Abstract
Hepatitis E virus (HEV) infection is now established as an emerging enteric viral hepatitis. Standard treatments in acute and chronic hepatitis E remain to be established. This study undertakes a review of the epidemiology, treatment implication and vaccine prevention from published literature. HEV infection is a worldwide public health problem and can cause acute and chronic hepatitis E. HEV genotypes 1 and 2 are primarily found in developing countries due to waterborne transmission, while the zoonotic potential of genotypes 3 and 4 affects mostly industrialized countries. An awareness of HEV transmission through blood donation, especially in the immunocompromised and solid organ transplant patients, merits an effective anti-viral therapy. There are currently no clear indications for the treatment of acute hepatitis E. Despite concerns for side effects, ribavirin monotherapy or in combination with pegylated interferon alpha for at least 3 mo appeared to show significant efficacy in the treatment of chronic hepatitis E. However, there are no available treatment options for specific patient population groups, such as women who are pregnant. Vaccination and screening of HEV in blood donors are currently a global priority in managing infection. New strategies for the treatment and control of hepatitis E are required for both acute and chronic infections, such as prophylactic use of medications, controlling large outbreaks, and finding acceptable antiviral therapy for pregnant women and other patient groups for whom the current options of treatment are not viable.
Keywords: Treatments, Blood donors, Adverse effects, Vaccination, Pegylated-interferon, Ribavirin, Hepatitis E
Core tip: Hepatitis E virus (HEV) infection affects individuals in both industrialized and developing countries and can cause acute and chronic hepatitis E. HEV genotypes 1 and 2 are primarily found in developing countries due to waterborne transmission, while the zoonotic potential of genotypes 3 and 4 affects mostly industrialized countries. An awareness of HEV transmission through blood donation, especially in the immunocompromised and solid organ transplant patients, merits an effective anti-viral therapy. The current treatment for HEV infection involving ribavirin and pegylated interferon-α therapy has shown limited efficacy. Although not widely used, an HEV vaccine is available for immunization in China.
INTRODUCTION
Hepatitis E infection is now regarded as a major cause of fecal-orally transmitted non-A, non-B hepatitis. Hepatitis E virus (HEV) is a non-enveloped, single-stranded RNA virus that contains three open reading frames (ORFs) that encode structural and non-structural proteins[1]. There are 4 major HEV genotypes (1, 2, 3 and 4). Symptomatic HEV infections produce varying clinical presentations depending on the HEV genotypes. Fulminant hepatic failures are caused mainly by genotype 1, while chronic infections have been observed only with genotype 3 thus far[2]. Recent reports of sporadic cases from developed countries in Europe and in the United States resulted from HEV genotype 3, which is zoonotic[3]. In a 2015 survey on the seroprevalence and risk factors for HEV infection among slaughterhouse workers in South Korea, the seropositive rate for HEV IgG was 33.5%[4]. Another report from Thailand in 2014 found that HEV strains identified from acute symptomatic hepatitis E infections were all genotype 3f, which was genetically closely related to a strain isolated in swine[5].
The clinical course of HEV infection among pregnant women is known to be more severe and often led to fulminant hepatic failure and death in up to 20%-25%, specifically in those living in developing countries[6]. The mortality rate has been found to be higher during the 2nd and the 3rd trimester[7]. However, underlying factors influencing high mortality during pregnancy are poorly understood.
Studies in chimpanzees to examine HEV and hepatitis C virus (HCV) infection revealed that HEV showed a lower frequency and a shorter duration of differentially expressed genes compared to HCV in intrahepatic transcriptome analysis. This suggests that HEV may be more susceptible to the innate immunity induced by interferon alpha[8]. However, HEV showed suppression of interferon alpha signaling in vitro[9].
MOLECULAR VIROLOGY OF HEV
HEV belongs to the family Hepeviridae[10]. It is a small non-enveloped virus 27-34 nm in diameter. The single-stranded positive-sense RNA genome of HEV is approximately 7.2 kb, 7-methylguanine capped at the 5’ terminus, and polyadenylated at the 3’ terminus[11]. Both ends of the genome consist of short 5’ and 3’ untranslated regions, which fold into stem-loop structures and are approximately 58 and 68 nucleotides, respectively[12]. The viral genome contains 3 open reading frames (ORF)[10,13]. ORF1 encodes a 1693 amino acid non-structural polyprotein including: Methyltransferase (MeT), which catalyses the capping of viral RNA; a papain like cysteine protease (PCP) with presumed post-translational protein processing; a helicase (Hel), which supports MeT by catalyzing the first step of RNA capping; an RNA-dependent RNA polymerase (RdRp) required for the synthesis of the genomic RNA; and several uncharacterized domains such as X or Macro, Y and V domain[1,12]. ORF2 located near the 3’ end encodes 599 to 660 amino acids capsid protein[1,10]. It is involved in virion assembly, cell attachment, and immunogenicity. ORF3, which overlaps ORF2, encodes a protein of 114 amino acids shown to be associated with subcellular localization and virion morphogenesis[11,14] (Figure 1).
Figure 1.
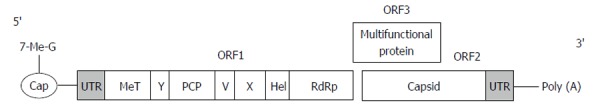
Schematic representation of hepatitis E virus genome[1,12,14]. ORF: Open reading frame; UTR: Untranslated region; MeT: Methyltransferase; PCP: A papain like cysteine protease; Hel: Helicase.
Replication steps in the HEV life cycle are not easily elucidated due to lack of a suitable cell culture system. However, proposed replication cycle commences with the viral attachment to the surface of target cells (hepatocyte) and binding to the unknown receptor(s). Next, the virus penetrates, uncoats, and releases the genomic RNA. Translation of the nonstructural proteins occurs in the cytoplasm. RdRp synthesizes the negative-sense intermediate RNA from the positive-sense genome, which subsequently acts as a template for the synthesis of subgenomic RNA and the full-length positive-sense transcripts. The subgenomic RNA is then translated into ORF2 and ORF3 proteins, which facilitates viral assembly and optimizes the host cell environment for viral replication[11,12]. The mechanism of viral egress from the host cells remains unclear[15,16].
IMMUNE RESPONSES IN HEV INFECTION
Investigation of host immune responses against virus infection are essential to understand host immunity and for vaccine development. Several studies have examined the host immune responses following HEV infection and the roles of immune components responsible for causing liver damage as a consequence of HEV infection. Although the mechanisms of hepatic injury enhanced by the host immune responses to HEV infection are still unclear[17-19], we review here the available literatures on HEV infection and how it affects host immunity and induction of liver injury.
Once HEV enters the body, viral clearance involves recruitment of immune cells against infection. These cells recognize HEV in the early stages through pattern recognition receptors. HEV components are then detected by toll-like receptors and retinoic acid-inducible gene-I like receptors. Subsequent recruitment of adapter proteins MyD88 and TRIF mediate interferon regulatory transcription factor 3 (IRF3) and nuclear factor κB (NF-κB) to produce type I interferons and pro-inflammatory cytokines vital to antiviral response[20].
Type I interferons are cytokines, which play important roles in innate immunity against virus infection. Interferon-inducible genes are up-regulated in HEV infected PLC/PRF/5 cells. Replication of HEV in this cell type was inhibited when cells were treated with interferon (IFN)-α2b[21]. A549 cells infected with HEV genotype 3 showed inhibition of IFN-α induced the signal transducer and activator of transcription 1 (STAT1) phosphorylation. Additionally, HEV ORF3 protein enhances IFN-β production induced by poly (I·C), a double-stranded RNA analogue[22]. HEV ORF3 was also shown to bind STAT1 resulting in inhibition of STAT1 phosphorylation[9]. A study in hepatocyte cells demonstrated that HEV infection inhibited IFN-β production induced by poly (I·C). Meanwhile, HEV ORF1 was identified as an IFN antagonist. It inhibited phosphorylation and ubiquitination of various proteins involving in interferon production such as IRF3, retinoic acid-inducible gene 1 and TANK-binding kinase 1[23]. ORF1 also activates the promoter activity of a chemokine, chemokine (C-X-C motif) ligand 8[24]. These reports collectively demonstrated that HEV proteins are involved in the activation of host antiviral cytokines, although some viral components could also inhibit interferon signalling, which facilitate HEV evasion from the host innate immune defenses.
Natural killer (NK) cells and NKT cells are responsible for killing virus-infected cells. Comparison of the numbers and function of NK/NKT cells from HEV infected patients and healthy controls showed that the percentage of activated NK cells was higher in patients than in controls[18]. However, there was no difference in the target cell cytotoxicity of NK cells from both groups. A study of lymphocytes taken from liver biopsy demonstrated that CD56 cell counts were higher in HEV patients than in patients with hepatitis A, B and C virus infections[25]. This group also showed increased CD8+ cells in liver failure cases caused by HEV and other viruses.
IFN-γ is a cytokine responsible for activation of NK and T cell function. IFN-γ expression was up-regulated in peripheral blood mononuclear cells (PBMCs) from HEV infected patients following HEV ORF2 stimulation[26]. In addition, PBMCs from acute viral hepatitis cases elicited higher responses to recombinant HEV ORF2 peptides than PBMCs from the liver failure group[27]. Studies in hepatocytes demonstrated that HEV ORF2 inhibited NF-κB activation[28].
Besides the innate immune responses, the alteration of cell-mediated immune responses by HEV infection had also been reported. HEV infected patients had increased CD8+ and CD4+CD8+ cells compared with healthy individuals. In addition, proportion of IFN-γ secreting cells in response to recombinant ORF2 and ORF3 were higher in patients than in controls. These data provided evidence of effector T cell responses induced by HEV components[29].
The studies of clinical manifestations in patients with self-limited acute viral hepatitis and acute liver failure support the roles of immune responses in liver injury. HEV infected patients with acute liver failure had higher antibody titers and higher levels of cytokines such as tumor necrosis factor-α, IFN-γ, interleukin-2 (IL-2) and IL-10. HEV RNA was detected in patients with self-limited acute hepatitis group but not in liver failure cases. This observation suggests that the immune responses rather than the virus are responsible for liver damage[30].
For humoral immune response, the incubation period is around 15-60 d after infection[31]. IgM antibodies increase rapidly and then begin to subside after 3 mo[32,33]. Anti-HEV IgG antibodies start to increase when anti-HEV IgM is first detected and may persist for years[17,34,35]. Presently, the viral detection by serology tests has been developed for HEV. Examination of blood and stool of 10 patients with acute HEV infection can identify HEV RNA, but only for a short period[36]. Experimental infection in chimpanzee demonstrated that HEV RNA was detectable in blood around 22 d after inoculation. In human volunteer, HEV RNA could be detected in blood sooner than in feces[37]. Naturally acquired humoral immune response in the body not only increased rapidly after infection, but also afforded protect from infection during an outbreak[38].
Regulatory T cells (Treg) and IL-10, components of the immune system which modulate immune response, were examined in acute hepatitis E patients, recovered individuals and healthy controls. Both percentage of Treg cells and IL-10 levels were higher in acute hepatitis patients than in recovered and healthy individual groups[39,40], implicating a role for Treg in the immune response induction as a result of HEV infection.
The regulation of protection in the body involves a complex network of innate and adaptive immune response. Immune responses are shown to promote tissue injury in various infectious diseases. During viral infection, both innate and adaptive immune responses are elicited in order to eliminate pathogens. Even so, several viruses are able to evade eradication by the host immunity. Prolonged or hyper-immune responses to eradicate persistent infection, however, could result in tissue injury. Knowledge gained from HEV studies thus far have mostly relied on recombinant HEV proteins instead of viral infections due to the lack of an efficient cell culture system. Moreover, most HEV infections are self-limited. Future studies including patients presented with various degrees of clinical manifestations should allow better understanding of the immunity or immunopathology induced by HEV infection.
EPIDEMIOLOGY OF HEV
HEV is a leading cause of hepatitis transmitted via fecal-oral route[41]. The first outbreak of HEV occurred in India around 1955-56, which affected as many as 29000 persons[42]. Other large outbreaks were reported in China, India, Somalia and Uganda[1]. Outbreaks were observed to occur after heavy rainfall and floods, which allowed contamination of human excreta with drinking water sources. Alternatively, outbreaks sometimes follow the dry summer season when water in the rivers or streams is reduced, resulting in increased concentration of fecal contamination in water[43].
It is known that humans could be infected with all 4 HEV genotypes[44]. In developing countries where HEV is endemic (Indian subcontinent, Asia, Middle East and Africa)[11], HEV genotypes 1 and 2 are common and restricted to human. In developed countries, these two genotypes were diagnosed only in persons who had recently traveled to highly endemic areas[45]. It is estimated that 21.1 million people, or 71% of the world population, are infected with HEV genotypes 1 and 2. Furthermore, HEV infection results in approximately 3 million symptomatic acute cases each year and 70000 deaths worldwide[1,44,46]. Individuals could be infected with HEV genotypes 1 and 2 from drinking contaminated water[47], and unfortunately people affected in large HEV outbreaks were found using water from a common source for cooking, drinking, and bathing. HEV RNA has also been detected in the sewage-contaminated water source. The limitation of inadequate public infrastructure may facilitate the spread of HEV infection, such as in refugee or military camps[43]. Areas affected by natural disasters such as earthquakes and monsoon storms are also at risk of HEV epidemic. Displaced populations with limited access to clean drinking water, lack of sanitary facilities, overburdened health-care infrastructure, and immunologically naive population lacking protective antibodies combined will increase the likelihood of HEV transmission[48]. Large outbreaks of HEV have occurred in Nepal, which in 2014 involved more than 10000 cases[49].
HEV infection does not only affect developing countries. HEV genotypes 3 and 4 are autochthonous in several industrialized countries. Occasional foodborne outbreaks have occurred in Europe, North America, Japan and New Zealand from consuming undercooked meat contaminated with HEV[50-53]. HEV genotypes 3 and 4 are found in human and other animal species such as pig, wild boar, and shellfish[54-56]. Specifically, HEV genotype 3 is zoonotic in developed countries from reports in pig farmers, individuals who came into close contact with animal reservoir and who consumed raw meat or meat products from deer, wild boar, and pig. HEV RNA could also be detected in food products such as liver and sausage. Viral sequence extracted from uncooked meat or sausage were very similar (99.7%-100% identity) to sequences of virus recovered from HEV-infected patients. In addition, HEV genotype 4 infection could be detected in both human and swine in Eastern Asia and Europe[57,58]. These evidence supported foodborne zoonotic HEV transmission, which occurred from consuming infected meat[59-63].
Several studies suggest that HEV genotypes 3 and 4 could be transmitted across multiple species. In animal models, pathogen-free pigs could be experimentally infected with human HEV genotypes 3 and 4. Furthermore, swine HEV genotypes 3 and 4 inoculated in rhesus monkey and chimpanzee resulted in seroconversion and virus shedding in feces. Anti-HEV IgG seroconversion in the pigs was observed within 2 wk. These experiments suggest that human HEV genotypes 3 and 4 could replicate in pigs and likely originated from swine[64]. In contrast, pigs inoculated with HEV genotypes 1 and 2 were not infected. This suggests that HEV genotypes 1 and 2 were host-restricted compared to genotypes 3 and 4[65]. Therefore, zoonotic transmission to humans is possible for HEV genotypes 3 and 4[65-67]. Other routes of transmission including HEV-infected blood transfusion, mother-to-child, person-to-person, and sexual intercourse were also documented but relatively uncommon[68-70].
Seroprevalence of anti-HEV IgG varies in different parts of the world. In developing countries, positive anti-HEV IgG serology might reach as high as 70%[71]. Being male, having pet animals or frequent contact with animal reservoirs, residing in the endemic area, or consuming liver or other organ meats were highly associated with positive HEV serology. Veterinary and slaughterhouse workers also have high seropositive rates for anti-HEV IgG compared to individuals with no occupational exposure to swine[72,73]. In swine-dense areas, individuals were more likely to be HEV-seropositive compared to those living in areas with few pig farms[74,75]. The seroprevalence of anti-HEV IgG antibodies is shown in Table 1.
Table 1.
Global prevalence of anti-hepatitis E virus IgG in different populations
| Regions | Prevalence (%) | Ref. |
| Low-to-medium income | ||
| Kashmir region | 49.6 | Khuroo et al[35] |
| India | 23.8-28.7 | Mathur et al[76] |
| Myanmar | 32.0 | Nakai et al[77] |
| Egypt | 67.7 | Stoszek et al[71] |
| Bangladesh | 22.5 | Labrique et al[78] |
| China | 19.7 | Dong et al[79] |
| Mexico | 36.6 | Alvarado-Esquivel et al[80] |
| Thailand | 14.0 | Gonwong et al[75] |
| Nigeria | 42.7 | Junaid et al[81] |
| Industrialized | ||
| Germany | 17.0 | Wenzel et al[82] |
| United States | 6.0 | Teshale et al[83] |
CLINICAL MANIFESTATIONS AND DIAGNOSTIC CRITERIA
HEV infection produces wide-ranging clinical manifestations. Most cases of HEV-related acute viral hepatitis resolve within 1 to 2 mo. However, HEV sometimes leads to acute liver failure, chronic infection, or extrahepatic symptoms[17]. An acute hepatitis from HEV is indistinguishable from other forms of acute viral hepatitis and is usually asymptomatic or self-limiting in most individuals[34]. However, acute hepatitis E infection can be severe and prolonged among the immunocompromised and even healthy individuals[84]. Severe acute hepatitis E infections have been described in pregnant women, elderly men, and persons with pre-existing chronic liver disease[85-87]. Among people with the chronic liver disease, acute hepatitis E can adversely lead to acute-on-chronic liver failure[88]. Acute HEV infection with pronounced symptoms resulted in higher mortality rates of 1%-4% and up to 11%, compared to the mortality rates of acute HAV (1%) or HBV (1.5%) infection based on CDC viral hepatitis surveillance data (2010)[84]. Extrahepatic manifestations including pancreatitis, arthritis, aplastic anemia, and neurologic complications have all been reported[11,89,90].
Hepatitis E, especially genotype 3, is known to cause chronic infection among the immunocompromised, especially solid organ recipients. Recently, however, there are reports of chronic hepatitis E infection in elderly immunocompetent patients[91,92]. Diagnosis of acute hepatitis E infection is established in patients with clinically relevant symptoms of acute hepatitis with positive level of anti-HEV IgM or a level of anti-HEV IgG that is twice the baseline concomitant with detectable HEV RNA in the serum and/or stool[93]. Chronic hepatitis E is defined by the persistent increase in liver enzyme levels and polymerase chain reaction-detectable HEV in the serum and/or stool over 6 mo[94].
TREATMENT OF ACUTE HEPATITIS E
Currently, there is no indicated treatment of acute hepatitis E. Nevertheless, treatment with ribavirin showed significant clinical improvements by reducing the symptomatic period (Table 2). Considering the high mortality caused by acute hepatitis E in acute-on-chronic liver failure, ribavirin therapy provides significant benefits for those with poor prognosis or at high risk of fulminant liver failure such as underlying chronic liver disease. Moreover, a high percentage of immunosuppressed individuals [such as human immunodeficiency virus (HIV)-positive individuals or recipients of organ transplant] who eventually develop chronic hepatitis E necessitate early and effective treatment of acute hepatitis E[84,88,95-98].
Table 2.
Recent evidences on the outcome of therapies against acute hepatitis E infection
| Ref. | Type of study | Patient profile | HEV genotype | Ribavirin regimen | Results |
| Gerolami et al[84] | Case report | 61-year-old man, 7 d after admission ALT 4565 IU/L | 3 | 1200 mg/d for 21 d | At day 21 of treatment, ALT normalized, RNA almost undetectable |
| Péron et al[88] | Case report | 79-year-old man with chronic liver disease, acute kidney failure | 3f | 200 mg/d for 3 mo | Serum HEV RNA negative at 1 mo therapy, stopped dialysis at 2 mo |
| A patient with chronic liver disease | 3f | 1000 mg/d for 10 d | Viral load 4.07 log copies/mL declined to 2.54 log copies/mL at day 6, Hgb 12.6 g/dL declined to 11.6 g/dL at day 6 of treatment | ||
| Del Bello et al[95] | Case report | 65-year-old man, liver transplant recipient Guillain-Barré syndrome with severe necrotizing myositis | 3f | 400 mg/d adapted to GFR (40 mL/min) for 3 mo | HEV RNA undetectable by day 15, progressive recovery of mobility |
| Pischke et al[96] | Case from prospective case series | 42-year-old woman had traveled to Eritrea and acquired severe acute hepatitis E | 1e | For 6 wk (dose: Undefined) | Rapidly improved liver function and cleared HEV |
| Robbins et al[97] | Case report | 39-year-old man HIV (+) CD4 51/mm3 prothrombin index 45% | 3c | 1200 mg/d (15 mg/kg per day) for 12 wk | Gradual normalization of LFT-HEV RNA decreased to < 100 copies/mL at 1 mo of treatment |
| Riveiro-Barciela et al[98] | Case report | 68-year-old man with Waldenström's macroglobulinemia | 3f | 800 mg/d for 12 wk | Achieved SVR after 12 wk; no ribavirin-related side effect reported |
LFT: Liver function test; ALT: Alanine transaminase; Hgb: Haemoglobin; GFR: Glomerular filtration rate; SVR: Sustained virological response; HIV: Human immunodeficiency virus; HEV: Hepatitis E virus.
TREATMENT OF CHRONIC HEPATITIS E
In a case-control study of solid-organ transplant recipients performed in France, 22 out of 38 individuals who tested positive for HEV genotype 3 (58%) developed chronic infection. Compared to the control group of 148 individuals who had no markers for HEV infection, one independent factor associated with HEV infection was the consumption of game meat (68% vs 47%, OR = 2.32)[3]. Moreover, a retrospective analysis of data from Europe and the United States found that among 85 recipients of solid organ transplants who had HEV infection, 56 patients (65.9%) developed chronic hepatitis E[94]. The main factor associated with developing chronicity assessed by multivariate analysis in this study was tacrolimus use for immune-suppression (OR = 1.87; 95%CI: 1.49-1.97). The reduction of immunosuppressive drugs, however, enabled HEV clearance in one-third of the individuals.
When reduction of immunosuppression is impossible or when clearing HEV by immunosuppression could not be achieved, 2 alternative therapies for chronic hepatitis E may be pursued: (1) Ribavirin monotherapy (dose 29-1200 mg/d, for 1-18 mo)[96,99,100]; and (2) Pegylated (Peg)-IFN-α for 3-12 mo[101,102].
It was reported that among 59 patients with chronic HEV after solid organ transplantation, HEV clearance was observed in 95% of the patients at the end of ribavirin therapy and that 78% of the patients achieved sustained virologic response (defined by undetectable serum HEV RNA at least 6 mo after cessation of therapy). These patients received ribavirin for a median of 3 mo. Longer treatment duration allowed 4 patients who had recurrence to achieve sustained virologic response[100]. The mechanism that ribavirin acts against HEV is not clearly understood[103]. However, a recent study suggests that ribavirin exerts an antiviral effect against HEV by depleting intracellular guanosine 5'-triphosphate pools[104]. Further research on this mechanism as well as other possible mechanisms of action of ribavirin is to be revealed. Meanwhile, overall data showed that ribavirin provided therapeutic effect in the treatment of chronic hepatitis E with the only significant adverse effect being anemia (Table 3).
Table 3.
Treatment of chronic hepatitis E virus with ribavirin regimen
| Ref. | Type of study | Patient profile | Ribavirin regimen | Result | Adverse effects |
| Kamar et al[99] | Prospective case series | 6 kidney transplant recipients, HEV RNA (+) for median of 36.5 mo | 600-800 mg/d for 3 mo adapted to GFR, Hgb | SVR in 4/6 patients; relapse in 2/6; AST, ALT normalized all | Anemia led to blood transfusion and RBV dose reduction in 2/6 patients |
| Mallet et al[105] | Case report | A kidney and pancreas transplanted man, a women with idiopathic CD4+ T lymphocytopenia | 12 mg/kg daily for 12 wk | Both cleared HEV after 4 wk of treatment and remained undetectable, LFT normalized | Anemia in 1st patient led to Ribavirin dose reduction to 200 mg/d |
| Pischke et al[96] | Prospective case series | Organ transplant recipients 11 subjects | 600-1000 mg/d for 5 mo, dose reduction according to Hgb or anemia | 9/11 showed SVR | Anemia, the mean Hgb decline was 3.4 g/dL (range 0-7.9 g/dL) |
| Neukam et al[106] | Case report | 2 HIV (+) male with liver cirrhosis with severe immunosuppression | Oral ribavirin 1200 mg/d (case 1) 1000 mg/d (case 2) for 24 wk | LFT normalized-Liver stiffness improved HEV RNA was detected after the end of treatment in both patients | - |
| Giordani et al[107] | Case report | 60-year-old man with lymphocytic leukemia | 1000 mg/d in 2 doses (400 and 600 mg), for 3 mo | HEV cleared and sustained over 6 mo after therapy | Mild anemia (Hgb 10.5 mg/dL) |
| Kamar et al[100] | Retrospective, multicentre case series | 37 kidney, 10 liver, 5 heart, 5 kidneys and pancreas, and 2 lung transplant recipients with chronic HEV | Median dose of 600 mg/d (range 29-1200), for a median of 3 mo (range 1-18 mo) | At the end of the therapy, 95% cleared HEV, 18% recurred after cessation of therapy is stopped, 78% showed SVR | Anemia required dose reduction (29%); use of erythropoietin (54%); required blood transfusion (12%) |
LFT: Liver function test; AST: Aspartate aminotransferase; ALT: Alanine transaminase; SVR: Sustained virological response; Hgb: Haemoglobin; GFR: Glomerular filtration rate; HEV: Hepatitis E virus; HIV: Human immunodeficiency virus.
Treatment with Peg-IFN-α has been reported (Table 4). The duration of therapy ranged from 3 mo to 1 year. Most of the cases are from solid organ transplant recipients, all of them showed the favourable outcome in liver enzyme levels as well as viral RNA suppression. However, 2 out of 6 transplanted patients developed acute allograft rejection after 3-mo Peg-IFN therapy[101,109].
Table 4.
Treatment of chronic hepatitis E virus with pegylated interferon-α therapy
| Ref. | Patient profile | Peg-IFN-α regimen | Result | Adverse effects |
| Kamar et al[101] | 29-year-old man with liver transplantation | Peg-IFN-α-2a for 12 wk (135 μg/wk) | Liver enzyme levels decreased. HEV RNA levels remained undetectable until week 12 | At week 12, signs of acute humoral rejection in liver biopsy |
| 26-year-old man with liver transplantation | Peg-IFN-α-2a for 12 wk (135 μg/wk) | HEV RNA levels undetectable by week 12; liver enzyme levels normalized by week 12 | ||
| 58-year-old man with liver transplantation liver cirrhosis from chronic HEV infection | Peg-IFN-α-2a for 12 wk (135 μg/wk) | HEV RNA was redetected 2 wk after completion of treatment; Liver enzyme levels normalized by 3 mo of therapy | ||
| Haagsma et al[102] | 37-year-old woman with liver transplantation | Peg-IFN-α-2b for 52 wk (80 μg/wk declined to 60 μg/wk) | Serum HEV RNA sustained undetectable during 3 mo follow-up; serum liver enzyme became normalized | |
| 59-year-old man with liver transplantation | Peg-IFN-α-2b 150 μg/wk, dose reduction due to leukopenia | HEV viral load and aminotransferases declined, but Peg-IFN discontinued from lack of further efficacy, HEV RNA level undetectable at 4 wk after the discontinuation of Peg-IFN and aminotransferase normalized | Leukopenia | |
| Alric et al[108] | 57-year-old man with hairy cell leukemia | Discontinued at week 16, Peg-IFN-α-2b 1 μg/kg per week for 3 mo | Achieved a complete virologic response by week 4 | |
| Kamar et al[109] | 24-year-old man with kidney transplantation, kidney failure from chronic HEV infection | 3-mo Peg-IFN-α-2a 135 μg/wk | Serum RNA undetectable after 5 mo, SVR for 6 mo after treatment | Acute rejection of the kidney allograft by month 3 of Peg-IFN therapy |
Peg-IFN: Pegylated interferon; SVR: Sustained virological response; HEV: Hepatitis E virus.
The slight synergistic effect for ribavirin combined with Peg-IFN-α was observed in a recent study in vitro[104]. Successful combination therapy had also been reported for a chronic HEV infection in an HIV-positive patient[110]. Decreasing the ribavirin dosage may help reduce anemia and other treatment-associated side effects[111].
HEPATITIS E VACCINE
Current data demonstrated that immunity acquired from infection or vaccination can protect individuals from symptomatic hepatitis E. In a large cohort study, the risk of infection was highest among the baseline seronegative placebo group participants (2.04%). The risks of HEV infection in population with pre-existing immunity or vaccine-induced immunity were significantly reduced to 0.52% and 0.30%, respectively[112]. Two recombinant hepatitis E vaccines developed from HEV genotype 1, by Glaxo SmithKline and Xiamen Innovax Biotech, have had short-term efficacy in clinical trials[113,114]. The latter vaccine, commercially available as Hecolin, has been in use in China since 2012. Its long-term efficacy was 86.8% during the 4.5-year follow-up period[93]. Estimated long-term persistence of anti-HEV IgG from hepatitis E vaccine is predicted to be from 8 years to nearly life-long based on mathematical assumptions[115]. The only currently licensed hepatitis E vaccine (Hecolin) is approved for use in China in those aged 16-65 years available in prefilled syringe for intramuscular injection at 0, 1, and 6 mo. Expansion of vaccine coverage to other HEV endemic country is necessary and might significant decrease burden of the disease[85]. In addition to improved personal hygiene, sanitation, and health education, vaccination might play a crucial role in the future prevention and control of HEV infection.
HEV IN BLOOD DONORS: CLINICAL IMPLICATIONS
Experiments involving the transfusion of blood plasma from anti-HEV IgM positive and anti-HEV IgG negative blood donors to rhesus monkey demonstrated that the virus was transmissible[116]. Therefore, many developed countries are now focused on studying the prevalence of viral transmission from blood donation[117-124] (Table 5). The demand for pathogen-free blood and blood components is highly needed for hospital patients and individuals requiring continuous blood transfusion (e.g., thalassemia patients). Thus, these patients are at-risk for being infected with HEV from donated blood. The development of screening methods for detecting HEV in donated blood involves both serology test and nucleic acid test. Novel techniques are being developed to increase the efficiency and sensitivity to identify HEV rapidly even at low viral concentration[125-127]. Many countries are aware of the necessity to screen the blood supply for HEV among blood donors and have begun to implement diagnostic tests for HEV. For example, Japan has started monitoring for HEV by comparing the increase in the alanine aminotransferase as a biomarker in the surveillance of HEV[128]. Germany is looking for a new approach to detecting HEV to find alternative ways to blood screening test[120]. Other countries have also implemented screening test but only focus on the suspected cases[129]. In Thailand, there were two reports on the incidence of HEV among blood donors. First, a study in 1996 reported the prevalence of HEV transmission among adults in different parts of Thailand, in which the studied population also included blood donors. The study found 9%-22% positive rates for anti-HEV IgG[130]. Another study found HEV seroprevalence around 8.7% among blood donors in 4 Northern provinces of Thailand[131], which was similar to those in other countries (Table 5). Thus, HEV poses a significant public health problem especially in blood donation even if the prevalence and virulence of the disease are lower than other infections[132].
Table 5.
Incidence of detectable hepatitis E virus in blood donors (hepatitis E virus-RNA)
| Year of study | Countries | Technique used for detection | No. of tests | Ratio of positive detections | Ref. |
| 2005 | China | Real-time fluorescence RT-PCR | 10741 | 1:1094 | Ren et al[117] |
| 2011 | England | PCR | 42000 | 1:7000 | Ijaz et al[118] |
| 2011 | German | Real-time RT-PCR | 18100 | 1:4525 | Baylis et al[119] |
| 2011 | Sweden | Real-time RT-PCR | 95835 | 1:7986 | Baylis et al[119] |
| 2011 | United States | Real-time RT-PCR | 51075 | None detected | Baylis et al[119] |
| 2011 | German | Real-time RT-PCR | 16125 | 1:1241 | Vollmer et al[120] |
| 2011-2012 | The Netherlands | Real-time PCR | 45415 | 1:2672 | Slot et al[121] |
| 2012-2013 | England | RT-PCR | 225000 | 1:2848 | Hewitt et al[122] |
| 2012 | France | RT-PCR | 53234 | 1:2218 | Gallian et al[123] |
| 2013 | Spain | Transcription-mediated amplification assay | 9998 | 1:3333 | Sauleda et al[124] |
RT-PCR: Reverse transcription-polymerase chain reaction.
CHALLENGES IN THE TREATMENT OF HEPATITIS E
Although the recent reports of treatment of HEV infection are showing beneficial outcomes, there are still areas to be overcome in the treatment of HEV infection. First of all, as described by previous articles, there are known severe side effects of current regimen with Peg-IFN-α and ribavirin. For Peg-IFN-α, the severe side effects include influenza-like symptoms[133] and acute rejection of allografts for solid organ transplant recipients[101]. For ribavirin monotherapy, it has the side effect of severe hemolytic anemia, sometimes resulting in treatment failures probably caused by dose reduction[100,111]. Furthermore, both ribavirin and Peg-IFN-α cannot be administered in pregnancy. Also, ribavirin use requires close monitoring of hemoglobin levels and other hematological parameters that make it difficult to apply in developing countries[111].
There has been a case report showing resolution of acute liver injury cause by hepatitis E with steroid use that was initially intended for immunosuppression[134]. Also, there have been reports of fulminant hepatic failure from HEV in women taking oral contraceptives[135]. Known that immunosuppression can cause persistent infection of hepatitis E, the relation between steroid hormone use and the clinical course of hepatitis E is not clear as the immune pathogenesis of hepatitis E infection itself being not explained thoroughly[2]. The various manifestations of hepatitis E according to the potential immune and hormonal status including pregnancy need to be explored precisely in their relation and the pathogenesis for the future direction of developing treatment regimen of hepatitis E. Larger research for establishing appropriate standard treatment as well as supportive treatment and steroid use are still in need in order to minimize limitations and side effects of the current administration of ribavirin and Peg-IFN-α monotherapy, with appropriate vaccination for the high-risk populations for controlling epidemics in resource-limited settings, and for pregnant women living in developing countries where the acute infections are threatening extremely great number of women and new-borns.
ACKNOWLEDGMENTS
We would like to express our gratitude to the staff in the Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University and the Center of Excellence in Clinical Virology, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University.
Footnotes
P- Reviewer: Berardinis PD, Kamal SA S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Supported by The National Research University Project, Office of Higher Education Commission, Nos. WCU001-HR-57, WCU007-HR-57, and WCU-58-006-HR; The National Research Council of Thailand (NRCT); The Research Chair Grant from the National Science and Technology Development Agency, Chulalongkorn University Centenary Academic Development Project, No. CU56-HR01; Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University, No. RES560530093; The Outstanding Professor of Thailand Research Fund, No. DPG5480002; The Doctoral Degree Chulalongkorn University 100th Year Birthday Anniversary to Duangnapa Intharasongkroh; and The Rachadapisek Sompote Fund of Chulalongkorn University for Postdoctoral Fellowship to Pattaratida Sa-nguanmoo.
Conflict-of-interest statement: The authors declare no conflicts of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 26, 2015
First decision: July 27, 2015
Article in press: September 18, 2015
References
- 1.Kumar S, Subhadra S, Singh B, Panda BK. Hepatitis E virus: the current scenario. Int J Infect Dis. 2013;17:e228–e233. doi: 10.1016/j.ijid.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Wedemeyer H, Rybczynska J, Pischke S, Krawczynski K. Immunopathogenesis of hepatitis E virus infection. Semin Liver Dis. 2013;33:71–78. doi: 10.1055/s-0033-1338118. [DOI] [PubMed] [Google Scholar]
- 3.Legrand-Abravanel F, Kamar N, Sandres-Saune K, Garrouste C, Dubois M, Mansuy JM, Muscari F, Sallusto F, Rostaing L, Izopet J. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis. 2010;202:835–844. doi: 10.1086/655899. [DOI] [PubMed] [Google Scholar]
- 4.Kim BS, Lim HS, Lee K, Min YS, Yoon YS, Jeong HS. A survey on the status of hepatitis e virus infection among slaughterhouse workers in South Korea. J Prev Med Public Health. 2015;48:53–61. doi: 10.3961/jpmph.14.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poovorawan K, Jitmitrapab S, Treeprasertsuk S, Thongmee T, Theamboonlers A, Tangkijvanich P, Komolmit P, Poovorawan Y. Risk factors and molecular characterization of acute sporadic symptomatic hepatitis E virus infection in Thailand. Asian Pac J Trop Med. 2014;7:709–714. [Google Scholar]
- 6.Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–138. doi: 10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61–69. doi: 10.1046/j.1365-2893.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- 8.Yu C, Boon D, McDonald SL, Myers TG, Tomioka K, Nguyen H, Engle RE, Govindarajan S, Emerson SU, Purcell RH. Pathogenesis of hepatitis E virus and hepatitis C virus in chimpanzees: similarities and differences. J Virol. 2010;84:11264–11278. doi: 10.1128/JVI.01205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong C, Zafrullah M, Mixson-Hayden T, Dai X, Liang J, Meng J, Kamili S. Suppression of interferon-α signaling by hepatitis E virus. Hepatology. 2012;55:1324–1332. doi: 10.1002/hep.25530. [DOI] [PubMed] [Google Scholar]
- 10.Guu TS, Liu Z, Ye Q, Mata DA, Li K, Yin C, Zhang J, Tao YJ. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc Natl Acad Sci USA. 2009;106:12992–12997. doi: 10.1073/pnas.0904848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 12.Holla RP, Ahmad I, Ahmad Z, Jameel S. Molecular virology of hepatitis E virus. Semin Liver Dis. 2013;33:3–14. doi: 10.1055/s-0033-1338110. [DOI] [PubMed] [Google Scholar]
- 13.Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao D, Meng XJ. Molecular biology and replication of hepatitis E virus. Emerg Microbes Infect. 2012;1:e17. doi: 10.1038/emi.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worm HC, van der Poel WH, Brandstätter G. Hepatitis E: an overview. Microbes Infect. 2002;4:657–666. doi: 10.1016/s1286-4579(02)01584-8. [DOI] [PubMed] [Google Scholar]
- 16.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–154. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 17.Krain LJ, Nelson KE, Labrique AB. Host immune status and response to hepatitis E virus infection. Clin Microbiol Rev. 2014;27:139–165. doi: 10.1128/CMR.00062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava R, Aggarwal R, Bhagat MR, Chowdhury A, Naik S. Alterations in natural killer cells and natural killer T cells during acute viral hepatitis E. J Viral Hepat. 2008;15:910–916. doi: 10.1111/j.1365-2893.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 19.TrehanPati N, Sukriti S, Geffers R, Hissar S, Riese P, Toepfer T, Guzman CA, Sarin SK. Gene expression profiles of T cells from hepatitis E virus infected patients in acute and resolving phase. J Clin Immunol. 2011;31:498–508. doi: 10.1007/s10875-010-9506-2. [DOI] [PubMed] [Google Scholar]
- 20.Devhare PB, Chatterjee SN, Arankalle VA, Lole KS. Analysis of antiviral response in human epithelial cells infected with hepatitis E virus. PLoS One. 2013;8:e63793. doi: 10.1371/journal.pone.0063793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Qi Y, Harrison TJ, Luo B, Zhou Y, Li X, Song A, Huang W, Wang Y. Hepatitis E genotype 4 virus from feces of monkeys infected experimentally can be cultured in PLC/PRF/5 cells and upregulate host interferon-inducible genes. J Med Virol. 2014;86:1736–1744. doi: 10.1002/jmv.24014. [DOI] [PubMed] [Google Scholar]
- 22.Nan Y, Ma Z, Wang R, Yu Y, Kannan H, Fredericksen B, Zhang YJ. Enhancement of interferon induction by ORF3 product of hepatitis E virus. J Virol. 2014;88:8696–8705. doi: 10.1128/JVI.01228-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nan Y, Yu Y, Ma Z, Khattar SK, Fredericksen B, Zhang YJ. Hepatitis E virus inhibits type I interferon induction by ORF1 products. J Virol. 2014;88:11924–11932. doi: 10.1128/JVI.01935-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Chen L, Liu Q. Activation of CXCL-8 Transcription by Hepatitis E Virus ORF-1 via AP-1. Mediators Inflamm. 2015;2015:495370. doi: 10.1155/2015/495370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhu SB, Gupta P, Durgapal H, Rath S, Gupta SD, Acharya SK, Panda SK. Study of cellular immune response against Hepatitis E virus (HEV) J Viral Hepat. 2011;18:587–594. doi: 10.1111/j.1365-2893.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava R, Aggarwal R, Jameel S, Puri P, Gupta VK, Ramesh VS, Bhatia S, Naik S. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol. 2007;20:56–65. doi: 10.1089/vim.2006.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majumdar M, Ratho R, Chawla Y, Singh MP. Evaluation of antigenicity and cell mediated immunity of hepatitis E virus patients: using non radioactive MTT assay. Indian J Med Microbiol. 2013;31:64–68. doi: 10.4103/0255-0857.108725. [DOI] [PubMed] [Google Scholar]
- 28.Surjit M, Varshney B, Lal SK. The ORF2 glycoprotein of hepatitis E virus inhibits cellular NF-κB activity by blocking ubiquitination mediated proteasomal degradation of IκBα in human hepatoma cells. BMC Biochem. 2012;13:7. doi: 10.1186/1471-2091-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husain MM, Aggarwal R, Kumar D, Jameel S, Naik S. Effector T cells immune reactivity among patients with acute hepatitis E. J Viral Hepat. 2011;18:e603–e608. doi: 10.1111/j.1365-2893.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 30.Saravanabalaji S, Tripathy AS, Dhoot RR, Chadha MS, Kakrani AL, Arankalle VA. Viral load, antibody titers and recombinant open reading frame 2 protein-induced TH1/TH2 cytokines and cellular immune responses in self-limiting and fulminant hepatitis e. Intervirology. 2009;52:78–85. doi: 10.1159/000214862. [DOI] [PubMed] [Google Scholar]
- 31.Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis. 2010;51:328–334. doi: 10.1086/653943. [DOI] [PubMed] [Google Scholar]
- 32.Favorov MO, Fields HA, Purdy MA, Yashina TL, Aleksandrov AG, Alter MJ, Yarasheva DM, Bradley DW, Margolis HS. Serologic identification of hepatitis E virus infections in epidemic and endemic settings. J Med Virol. 1992;36:246–250. doi: 10.1002/jmv.1890360403. [DOI] [PubMed] [Google Scholar]
- 33.Arankalle VA, Chadha MS, Tsarev SA, Emerson SU, Risbud AR, Banerjee K, Purcell RH. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc Natl Acad Sci USA. 1994;91:3428–3432. doi: 10.1073/pnas.91.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237–1244. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- 35.Khuroo MS, Kamili S, Dar MY, Moecklii R, Jameel S. Hepatitis E and long-term antibody status. Lancet. 1993;341:1355. [PubMed] [Google Scholar]
- 36.Aggarwal R, Kini D, Sofat S, Naik SR, Krawczynski K. Duration of viraemia and faecal viral excretion in acute hepatitis E. Lancet. 2000;356:1081–1082. doi: 10.1016/S0140-6736(00)02737-9. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan A, Jameel S, Dilawari JB, Chawla YK, Kaur U, Ganguly NK. Hepatitis E virus transmission to a volunteer. Lancet. 1993;341:149–150. doi: 10.1016/0140-6736(93)90008-5. [DOI] [PubMed] [Google Scholar]
- 38.Shata MT, Daef EA, Zaki ME, Abdelwahab SF, Marzuuk NM, Sobhy M, Rafaat M, Abdelbaki L, Nafeh MA, Hashem M, et al. Protective role of humoral immune responses during an outbreak of hepatitis E in Egypt. Trans R Soc Trop Med Hyg. 2012;106:613–618. doi: 10.1016/j.trstmh.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Tripathy AS, Das R, Rathod SB, Gurav YK, Arankalle VA. Peripheral T regulatory cells and cytokines in hepatitis E infection. Eur J Clin Microbiol Infect Dis. 2012;31:179–184. doi: 10.1007/s10096-011-1291-1. [DOI] [PubMed] [Google Scholar]
- 40.Rathod SB, Das R, Thanapati S, Arankalle VA, Tripathy AS. Suppressive activity and altered conventional phenotype markers/mediators of regulatory T cells in patients with self-limiting hepatitis E. J Viral Hepat. 2014;21:141–151. doi: 10.1111/jvh.12125. [DOI] [PubMed] [Google Scholar]
- 41.Miyamura T. Hepatitis E virus infection in developed countries. Virus Res. 2011;161:40–46. doi: 10.1016/j.virusres.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Nelson KE, Panzner U, Kasture Y, Labrique AB, Wierzba TF. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect Dis. 2014;14:308. doi: 10.1186/1471-2334-14-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal R. Hepatitis e: epidemiology and natural history. J Clin Exp Hepatol. 2013;3:125–133. doi: 10.1016/j.jceh.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wedemeyer H, Pischke S, Manns MP. Pathogenesis and treatment of hepatitis e virus infection. Gastroenterology. 2012;142:1388–1397.e1. doi: 10.1053/j.gastro.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention (CDC) Hepatitis E among US travelers, 1989-1992. MMWR Morb Mortal Wkly Rep. 1993;42:1–4. [PubMed] [Google Scholar]
- 46.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 47.Corwin AL, Tien NT, Bounlu K, Winarno J, Putri MP, Laras K, Larasati RP, Sukri N, Endy T, Sulaiman HA, et al. The unique riverine ecology of hepatitis E virus transmission in South-East Asia. Trans R Soc Trop Med Hyg. 1999;93:255–260. doi: 10.1016/s0035-9203(99)90014-7. [DOI] [PubMed] [Google Scholar]
- 48.Basnyat B, Dalton HR, Kamar N, Rein DB, Labrique A, Farrar J, Piot P. Nepali earthquakes and the risk of an epidemic of hepatitis E. Lancet. 2015;385:2572–2573. doi: 10.1016/S0140-6736(15)61110-2. [DOI] [PubMed] [Google Scholar]
- 49.Shrestha A, Lama TK, Karki S, Sigdel DR, Rai U, Rauniyar SK, Al-Mahtab M, Takahashi K, Arai M, Akbar SM, et al. Hepatitis E epidemic, Biratnagar, Nepal, 2014. Emerg Infect Dis. 2015;21:711–713. doi: 10.3201/eid2104.141512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansuy JM, Peron JM, Abravanel F, Poirson H, Dubois M, Miedouge M, Vischi F, Alric L, Vinel JP, Izopet J. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol. 2004;74:419–424. doi: 10.1002/jmv.20206. [DOI] [PubMed] [Google Scholar]
- 51.Wichmann O, Schimanski S, Koch J, Kohler M, Rothe C, Plentz A, Jilg W, Stark K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198:1732–1741. doi: 10.1086/593211. [DOI] [PubMed] [Google Scholar]
- 52.Dalton HR, Fellows HJ, Gane EJ, Wong P, Gerred S, Schroeder B, Croxson MC, Garkavenko O. Hepatitis E in new zealand. J Gastroenterol Hepatol. 2007;22:1236–1240. doi: 10.1111/j.1440-1746.2007.04894.x. [DOI] [PubMed] [Google Scholar]
- 53.Tsang TH, Denison EK, Williams HV, Venczel LV, Ginsberg MM, Vugia DJ. Acute hepatitis E infection acquired in California. Clin Infect Dis. 2000;30:618–619. doi: 10.1086/313730. [DOI] [PubMed] [Google Scholar]
- 54.Kaci S, Nöckler K, Johne R. Detection of hepatitis E virus in archived German wild boar serum samples. Vet Microbiol. 2008;128:380–385. doi: 10.1016/j.vetmic.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 55.Goens SD, Perdue ML. Hepatitis E viruses in humans and animals. Anim Health Res Rev. 2004;5:145–156. doi: 10.1079/ahr200495. [DOI] [PubMed] [Google Scholar]
- 56.Said B, Ijaz S, Kafatos G, Booth L, Thomas HL, Walsh A, Ramsay M, Morgan D. Hepatitis E outbreak on cruise ship. Emerg Infect Dis. 2009;15:1738–1744. doi: 10.3201/eid1511.091094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W, Shen Q, Mou J, Gong G, Yang Z, Cui L, Zhu J, Ju G, Hua X. Hepatitis E virus infection among domestic animals in eastern China. Zoonoses Public Health. 2008;55:291–298. doi: 10.1111/j.1863-2378.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 58.Hakze-van der Honing RW, van Coillie E, Antonis AF, van der Poel WH. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in the Netherlands and Belgium. PLoS One. 2011;6:e22673. doi: 10.1371/journal.pone.0022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 60.Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Heyries L, Raoult D, Gerolami R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–834. doi: 10.1086/655898. [DOI] [PubMed] [Google Scholar]
- 61.Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–505. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 64.Xia H, Wahlberg N, Belák S, Meng XJ, Liu L. The emergence of genotypes 3 and 4 hepatitis E virus in swine and humans: a phylogenetic perspective. Arch Virol. 2011;156:121–124. doi: 10.1007/s00705-010-0818-6. [DOI] [PubMed] [Google Scholar]
- 65.Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23–30. doi: 10.1016/j.virusres.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arankalle VA, Chobe LP, Chadha MS. Type-IV Indian swine HEV infects rhesus monkeys. J Viral Hepat. 2006;13:742–745. doi: 10.1111/j.1365-2893.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- 68.Aggarwal R, Naik SR. Hepatitis E: does person-to-person spread occur? Indian J Gastroenterol. 1992;11:109–112. [PubMed] [Google Scholar]
- 69.Montella F, Rezza G, Di Sora F, Pezzotti P, Recchia O. Association between hepatitis E virus and HIV infection in homosexual men. Lancet. 1994;344:1433. doi: 10.1016/s0140-6736(94)90598-3. [DOI] [PubMed] [Google Scholar]
- 70.Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol. 2005;76:341–349. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 71.Stoszek SK, Engle RE, Abdel-Hamid M, Mikhail N, Abdel-Aziz F, Medhat A, Fix AD, Emerson SU, Purcell RH, Strickland GT. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg. 2006;100:89–94. doi: 10.1016/j.trstmh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Withers MR, Correa MT, Morrow M, Stebbins ME, Seriwatana J, Webster WD, Boak MB, Vaughn DW. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am J Trop Med Hyg. 2002;66:384–388. doi: 10.4269/ajtmh.2002.66.384. [DOI] [PubMed] [Google Scholar]
- 74.Drobeniuc J, Favorov MO, Shapiro CN, Bell BP, Mast EE, Dadu A, Culver D, Iarovoi P, Robertson BH, Margolis HS. Hepatitis E virus antibody prevalence among persons who work with swine. J Infect Dis. 2001;184:1594–1597. doi: 10.1086/324566. [DOI] [PubMed] [Google Scholar]
- 75.Gonwong S, Chuenchitra T, Khantapura P, Islam D, Sirisopana N, Mason CJ. Pork consumption and seroprevalence of hepatitis E virus,Thailand, 2007-2008. Emerg Infect Dis. 2014;20:1531–1534. doi: 10.3201/eid2009.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mathur P, Arora NK, Panda SK, Kapoor SK, Jailkhani BL, Irshad M. Sero-epidemiology of hepatitis E virus (HEV) in urban and rural children of North India. Indian Pediatr. 2001;38:461–475. [PubMed] [Google Scholar]
- 77.Nakai K, Win KM, Oo SS, Arakawa Y, Abe K. Molecular characteristic-based epidemiology of hepatitis B, C, and E viruses and GB virus C/hepatitis G virus in Myanmar. J Clin Microbiol. 2001;39:1536–1539. doi: 10.1128/JCM.39.4.1536-1539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Labrique AB, Zaman K, Hossain Z, Saha P, Yunus M, Hossain A, Ticehurst J, Nelson KE. Population seroprevalence of hepatitis E virus antibodies in rural Bangladesh. Am J Trop Med Hyg. 2009;81:875–881. doi: 10.4269/ajtmh.2009.09-0352. [DOI] [PubMed] [Google Scholar]
- 79.Dong C, Dai X, Liang J, Dong M, Meng J. Seroprevalence of hepatitis e virus varies considerably among chinese provinces. Hepat Mon. 2012;12:386–390. doi: 10.5812/hepatmon.6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvarado-Esquivel C, Sanchez-Anguiano LF, Hernandez-Tinoco J. Seroepidemiology of hepatitis e virus infection in general population in rural durango, Mexico. Hepat Mon. 2014;14:e16876. doi: 10.5812/hepatmon.16876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Junaid SA, Agina SE, Abubakar KA. Epidemiology and associated risk factors of hepatitis e virus infection in plateau state, Nigeria. Virology (Auckl) 2014;5:15–26. doi: 10.4137/VRT.S15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wenzel JJ, Sichler M, Schemmerer M, Behrens G, Leitzmann MF, Jilg W. Decline in hepatitis E virus antibody prevalence in southeastern Germany, 1996-2011. Hepatology. 2014;60:1180–1186. doi: 10.1002/hep.27244. [DOI] [PubMed] [Google Scholar]
- 83.Teshale EH, Denniston MM, Drobeniuc J, Kamili S, Teo CG, Holmberg SD. Decline in hepatitis E virus antibody prevalence in the United States from 1988-1994 to 2009-2010. J Infect Dis. 2015;211:366–373. doi: 10.1093/infdis/jiu466. [DOI] [PubMed] [Google Scholar]
- 84.Gerolami R, Borentain P, Raissouni F, Motte A, Solas C, Colson P. Treatment of severe acute hepatitis E by ribavirin. J Clin Virol. 2011;52:60–62. doi: 10.1016/j.jcv.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Labrique AB, Sikder SS, Krain LJ, West KP, Christian P, Rashid M, Nelson KE. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18:1401–1404. doi: 10.3201/eid1809.120241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu FC, Huang SJ, Wu T, Zhang XF, Wang ZZ, Ai X, Yan Q, Yang CL, Cai JP, Jiang HM, et al. Epidemiology of zoonotic hepatitis E: a community-based surveillance study in a rural population in China. PLoS One. 2014;9:e87154. doi: 10.1371/journal.pone.0087154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar Acharya S, Kumar Sharma P, Singh R, Kumar Mohanty S, Madan K, Kumar Jha J, Kumar Panda S. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46:387–394. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 88.Péron JM, Dalton H, Izopet J, Kamar N. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: a role for ribavirin? J Hepatol. 2011;54:1323–1324; author reply 1324-1325. doi: 10.1016/j.jhep.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Rianthavorn P, Thongmee C, Limpaphayom N, Komolmit P, Theamboonlers A, Poovorawan Y. The entire genome sequence of hepatitis E virus genotype 3 isolated from a patient with neuralgic amyotrophy. Scand J Infect Dis. 2010;42:395–400. doi: 10.3109/00365540903496551. [DOI] [PubMed] [Google Scholar]
- 90.Kamar N, Bendall RP, Peron JM, Cintas P, Prudhomme L, Mansuy JM, Rostaing L, Keane F, Ijaz S, Izopet J, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17:173–179. doi: 10.3201/eid1702.100856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grewal P, Kamili S, Motamed D. Chronic hepatitis E in an immunocompetent patient: a case report. Hepatology. 2014;59:347–348. doi: 10.1002/hep.26636. [DOI] [PubMed] [Google Scholar]
- 92.González Tallón AI, Moreira Vicente V, Mateos Lindemann ML, Achécar Justo LM. [Chronic hepatitis E in an immunocompetent patient] Gastroenterol Hepatol. 2011;34:398–400. doi: 10.1016/j.gastrohep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914–922. doi: 10.1056/NEJMoa1406011. [DOI] [PubMed] [Google Scholar]
- 94.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 95.Del Bello A, Arné-Bes MC, Lavayssière L, Kamar N. Hepatitis E virus-induced severe myositis. J Hepatol. 2012;57:1152–1153. doi: 10.1016/j.jhep.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Pischke S, Hardtke S, Bode U, Birkner S, Chatzikyrkou C, Kauffmann W, Bara CL, Gottlieb J, Wenzel J, Manns MP, et al. Ribavirin treatment of acute and chronic hepatitis E: a single-centre experience. Liver Int. 2013;33:722–726. doi: 10.1111/liv.12114. [DOI] [PubMed] [Google Scholar]
- 97.Robbins A, Lambert D, Ehrhard F, Brodard V, Hentzien M, Lebrun D, Nguyen Y, Tabary T, Peron JM, Izopet J, et al. Severe acute hepatitis E in an HIV infected patient: Successful treatment with ribavirin. J Clin Virol. 2014;60:422–423. doi: 10.1016/j.jcv.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Riveiro-Barciela M, Mínguez B, Gironés R, Rodriguez-Frías F, Quer J, Buti M. Phylogenetic demonstration of hepatitis E infection transmitted by pork meat ingestion. J Clin Gastroenterol. 2015;49:165–168. doi: 10.1097/MCG.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 99.Kamar N, Rostaing L, Abravanel F, Garrouste C, Lhomme S, Esposito L, Basse G, Cointault O, Ribes D, Nogier MB, et al. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis e virus infection. Gastroenterology. 2010;139:1612–1618. doi: 10.1053/j.gastro.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D’Alteroche L, et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 101.Kamar N, Rostaing L, Abravanel F, Garrouste C, Esposito L, Cardeau-Desangles I, Mansuy JM, Selves J, Peron JM, Otal P, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis. 2010;50:e30–e33. doi: 10.1086/650488. [DOI] [PubMed] [Google Scholar]
- 102.Haagsma EB, Riezebos-Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010;16:474–477. doi: 10.1002/lt.22014. [DOI] [PubMed] [Google Scholar]
- 103.Kamar N, Abravanel F, Lhomme S, Rostaing L, Izopet J. Hepatitis E virus: chronic infection, extra-hepatic manifestations, and treatment. Clin Res Hepatol Gastroenterol. 2015;39:20–27. doi: 10.1016/j.clinre.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Debing Y, Emerson SU, Wang Y, Pan Q, Balzarini J, Dallmeier K, Neyts J. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob Agents Chemother. 2014;58:267–273. doi: 10.1128/AAC.01795-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mallet V, Nicand E, Sultanik P, Chakvetadze C, Tessé S, Thervet E, Mouthon L, Sogni P, Pol S. Brief communication: case reports of ribavirin treatment for chronic hepatitis E. Ann Intern Med. 2010;153:85–89. doi: 10.7326/0003-4819-153-2-201007200-00257. [DOI] [PubMed] [Google Scholar]
- 106.Neukam K, Barreiro P, Macías J, Avellón A, Cifuentes C, Martín-Carbonero L, Echevarría JM, Vargas J, Soriano V, Pineda JA. Chronic hepatitis E in HIV patients: rapid progression to cirrhosis and response to oral ribavirin. Clin Infect Dis. 2013;57:465–468. doi: 10.1093/cid/cit224. [DOI] [PubMed] [Google Scholar]
- 107.Giordani MT, Fabris P, Brunetti E, Goblirsch S, Romanò L. Hepatitis E and lymphocytic leukemia in Man, Italy. Emerg Infect Dis. 2013;19:2054–2056. doi: 10.3201/eid1912.130521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alric L, Bonnet D, Laurent G, Kamar N, Izopet J. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-alpha therapy. Ann Intern Med. 2010;153:135–136. doi: 10.7326/0003-4819-153-2-201007200-00256. [DOI] [PubMed] [Google Scholar]
- 109.Kamar N, Abravanel F, Garrouste C, Cardeau-Desangles I, Mansuy JM, Weclawiak H, Izopet J, Rostaing L. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant. 2010;25:2792–2795. doi: 10.1093/ndt/gfq282. [DOI] [PubMed] [Google Scholar]
- 110.Dalton HR, Keane FE, Bendall R, Mathew J, Ijaz S. Treatment of chronic hepatitis E in a patient with HIV infection. Ann Intern Med. 2011;155:479–480. doi: 10.7326/0003-4819-155-7-201110040-00017. [DOI] [PubMed] [Google Scholar]
- 111.Debing Y, Neyts J. Antiviral strategies for hepatitis E virus. Antiviral Res. 2014;102:106–118. doi: 10.1016/j.antiviral.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Yao X, Liang ZL, Wu T, Li JX, Yan Q, et al. Protection against hepatitis E virus infection by naturally acquired and vaccine-induced immunity. Clin Microbiol Infect. 2014;20:O397–O405. doi: 10.1111/1469-0691.12419. [DOI] [PubMed] [Google Scholar]
- 113.Shrestha MP, Scott RM, Joshi DM, Mammen MP, Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 114.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 115.Chen S, Zhou Z, Wei FX, Huang SJ, Tan Z, Fang Y, Zhu FC, Wu T, Zhang J, Xia NS. Modeling the long-term antibody response of a hepatitis E vaccine. Vaccine. 2015;33:4124–4129. doi: 10.1016/j.vaccine.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 116.Xia NS, Zhang J, Zheng YJ, Qiu Y, Ge SX, Ye XZ, Ou SH. [Detection of hepatitis E virus on a blood donor and its infectivity to rhesus monkey] Zhonghua Gan Zang Bing Za Zhi. 2004;12:13–15. [PubMed] [Google Scholar]
- 117.Ren F, Zhao C, Wang L, Wang Z, Gong X, Song M, Zhuang H, Huang Y, Shan H, Wang J, et al. Hepatitis E virus seroprevalence and molecular study among blood donors in China. Transfusion. 2014;54:910–917. doi: 10.1111/trf.12530. [DOI] [PubMed] [Google Scholar]
- 118.Ijaz S, Szypulska R, Tettmar KI, Kitchen A, Tedder RS. Detection of hepatitis E virus RNA in plasma mini-pools from blood donors in England. Vox Sang. 2012;102:272. doi: 10.1111/j.1423-0410.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 119.Baylis SA, Gärtner T, Nick S, Ovemyr J, Blümel J. Occurrence of hepatitis E virus RNA in plasma donations from Sweden, Germany and the United States. Vox Sang. 2012;103:89–90. doi: 10.1111/j.1423-0410.2011.01583.x. [DOI] [PubMed] [Google Scholar]
- 120.Vollmer T, Diekmann J, Johne R, Eberhardt M, Knabbe C, Dreier J. Novel approach for detection of hepatitis E virus infection in German blood donors. J Clin Microbiol. 2012;50:2708–2713. doi: 10.1128/JCM.01119-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Slot E, Hogema BM, Riezebos-Brilman A, Kok TM, Molier M, Zaaijer HL. Silent hepatitis E virus infection in Dutch blood donors, 2011 to 2012. Euro Surveill. 2013;18:pii: 20550. doi: 10.2807/1560-7917.es2013.18.31.20550. [DOI] [PubMed] [Google Scholar]
- 122.Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–1773. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 123.Gallian P, Lhomme S, Piquet Y, Sauné K, Abravanel F, Assal A, Tiberghien P, Izopet J. Hepatitis E virus infections in blood donors, France. Emerg Infect Dis. 2014;20:1914–1917. doi: 10.3201/eid2011.140516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sauleda S, Ong E, Bes M, Janssen A, Cory R, Babizki M, Shin T, Lindquist A, Hoang A, Vang L, et al. Seroprevalence of hepatitis E virus (HEV) and detection of HEV RNA with a transcription-mediated amplification assay in blood donors from Catalonia (Spain) Transfusion. 2015;55:972–979. doi: 10.1111/trf.12929. [DOI] [PubMed] [Google Scholar]
- 125.Baylis SA, Hanschmann KM, Blümel J, Nübling CM. Standardization of hepatitis E virus (HEV) nucleic acid amplification technique-based assays: an initial study to evaluate a panel of HEV strains and investigate laboratory performance. J Clin Microbiol. 2011;49:1234–1239. doi: 10.1128/JCM.02578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 127.Dodd RY. Emerging pathogens and their implications for the blood supply and transfusion transmitted infections. Br J Haematol. 2012;159:135–142. doi: 10.1111/bjh.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sakata H, Matsubayashi K, Takeda H, Sato S, Kato T, Hino S, Tadokoro K, Ikeda H. A nationwide survey for hepatitis E virus prevalence in Japanese blood donors with elevated alanine aminotransferase. Transfusion. 2008;48:2568–2576. doi: 10.1111/j.1537-2995.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- 129.Bajpai M, Gupta E. Transfusion-transmitted hepatitis E: is screening warranted? Indian J Med Microbiol. 2011;29:353–358. doi: 10.4103/0255-0857.90158. [DOI] [PubMed] [Google Scholar]
- 130.Poovorawan Y, Theamboonlers A, Chumdermpadetsuk S, Komolmit P. Prevalence of hepatitis E virus infection in Thailand. Ann Trop Med Parasitol. 1996;90:189–196. doi: 10.1080/00034983.1996.11813043. [DOI] [PubMed] [Google Scholar]
- 131.Jutavijittum P, Jiviriyawat Y, Jiviriyawat W, Yousukh A, Hayashi S, Toriyama K. Present epidemiological pattern of antibody to hepatitis a virus among Chiang Mai children, Northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33:268–271. [PubMed] [Google Scholar]
- 132.Bureau of Epidemiology DoDC, Ministry of Public Health, Thailand. Case-rate and deaths-rate by year, Thailand, 2003–2012. Annual Epidemiological Surveillance Report, 2012. [Accessed 2015 Apr 1] Available from: http://www.boe.moph.go.th/Annual/AESR2012/main/AESR55_Part2/table6.pdf. [Google Scholar]
- 133.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sebode M, Pischke S, Lütgehetmann M, Polywka S, Quaas A, Lohse AW, Wege H. New foe treated with old guns - supportive role of steroids in the treatment of acute severe hepatitis E. BMC Gastroenterol. 2014;14:191. doi: 10.1186/s12876-014-0191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mateos Lindemann ML, Morales JG, Fernández-Barredo S, Domínguez MR, García de la Hoz F, Halfon P, Pérez Gracia MT. Fulminant hepatitis E in a woman taking oral contraceptive medication. Am J Trop Med Hyg. 2010;82:12–15. doi: 10.4269/ajtmh.2010.09-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]


