually become more ambiguous. Therefore, implant-based prosthetics were evaluated clinically and on conventional radiographic films. Only 3 implants out of the 267 total had undergone mild marginal bone loss after 24.4±14.3 months. The success rate was 98.8%.
In this study, age, sex, grafting direction and location, and whether or not the sinus lift procedure was used, were factors selected that can affect volumetric change. However, only grafting direction and location were significant factors. Vertical onlay-grafts were more susceptible to strong tension because of direction and were more likely to be uncovered by the gingiva, owing to inadequate flap length compared with the horizontal only-grafts. The reason that the anterior part of the jaw maintained more grafted bone than the posterior part is that the former had more horizontal onlay-grafts than the latter. These results are universally accepted; however, more data and further studies are needed to determine volumetric stability, when viewed as a varying factor.
V. Conclusion
The high residual rate (74.6%) of the grafted bone and the perfect survival rate of dental implants on grafted bone makes our bone grafting technique (using mandibular block bone and supplementary particulate bone) a reliable method. Long-term 3D volumetric studies after implant-placement surgery require exclusive software that are optimized for the calculation of the 3D volume, due to artifacts and distortion from metal and difficulties distinguishing grafted bone from existing bone.
Footnotes
This work was supported by a 2-Year Research Grant of Pusan National University.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347–359. [PubMed] [Google Scholar]
- 2.Jemt T, Lekholm U, Adell R. Osseointegrated implants in the treatment of partially edentulous patients: a preliminary study on 876 consecutively placed fixtures. Int J Oral Maxillofac Implants. 1989;4:211–217. [PubMed] [Google Scholar]
- 3.McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78:377–396. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]
- 4.Barone A, Covani U. Maxillary alveolar ridge reconstruction with nonvascularized autogenous block bone: clinical results. J Oral Maxillofac Surg. 2007;65:2039–2046. doi: 10.1016/j.joms.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Sjöström M, Lundgren S, Sennerby L. A histomorphometric comparison of the bone graft-titanium interface between interpositional and onlay/inlay bone grafting techniques. Int J Oral Maxillofac Implants. 2006;21:52–62. [PubMed] [Google Scholar]
- 6.Reinert S, König S, Bremerich A, Eufinger H, Krimmel M. Stability of bone grafting and placement of implants in the severely atrophic maxilla. Br J Oral Maxillofac Surg. 2003;41:249–255. doi: 10.1016/s0266-4356(03)00078-0. [DOI] [PubMed] [Google Scholar]
- 7.Zouhary KJ. Bone graft harvesting from distant sites: concepts and techniques. Oral Maxillofac Surg Clin North Am. 2010;22:301–316. doi: 10.1016/j.coms.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Brugnami F, Caiazzo A, Leone C. Local intraoral autologous bone harvesting for dental implant treatment: alternative sources and criteria of choice. Keio J Med. 2009;58:24–28. doi: 10.2302/kjm.58.24. [DOI] [PubMed] [Google Scholar]
- 9.Misch CM. Maxillary autogenous bone grafting. Oral Maxillofac Surg Clin North Am. 2011;23:229–238. doi: 10.1016/j.coms.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg JA, Wiltz MJ, Kraut RA. Augmentation of the anterior maxilla with intraoral onlay grafts for implant placement. Implant Dent. 2012;21:21–24. doi: 10.1097/ID.0b013e3182435ffd. [DOI] [PubMed] [Google Scholar]
- 11.Zins JE, Whitaker LA. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72:778–785. doi: 10.1097/00006534-198312000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Borstlap WA, Heidbuchel KL, Freihofer HP, Kuijpers-Jagtman AM. Early secondary bone grafting of alveolar cleft defects. A comparison between chin and rib grafts. J Craniomaxillofac Surg. 1990;18:201–205. doi: 10.1016/s1010-5182(05)80411-1. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki W, Buchman SR. Volume maintenance of onlay bone grafts in the craniofacial skeleton: micro-architecture versus embryologic origin. Plast Reconstr Surg. 1998;102:291–299. doi: 10.1097/00006534-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal AH, Buchman SR. Volume maintenance of inlay bone grafts in the craniofacial skeleton. Plast Reconstr Surg. 2003;112:802–811. doi: 10.1097/01.PRS.0000069713.62687.F5. [DOI] [PubMed] [Google Scholar]
- 15.Keller EE, Tolman DE, Eckert S. Surgical-prosthodontic reconstruction of advanced maxillary bone compromise with autogenous onlay block bone grafts and osseointegrated endosseous implants: a 12-year study of 32 consecutive patients. Int J Oral Maxillofac Implants. 1999;14:197–209. [PubMed] [Google Scholar]
- 16.Nyström E, Legrell PE, Forssell A, Kahnberg KE. Combined use of bone grafts and implants in the severely resorbed maxilla. Postoperative evaluation by computed tomography. Int J Oral Maxillofac Surg. 1995;24:20–25. doi: 10.1016/s0901-5027(05)80851-3. [DOI] [PubMed] [Google Scholar]
- 17.Waitzman AA, Posnick JC, Armstrong DC, Pron GE. Craniofacial skeletal measurements based on computed tomography: part I. Accuracy and reproducibility. Cleft Palate Craniofac J. 1992;29:112–117. doi: 10.1597/1545-1569_1992_029_0112_csmboc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 18.Dasmah A, Thor A, Ekestubbe A, Sennerby L, Rasmusson L. Particulate vs. block bone grafts: three-dimensional changes in graft volume after reconstruction of the atrophic maxilla, a 2-year radiographic follow-up. J Craniomaxillofac Surg. 2012;40:654–659. doi: 10.1016/j.jcms.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Johansson B, Grepe A, Wannfors K, Hirsch JM. A clinical study of changes in the volume of bone grafts in the atrophic maxilla. Dentomaxillofac Radiol. 2001;30:157–161. doi: 10.1038/sj/dmfr/4600601. [DOI] [PubMed] [Google Scholar]
- 20.Honma K, Kobayashi T, Nakajima T, Hayasi T. Computed tomographic evaluation of bone formation after secondary bone grafting of alveolar clefts. J Oral Maxillofac Surg. 1999;57:1209–1213. doi: 10.1016/s0278-2391(99)90488-3. [DOI] [PubMed] [Google Scholar]
- 21.White SC, Pharoah MJ. Oral radiology: principles and interpretation. London: Elsevier Health Sciences; 2008. [Google Scholar]
- 22.Razi T, Niknami M, Alavi Ghazani F. Relationship between Hounsfield unit in CT scan and gray scale in CBCT. J Dent Res Dent Clin Dent Prospects. 2014;8:107–110. doi: 10.5681/joddd.2014.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iizuka T, Smolka W, Hallermann W, Mericske-Stern R. Extensive augmentation of the alveolar ridge using autogenous calvarial split bone grafts for dental rehabilitation. Clin Oral Implants Res. 2004;15:607–615. doi: 10.1111/j.1600-0501.2004.01043.x. [DOI] [PubMed] [Google Scholar]
- 24.Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants. 1997;12:767–776. [PubMed] [Google Scholar]
- 25.Smolka W, Eggensperger N, Carollo V, Ozdoba C, Iizuka T. Changes in the volume and density of calvarial split bone grafts after alveolar ridge augmentation. Clin Oral Implants Res. 2006;17:149–155. doi: 10.1111/j.1600-0501.2005.01182.x. [DOI] [PubMed] [Google Scholar]
- 26.Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;(174):28–42. [PubMed] [Google Scholar]
- 27.von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006;17:359–366. doi: 10.1111/j.1600-0501.2005.01234.x. [DOI] [PubMed] [Google Scholar]
- 28.Dasmah A, Thor A, Ekestubbe A, Sennerby L, Rasmusson L. Marginal bone-level alterations at implants installed in block versus particulate onlay bone grafts mixed with platelet-rich plasma in atrophic maxilla. a prospective 5-year follow-up study of 15 patients. Clin Implant Dent Relat Res. 2013;15:7–14. doi: 10.1111/j.1708-8208.2011.00377.x. [DOI] [PubMed] [Google Scholar]