Abstract
Up-regulation of cell adhesion molecules and proinflammatory cytokines contributes to enhanced monocyte adhesiveness and infiltration into the skin, during the pathogenesis of various inflammatory skin diseases, including atopic dermatitis. In this study, we examined the anti-inflammatory effects of butein, a tetrahydroxychalcone, and its action mechanisms using TNF-α-stimulated keratinocytes. Butein significantly inhibited TNF-α-induced ICAM-I expression and monocyte adhesion in human keratinocyte cell line HaCaT. Butein also decreased TNF-α-induced pro-inflammatory mediators, such as IL-6, IP-10 and MCP-1, in HaCaT cells. Butein decreased TNF-α-induced ROS generation in a dose-dependent manner in HaCaT cells. In addition, treatment of HaCaT cells with butein suppressed TNF-α-induced MAPK activation. Furthermore, butein suppressed TNF-α-induced NF-kappaB activation. Overall, our results indicate that butein has immunomodulatory activities by inhibiting expression of proinflammatory mediators in keratinocytes. Therefore, butein may be used as a therapeutic agent for the treatment of inflammatory skin diseases. [BMB Reports 2015; 48(9): 495-500]
Keywords: Butein, ICAM-1, Inflammation, Keratinocyte, TNF-alpha
INTRODUCTION
One of the characteristic features of skin inflammation is the infiltration of various immune cells including monocytes into the inflamed skin area (1). Dysregulation of adhesion molecules and cytokines/chemokines increases the infiltration of immune cells into the site of inflammation in the skin (2,3). Upon stimulation with inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interferon-γ, keratinocytes can express adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1), and various cytokines/chemokines (2,4).
Major intracellular signaling regulators mediating pro-inflammatory responses include mitogen-activated protein kinase (MAPK) signaling pathways. The MAPK activation plays important roles in the signaling cascades leading to the expression of various pro-inflammatory mediators during the inflammation process (5). Upon stimulation with TNF-α, MAPKs such as extracellular signal-regulated kinase (ERK), p38 and c-Jun NH2-terminal kinase (JNK), are activated in the phosphorylated forms, mediating the signaling cascades leading to activation of various transcription factors including nuclear factor-kappa B (NF-κB) in keratinocytes (6). NF-κB is a principal transcription factor involved in the regulation of pro-inflammatory gene expression (7). In unstimulated cells, inhibitor of kappa B alpha (IκBα) sequestrates NF-κB in the cytosol. Upon stimulation with cytokines such as TNF-α, IκBα is phosphorylated, and subsequently ubiquitinated, leading to its proteasomal degradation (8). Dissociated NF-κB from IκBα translocates to the nucleus, where it activates the transcription of pro-inflammatory genes.
Butein (3, 4, 2', 4'-tetrahydroxychalcone; Fig. 1A), a polyphenolic compound, is present in medicinal plants including Semecarpus anacardium and Rhus verniciflua (9). Butein has been shown to exert various biological activities, such as antioxidant, anti-inflammatory, and anti-tumor activities (10,11,12). Butein inhibited lipopolysaccharide-induced expression of inducible nitric oxide synthase, by blocking activation of NF-κB and ERK MAPK in RAW 264.7 cells (11). Butein was shown to down-regulate phorbol 12-myristate 13-acetate-induced cyclooxygenase-2 expression, by suppressing ERK activation, in both cancerous and non-cancerous breast cells (13). Butein also suppressed TNF-α-mediated ICAM-1 and VCAM-1 expression and monocyte adhesion via blocking NF-κB, MAPK and Akt signaling pathways in human lung epithelial A549 cells (14). However, very little is known about the protective effects of butein and its mechanism of action in keratinocytes.
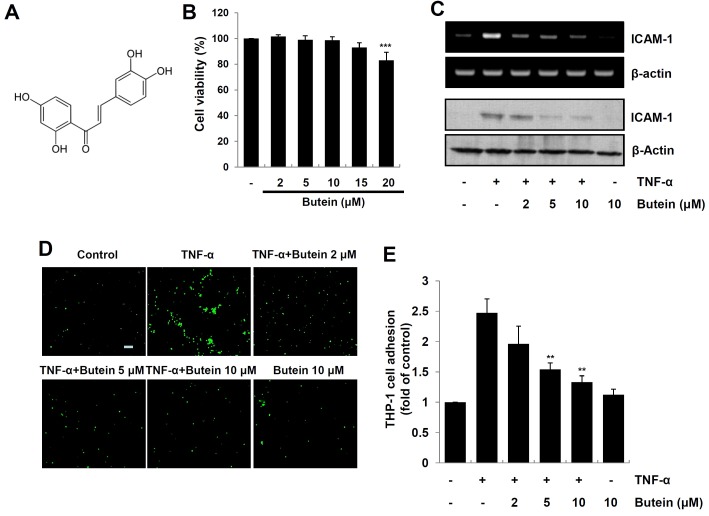
Fig. 1. Effect of butein on TNF-α-induced expression of ICAM-1 and monocyte adhesion in HaCaT cells. (A) Chemical structure of butein. (B) HaCaT cells were incubated with various concentrations of butein for 24, and then cell viability was evaluated by MTT assay. The results are expressed as mean ±SD of three independent experiments. Statistical significance: ***P < 0.001 compared to control group. (C) Cells pretreated with 2, 5 and 10 μM butein for 4 h were exposed to 10 ng/ml TNF-α for 1 h (for RNA), or 12 h (for protein). Total RNA and protein were analyzed by RTPCR (upper panel), and Western blotting (bottom panel), respectively. (D) HaCaT cells were incubated with 2, 5 and 10 μM butein for 4 h, and then exposed to 10 ng/ml TNF-α for 12 h. Calcein-AM-labeled THP-1 monocytes were added, and incubated with HaCaT cells for 1 h. Microscopic images were obtained using a fluorescence microscopy (scale bar=50 μm). (E) Calcein-AM fluorescent intensity was quantified using a fluorescence plate reader. The results are expressed as mean ± SD of three independent experiments. Statistical significance: **P < 0.01 compared to TNF-α alone.
In this study, we investigated the anti-inflammatory effects of butein on TNF-α-stimulated HaCaT cells. We observed that butein inhibited TNF-α-induced ICAM-1 expression, as well as the subsequent monocyte adhesiveness in HaCaT cells. Butein also suppressed TNF-α-induced pro-inflammatory cytokines, such as interleukin 6 (IL-6), IFN-γ-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1). Butein decreased TNF-α-induced ROS generation in HaCaT cells. In addition, butein significantly inhibited TNF-α-induced activation of MAPK and NF-κB in HaCaT cells.
RESULTS
Butein inhibits ICAM-1 expression and subsequent monocyte adhesiveness in TNF-α-stimulated HaCaT cells
To ensure that the anti-inflammatory effect of butein is not due to cell death, we first evaluated the cytotoxicity of butein (Fig. 1A) on HaCaT cells using an MTT assay. Because butein did not show any cytotoxic effects at concentrations up to 15 μM, we used butein in the subsequent experiments at the concentration of 0-10 μM (Fig. 1B). To examine the suppressive effects of butein on ICAM-1 expression, cells were pretreated with various concentrations of butein for 4 h, and exposed to TNF-α for 1 h, and then the mRNA and protein levels of ICAM-1 were measured by RT-PCR and Western blot analysis, respectively. As shown in Fig. 1C, butein significantly suppressed TNF-α-induced ICAM-1 expression at the mRNA and protein levels in HaCaT cells. Because the previous study has reported that up-regulation of ICAM-1 is involved in increased monocyte adhesiveness in the human keratinocytes (15), we next examined the inhibitory effect of butein on TNF-α-induced monocyte adhesion to HaCaT cells. As shown in Fig. 1D and E, butein significantly inhibited monocyte adhesiveness in TNF-α-stimulated HaCaT cells.
Butein inhibits the production of pro-inflammatory cytokines in TNF-α-stimulated HaCaT cells
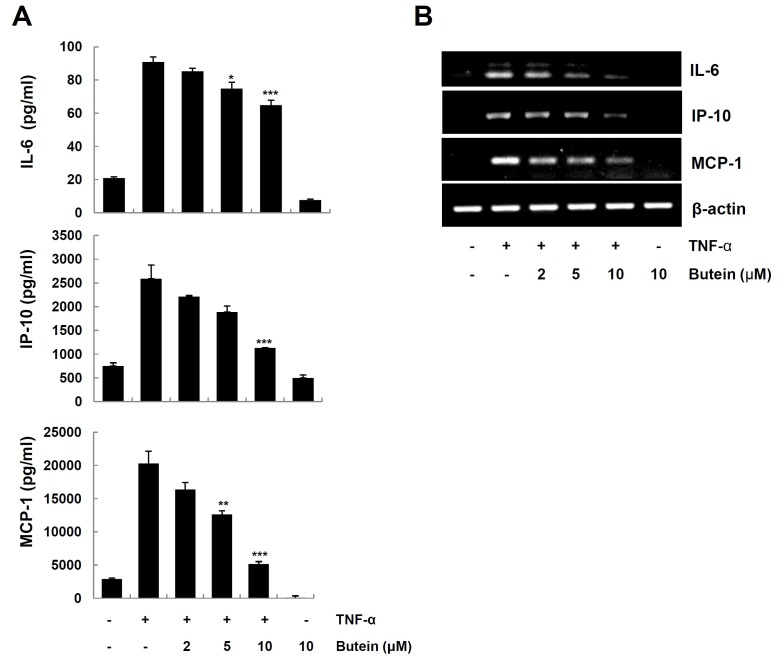
Since the up-regulation of pro-inflammatory cytokines/chemokines contributes to the development of skin inflammation (2,3), we further examined the effect of butein on the production of IL-6, IP-10 and MCP-1 in TNF-α-stimulated HaCaT cells. Cells pretreated with butein for 4 h were exposed to TNF-α. We analyzed the levels of cytokines/chemokines protein and mRNA by ELISA and RT-PCR, respectively. Butein significantly decreased TNF-α-induced expression of IL-6, IP-10 and MCP-1 protein (Fig. 2A) and mRNA (Fig. 2B), in a dose-dependent manner.
Fig. 2. Inhibitory effects of butein on TNF-α-induced expression of IL-6, IP-10 and MCP-1 in HaCaT cells. HaCaT cells were pretreated with 2, 5 and 10 μM butein for 4 h, and then exposed to 10 ng/ml TNF-α for 24 h (for protein), or 6 h (for mRNA). (A) The levels of IL-6, IP-10 and MCP-1 in the culture medium were determined by ELISA. The results are expressed as mean ±SD of three independent experiments. Statistical significance: *P < 0.05, **P < 0.01 and ***P < 0.001 compared to TNF-α alone. (B) Total RNA was prepared from cells, and analyzed for mRNA expression of IL-6, IP-10, MCP-1, and β-actin by RT-PCR, using specific primers.
Butein inhibits TNF-α-induced ROS generation in HaCaT cells
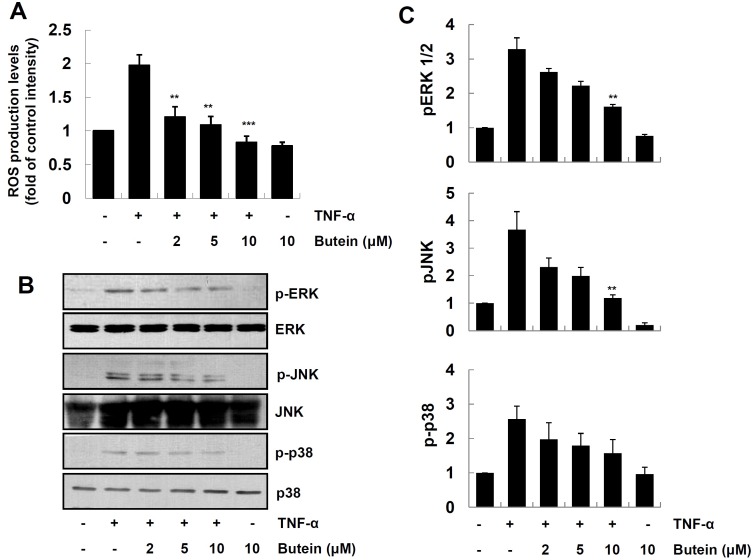
Exposure of the keratinocytes to a variety of stimuli, such as cytokines and TPA, leads to the excessive generation of reactive oxygen species (ROS) (4) We further examined the effect of butein on TNF-α-induced ROS generation. Cells were pretreated with butein, exposed to TNF-α for 1 h and then the levels of ROS in cells were determined, using DCF-DA as a probe. As shown in Fig. 3A, butein significantly inhibited TNF--α-induced ROS production in HaCaT cells.
Fig. 3. Inhibitory effect of butein on TNF-α-induced ROS generation and MAPK activation in HaCaT cells. (A) HaCaT cells were pretreated with 2, 5 and 10 μM butein for 4 h, and then exposed to 10 ng/ml TNF-α for 15 min. Intracellular ROS levels were assessed by staining with DCF-DA using an ELISA plate reader. The results are expressed as mean ±SD of three independent experiments. Statistical significance: **P < 0.01 and ***P < 0.001 compared to TNF-α alone. (B) Cells were pretreated with 2, 5 and 10 μM butein for 4 h, and then exposed to 10 ng/ml TNF-α for 15 min. Cells extracts were prepared, and analyzed for MAPK activation by Western blot analysis, using specific antibodies. (C) Relative protein levels in the (B) panel were quantified by scanning densitometry, and normalized to control protein levels. The results are expressed as mean ±SD of three independent experiments. Statistical significance: **P < 0.01 compared to TNF-α alone.
Butein inhibits TNF-α-induced MAPK activation in HaCaT cells
Previous studies have reported that the activation of MAPKs, such as JNK, ERK and p38 MAPK, is involved in TNF-α-induced NF-κB activation, and subsequent expression of pro-inflammatory mediators in the keratinocytes (6). We further examined the effect of butein on TNF-α-induced phosphorylation of MAPKs, by Western blot analysis using phosphor-specific antibodies. As shown in Fig. 3B and C, butein inhibited TNF-α-induced phosphorylation of ERK and JNK in a dose-dependent manner, whereas it has minimal effect on p38 MAPK. These results suggest that butein inhibits TNF-α-induced expression of pro-inflammatory mediators, by decreasing the phosphorylation levels of ERK and JNK MAPKs in HaCaT cells.
Butein inhibits TNF-α-induced NF-κB activation in HaCaT cells
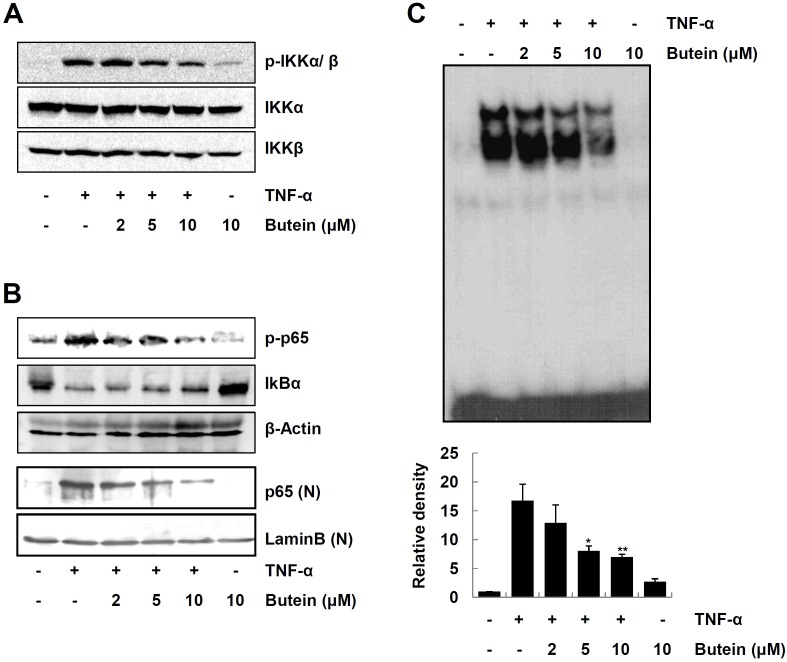
NF-κB is the principal regulator involved in the gene expression of various proinflammatory mediators, and is activated upon stimulation with cytokines, including TNF-α (6,8). We next investigated the effect of butein on the signaling cascades leading to NF-κB activation in TNF-α-stimulated HaCaT cells. We first examined the effect of butein on IKKα/β activation in TNF-α-stimulated HaCaT cells. As shown in Fig. 4A, butein efficiently inhibited TNF-α-induced IKKα/β phosphorylation. In addition, butein suppressed TNF-α-induced IκBα degradation, and phosphorylation/nuclear translocation of NF-κB p65, in a dose-dependent manner (Fig. 4B). Furthermore, butein significantly decreased NF-κB DNA binding activity in TNF-α-stimulated HaCaT cells, as judged by EMSA (Fig. 4C). These results suggest that butein inhibits TNF-α-induced expression of pro-inflammatory mediators, through inhibition of NF-κB activation.
Fig. 4. Inhibitory effect of butein on NF-κB signaling pathways in TNF-α-stimulated HaCaT cells. Cells were pretreated with butein for 4 h, and then exposed to 10 ng/ml TNF-α for 15 min. (A) Whole cell lysates were prepared and analyzed for IKKα/β activation by Western blotting, using phospho-specific antibodies. (B) Cytoplasmic or nuclear extracts prepared from cells stimulated with TNF-α were analyzed by Western blot analysis, for the levels of signaling molecules leading to NF-κB activation. (C) DNA-binding activity of NF-κB in the nuclear extracts of the cells was measured by EMSA. Intensity of each band was quantified by scanning densitometry and presented as the bar graphs in the bottom panel. The results are expressed as mean ±SD of three independent experiments. Statistical significance: *P < 0.05 and **P < 0.01 compared to TNF-α alone.
DISCUSSION
Dysregulated inflammatory responses lead to various pathological conditions, such as inflammatory diseases. Up-regulation of pro-inflammatory mediators in the inflamed skin area plays an important role during the inflammation process. Therefore, down-regulation of pro-inflammatory mediators in the area of the inflamed skin presents an important strategy to modulate various inflammatory skin diseases (16). In this study, we demonstrate that butein can exert anti-inflammatory effects in keratinocytes.
ICAM-1 belongs to a class of cell adhesion molecules involved in the infiltration of leukocytes into the skin, which represents one of the early steps during the inflammatory immune response (17). Keratinocytes express ICAM-1 molecules at a low basal level; but upon stimulation, ICAM-1 expression is increased. Along with adhesion molecules, pro-inflammatory cytokines/chemokines, such as IL-6, IP-10 and MCP-1, play important roles in leukocyte infiltration, during immune responses (1,17). Stimulation with TNF-α increased mRNA and protein levels of ICAM-1 in HaCaT cells. However, treatment with butein suppressed TNF-α-induced ICAM-1 expression, and subsequent monocyte adhesion to HaCaT cells (Fig. 1). Similar to the results of ICAM-1 expression, butein significantly inhibited production of IL-6, IP-10 and MCP-1, in TNF-α-stimulated HaCaT cells (Fig. 2).
Growing evidence suggested that ROS generation is involved in the pathogenesis of various inflammatory diseases (18). Stimulation with TNF-α can increase ROS generation directly and indirectly in the keratinocytes (7,19). Therefore, antioxidant enzymes have been considered to be potential therapeutic agents for ROS-mediated inflammatory skin diseases. In supporting this notion, we previously demonstrated that a cell permeable superoxide dismutase exerted protective effects, in a skin inflammation model (4). Previous studies demonstrated that butein has an antioxidant activity (10). As shown in Fig. 3A, butein efficiently decreased TNF-α-induced ROS generation in HaCaT cells. It was reported that ROS influences various cellular signaling pathways, such as MAPK and NF-κB activation, leading to expression of pro-inflammatory mediators (14,18).
Growing evidence suggested that one or more MAPK signaling pathways mediated expression of pro-inflammatory mediators in skin inflammation (22). Consistent with previous results (6), TNF-α induced all three MAPKs in HaCaT cells. We previously reported that activation of ERK and p38, but not JNK, are partially involved in TNF-α-induced ICAM-1 in HaCaT cells (6), suggesting the functional relationship between MAPK activation and ICAM-1 expression. As shown in Fig. 3B, butein significantly abolished TNF-α-induced activation of ERK and JNK, but not p38 MAPK. Taken together, these results indicate that butein inhibits TNF-α-induced expression of ICAM-1 and pro-inflammatory mediators, by suppressing ERK and JNK activation in HaCaT cells.
NF-κB is one of the major transcriptional factors that mediate expression of pro-inflammatory mediators during inflammatory immune responses (8). Upon stimulation with pro-inflammatory stimuli, activated IKK complex phosphorylates IκBα, which is then rapidly ubiquitinated, and subsequently degraded by proteasome. The free NF-κB dissociated from IκBα translocates into the nucleus, where it induces the expression of pro-inflammatory mediators. It was recently reported that butein inhibited TNF-α-induced NF-κB signaling pathways, via the direct inhibition of IKK (12). We performed experiments to assess the regulatory effects of butein on the signaling pathways leading to activation of NF-κB in HaCaT cells (Fig. 4). We observed that butein inhibited TNF-α-induced IKK phosphorylation, IκBα degradation, and translocation of p65 into the nucleus. Butein also suppressed p65 DNA-binding activity in TNF-α-stimulated HaCaT cells. These results indicate that butein exerts anti-inflammatory activities, by regulating the signaling cascades leading to activation of NF-κB.
In summary, our in vitro studies provide evidences that butein exerts its anti-inflammatory activity by down-regulation of pro-inflammatory mediators, via inhibition of ROS-MAPK-NF-κB signaling pathways in HaCaT cells. Our results suggest that butein may be used as a therapeutic compound against various inflammatory skin diseases.
MATERIALS AND METHODS
Cell culture and reagents
The immortalized human keratinocyte cell line, HaCaT, was maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin) at 37 ℃. Human THP-1 monocytic cells were maintained in RPMI 1640 medium, supplemented with 2 mM L-glutamine and 10% FBS. Butein, dichlorofluorescin diacetate (DCF-DA) and 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO, USA). Calcein acetoxymethyl ester (calcein-AM) was purchased from Molecular Probe (Eugene, OR, USA). Primary antibodies against ICAM-1, lamin B, and β-actin (Santa Cruz, CA, USA) and IκBα, phospho-ERK, ERK, phospho-p38, p38, phospho-JNK, and JNK (Cell Signaling Technology, Beverly, MA, USA) were obtained commercially. Oligonucleotide primers were purchased from Bioneer (Seoul, Korea). Butein was dissolved in dimethyl sulfoxide (DMSO) at 50 mM, and stored at −20 ℃
Cell viability assay
Cell viability was evaluated by MTT colorimetric assay (21). Briefly, HaCaT cells (1×106 cells/well) were seeded into each well of 6-well plates, and incubated with various concentrations of butein for 24 h in complete media, and then 1 mL of 1 mg/mL MTT reagent was added into each well for 2 h at 37 ℃. After removal of the MTT solution, 0.5 mL of DMSO was added to solubilize the blue formazan crystals. The absorbance was measured at 540 nm with a microplate reader (Labsystems Multiskan MCC/340, Pittsburg, PA, USA).
Measurement of intracellular ROS
ROS generation in the cells was assessed using the ROS sensitive dye DCF-DA (22). HaCaT cells were pretreated with butein for 4 h, and then exposed to 10 ng/ml TNF-α for 15 min. After washing with phosphate buffered saline twice, cells were incubated with 10 μM DCF-DA for 30 min. The fluorescence intensity was determined at 485 nm excitation and 538 nm emission wavelengths, using a Fluoroskan ELISA plate reader (Labsystems Oy, Helsinki, Finland).
Western blot analysis
Equal amounts of protein (20-30 μg) were separated by electrophoresis on 10% sodium dodecyl sulphate (SDS)-polyacrylamide gels. The proteins were transferred to nitrocellulose membranes. The membranes were probed with primary antibodies (1:1000 dilution), followed by horseradish peroxidase-conjugated secondary antibodies. The bound antibodies on the membranes were visualized by a chemiluminescence system (Millipore Corporation, Billerica, MA. USA) (6).
RT-PCR analysis
Total RNA was isolated from HaCaT cells or ear tissues of mice using a Trizol reagent kit (Invirogen, Gaithersburg, MD, USA), according to the manufacturer’s instructions. RNA concentrations were determined by measuring absorption at 260 nm in a spectrophotometer. cDNA was synthesized from 2 μg of total RNA, using 10,000 U of reverse transcriptase, and 0.5 μg/μl oligo-(dT)15 primer (Promega, Madison, WI, USA) (21). cDNA was amplified by polymerase chain reaction (PCR), using the following sense and antisense primers (5'→3'): human ICAM-1 sense, CAC CCT AGA GCC AAG GTG AC; human ICAM-1 antisense, CAT TGG AGT CTG CTG GGA AT; human IP-10 sense, GAA CCT CCA GTC TCA GCA CC; human IP-10 antisense, GCT CCC CTC TGG TTT TAA GGA GAT; human MCP-1 sense, AGT CTC TGC CGC CCT TCT GTG; human MCP-1 antisense, TGC TGC TGG TGA TTC TTC TAT; human IL-6 sense, AGA GTA GTG AGG AAC AAG CC; human IL-6 antisense, TAC ATT TGC CGA AGA GCC CT; human β-actin sense, GCG GGA AAT CGT GCG TGA CAT T; human β-actin antisense, GAT GGA GTT GAA GGT AGT TTC GTG. PCR products were separated on a 1% agarose gel, and visualized with UV light, after staining with ethidium bromide.
Cell adhesion assay
To analyze the adhesiveness of THP-1 cells to HaCaT cells, we performed a cell-cell adhesion assay, as described elsewhere (23). Briefly, HaCaT cells (7.0×104) were seeded in a 12-well plate. After 12 h incubation, HaCaT cell were pre-treated with butein at the indicated concentrations for 4 h, and then exposed to 10 ng/ml TNF-α for 12 h. Calcein-AM labeled THP-1 (7.0×105) cells were co-cultured with HaCaT cells for 1 h. After washing co-cultured cells with PBS, the fluorescence images were obtained at 485 nm excitation and 538 nm emission wavelengths, using a SPOT II digital camera-attached fluorescence microscope (Diagnostic instrument, Livingston, UK). The fluorescence intensities were quantitated, using a Fluoroskan ELISA plate reader (Labsystems Oy, Helsinki, Finland), at 485 nm excitation and 538 nm emission.
Enzyme-linked immunosorbent assay (ELISA)
To analyze the production of IL-6, IP-10 and MCP-1 in TNF-α-stimulated HaCaT cells, cells were pretreated with butein for 4 h, and then stimulated with 10 ng/ml TNF-α for 24 h. The supernatants from cell culture media were analyzed for the levels of cytokines or chemokines, using ELISA kits (R & D Systems, Minneapolis, MN), according to the manufacturer’s instructions (4).
Electrophoretic mobility shift assay (EMSA)
HaCaT cells pre-treated with butein for 4 h were exposed to 10 ng/ml TNF-α for 15 min. The nuclear extracts were prepared from HaCaT cells and analyzed for NF-κB DNA binding activity by EMSA as described previously (24). Double-stranded oligonucleotide corresponding to an NF-κB consensus sequence (5'-AGT TGA GGG GAC TTT CCC AGG C-3'; Promega), was end-labeled with [γ- 32 P]ATP, using T4 polynucleotide kinase. 32P-labeled oligonucleotide probe was incubated with nuclear extracts (5 μg) for 20 min on ice, in 20 μl of binding buffer (10 mM Tris-HCl, pH 8.0, 75 mM KCl, 2.5 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 0.25 mM DTT), containing 1 μg of poly dI/dC. Free DNA and DNA-protein complexes were then resolved by electrophoresis on a 6 % native polyacrylamide gel in TBE buffer (89 mM Tris-HCl, 89 mM boric acid, and 2 mM EDTA). Then, the gels were dried, and examined by autoradiography.
Statistical analysis
The results were expressed as the means ± SD from at least 3 independent experiments. The values were evaluated by oneway analysis of variance (ANOVA), followed by Duncan’s multiple range tests, using GraphPad Prism 4.0 software (GraphPad Software, Inc., San Diego, CA, USA). Differences were considered to be significant at P < 0.05.
Acknowledgments
This work was supported by the Priority Research Centers Program Grant (2009-0093812) and by a Grant (2012R1A-1A4A01007173) through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology. This work was also supported by the Hallym University Specialization Fund (HRF-S-12).
References
- 1.Gröne A. Keratinocytes and cytokines. Vet Immunol Immunopathol. (2002);88:1–12. doi: 10.1016/S0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 2.Dustin ML, Singer KH, Tuck DT, Springer TA. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. (1988);167:1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastiani S, Albanesi C, De PO, Puddu P, Cavani A, Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. (2002);293:552–559. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- 4.Song HY, Lee JA, Ju SM, et al. Topical transduction of superoxide dismutase mediated by HIV-1 Tat protein transduction domain ameliorates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice. Biochem Pharmacol. (2008);75:1348–1357. doi: 10.1016/j.bcp.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. (2005);1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Kwon DJ, Bae YS, Ju SM, Goh AR, Choi SY, Park J. Casuarinin suppresses TNF-α-induced ICAM-1 expression via blockade of NF-κB activation in HaCaT cells. Biochem. (2011);409:780–785. doi: 10.1016/j.bbrc.2011.05.088. [DOI] [PubMed] [Google Scholar]
- 7.Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. (2008);128:2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. (2006);72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Yadav VR, Prasad S, Sung B, Aggarwal BB. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int Immunopharmacol. (2011);11:295–309. doi: 10.1016/j.intimp.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng ZJ, Kuo SC, Chan SC, Ko FN, Teng CM. Antioxidant properties of butein isolated from Dalbergia odorifera. Biochim Biophys Acta. (1998);1392:291–299. doi: 10.1016/S0005-2760(98)00043-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Seo GS, Sohn DH. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by butein in RAW 264.7 cells. Biochem Biophys Res Commun. (2004);323:125–132. doi: 10.1016/j.bbrc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 12.Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaBregulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue. J Biol Chem. (2007);282:17340–17350. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 13.Lau GT, Huang H, Lin SM, Leung LK. Butein downregulates phorbol 12-myristate 13-acetate-induced COX-2 transcriptional activity in cancerous and non-cancerous breast cells. Eur J Pharmacol. (2010);648:24–30. doi: 10.1016/j.ejphar.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Jang JH, Yang ES, Min KJ, Kwon TK. Inhibitory effect of butein on tumor necrosis factor-α-induced expression of cell adhesion molecules in human lung epithelial cells via inhibition of reactive oxygen species gen- eration, NF-κB activation and Akt phosphorylation. Int J Mol Med. (2012);30:1357–1364. doi: 10.3892/ijmm.2012.1158. [DOI] [PubMed] [Google Scholar]
- 15.Bito T, Roy S, Sen CK, Packer L. Pine bark extract pycnogenol downregulates IFN-gamma-induced adhesion of T cells to human keratinocytes by inhibiting inducible ICAM-1 expression. Free Radic Biol Med. (2000);28:219–227. doi: 10.1016/S0891-5849(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 16.Albanesi C, Pastore S. Pathobiology of chronic inflammatory skin diseases: interplay between keratinocytes and immune cells as a target for anti-inflammatory drugs. Curr Drug Metab. (2010);11:210–227. doi: 10.2174/138920010791196328. [DOI] [PubMed] [Google Scholar]
- 17.Nickoloff BJ, Griffiths CE, Barker JN. The role of adhesion molecules, chemotactic factors, and cytokines in inflammatory and neoplastic skin disease--1990 update. J Invest Dermatol. (1990);94:151S–157S. doi: 10.1111/1523-1747.ep12876134. [DOI] [PubMed] [Google Scholar]
- 18.Dhar A, Young MR, Colburn NH. The role of AP-1, NF-kappaB and ROS/NOS in skin carcinogenesis: the JB6 model is predictive. Mol Cell Biochem. (2002);234-235:185–193. doi: 10.1023/A:1015948505117. [DOI] [PubMed] [Google Scholar]
- 19.K#246;hler HB, Huchzermeyer B, Martin M, De Bruin A, Meier B, Nolte I. TNF-alpha dependent NF-kappa B activation in cultured canine keratinocytes is partly mediated by reactive oxygen species. Vet Dermatol. (2001);12:129–137. doi: 10.1046/j.1365-3164.2001.00237.x. [DOI] [PubMed] [Google Scholar]
- 20.Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. (2005);174:5047–5056. doi: 10.4049/jimmunol.174.8.5047. [DOI] [PubMed] [Google Scholar]
- 21.Kwon DJ, Bae YS, Ju SM, Youn GS, Choi SY, Park J. Salicortin suppresses lipopolysaccharide-stimulated inflammatory responses via blockade of NF-κB and JNK activation in RAW 264.7 macrophages. BMB Rep. (2014);47:318–323. doi: 10.5483/BMBRep.2014.47.6.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ha SC, Han AR, Kim DW, et al. Neuroprotective effects of the antioxidant action of 2-cyclopropylimino-3-methyl-1,3-thiazoline hydrochloride against ischemic neuronal damage in the brain. BMB Rep. (2013);46:370–375. doi: 10.5483/BMBRep.2013.46.7.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youn GS, Kwon DJ, Ju SM, Choi SY, Park J. Curcumin ameliorates TNF-α-induced ICAM-1 expression and subsequent THP-1 adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. BMB Rep. (2013);46:410–415. doi: 10.5483/BMBRep.2013.46.8.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin SY, Kim CG, Lee YH. Egr-1 regulates the transcription of the BRCA1 gene by etoposide. BMB Rep. (2013);46:92–96. doi: 10.5483/BMBRep.2013.46.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]