Abstract
Objective
Although pain in knee osteoarthritis (OA) commonly affects activity engagement, the daily pain experience has not been fully-characterized. Specifically, the nature and impact of pain flares is not well-understood. This study characterized pain flares, defined by participants with knee OA; pain flare occurrence and experience were measured over 7 days.
Methods
This was a multiple methods study; qualitative methods were dominant. Data were collected during the baseline portion of a randomized controlled trial. Participants met criteria for knee OA and had moderate to severe pain. They completed questionnaires and a 7-day home monitoring period that captured momentary symptom reports simultaneously with physical activity via accelerometry (N = 45). Participants also provided individual definitions of pain flare which were used throughout the home monitoring period to indicate whether a pain flare occurred.
Results
Pain flares were described most often by quality (often sharp), followed by timing (seconds-minutes), and by antecedents and consequences. When asked if their definition of a flare agreed with a supplied definition, 49% of the sample reported only “somewhat”, “a little” or “not at all”. Using individual definitions, 78% experienced at least one daily pain flare over the home monitoring period; 24% had a flare on over 50% of the monitored days.
Conclusions
Pain flares were common, fleeting, and often experienced in the context of activity engagement. Participants’ views on what constitutes a pain flare differ from commonly accepted definitions. Pain flares are an understudied aspect of the knee OA pain experience and require further characterization.
In osteoarthritis (OA), pain is the main symptom that contributes to disability and reduced quality of life. Pain drives people to seek treatment; however, it is unclear whether current treatments address the most salient or distressing aspects of the pain experience for individuals with OA. Although treatments and clinical trials typically focus on pain intensity (1), adults with OA have identified other aspects of the pain experience as being important including pain qualities (e.g. stabbing, shooting) (2–7) and timing (5, 8, 9). These additional aspects may provide insight into mechanisms of pain and can help enhance treatment selection and assessment of treatment effectiveness. For instance, some descriptions of OA pain quality are more consistent with characteristics of nociceptive pain (e.g., sharp, stabbing) while others are more consistent with neuropathic pain (e.g., burning, spread of heat). Further, timing of pain episodes or “flares” has become an important element of pain experience to study to understand the pain’s impact on functioning. Previous research has characterized timing of pain severity through diurnal variations (10–12), seasonal effects (13, 14), and pain during movements (15, 16). The fluctuating nature of OA pain has been described by patients as being disruptive in daily life (5, 17, 18), however little is known about this day-to-day pain experience in OA.
Only one study combined aspects of pain intensity, qualities, and timing to understand OA pain experience. Hawker and colleagues identified two distinct OA pain profiles—intermittent periods of intense pain brought on by a trigger which then resolve, and a dull aching constant pain (background pain) that is increasingly punctuated by short intense pain episodes. Of these two pain types (intermittent and constant), intermittent, intense pain with an unpredictable trigger tends to affect mood and function most (8). Although characterized as distinct, periods of intense pain are present in both profiles. Little is known about the nature of these momentary pain “flares” in OA.
Few studies discuss pain flares in OA except in the context of research design in which potential participants of clinical trials must have a predefined increase in pain to indicate a flare, which may not have clinical relevance for patients (19–21). Although not well-characterized, flares have been described as inflammatory in nature (22) and may be experienced during or after a period of being active (23). For instance, in behavioral treatments, such as activity pacing, people with OA are thought to have pain increases (or have a flare-up) by engaging in too much activity (24–26). The goal of activity pacing is to dissociate the pain flare with the behavior of limiting activity that could reinforce a cycle of disuse and disability over time. While these studies have used the term ‘pain flare’, little is known about the actual experiences and nature of these periods of more intense pain in people with OA as they go about their daily lives. The purpose of this mixed methods study was to begin to characterize pain flares in individuals with knee OA. Our research design was a multilevel daily process study in which the cohort underwent a prospective 7 day data collection. Both qualitative and quantitative methods were used with qualitative methods being dominant in this study.
PATIENTS AND METHODS
Participants
Community-living adults aged ≥50 years were recruited through pain clinics at the University of Michigan. The study was approved by the University of Michigan Hospital Institutional Review Board. Participants were included in the larger study if they met criteria for knee OA as defined by American College of Rheumatology (ACR) criteria (27). Every patient enrolled was examined by a rheumatologist and had at least moderate knee pain (VAS of ≥40/100mm in the target knee or ≥4 of 10 on a NRS). OA severity was confirmed by radiographs. If both knees had OA, the target knee was the one with the highest pain.
Participants were excluded if they had a recent history of severe anemia, history of chronic severe kidney disease or moderate to severe hepatic impairment, had an allergy to or were previous or recent users of either study medication, were currently on chronic opioids, antidepressants, or centrally-acting chronic knee pain medications which were deemed by the investigators to potentially interfere with the study drugs or outcome assessments. Participants were also excluded if they had knee joint injections within the last 12 weeks. Participants could not be taking any supplements (e.g., glucosamine, chondroitin sulfate) during the study period. They were also excluded if having concurrent treatments for knee pain (e.g., physical and occupational therapy, acupuncture, cognitive behavioral therapy). Night shift workers or people with an extremely variable sleep schedule were excluded because of differential effects on accelerometry. All participants were ambulatory with or without an assistive device.
Procedure
After obtaining verbal and written consent, participants underwent the baseline assessment including questionnaires, a brief interview about pain flares, and instruction in the use of the Actiwatch-Score (Actiwatch-S; Philips Respironics, Bend, OR) accelerometer with an accompanying logbook for use in the home monitoring period. Participants were instructed to wear the Actiwatch-S on their non-dominant wrist for 7 days and to take it off only when there was a possibility of the device becoming wet. Participants were instructed to input pain severity ratings 8 times/day and to record ratings in the logbook along with wake and bedtimes each day. There were also end-of-day reports in which participants were asked about their pain flare experiences. After 7 days, participants returned the Actiwatch-S and logbook by mail.
Measures
Pain Flares
The study personnel introduced the concept of pain flares by saying:
“One of the goals of this project is to understand the daily pain experiences of people with knee osteoarthritis. We know that some people’s pain goes up and down across the day, and we know that the term ‘pain flare’ means different things to different people.”
The study personnel then asked “in your own words, can you please tell me what ‘pain flare’ means to you?” Based on the response, additional descriptors were probed in order to come up with an individual definition. Study personnel recorded responses verbatim. Participants were told to use their definition to determine if they experienced a pain flare during the 7 day period. The occurrence of pain flares was recorded daily in the logbook. Participants were also asked to describe the circumstances around the pain flare, such as the time, and what they were doing, and how it was different than their typical pain experience.
In addition to examining participant definitions of pain flare, the degree of concordance between the participant and investigator definitions of pain flare was assessed. The investigator definition —“inadequate pain relief for an episode of intense pain that is usually brought on by too much activity” was derived from the following sources (19, 20) as well as the research team’s experience. After presenting the definition, participants were asked to rate how well the definition captured their experience of pain flare on a scale of 0 = not at all to 4 = very much. Participants were also asked if there was anything missing from the investigator definition that should be included and were allowed to discuss their concerns about the definition.
Surveys and Background Variables
Demographics included age, gender, race/ethnicity, and marital status. Health status variables included pain severity in each joint with OA and body mass index (BMI). The Hospital Anxiety and Depression Scale (HADS) was used to measure anxiety and depressive symptoms (28); the scales range from 0 – 21 and ≥8 is considered clinically-relevant (29). Fatigue and sleep impairment were measured using PROMIS Scales which are measured on a standardized T scores metric (normative sample M = 50 ±10) (30). Pain was measured at baseline with the following: 1) Brief Pain Inventory (BPI) which has severity and interference subscales measured on 0 – 10 scales (31); 2) PainDETECT, which examines neuropathic pain (32), ranging from −1 – 38 with a higher score indicating more neuropathic pain; 3) and the Pain Quality Assessment Scale (PQAS) (33) which examines intensity of various pain qualities alone and grouped under different types--paroxysmal pain (shooting, sharp, electric, hot, and radiating), superficial pain (itchy, cold, numb, sensitive, and tingling), and deep pain (aching, heavy, dull, cramping, and throbbing) (34), all on scales of 0 – 10.
Measures from the Actiwatch Accelerometer
Daily Pain
Participants input ratings of pain severity into the Actiwatch-S 8 times a day (wakeup and approximately every 2 hours over the waking hours) for 7 days. Pain was rated from 0 = “no pain,” to 10 = “pain as bad as you can imagine.”
Daily Physical Activity
Physical activity was measured using the wrist-worn Actiwatch-S, an increasingly-used placement in population-based studies to overcome compliance issues with devices worn on the hip or waist (35). Although there are no gold standard methods to classify activity type or intensity for wrist accelerometers (36, 37), the overall raw activity counts indicate general physical activity levels (38). Studies support reliability and criterion validity of the Actiwatch-S (39) as well as discriminative validity between controls versus disease groups (12, 40). The accelerometer recorded activity over 30-second epochs. Our primary physical activity variable was average activity counts per min (AC/min) over the 7 days. A greater average AC/min indicates higher levels of activity. We also calculated pain variability over the 7 days by examining the average standard deviation of average AC/min.
Data Analysis
Qualitative analyses to conceptualize pain flares
We included participants’ descriptions of their pain experiences throughout the week (recorded in the log book), their verbatim responses to the open-ended question of “what does pain flare mean to you?” and to the investigator pain flare definition in the qualitative analysis. All data were transferred into an Excel spreadsheet prior to coding. Because the purpose of the qualitative component of the study was to investigate how participants conceptualized a pain flare, authors SM and HF independently conducted the qualitative content analysis (41, 42) using the following a priori categories: (a) pain descriptors, (b) antecedents to a pain flare, (c) temporal characteristics of a flare (onset and duration) and (e) any consequences of the flare (e.g., stopping or changing activities, mood changes, or actions taken for relief). After the initial round of coding, SM and HF jointly reviewed the coded data. Interrater reliability of 91% was achieved on the responses.
Quantitative analyses
Descriptive statistics were used to examine number of daily pain flares experienced by participants over the home monitoring period.
RESULTS
Eighty patients were screened for study participation. Twenty-eight were ineligible due to use of exclusionary medications or missed baseline assessments. Of 52 people who completed baseline assessments, 7 did not complete the home monitoring period leaving 45 participants. Participants were 55% female, mostly White (86%), mean age of 64 years (range 37 – 83), and 91% had bilateral knee OA. They reported mild - moderate pain on the Brief Pain Inventory (see Table 1). The sample had a mean score of 8.3 on the PainDETECT indicating low levels of neuropathic pain. Participants had low levels of depression and anxiety and were only slightly below population-based means for fatigue and sleep impairment. On the PQAS, the sample reported the highest level of intensity in those qualities indicative of deep pain (3.1±2.1), followed by paroxysmal pain (2.9±2.0), and surface pain (1.0±1.3). Unpleasantness was the most highly rated item on the PQAS (5.9±2.6). Of all pain qualities assessed, sharp (4.8±3.1) and intense pain (4.8±2.5) were reported to be the highest. In the home monitoring period, participants reported pain severity at mild to moderate levels (3.1) with an average pain variability of 1.1 standard deviations. Their average physical activity (AC/min) was slightly higher than in other samples of people with OA using this method (43, 44).
Table 1.
Participant Characteristics at Baseline n = 45
| Variable | Mean (sd) | Range |
|---|---|---|
| Age | 64.1 (10.0) | 37–83 |
| % Female (n) | 54.5 (24) | |
| Race | ||
| White | 86.4 (38) | |
| Black | 6.8 (3) | |
| Unknown | 6.8 (3) | |
| Body Mass Index (n = 40) | 30.9 (6.1) | 20.7 – 45.4 |
| PainDETECT(neuropathic pain scale) | 8.3 (6.3) | 0 – 20 |
| Brief Pain Inventory Severity Subscale | 3.7 (2.0) | .3 – 8.3 |
| Brief Pain Inventory Interference Subscale | 3.1 (2.3) | .1 – 8.7 |
| PROMIS Fatigue scale (T score) | 49.4 (7.5) | 33.4 – 64.8 |
| PROMIS Sleep Impairment scale (T score) | 46.3 (8.4) | 30 – 64.3 |
| HADS Depression | 2.7 (2.3) | 0 – 10 |
| HADS Anxiety | 4.1 (2.8) | 0 – 12 |
| PQAS (individual items) | ||
| Unpleasant | 5.9 (2.6) | 0 – 10 |
| Sharp | 4.8 (3.1) | 0 – 10 |
| Intense | 4.8 (2.5) | 1 – 9 |
| Aching | 4.3 (3.2) | 0 – 10 |
| Dull | 3.6 (2.4) | 0 – 8 |
| Shooting | 3.4 (3.0) | 0 – 10 |
| Tender when something pressed against skin | 2.8 (2.9) | 0 – 9 |
| Throbbing | 2.8 (2.8) | 0 – 9 |
| Heavy | 2.5 (2.9) | 0 – 9 |
| Cramping | 2.5 (2.7) | 0 – 8 |
| Hot | 2.1 (2.7) | 0 – 9 |
| Radiating | 2.1 (2.5) | 0 – 9 |
| Electrical | 2.0 (2.6) | 0 – 10 |
| Numb | 1.7 (2.5) | 0 – 8 |
| Tingling | 1.6 (2.5) | 0 – 9 |
| Sensitive to light touch | .6 (1.3) | 0 – 5 |
| Itchy | .5 (1.6) | 0 – 9 |
| Cold | .5 (1.3) | 0 – 7 |
| Weekly Average Pain | 3.1 (2.0) | .22 – 8.24 |
| Weekly Average Pain Variability | 1.1 (.5) | .4 – 2.4 |
| Weekly Average activity counts per minute (AC/min) | 355.2 (131.4) | 179.2 – 798.5 |
How are pain flares conceptualized by participants?
In response to the question, “What does a pain flare mean to you?”, participants described flares in terms of the pain quality, timing (e.g., onset and duration), and antecedents and consequences. Table 2 shows participants’ responses grouped under each theme. Participants used various terms to describe the quality of pain experienced during flares with some participants using more than one descriptor. The majority of participants described their pain flare experience using terms associated with pain quality; 36% of descriptors of pain flares were ‘sharp,’ and 42% included other qualities: ‘shooting’, ‘spark’, ‘electrical’, ‘achy’, ‘spike’, ‘needles’, ‘burning’, ‘burst’, ‘pulsating’, and ‘spread of heat’. In contrast, some descriptions focused on pain magnitude rather than quality. For example, 22% defined the pain flare experience as a general increase in pain while 13% characterized it as an ‘intense’ or ’severe’ level of pain.
Table 2.
What does a pain flare mean to you? N = 45
| Theme | Description | Frequency of Responses | Participant Quotes |
|---|---|---|---|
| Pain Qualities | Sharp | 16 |
|
| Increase in pain/higher pain | 10 |
|
|
| Intense (severe) | 6 |
|
|
| Other—Electrical, glitch, twinge, stabbing, burning | 19 |
|
|
|
| |||
| Timing | Short duration | 11 |
|
| Sudden onset | 7 |
|
|
| Variable | 5 |
|
|
|
| |||
| Antecedents | Specific Activities or increase in usual activity | 17 |
|
| Sitting/staying in one position too long/sleeping at night | 5 |
|
|
|
| |||
| Consequences | Activity interference | 2 |
|
| Seeks remedies | 2 |
|
|
| Lasting pain | 1 |
|
|
| Cues about changing position/function | 2 |
|
|
The terms that participants used to describe pain flares also reflected the variable onset and duration of the flare. Sixteen percent of participants described flares that had a ‘quick’ or ‘sudden’ onset, and 24% described timing of the flares as having lasted from a few seconds to 10–15 minutes. For example, one participant described a pain flare as ‘an electrical shock’ with pain lasting only ~10 seconds. However, not all flares were experienced as brief events; 11% of participants experienced flares of variable intensity and duration.
Of participants who mentioned antecedents to pain flares, antecedent events were most often reported as activity-related (reported by 38% of participants). However, two participants mentioned that there was no particular preceding event to pain flares. The activities most commonly associated with a pain flare were typical daily activities (standing, walking, or getting up from sitting). Eleven percent of participants reported that flares were due to staying in one position too long. The two most common actions taken in response to flares were to rest, and to take additional pain medication. Thirteen percent of participants discussed the consequences of pain flares. Consequences of having a pain flare pertained to interfering with activity performance, seeking pain remedies, having lasting pain, or providing a personal cue about their own function.
Investigator vs. participant definitions of pain flare
When asked how well the supplied research definition of pain flare captured their experience, 51% of the sample said “very much” or “quite a bit” (11% and 40% respectively); 33% said “somewhat”; and 16% said “a little” or “not at all” (9% and 7% respectively). Across participants, 34 (76%) suggested modifications to the investigator definition of pain flares to better match their pain flare experience. Their responses (in Table 3) could be grouped under the same categories as in Table 2—Pain Qualities, Timing, Antecedents, and Consequences.
Table 3.
Participant responses regarding what is missing from the investigator definition of pain flare - Inadequate pain relief for an episode of intense pain that is usually brought about by too much activity
| Participant Quotes | ||
|---|---|---|
| Pain Qualities |
|
|
| Timing |
|
|
| Antecedents |
|
|
| Consequences |
|
|
The most commonly reported area of improvement was the antecedent of pain flare. There were 19 participants who commented on activity not being a trigger or not being the only trigger for pain flares. Responses and suggestions regarding antecedents to pain flares are in Table 3. Some participants also suggested that being sedentary or being in one position (sitting or sleeping) or other reasons (weather or pressure) may cause a pain flare. Others suggested that “too much” activity did not capture pain associated with routine daily activities (e.g., walking or standing) and some suggested including specific activities. In addition to antecedents, some participants suggested additional descriptors of pain quality (burning, heaviness, intense pain, spikes). Some participants commented on adding aspects of timing to the investigator definition of pain flare, such as onset (e.g., suddenly), frequency, or duration of flare (e.g., short to more long lasting e.g., up to 24 hours). A few participants mentioned clarifying consequences such as what is meant by pain relief, use of pain medications, or how flares impact activity.
How are pain flares experienced in daily life?
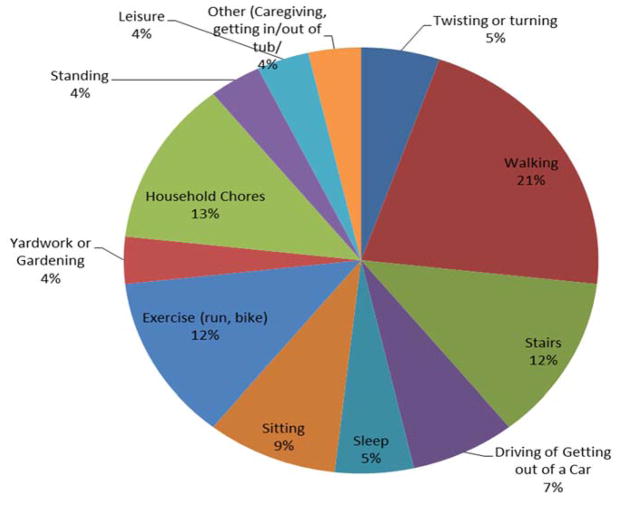
Over the 7-day home monitoring period, daily pain flares were common; 78% of the sample who had data on pain flares experienced at least one pain flare according to their individual definitions. Almost one quarter of the sample (24%) experienced daily pain flares on over 50% of the monitored days. The mean number of pain flares for the sample was 2.2 (± 1.9). Participants identified numerous possible flare antecedents (see Figure 1). When asked what they were doing when the pain flare occurred, participants most often reported walking and going up or down stairs, followed by exercise and household chores (e.g., cleaning, laundry, or yard work). Some participants identified ‘sedentary’ antecedents to flares, such as sitting while watching television, driving, or sleeping.
Figure 1.
Reported Antecedents of Pain Flares Over 7 Days (N = 31)
Nearly all participants identified more than one antecedent and antecedents could change daily. For example, one participant reported flares on three consecutive days, and each was associated with a different antecedent. In addition, across the sample there was no clear association between sudden changes in usual daily activities (e.g., sudden increases in time spent sitting, or increases in physical exertion) reported by participants and incidence of pain flares.
DISCUSSION
We sought to better understand pain flares from the perspectives of people with knee OA. When asked to describe a pain flare, participants reported one or more of the following aspects: pain quality, timing, antecedents, or consequences; which are similar to what has been reported in earlier qualitative studies on overall pain experience in OA (6–8). Aspects of pain experience related to timing (i.e., pain pattern) and predictability of antecedents to pain are important to understand as they are differentially associated with mood, mobility, and sleep (8) and symptom acceptability (45). Thus, further insight into pain experience can ultimately guide the tailoring of knee OA treatments that will be most relevant to individuals. Further study of pain flares, in particular, can provide additional insight into momentary aspects of pain experience focused on timing or patterns (intermittent or constant) which are both described as involving flare-like experiences and on predictability of antecedents to pain in daily life (8).
The majority of participants described pain flares as a sudden onset of sharp pain which was of short duration (seconds to a few minutes). Interestingly the participants’ descriptions of pain flares were in contrast with the supplied definition; almost half of participants felt that the supplied definition of pain flare did not match their own personal definition well. There were missing elements that needed further specification, such as broadening pain quality descriptors and antecedents. In order to better capture pain flare experiences, further studies will be needed to develop and validate a tool to for this purpose.
In this study, participants were asked to use their own definition of pain flares and report on any pain flare experience over 7 days. During that time, pain flares were commonly reported and resulted from multiple antecedents. Antecedents to pain flares were commonly described and were most often activity-related, such as from walking or stair-climbing, or resulted from sedentary activities. Although predictability of the antecedent was not specifically measured, participants’ description of ‘quick’ or ‘sudden’ onset may be indicative of unpredictability.
In addition to the potential of better understanding aspects of pain experience to tailor interventions to patients, pain flares in knee OA requires further study to examine the chronic pain experience over time in relation to disease progression (46). Neogi found that consistency of pain (pain on most days in the past month) was associated with more radiographic severity of knee OA compared to inconsistent pain, which was associated with more mobility and perhaps earlier disease progression (9). The examination of pain flares may provide insight into the real-life pain experience of people at different stages of OA disease. One aspect in which they may differ is in terms of antecedents. Although different activities were mentioned, there may be common characteristics, such as bone-loading intensity of activity (i.e., stairs) (47), and bone-loading is associated with nociceptive pain (48). In addition, knee instability will be important to measure in relation to pain flares. It is possible that pain flares for some people are associated with knee locking (as mentioned by a few participants) or with knee buckling during activities. Although no participants used the term ‘buckling’, the end of day questions regarding pain flares only pertained to what activities they were doing and not on their physical reactions. Buckling, which is associated with reduced function and low quadriceps strength (49), could occur in conjunction with pain flares. Because many flares happened during walking, gait issues of people with and without pain flares may also be important to examine.
Limitations
Because so little was known regarding the frequency of pain flares in this population prior to this study, we designed this study to capture daily pain flares. Some participants included extra accounts of pain flares within a day which was not expected. Given the variability in reporting, we collapsed these extraneous entries into a daily measure of pain flare (yes/no); however, future studies could include more frequent ascertainment of pain flares and could utilize event-based sampling instead of end of day recall. Further, we did not assess other aspects of the pain flare experience in the monitoring period such as the momentary pain quality, momentary pain severity, reported consequences or predictability of the flare. Another study limitation is that the participants were mainly white, and other racial and ethnic groups may have different experiences. Further, the participants had only mild to moderate pain, had very low levels of anxiety or depressive symptoms, and mild to moderate intensity of individual pain qualities. Pain flares may be different in cohorts with higher symptom levels and mood disturbance. Finally a larger sample is needed to understand differences of people with and without pain flares. Despite limitations, this study provided a rich source of data on the pain flare experience in knee OA. Further, the roughly equal representation of males and females was a strength in this study. Having a substantial proportion of men in the study ensured that a wide range of male experiences were represented.
Future directions
One direction is to develop and validate a measure of pain flares and to examine whether the characterization of pain flares varies at different stages of disease or with different symptom burdens. This will require additional qualitative studies with people from across the disease and symptom burden spectrum. Because pain has a reciprocal relationship with social, emotional, physical, functional well-being, there is a need to better understand how pain flares fit into these processes. The actual experience of pain flares, as to when pain occurs during a pain flare (such as at the beginning of a movement, when changing positions, or when sedentary), can also be further examined and may provide insight to underlying biomechanical and disease processes. The consequences of pain flares need to be understood more fully and future studies should examine the effect of pain flares on activity engagement, medication usage, and on later mood, activity, and sleep. Particular characteristics, such as pain catastrophizing, that could contribute to maladaptive patterns of functioning (e.g., limiting daily activities and reducing activity) after pain flare experiences should also be investigated.
While our study used both quantitative and qualitative methods, with a dominant focus on qualitative approaches to begin to characterize pain flares from the participants’ perspective, further quantitative analyses of these pain flare data will be needed. An important aspect which we will examine in a future study with these data is the day-to-day associations of pain flares to pain variability, physical activity, and symptoms over the 7 days. These analyses will enable us to better examine if and how pain flares are associated with later day or next day symptoms and activity.
Conclusion
In this study, we began to characterize pain flares in participants with knee OA. We found that pain flares were commonly experienced and typically of sudden onset and short duration and had negative consequences on daily routines. We also found that the current literature definitions of pain flare do not align with participants’ perspectives. Pain flares require further study in order to better understand the daily pain experience of people with knee OA.
SIGNIFICANCE AND INNOVATION.
Among adults with painful knee osteoarthritis, daily pain flares were common and tended to be an intense, transient pain experience.
Participants did not agree that commonly used definitions of pain flare in the literature represented their experience.
This study contributes to the understanding of pain flares in knee OA, an experience not yet characterized in this population.
Acknowledgments
Supported by: The project described was supported by an Investigator Initiated Grant from Nuvo/Zars Pharmaceuticals and also by Merck Pharmaceuticals. KP was supported by a National Institutes of Health K23 AR 060241.
We thank Ryan Scott for assistance with data collection and data management.
Footnotes
Conflict of Interest: At the time this study was conducted, Dr. Gammaitoni was an employee of Nuvo research, but no longer works there and has no monetary connection with the company.
References
- 1.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Hochman JR. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–54. doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1236–42. doi: 10.1016/j.joca.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Hochman J, French M, Bermingham S, Hawker G. The nerve of osteoarthritis pain. Arthritis Care Res. 2010;62:1019–23. doi: 10.1002/acr.20142. [DOI] [PubMed] [Google Scholar]
- 5.Gignac M, Davis A, Hawker G, Wright J, Mahomed N, Fortin P. “What do you expect? You’re just getting older”: A comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum. 2006;55:905–12. doi: 10.1002/art.22338. [DOI] [PubMed] [Google Scholar]
- 6.Wagstaff S, Smith OV, Wood PH. Verbal pain descriptors used by patients with arthritis. Ann Rheum Dis. 1985;44(4):262–5. doi: 10.1136/ard.44.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papageorgiou AC, Badley EM. The quality of pain in arthritis: The words patients use to describe overall pain and pain in individual joints at rest and on movement. J Rheumatol. 1989;16(1):106–12. [PubMed] [Google Scholar]
- 8.Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, et al. Understanding the pain experience in hip and knee osteoarthritis - an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:415–22. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Neogi T, Nevitt MC, Yang M, Curtis JR, Torner J, Felson DT. Consistency of knee pain: correlates and association with function. Osteoarthritis Cartilage. 2010;18:1250–5. doi: 10.1016/j.joca.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen KD, Coffman CJ, Golightly Y, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis Cartilage. 2009;17(10):1275–82. doi: 10.1016/j.joca.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy N, Sothern RB, Campbell J. Rhythmic variations in pain perception in osteoarthritis of the knee. J Rheumatol. 1990;17(3):364–72. [PubMed] [Google Scholar]
- 12.Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Rheum. 2008;59(6):849–56. doi: 10.1002/art.23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorleijn DMJ, Luijsterburg PAJ, Burdorf A, Rozendaal RM, Verhaar JAN, Bos PK, et al. Associations between weather conditions and clinical symptoms in patients with hip osteoarthritis: A 2-year cohort study. Pain. 2014;155(4):808–13. doi: 10.1016/j.pain.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Hawley DJ, Wolfe F, Lue FA, Moldofsky H. Seasonal symptom severity in patients with rheumatic diseases: A study of 1424 patients. J Rheumatol. 2001;28(8):1900–9. [PubMed] [Google Scholar]
- 15.Keefe FJ, Caldwell DS, Queen K, Gil KM, Martinez S, Crisson JE, et al. Osteoarthritic knee pain: a behavioral analysis. Pain. 1987;28(3):309–21. doi: 10.1016/0304-3959(87)90066-2. [DOI] [PubMed] [Google Scholar]
- 16.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87(3):325–34. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 17.Ong BN, Hooper H, Jinks C, Dunn K, Croft P. ’I suppose that depends on how I was feeling at the time’: Perspectives on questionnaires measuring quality of life and musculoskeletal pain. J Health Services Res Policy. 2006;11(2):81–8. doi: 10.1258/135581906776318938. [DOI] [PubMed] [Google Scholar]
- 18.Sanders C, Donovan J, Dieppe P. The significance and consequences of having painful and disabled joints in older age: Co-existing accounts of normal and disrupted biographies. Sociol Health Illn. 2002;24(2):227–53. [Google Scholar]
- 19.Battisti WP, Katz NP, Weaver AL, Matsumoto AK, Kivitz AJ, Polis AB, et al. Pain management in osteoarthritis: A focus on onset of efficacy—a comparison of rofecoxib, celecoxib, acetaminophen, and nabumetone across four clinical trials. J Pain. 2004;5:511–20. doi: 10.1016/j.jpain.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Pareek A, Chandurkar N, Ambade R, Chandanwale A, Bartakke G. Efficacy and safety of etodolac-paracetamol fixed dose combination in patients with knee osteoarthritis flare-up: a randomized, double-blind comparative evaluation. Clin J Pain. 2010;26:561–7. doi: 10.1097/AJP.0b013e3181e15bba. [DOI] [PubMed] [Google Scholar]
- 21.Trijau S, Avouac J, Escalas C, Gossec L, Dougados M. Influence of flare design on symptomatic efficacy of non-steroidal anti-inflammatory drugs in osteoarthritis: A meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2010;18:1012–8. doi: 10.1016/j.joca.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Bliddal H. Guidelines for the use of nonsurgical interventions in osteoarthritis management. Expert Rev Clin Immunol. 2008;4(5):583–90. doi: 10.1586/1744666X.4.5.583. [DOI] [PubMed] [Google Scholar]
- 23.Lane NE, Hochberg MC, Pressman A, Scott JC, Nevitt MC. Recreational physical activity and the risk of osteoarthritis of the hip in elderly women. J Rheumatol. 1999;26:849–54. [PubMed] [Google Scholar]
- 24.Fordyce W. Behavioral Methods for Chronic Pain and Illness. St. Louis: Mosby; 1976. [Google Scholar]
- 25.Birkholtz M, Aylwin L, Harman RM. Activity pacing in chronic pain management: One aim, but which method? Part one: Introduction and literature review. Br J Occup Ther. 2004;67:447–52. [Google Scholar]
- 26.Murphy SL, Lyden AK, Clary M, Geisser ME, Yung RL, Clauw DJ, Williams DA. Activity pacing for osteoarthritis symptom management: study design and methodology of a randomized trial testing a tailored clinical approach using accelerometers for veterans and non-veterans. BMC Musculoskelet Disord. 2011;12:177. doi: 10.1186/1471-2474-12-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale-A review of validation data and clinical results. J Psychosom Res. 1997;42:17–41. doi: 10.1016/s0022-3999(96)00216-4. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11:304–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Keller SB, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–20. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Gammaitoni AR, Olaleye DO, Oleka N, Nalamachu SR, Galer BS. The Pain Quality Assessment Scale: Assessment of pain quality in carpal tunnel syndrome. J Pain. 2006;7:823–32. doi: 10.1016/j.jpain.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS. Dimensions of pain quality: Factor analysis of the pain quality assessment scale. Clin J Pain. 2008;24:550–5. doi: 10.1097/AJP.0b013e31816b1058. [DOI] [PubMed] [Google Scholar]
- 35.Troiano R, Berrigan D, Dodd K, Masse L, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 36.Mannini A, Intille SS, Rosenberger M, Sabatini AM, Haskell W. Activity recognition using a single accelerometer placed at the wrist or ankle. Med Sci Sports Exerc. 2013;45(11):2193–203. doi: 10.1249/MSS.0b013e31829736d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberger ME, Haskell WL, Albinali F, Mota S, Nawyn J, Intille S. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med Sci Sports Exerc. 2013;45:964–75. doi: 10.1249/MSS.0b013e31827f0d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westerterp KR, Plasqui G. Physical activity and human energy expenditure. Curr Opin Clin Nutr Metab Care. 2004;7:607–13. doi: 10.1097/00075197-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Gironda RJ, Lloyd J, Clark ME, Walker RL. Preliminary evaluation of reliability and criterion validity of Actiwatch-Score. J Rehabil Res Dev. 2007;44:223–30. doi: 10.1682/jrrd.2006.06.0058. [DOI] [PubMed] [Google Scholar]
- 40.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 41.Miles M, Huberman AM. Qualitative Data Analysis. Thousand Oaks, CA: Sage; 1994. [Google Scholar]
- 42.Saldana J. The Coding Manual for Qualitative Research. Thousand Oaks, California: Sage; 2009. [Google Scholar]
- 43.Murphy SL, Alexander NB, Levoska M, Smith DM. Relationship between fatigue and subsequent physical activity among older adults with symptomatic osteoarthritis. Arthritis Care Res. 2013;65:1617–24. doi: 10.1002/acr.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy SL, Smith DM, Alexander NB. Measuring activity pacing in women with lower-extremity osteoarthritis: A pilot study. Am J Occup Ther. 2008;62:329–34. doi: 10.5014/ajot.62.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu A, Kendzerska T, Stanaitis I, Hawker GA. The relationship between knee pain characteristics and symptom state acceptability in people with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:178–83. doi: 10.1016/j.joca.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Hawker GA. The challenge of pain for patients with OA. HSS J. 2012;8:42–4. doi: 10.1007/s11420-011-9254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelley S, Hopkinson G, Strike S, Luo J, Lee R. An accelerometry-based approach to assess loading intensity of physical activity on bone. Res Q Exerc Sport. 2014;85:245–50. doi: 10.1080/02701367.2014.897680. [DOI] [PubMed] [Google Scholar]
- 48.Felson DT. The sources of pain in knee osteoarthritis. Current Opin Rheumatol. 2005;17:624–8. doi: 10.1097/01.bor.0000172800.49120.97. [DOI] [PubMed] [Google Scholar]
- 49.Felson DT, Niu J, McClennan C, Sack B, Aliabadi P, Hunter DJ, et al. Knee buckling: Prevalence, risk factors, and associated limitations in function. Ann Intern Med. 2007;147:534–40. doi: 10.7326/0003-4819-147-8-200710160-00005. [DOI] [PubMed] [Google Scholar]