Abstract
Pseudotumor cerebri syndrome (PTCS) is defined by the presence of elevated intracranial pressure (ICP) in the setting of normal brain parenchyma and cerebrospinal fluid (CSF). Headache, vision changes, and papilledema are common presenting features. Up to 10% of appropriately treated patients may experience permanent visual loss. The mechanism(s) underlying PTCS is unknown. PTCS occurs in association with a variety of conditions, including kidney disease, obesity, and adrenal insufficiency, suggesting endocrine and/or metabolic derangements may occur. Recent studies suggest that fluid and electrolyte balance in renal epithelia is regulated by a complex interaction of metabolic and hormonal factors; these cells share many of the same features as the choroid plexus cells in the central nervous system (CNS) responsible for regulation of CSF dynamics. Thus, we posit that similar factors may influence CSF dynamics in both types of fluid-sensitive tissues. Specifically, we hypothesize that, in patients with PTCS, mitochondrial metabolites (glutamate, succinate) and steroid hormones (cortisol, aldosterone) regulate CSF production and/or absorption. In this integrated mechanism review, we consider the clinical and molecular evidence for each metabolite and hormone in turn. We illustrate how related intracellular signaling cascades may converge in the choroid plexus, drawing on evidence from functionally similar tissues.
Pseudotumor cerebri syndrome (PTCS) is defined by the presence of elevated intracranial pressure (ICP) in the setting of normal brain parenchyma and normal cytological and chemical analyses of the cerebrospinal fluid (CSF). Presenting signs and symptoms are varied, particularly in the pediatric population, but commonly include headache, visual disturbances (i.e., vision loss or double vision), and papilledema. Patients typically recover with appropriate medical or surgical treatments, but up to 10% of patients may experience permanent visual loss (1). Estimated annual incidence rates of PTCS are 0.9 per 100,000 in both the symptomatic pediatric and general adult populations (2,3). The classification and diagnostic criteria of PTCS have recently been revised in an effort to improve consistency in nomenclature, including distinguishing between apparently primary and secondary (e.g., medication-related) causes (4).
The mechanisms underlying PTCS are poorly understood (see reviews (5–7)); however, the central pathological feature of PTCS is the presence of elevated ICP. Occurring in an enclosed compartment, in the absence of a space-occupying mass, an increase in ICP may be the result of either increased CSF production and/or reduced CSF outflow. Many studies have used advanced neuro-imaging techniques to examine CSF dynamics in PTCS. Many have demonstrated an increased resistance to CSF absorption within the cerebral venous system (8,9), related to increased cerebral sinus pressure or increased central systemic venous pressure (9), for example. However, questions remain about the relative contributions, and regulation, of accessory CSF outflow routes and the specific site of resistance. Impaired CSF absorption is not likely to explain the pathogenesis of PTCS in its entirety. Rather, there is evidence that primary (obesity-associated) and secondary PTCS may have differing CSF flux disturbances (8). Relevant to focus of the current review, PTCS occurring in the setting of associated endocrinopathies appears to be related to increased CSF production and secondarily raised resistance to CSF absorption (8). As many cases of pediatric PTCS are associated with endocrine or metabolic disorders, this observation may be particularly relevant to this population. An altered balance of CSF production and absorption is further supported when considering the actions of pharmacologic agents used to lower ICP. Acetazolamide and furosemide reduce CSF production (10). Prednisone is a steroid medication used to lower ICP and manage PTCS at elevated ICPs, prednisone limits CSF production and absorption (11).
A Proposed Integrated Mechanism
We propose that a range of metabolic and hormonal signals regulates CSF dynamics not only by influencing the resistance to CSF absorption but also by acting at the level of the choroid plexus epithelial cells to regulate CSF secretion. The choroid plexus and its constituent epithelial cells are the key site of CSF production and many of the secretory regulatory mechanisms in the choroid plexus are analogous to those within the renal medullary epithelial cells (12). Thus, we further suggest that one way to gain additional novel insights into the mechanisms of PTCS is to translate new insights from renal physiology. PTCS does occur in adult and pediatric patients with renal disease (13) and may complicate management of post-renal-transplant patients, although this observation historically has not been felt to be a direct association but rather attributed to medication effects or secondary changes in body weight and/ or hypercoagulability (14).
Recent laboratory investigations demonstrate that complementary transporters regulate fluid balance in the kidney and central nervous system (CNS) (see Figure 1). For example, electro-neutral Na+-HCO3- cotransporters are expressed in the medullary collecting duct epithelial cells of the kidney, as well as the epithelial cells of the choroid plexus in the CNS (12). Studies in animals engineered to lack these key transporters (15) lend additional support to this contention. Knock-out of the SCL4 family of bicarbonate transporter leads to reduced brain ventricle size, perhaps due to decreased CSF production (15). Carbonic anhydrase inhibitors influence the activity of this transporter system (see Figure 1) and are effective in the treatment of patients with PTCS (10). Finally, mice lacking aquaporin-1 (AQP, see Figure 1), another important water transport channel, also had reduced ICP and CSF production (15). Specifically, we hypothesize that in patients with PTCS, mitochondrial metabolites (glutamate and succinate), along with insulin, and steroid hormones (cortisol, 11-deoxycortisol, aldosterone, 11-deoxycorticosterone; see below), regulate CSF production, by the choroid plexus, and CSF absorption, at the arachnoid villi, ultimately leading to raised ICP. Together, these factors represent components of an integrated neuroendocrine pathophysiology that underlies PTCS. Our summary mechanistic model is shown in the Figure 1 (16). Where evidence is available, we have focused our discussion on pediatric PTCS.
Figure 1.
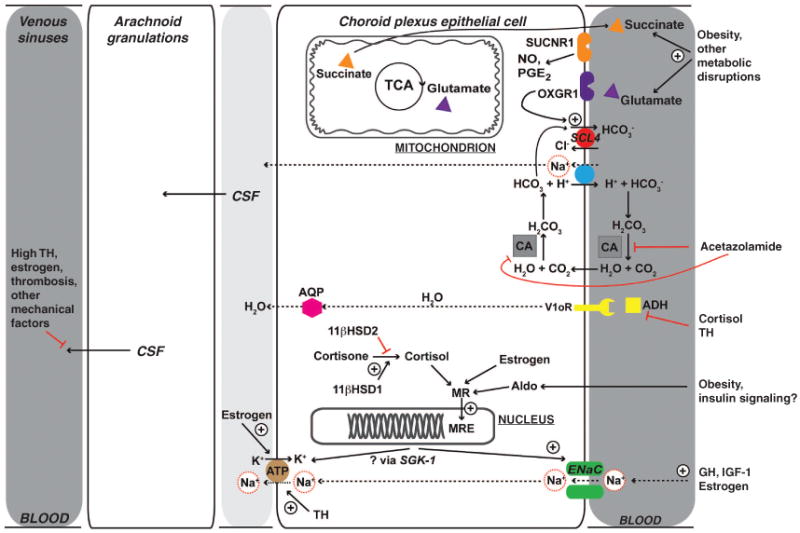
Proposed metabolic and hormonal mechanisms underlying pediatric PTCS demonstrate some of the potential mechanisms underlying pediatric PTCS related to CSF dynamics. Of note, much of this remains to be tested. CSF production and absorption are illustrated. At the choroid plexus epithelium, the site of CSF production, apical Na+-dependent transporters, apical Na+ channels, and the basolateral Na+/K+ ATPase allow transcellular Na+ flow, in turn, facilitating water movement and CSF production. For example, carbonic anhydrase activity (gray box) produces intracellular H+ and HCO3-, the former of which exits the cell via the Na+/H+ exchanger (blue circle), allowing Na+ flux into the cell. Acetazolamide blocks carbonic anhydrase and limits this capacity. Cortisol (unless inactivated by 11βHSD2) acts at the mineralocorticoid response element (MRE) to promote Na+/K+ ATPase activity, as well as Na+ flux into the cell by ENaC. Growth hormone (GH) may also stimulate Na+ flux into the cell by ENaC. Both of these facilitate ongoing Na+ movement into CSF. Insulin has an antinatriuretic effect, which may exacerbate this in the setting of obesity-related insulin resistance and hyperinsulinemia. (Of note, initiation of insulin therapy can be associated with fluid retention, so-called “insulin edema.” This may be mediated by serum- and glucocorticoid-inducible kinase, SGK-1, which has been posited to integrate the various endocrine signals that affect sodium and water balance.) Na+ and Ca++ transport are closely related in the kidney, and may also be important in the choroid, which could account for the association of PTCS with hypoparathyroidism (not shown). Cortisol and thyroid hormone may also affect anti-diuretic hormone related free water clearance. Metabolites reflecting energy balance and acid-bases status (postulated from the renal literature, as a result of similarities between medullary collecting duct epithelial cells of the kidney and the epithelial cells of the choroid plexus) are also shown. The TCA cycle intermediate α-ketoglutarate is in equilibrium with glutamate. Acting at its receptor, OXGR1, glutamate stimulates Cl--dependent HCO3- secretion, which allows movement of Na+ into the cell. Succinate acts to stimulate vasoactive factors that may also affect both blood flow and ultimately salt and water balance. CSF absorption: The role of altered CSF absorption is less well-understood (see review (7)). Mechanical factors, including, for example, venous sinus thrombosis, would be expected to impair CSF absorption and lead to increase pressure. Historically, CSF absorption has been viewed as a “passive” process, although it may be that there are regulatory aspects that remain to be explained. Net osmotic flow of Na+ and water is indicated by dashed lines. Red lines indicate inhibitory effects, black arrows, stimulatory effects. 11βHSD1/2, 11β-hydroxysteroid dehydrogenases, type 1 and 2; ADH, antidiuretic hormone or vasopressin; AQP, aquaporin; CA, carbonic anhydrase; CSF, cerebrospinal fluid; ENaC, epithelial sodium channel; GH, growth hormone; MRE, mineralocorticoid response element; NO, nitric oxide; OXGR1, glutamate receptor; PGE2, prostaglandin E2; PTCS, pseudotumor cerebri syndrome; SUCNR1, succinate receptor; TCA, citrate cycle; V1oR, vasopressin receptor, type V1. Adapted with permission from ref. (16). Copyright @ 2010, The Endocrine Society.
Role of Metabolic Metabolites in PTCS: Glutamate/α-Ketoglutarate and Succinate
Although the details remain unclear, at least a few studies in humans have supported the contention that global metabolic changes may be involved in the pathophysiology of PTCS. In a study of adult patients (17), CSF metabolomics revealed elevated lactate and evidence of tricarboxylic acid cycle disruption; these initial findings deserve follow-up study. Preliminary metabolomics studies have also generated hypotheses about the metabolic contributions to other neuro-ophthalmologic conditions related to PTCS (18).
In the mitochondria, nutrients from the diet are processed through the tricarboxylic acid cycle to generate reducing equivalents. These reducing equivalents are ultimately used to produce ATP, the energy “currency” of the cell. Glutamate and succinate are two important tricarboxylic acid cycle intermediates. In the kidney, these metabolites act via specific cellular receptors to affect fluid balance; this may reflect a homeostatic response to changes in energy balance, although the significance of this signaling system is just beginning to be explored.
Glutamate is in equilibrium with α-ketoglutarate, a metabolite that has many important functions. It is a component of the tricarboxylic acid cycle, and so contributes to the generation of energy from nutrients. α-ketoglutarate is also a cofactor for enzymatic reactions, influenced by the metabolic status of the cell. The α-ketoglutarate receptor (OXGR1) is expressed in the kidney, and in the setting of increased glutamate, stimulates chloride-dependent bicarbonate secretion as shown in Figure 1 (19). Variation in blood levels of glutamate have been seen in PTCS-associated conditions, including obesity, and may be related to altered cellular redox balance in the setting of chronic overnutrition and inactivity (20). For example, we have previously found that in children, BMI is associated with higher circulating concentrations of glutamate (21). Markedly elevated blood glutamate levels are also found in obese adults (22). Outlined in Figure 1, we suggest that when glutamate/α-ketoglutarate levels are elevated in the blood, this leads to activation of chloride-dependent bicarbonate secretion, and an influx of sodium into the choroid plexus cell. This influx of sodium is expected to be accompanied by inflow of water. The sodium and water that enter the cell support ongoing CSF production (via AQP and Na+/K+-dependent ATPases, as shown). This is the same process that is inhibited by carbonic anhydrase inhibitors also used in the treatment of PTCS.
Succinate is another important metabolic signal that may affect CSF balance. When infused into mice, it leads to hypertension, via paracrine release of vascular factors including nitric oxide and prostaglandin E2 that, in the kidney, lead to activation of the rennin-angiotensin-aldosterone axis (23). Higher concentrations also have been found in rodent models of metabolic syndrome; that these levels have not been consistently demonstrated in humans may be evidence for negative feedback mechanisms that alter its production. These and other tissue-specific activities of the succinate receptor (SUCNR1) are reviewed in ref. (23). In addition, succinate acting on succinate receptors located in retinal ganglion neurons is increased in ischemic stress, and modulates associated retinal neovascularization (24). This study also suggests that succinate may also mediate iron-dependent effects on CSF pressure, which could provide a mechanism for the connection between anemia and PTCS.
Association of PTCS and Endocrinopathies
The Hypothalamic Pituitary-Adrenal Axis
Multiple lines of evidence provide support for the hypothesis that the pathophysiology of PTCS involves aberrant glucocorticoid metabolism. Case reports have illustrated that PTCS can occur following surgical resection of the pituitary tumors for Cushing's disease (16,25), as an initial presentation of Addison's disease (26) or after withdrawal/tapering of chronic steroid medications (27). In a large retrospective review of over 700 adults and 200 children who underwent surgical resection of adrenocorticotropic hormone-secreting pituitary tumors, no adult developed PTCS following resection while 3% of the pediatric group did (25). No difference was found in the pre-operative, absolute 24h urine-free cortisol, adjusted for body surface area, between those individuals that did and did not develop PTCS (25). Thus, in the pediatric population, relative reductions in cortisol levels may be an inciting factor for PTCS, at least some vulnerable populations, a suggestion that is further supported by the fact that, in the acute management of vision-threatening PTCS, a short-course of high-dose methylprednisolone is a treatment option (28).
In the absence of steroid withdrawal or a surgical intervention, how could relative cortisol deficiency occur in PTCS? Recent studies have suggested that this may involve the enzyme complex of 11-β-hydroxysteroid dehydrogenase type 1 (HSD1) and type 2 (HSD2) that, together, modulate the local, tissue availability of cortisol. HSD1 catalyzes the conversion of cortisone to cortisol, thus increasing local cortisol availability while HSD2 catalyzes the reverse reaction, inactivating cortisol to its inert counterpart, cortisone. In some tissues, including liver and adipose tissue, increased HSD1 activity (29) may contribute to metabolic disease (e.g., insulin resistance, dyslipidemia, and hypertension), while, in other tissues, such as insulin-producing pancreatic β cells (30) and airway smooth muscle (31), increased HSD1 activity may be an adaptive response to preserve local cortisol levels. The role of this system in the dynamics of CSF production and absorption is incompletely understood; however, we suggest that proportional upregulation of HSD1 is a compensatory measure to limit existing cortisol deficiencies. Through its actions at the mineralcorticoid receptor and possible down-stream effects on aquaporin water channels or sodium-dependent transport mechanisms, cortisol is well-poised to regulate brain edema and CSF production (32). In support of this contention, Sinclair et al. (33) illustrated that, in adult patients with PTCS, urinary HSD1 activity was elevated at the time of diagnosis and declined as ICP fell with weight loss. In the CSF, as weight fell, CSF cortisone rose, while cortisol remained constant, which could indirectly reflect decreased HSD1 activity, although the balance of cortisol metabolites were not measured in CSF in this study.
Renin-Angiotensin-Aldosterone
After the association was first identified in 2002 (ref. (34)), a recent retrospective series and literature review described 12 patients with PTCS and hyperaldosteronism (35). Two demographic groups emerged: (i) middle-aged, overweight adult patients with primary aldosteronism and (ii) pediatric patients with secondary aldosteronism. In cases of primary PTCS, activation of the renin-aldosterone system may be enhanced by PTCS-associated risk factors, including obesity, hypervitaminosis A and corticosteroids (36). Pediatric cases of PTCS involved subjects with secondary aldosteronism, and PTCS related to renal structural or transport dysfunction (e.g., SLC12A3 gene mutation, renal congenital hypoplasia or genetic renal tubular disorders). Providing further support for the role of aldosterone in these conditions, spironolactone, an aldosterone receptor antagonist, can assist in the management of PTCS (35).
With its actions on the renal tubular cells, aldosterone is a mineralcorticoid that promotes sodium resorption while simultaneously promoting potassium, magnesium, and calcium excretion. Many questions remain unanswered about aldosterone's actions within the CNS; however, it is generally accepted that aldosterone is present within the CSF in concentrations that correlate with plasma levels, and bind high-affinity aldosterone, mineralcorticoid receptor-binding sites within the choroid plexus and other central nuclei (ref. (37); see Figure 1). In a manner analogous to its actions at the level of kidney, aldosterone appears to augment the activities of epithelial sodium channels and Na+/K+ ATPase transporters (38), to reduce CSF potassium concentrations while increasing CSF sodium concentrations. This response will create an osmotic gradient and encourage CSF production.
The cellular receptors and signaling pathways mediating the downstream effects of glucocorticoid and aldosterone must reconcile the observations that mineralcorticoid receptors within the CNS are high-affinity binding sites for both glucocorticoids and mineralocorticoids. Salpietro et al. (39) have suggested that mineralcorticoid signaling, activated by aldosterone, may help explain how hypervitaminosis A, pediatric obesity, and recombinant GH (see below) trigger PTCS. This model must also reconcile the possible increase in HSD1 activity in PTCS (see above) and apparent lack of HSD2 activity in the choroid plexus (40), both of which would act to limit the aldosterone-mediated MR activation. HSD2, as discussed above, inactivates cortisol to the inactive metabolite cortisone, permitting aldosterone's action at the mineralocorticoid receptor.
Growth Hormone and Insulin-Like Growth Factor 1
The first reports of recombinant growth hormone (rGH) or IGF-1 therapy causing PTCS were published in the early 1990s (41). Since then, a number of studies have suggested a causal relationship between administration of rGH and PTCS (42,43). Specifically, these cases support a temporal relationship between GH treatment and features consistent with PTCS: signs and symptoms are seen after the start of treatment, are relieved after discontinuing the medication and, in many cases, return with its rechallenge (41). Genetech and Pfizer have both compiled the frequency of PTCS in children being treated with rGH (Nutropin and Genotropin, respectively) and have illustrated that the frequency of PTCS is increased by 23–100 times above that seen in the general pediatric population (42,44). Patients with Turner syndrome and renal disease were clearly at increased risk of developing PTCS with rGH treatment (42,44) while those with organic growth hormone deficiency or Prader-Willi syndrome may also be at increased risk (44).
The presentation of PTCS seen following rGH treatment may differ from the idiopathic form seen in adults and postpubertal children. Obesity and female gender are less convincingly risk factors. For example, obesity appears not to be a global risk-factor and only appears associated with an increased risk of rGH-related PTCS in children with renal failure and Prader-Willi syndrome. These differences may reflect, in part, the wide range of pediatric ages being treated with rGH, from pre- to postpubertal. In a younger population, < 12 y old, obesity is not a significant risk factor for PTCS and there is no gender predilection (45). Thus, unless the important effects of age, size, and/or pubertal stage are accounted for, significant relationships may be missed. In addition, multiple contributing factors may be at play. For example, while renal disease per se may predispose to PTCS, a retrospective review of PTCS seen in children following renal transplant identified other risk factors in the majority of cases (14), including the effects of immunosuppressant medications, tetracycline antibiotics and weight gain.
How rGH leads to PTCS is unclear but the answer will likely provide insights into the pathophysiology of pediatric PTCS, in particular. Both IGF-1 and rGH are able to gain access to the CSF and receptors are present in diverse CNS locations, including the choroid plexus and arachnoid villi. In a study involving prepubertal children with short stature, a 4-wk challenge with GH caused reproducible changes in body fluid handling: there was a decrease in fractional excretion of water and increase in serum aldosterone (46), an intriguing observation given the relationship between secondary aldosteronism and pediatric PTCS (see above). Another study illustrated that GH therapy suppressed basal and glucocorticoid-stimulated HSD1 activity which may directly induce a relative cortisol deficiency (47).
Hypothalamic-Pituitary-Thyroid Axis
A number of cases describe the potential relationship between thyroid dysfunction and PTCS. The majority describe a new presentation of PTCS as an initial presentation of hyperthyroidism (e.g., seropositive Grave's disease, Hashimoto's thyroiditis) (48,49) or in the early stages of L-thyroxine therapy in the management of primary hypothyroidism and hypothalamic hypothyroidism (50,51).
Both hyper- and hypothyroidism are associated with abnormal renal fluid handling, effects mediated by reduced protein abundance of renal sodium-dependent transporters and water channels (52). As discussed above, many analogous transporters are expressed in the renal tubules and choroid plexus. In this way, thyroid dysfunction may lead to dysregulation of CSF dynamics (Figure 1). While hyperthyroidism leads to decreased activities of apical Na-dependent transporters, hypothyroidism leads to the opposite, a finding which, on its own, cannot account for an increase in ICP. Alternatively, a common complication of hypothyroidism, hyponatremia, under these conditions may be, in part, related to nonosmotic arginine vasopressin release or ability to clear free water (52); these mechanisms remain incompletely understood, and studies have discrepant findings. Indeed arginine vasopressin levels may be elevated in patients in PTCS (see below). Thyroid hormone has further effects as a prothrombotic agent, which increases the risk of venous thrombosis secondary cause of PTCS (53,54) (Figure 1). Thus, although not completely understood, through a combination of actions, both hypo- and hyperthyroidism may contribute to the development of PTCS.
Hypothalamic-Pituitary-Gonadal Axis
Androgens and estrogens have diverse metabolic, influences on the CNS. In patients with PTCS, characteristic changes in sex hormones may occur. Indeed, there are clear demographic and anthropometric differences in the young, presumably prepubertal, pediatric population with PTCS compared with the adolescent with pediatric PTCS (45). However, studies aimed at clarifying the role of sex hormones involved have been limited to the adult population. In the CSF of both male and female adult patients with PTCS, estrone levels are increased while androstenedione levels are decreased (ref. (55,56) but see (57), differences not seen in plasma (55) and suggestive of a localized increase in aromatase activity. Indeed, exposure to exogenous (Levonorgestrel (58) and emergency contraceptives (59)) and endogenous (pregnancy (60,61) and polycystic ovarian syndrome (62)) estrogens have all been associated with PTCS. Increased androgens, namely basal testosterone and androstenedione, have been linked with a younger age at presentation of PTCS, in an adult, female population (63). It was speculated that increased levels of these sex hormones not only act as precipitating factors to trigger PTCS expression but that this hyperandrogenism may be the more direct link between tetracycline-derivatives and PTCS by promoting acne development and medication usage (63).
No clear pathophysiologic pathway has been identified for the effects of sex steroids. It has been speculated that estrogens may promote venous sinus thrombosis, impairing CSF resorption (e.g., ref. (64); Figure 1). Adding to this, it is becoming increasingly recognized that estrogen steroid hormones influence the expression and activity of variety of epithelial transport mechanisms and, in particular, promote sodium reabsorption and water retention in the distal nephron (ref. (65); Figure 1). It is interesting to suggest that the differential effects of sex hormones on CSF dynamics may, in part, explain the clear gender bias of PTCS in the older adolescent and adult patients.
The Posterior Pituitary and Antidiuretic Hormone/Vasopressin
Vasopressin is a hormone secreted from the posterior pituitary and other extra-pituitary sites whose function is to regulate extracellular fluid volume in the periphery and CNS. In renal collecting tubule cells, vasopressin's primary action is to traffic aquaporins to the apical membrane and, in turn, facilitate water permeability. Studies agree that CSF vasopressin levels (66,67) are increased in patients with PTCS. Clinically, patients with PTCS demonstrate abnormal urine water excretion, in a manner analogous to that seen in patients with idiopathic orthostatic edema, a condition in which there is abnormal peripheral water retention (68). However, it is not clear if elevations in vasopressin contribute to the pathophysiology of PTCS. On the one hand, volume receptors within the walls of the cerebral ventricles respond to changes in ICP by altering vasopressin excretion (69), suggesting that elevations in vasopressin may simply reflect the fact that ICP is elevated. Moreover, intraventricular infusion of vasopressin decreased production of CSF by 35% in one animal study (70). On the other hand, this observation must be reconciled with further observations that intraventricular infusions of vasopressin induce elevations in ICP in other animal studies (71), through mechanism that may involve an increased resistance to CSF outflow (72) (Figure 1).
Leptin and Other Adipokines
Leptin was first cloned 15 years ago, in studies using genetically-obese mice whose phenotype is related to homozygous mutations of the obese (ob) gene (73). Leptin, the hormone product of the ob gene, functions to regulate energy balance, body weight, and hypothalamic hormones (74). By its absence, leptin communicates to the brain that the body is starving, triggering the sense of hunger, reducing energy expenditure, and limiting reproductive capacity. Human obesity conditions associated with a relative leptin deficiency include congenital leptin deficiency and hypothalamic amenorrhea while, conversely, hyperleptinemia is seen in patients with diet-induced obesity.
It is well-established that PTCS, in the adult and older pediatric populations, is associated with obesity (45). Given that the pathophysiology of endogenous obesity may involve abnormal leptin levels, impaired access of leptin to its effector sites in the CNS, and/or impaired downstream leptin signaling cascades (75), studies have evaluated the possibility that leptin dysregulation is involved in the mechanism of disease in PTCS, with variable results. The first study measured fasting serum leptin levels in three cohorts of age-matched adult women: obese women with PTCS, obese women and nonobese women (76). As expected, leptin levels were elevated in both obese cohorts when compared with measurements made in nonobese women, however, there was a further elevation in leptin levels seen in the cohort of obese women with PTCS, compared with the unaffected obese women (76). These findings were not reproduced by two subsequent studies (77,78) where, in the first, fasting leptin levels were measured in a mixed cohort of adult obese and overweight men and women with PTCS and compared values to those obtained from an obese cohort (77). In the second, two cohorts were again compared: obese women with PTCS and a heterogeneous group of participants (78). Multivariable regression analysis was used to correct for the confounding effects of age, BMI, or gender. After accounting for leptin variability with BMI and gender, neither study illustrated a significant difference in serum leptin in patients with PTCS.
Peripheral leptin gains access to the CSF via a saturable high-affinity transport mechanism at the choroid plexus and blood–brain barrier (79). As an extension of one of the above studies, Ball and colleagues illustrated nonelevated serum levels coupled with elevated CSF levels, arguing that patients with PTCS have a relative leptin resistance (78). Others have not illustrated elevated levels of leptin within the CSF (e.g., ref. (80)).
Thus, whether serum or CSF leptin levels are altered in PTCS remain contentious. From the studies completed so far, we have learned that careful selection of the comparative cohorts remains critical. Potential cofounds include adiposity, age, BMI, and gender of the subjects. Leptin secretion also varies with menstrual phase and renal disease (74). These factors most certainly contribute to the variable results seen to date. Furthermore, measuring leptin levels in isolation may be too simplistic. Leptin functions in concert with other satiety hormones, including neuropeptide Y, ghrelin, kisspeptin, and adiponectin, and it may be that the dysregulation of this balance contributes to the pathogenesis of PTCS. However, plasma ghrelin levels measured in patients with PTCS were not different compared to an obese cohort (77), although measurements of ghrelin levels are influenced by anticoagulants used during sample collection and storage conditions. Questions also remain about the potential implications of a relative hyperleptinemia in obesity-associated PTCS and it is unclear if alterations in satiety hormones, if they exist, reflect an associated epiphenomenon or not. Nevertheless, chronically elevated levels of leptin in obesity have the capacity to increase renal salt retention (81), thus hyperleptinemia may achieve a similar result in the choroid (not shown). Further study into the relationship between leptin and PTCS is certainly needed to address these complex issues.
Conclusion
We present a comprehensive discussion of the hypothesis that pediatric PTCS is a neuroendocrine disorder. We illustrate that many metabolic and hormonal derangements have been associated with PTCS and, in this way, may present an opportunity to identify a unifying pathophysiology. Further, we suggest that understanding the different ways in which specific metabolites and hormones regulate choroid plexus water and ion transport will implicate final common cellular pathways by which a diverse range of conditions produces clinically similar elevations in ICP.
Acknowledgments
This work was supported in part by 5K12DK094723-02 (SEM, The Children's Hospital of Philadelphia, Philadelphia, PA, USA).
Footnotes
Statement of Financial Support: Disclosure: G.T.L. has consulted for Ipsen. None of the other authors have any financial ties to products in the study or potential/perceived conflicts of interest.
References
- 1.Soiberman U, Stolovitch C, Balcer LJ, Regenbogen M, Constantini S, Kesler A. Idiopathic intracranial hypertension in children: visual outcome and risk of recurrence. Childs Nerv Syst. 2011;27:1913–8. doi: 10.1007/s00381-011-1470-5. [DOI] [PubMed] [Google Scholar]
- 2.Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana Arch Neurol. 1988;45:875–7. doi: 10.1001/archneur.1988.00520320065016. [DOI] [PubMed] [Google Scholar]
- 3.Gordon K. Pediatric pseudotumor cerebri: descriptive epidemiology. Can J Neurol Sci. 1997;24:219–21. doi: 10.1017/s031716710002182x. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1–7. doi: 10.1212/WNL.0b013e3182a55f17. [DOI] [PubMed] [Google Scholar]
- 5.Ko MW, Liu GT. Pediatric idiopathic intracranial hypertension (pseudotumor cerebri) Horm Res Paediatr. 2010;74:381–9. doi: 10.1159/000321180. [DOI] [PubMed] [Google Scholar]
- 6.Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52:597–617. doi: 10.1016/j.survophthal.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 7.McGeeney BE, Friedman DI. Pseudotumor cerebri pathophysiology. Headache. 2014;54:445–58. doi: 10.1111/head.12291. [DOI] [PubMed] [Google Scholar]
- 8.Gasparian SS, Serova NK, Sherbakova EY, Belova TN. Compensatory mechanisms in patients with benign intracranial hypertension syndrome. Acta Neurochir Suppl. 2002;81:31–3. doi: 10.1007/978-3-7091-6738-0_8. [DOI] [PubMed] [Google Scholar]
- 9.Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology. 1996;46:198–202. doi: 10.1212/wnl.46.1.198. [DOI] [PubMed] [Google Scholar]
- 10.Melby JM, Miner LC, Reed DJ. Effect of acetazolamide and furosemide on the production and composition of cerebrospinal fluid from the cat choroid plexus. Can J Physiol Pharmacol. 1982;60:405–9. doi: 10.1139/y82-059. [DOI] [PubMed] [Google Scholar]
- 11.Guess HA, Charlton JD, Johnson RN, Mann JD. A nonlinear least-squares method for determining cerebrospinal fluid formation and absorption kinetics in pseudotumor cerebri. Comput Biomed Res. 1985;18:184–92. doi: 10.1016/0010-4809(85)90044-8. [DOI] [PubMed] [Google Scholar]
- 12.Boedtkjer E, Praetorius J, Füchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3- cotransporter NBCn1 (slc4a7) in mice. Am J Physiol, Cell Physiol. 2008;294:C591–603. doi: 10.1152/ajpcell.00281.2007. [DOI] [PubMed] [Google Scholar]
- 13.Guy J, Johnston PK, Corbett JJ, Day AL, Glaser JS. Treatment of visual loss in pseudotumor cerebri associated with uremia. Neurology. 1990;40:28–32. doi: 10.1212/wnl.40.1.28. [DOI] [PubMed] [Google Scholar]
- 14.Francis PJ, Haywood S, Rigden S, Calver DM, Clark G. Benign intracranial hypertension in children following renal transplantation. Pediatr Nephrol. 2003;18:1265–9. doi: 10.1007/s00467-003-1274-2. [DOI] [PubMed] [Google Scholar]
- 15.Keep RF, Smith DE. Choroid plexus transport: gene deletion studies. Fluids Barriers CNS. 2011;8:26. doi: 10.1186/2045-8118-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zada G, Tirosh A, Kaiser UB, Laws ER, Woodmansee WW. Cushing's disease and idiopathic intracranial hypertension: case report and review of underlying pathophysiological mechanisms. J Clin Endocrinol Metab. 2010;95:4850–4. doi: 10.1210/jc.2010-0896. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair AJ, Viant MR, Ball AK, et al. NMR-based metabolomic analysis of cerebrospinal fluid and serum in neurological diseases–a diagnostic tool? NMR Biomed. 2010;23:123–32. doi: 10.1002/nbm.1428. [DOI] [PubMed] [Google Scholar]
- 18.Young SP, Wallace GR. Metabolomic analysis of human disease and its application to the eye. J Ocul Biol Dis Infor. 2009;2:235–42. doi: 10.1007/s12177-009-9038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokonami N, Morla L, Centeno G, et al. α-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest. 2013;123:3166–71. doi: 10.1172/JCI67562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace DC. Mitochondria as chi. Genetics. 2008;179:727–35. doi: 10.1534/genetics.104.91769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariza AC, Deen PM, Robben JH. The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions. Front Endocrinol (Lausanne) 2012;3:22. doi: 10.3389/fendo.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sapieha P, Sirinyan M, Hamel D, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–76. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 25.Kiehna EN, Keil M, Lodish M, Stratakis C, Oldfield EH. Pseudotumor cerebri after surgical remission of Cushing's disease. J Clin Endocrinol Metab. 2010;95:1528–32. doi: 10.1210/jc.2009-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condulis N, Germain G, Charest N, Levy S, Carpenter TO. Pseudotumor cerebri: a presenting manifestation of Addison's disease. Clin Pediatr (Phila) 1997;36:711–3. doi: 10.1177/000992289703601208. [DOI] [PubMed] [Google Scholar]
- 27.Liu GT, Kay MD, Bienfang DC, Schatz NJ. Pseudotumor cerebri associated with corticosteroid withdrawal in inflammatory bowel disease. Am J Ophthalmol. 1994;117:352–7. doi: 10.1016/s0002-9394(14)73145-9. [DOI] [PubMed] [Google Scholar]
- 28.Liu GT, Glaser JS, Schatz NJ. High-dose methylprednisolone and acetazolamide for visual loss in pseudotumor cerebri. Am J Ophthalmol. 1994;118:88–96. doi: 10.1016/s0002-9394(14)72847-8. [DOI] [PubMed] [Google Scholar]
- 29.Masuzaki H, Flier JS. Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)–a promising drug target for the treatment of metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:255–62. doi: 10.2174/1568008033340135. [DOI] [PubMed] [Google Scholar]
- 30.Turban S, Liu X, Ramage L, et al. Optimal elevation of ß-cell 11ß-hydroxysteroid dehydrogenase type 1 is a compensatory mechanism that prevents high-fat diet-induced ß-cell failure. Diabetes. 2012;61:642–52. doi: 10.2337/db11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu A, Fatma S, Cao J, et al. Th2 cytokine-induced upregulation of 11beta-hydroxysteroid dehydrogenase-1 facilitates glucocorticoid suppression of proasthmatic airway smooth muscle function. Am J Physiol Lung Cell Mol Physiol. 2009;296:L790–803. doi: 10.1152/ajplung.90572.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–77. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinclair AJ, Walker EA, Burdon MA, et al. Cerebrospinal fluid corticosteroid levels and cortisol metabolism in patients with idiopathic intracranial hypertension: a link between 11beta-HSD1 and intracranial pressure regulation? J Clin Endocrinol Metab. 2010;95:5348–56. doi: 10.1210/jc.2010-0729. [DOI] [PubMed] [Google Scholar]
- 34.Weber KT, Singh KD, Hey JC. Idiopathic intracranial hypertension with primary aldosteronism: report of 2 cases. Am J Med Sci. 2002;324:45–50. doi: 10.1097/00000441-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Khan MU, Khalid H, Salpietro V, Weber KT. Idiopathic intracranial hypertension associated with either primary or secondary aldosteronism. Am J Med Sci. 2013;346:194–8. doi: 10.1097/MAJ.0b013e31826e3635. [DOI] [PubMed] [Google Scholar]
- 36.Rossi GP, Seccia TM. Changes in aldosterone and obesity-related cardio-metabolic risk factors with a 1-year weight loss intervention in normotensive overweight and obese young adults. Hypertens Res. 2013;36:856–8. doi: 10.1038/hr.2013.77. [DOI] [PubMed] [Google Scholar]
- 37.Weber KT. Aldosteronism revisited: perspectives on less well-recognized actions of aldosterone. J Lab Clin Med. 2003;142:71–82. doi: 10.1016/S0022-2143(03)00062-3. [DOI] [PubMed] [Google Scholar]
- 38.Leenen FH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–9. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Salpietro V, Polizzi A, Bertè LF, et al. Idiopathic intracranial hypertension: a unifying neuroendocrine hypothesis through the adrenal-brain axis. Neuro Endocrinol Lett. 2012;33:569–73. [PubMed] [Google Scholar]
- 40.Sinclair AJ, Onyimba CU, Khosla P, et al. Corticosteroids, 11beta-hydroxysteroid dehydrogenase isozymes and the rabbit choroid plexus. J Neuroendocrinol. 2007;19:614–20. doi: 10.1111/j.1365-2826.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 41.Malozowski S, Tanner LA, Wysowski D, Fleming GA. Growth hormone, insulin-like growth factor I, and benign intracranial hypertension. N Engl J Med. 1993;329:665–6. doi: 10.1056/NEJM199308263290917. [DOI] [PubMed] [Google Scholar]
- 42.Reeves GD, Doyle DA. Growth hormone treatment and pseudotumor cerebri: coincidence or close relationship? J Pediatr Endocrinol Metab. 2002;15(Suppl 2):723–30. doi: 10.1515/jpem.2002.15.s2.723. [DOI] [PubMed] [Google Scholar]
- 43.Rogers AH, Rogers GL, Bremer DL, McGregor ML. Pseudotumor cerebri in children receiving recombinant human growth hormone. Ophthalmology. 1999;106:1186–9. doi: 10.1016/S0161-6420(99)90266-X. discussion 1189–90. [DOI] [PubMed] [Google Scholar]
- 44.Darendeliler F, Karagiannis G, Wilton P. Headache, idiopathic intracranial hypertension and slipped capital femoral epiphysis during growth hormone treatment: a safety update from the KIGS database. Horm Res. 2007;68(Suppl 5):41–7. doi: 10.1159/000110474. [DOI] [PubMed] [Google Scholar]
- 45.Balcer LJ, Liu GT, Forman S, et al. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999;52:870–2. doi: 10.1212/wnl.52.4.870. [DOI] [PubMed] [Google Scholar]
- 46.Lampit M, Nave T, Hochberg Z. Water and sodium retention during short-term administration of growth hormone to short normal children. Horm Res. 1998;50:83–8. doi: 10.1159/000023239. [DOI] [PubMed] [Google Scholar]
- 47.Zuckerman-Levin N, Tsivlin L, Knopf C, et al. 11ß-Hydroxysteroid dehydrogenase type 1 activity in short small-for-GA children and in response to GH therapy. Pediatr Res. 2011;70:208–12. doi: 10.1203/PDR.0b013e3182226a0c. [DOI] [PubMed] [Google Scholar]
- 48.Merkenschlager A, Ehrt O, Müller-Felber W, Schmidt H, Bernhard MK. Reversible benign intracranial hypertension in a child with hyperthyroidism. J Pediatr Endocrinol Metab. 2008;21:1099–101. doi: 10.1515/jpem.2008.21.11.1099. [DOI] [PubMed] [Google Scholar]
- 49.Coutinho E, Silva AM, Freitas C, Santos E. Graves' disease presenting as pseudotumor cerebri: a case report. J Med Case Rep. 2011;5:68. doi: 10.1186/1752-1947-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campos SP, Olitsky S. Idiopathic intracranial hypertension after L-thyroxine therapy for acquired primary hypothyroidism. Clin Pediatr (Phila) 1995;34:334–7. doi: 10.1177/000992289503400608. [DOI] [PubMed] [Google Scholar]
- 51.Huseman CA, Torkelson RD. Pseudotumor cerebri following treatment of hypothalamic and primary hypothyroidism. Am J Dis Child. 1984;138:927–31. doi: 10.1001/archpedi.1984.02140480029010. [DOI] [PubMed] [Google Scholar]
- 52.Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23:22–6. doi: 10.1681/ASN.2010070766. [DOI] [PubMed] [Google Scholar]
- 53.Stuijver DJ, van Zaane B, Romualdi E, Brandjes DP, Gerdes VE, Squizzato A. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: a systematic review and meta-analysis. Tromb Haemost. 2012;108:1077–88. doi: 10.1160/TH12-07-0496. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q, Yao ZP, Zhou D, Zheng HB, Shang HF. Lateral sinus thrombosis and intracranial hypertension associated with primary hypothyroidism: case report. Neuro Endocrinol Lett. 2008;29:41–3. [PubMed] [Google Scholar]
- 55.Toscano V, Sancesario G, Bianchi P, Cicardi C, Casilli D, Giacomini P. Cerebrospinal fluid estrone in pseudotumor cerebri: a change in cerebral steroid hormone metabolism? J Endocrinol Invest. 1991;14:81–6. doi: 10.1007/BF03350271. [DOI] [PubMed] [Google Scholar]
- 56.Donaldson JO, Horak E. Cerebrospinal fluid oestrone in pseudotumour cerebri. J Neurol Neurosurg Psychiatr. 1982;45:734–6. doi: 10.1136/jnnp.45.8.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soelberg Sørensen P, Gjerris F, Svenstrup B. Endocrine studies in patients with pseudotumor cerebri. Estrogen levels in blood and cerebrospinal fluid. Arch Neurol. 1986;43:902–6. doi: 10.1001/archneur.1986.00520090038013. [DOI] [PubMed] [Google Scholar]
- 58.Alder JB, Fraunfelder FT, Edwards R. Levonorgestrel implants and intracranial hypertension. N Engl J Med. 1995;332:1720–1. [PubMed] [Google Scholar]
- 59.Ivancic R, Pfadenhauer K. Pseudotumor cerebri after hormonal emergency contraception. Eur Neurol. 2004;52:120. doi: 10.1159/000080269. [DOI] [PubMed] [Google Scholar]
- 60.Digre KB, Varner MW, Corbett JJ. Pseudotumor cerebri and pregnancy. Neurology. 1984;34:721–9. doi: 10.1212/wnl.34.6.721. [DOI] [PubMed] [Google Scholar]
- 61.Kesler A, Kupferminc M. Idiopathic intracranial hypertension and pregnancy. Clin Obstet Gynecol. 2013;56:389–96. doi: 10.1097/GRF.0b013e31828f2701. [DOI] [PubMed] [Google Scholar]
- 62.Glueck CJ, Aregawi D, Goldenberg N, Golnik KC, Sieve L, Wang P. Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia. J Lab Clin Med. 2005;145:72–82. doi: 10.1016/j.lab.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Klein A, Stern N, Osher E, Kliper E, Kesler A. Hyperandrogenism is associated with earlier age of onset of idiopathic intracranial hypertension in women. Curr Eye Res. 2013;38:972–6. doi: 10.3109/02713683.2013.799214. [DOI] [PubMed] [Google Scholar]
- 64.Dindar F, Platts ME. Intracranial venous thrombosis complicating oral contraception. Can Med Assoc J. 1974;111:545–8. [PMC free article] [PubMed] [Google Scholar]
- 65.Saint-Criq V, Rapetti-Mauss R, Yusef YR, Harvey BJ. Estrogen regulation of epithelial ion transport: Implications in health and disease. Steroids. 2012;77:918–23. doi: 10.1016/j.steroids.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Seckl J, Lightman S. Cerebrospinal fluid neurohypophysial peptides in benign intracranial hypertension. J Neurol Neurosurg Psychiatr. 1988;51:1538–41. doi: 10.1136/jnnp.51.12.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammer M, Sørensen PS, Gjerris F, Larsen K. Vasopressin in the cerebrospinal fluid of patients with normal pressure hydrocephalus and benign intracranial hypertension. Acta Endocrinol. 1982;100:211–5. doi: 10.1530/acta.0.1000211. [DOI] [PubMed] [Google Scholar]
- 68.Friedman DI, Streeten DH. Idiopathic intracranial hypertension and orthostatic edema may share a common pathogenesis. Neurology. 1998;50:1099–104. doi: 10.1212/wnl.50.4.1099. [DOI] [PubMed] [Google Scholar]
- 69.Satta A, Varoni MV, Palomba D, et al. Relationship between cerebrospinal fluid pressure and plasmatic ADH. Pharmacol Res. 1999;39:383–8. doi: 10.1006/phrs.1998.0452. [DOI] [PubMed] [Google Scholar]
- 70.Faraci FM, Mayhan WG, Heistad DD. Effect of vasopressin on production of cerebrospinal fluid: possible role of vasopressin (V1)-receptors. Am J Physiol. 1990;258(1 Pt 2):R94–8. doi: 10.1152/ajpregu.1990.258.1.R94. [DOI] [PubMed] [Google Scholar]
- 71.Seckl JR, Lightman SL. Intracerebroventricular arginine vasopressin causes intracranial pressure to rise in conscious goats. Brain Res. 1987;423:279–85. doi: 10.1016/0006-8993(87)90850-x. [DOI] [PubMed] [Google Scholar]
- 72.Seckl JR, Lightman SL. Intracerebroventricular vasopressin reduces CSF absorption rate in the conscious goat. Exp Brain Res. 1991;84:173–6. doi: 10.1007/BF00231772. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 74.Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–84. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 76.Lampl Y, Eshel Y, Kessler A, et al. Serum leptin level in women with idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatr. 2002;72:642–3. doi: 10.1136/jnnp.72.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Subramanian PS, Goldenberg-Cohen N, Shukla S, Cheskin LJ, Miller NR. Plasma ghrelin levels are normal in obese patients with idiopathic intracranial hypertension (pseudotumor cerebri) Am J Ophthalmol. 2004;138:109–13. doi: 10.1016/j.ajo.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Ball AK, Sinclair AJ, Curnow SJ, et al. Elevated cerebrospinal fluid (CSF) leptin in idiopathic intracranial hypertension (IIH): evidence for hypothalamic leptin resistance? Clin Endocrinol (Oxf) 2009;70:863–9. doi: 10.1111/j.1365-2265.2008.03401.x. [DOI] [PubMed] [Google Scholar]
- 79.Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL. Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology. 2000;141:1434–41. doi: 10.1210/endo.141.4.7435. [DOI] [PubMed] [Google Scholar]
- 80.Behbehani R, Mabrook A, Abbas JM, Al-Rammah T, Mojiminiyi O, Doi SA. Is cerebrospinal fluid leptin altered in idiopathic intracranial hypertension? Clin Endocrinol (Oxf) 2010;72:851–2. doi: 10.1111/j.1365-2265.2009.03722.x. [DOI] [PubMed] [Google Scholar]
- 81.Nasrallah MP, Ziyadeh FN. Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Semin Nephrol. 2013;33:54–65. doi: 10.1016/j.semnephrol.2012.12.005. [DOI] [PubMed] [Google Scholar]


