Abstract
9-Substituted phenanthrene-3-carboxylic acids have been reported to have allosteric modulatory activity at the NMDA receptor. This receptor is activated by the excitatory neurotransmitter L-glutamate and has been implicated in a range of neurological disorders such as schizophrenia, epilepsy and chronic pain and neurodegenerative disorders such as Alzheimer’s disease. Herein, the convenient synthesis of a wide range of novel 3,9-disubstituted phenanthrene derivatives starting from a few common intermediates is described. These new phenanthrene derivatives will help to clarify the structural requirements for allosteric modulation of the NMDA receptor.
Keywords: Phenanthrenes, NMDA receptor, allosteric modulators, palladium coupling, Wittig reaction
Graphical abstract
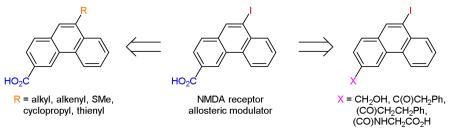
3,9-Disubstituted Phenanthrenes
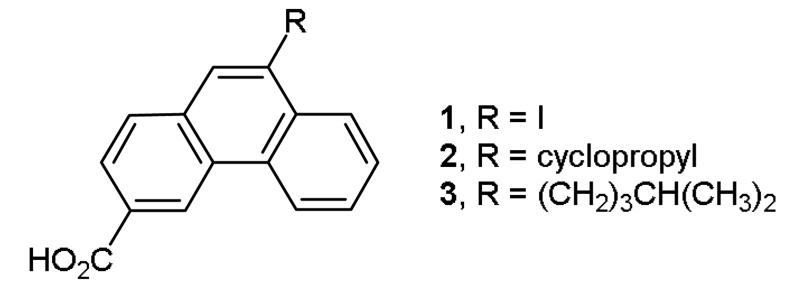
Phenanthrene is a naturally occurring polycyclic aromatic ring system which is found in a number of biologically active compounds.2 Recently, we reported that phenanthrene derivatives such as 1, 2, and 3 (Figure 1) are allosteric modulators of the N-methyl-D-aspartate (NMDA) family of ionotropic glutamate receptors (i-GluRs).3 NMDA receptors are tetrameric ligand-gated ion channels comprised of GluN1 and GluN2A-D subunits.3 NMDA receptors have been implicated in a range of neurological disorders such as epilepsy, schizophrenia and chronic pain and neurodegenerative disorders such as ischaemia, Alzheimer’s disease and Parkinson’s disease.3 Allosteric modulators have potential to treat these disorders, as they are less likely to interfere with the physiological roles of NMDA receptors compared to competitive antagonists or channel blockers.3 A convenient synthetic route for 3-carboxyphenanthrenes with a wide range of hydrophobic and hydrophilic substituents at the 9-position was required to conduct a structure-activity relationship (SAR) study surrounding allosteric modulators 1-3. A search of the literature revealed that with the exception of the 9-bromo, 9-chloro, and 9-carboxy derivatives, no 9-subtituted-3-carboxyphenanthrenes have previously been reported.4,5 Herein, we report the synthesis of 1-3 and a novel series of their derivatives starting from a few common intermediates.
Figure 1.
NMDA receptor allosteric modulators
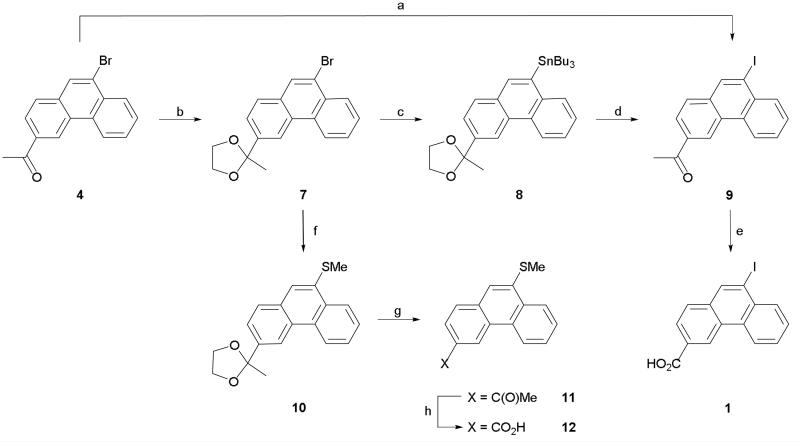
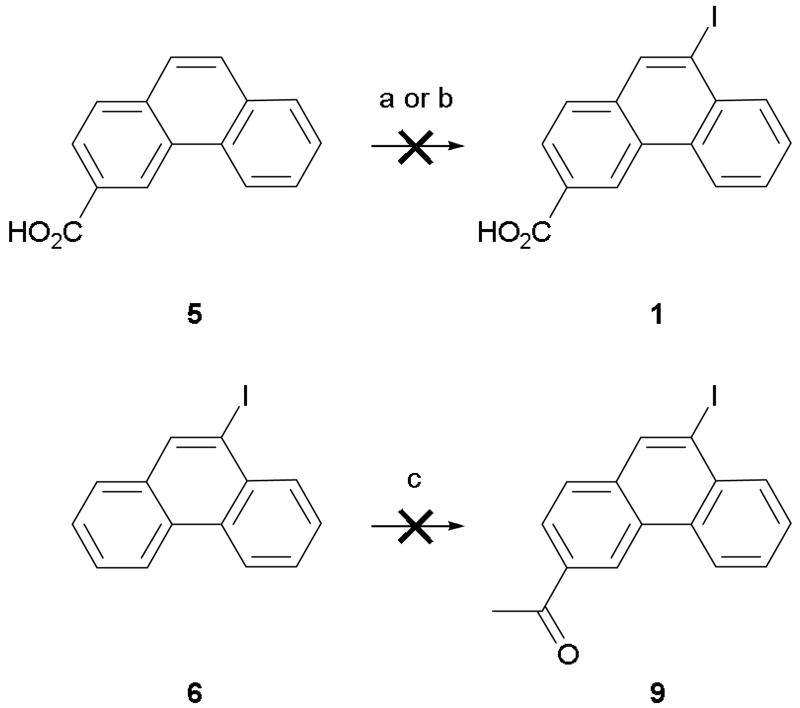
Initial studies suggested that 9-iodophenanthrene-3-carboxylic acid (1) had an interesting pharmacological profile3 and so we investigated suitable methods for larger scale production of this compound. We recently reported a 2-step route to 1 in which an aromatic Finkelstein reaction6 was utilized to convert 3-acetyl-9-bromophenanthrene (4) to its corresponding 9-iodo analogue (9).7 A haloform reaction was then employed to oxidize the acetyl group and give the desired acid (Scheme 1). However, whilst our initial experiments led to complete conversion, subsequent attempts to resynthesize 9 led only to in-separable mixtures of 4 and 9 being isolated. Despite investigation (e.g. different amine ligands, alternative solvents, purification of copper (I) iodide catalyst), the exact reason for the non-reproducibility of the aromatic Finkelstein reaction could not be determined. With the halogen conversion route proving unreliable an alternative and robust pathway to 1 was sought. Unfortunately, attempts to directly iodinate the 9-position of phenanthrene-3-carboxylic acid (5) using either iodine monochloride or sodium iodide and sodium hypochlorite (Scheme 2) led only to the recovery of un-reacted starting material. As a consequence, we decided to focus on developing an alternative route to ketone 9. The most obvious route to this compound is via Friedel-Crafts acylation. However, whilst Friedel-Crafts acylation can be used to synthesize the 3-acetyl derivatives of both 9-bromo and 9-chlorophenanthrene we found that employing the same reaction conditions on 9-iodophenanthrene (6) led only to the isolation of a black tar (Scheme 2).4,5 Attempts to modify the reaction conditions by using aluminum iodide instead of aluminum chloride or acetic anhydride instead of acetyl chloride led only to the same outcome. With all previous routes proving unsuccessful we decided to investigate lithiation as a possible way of introducing the iodo substituent (Scheme 1). After protecting ketone 4 as an acetal (7), lithiation at the 9-position followed by quenching with (n-Bu)3SnCl afforded stannane 8. The 9-iodo group was then readily introduced by stirring with a saturated solution of iodine in DCM at 0 °C. Subsequent de-protection gave ketone 9 which was then easily converted to 1 using the haloform reaction described previously. In theory, the iodo group could have been introduced by quenching the lithiated species with iodine. However, we were concerned that employing this route would lead to the formation of side products which could not be easily separated from the desired product. Whilst it added an additional step, utilizing stannane 8 allowed 1 to be synthesized both cleanly and in high yield.
Scheme 1.
Reagents and conditions: (a) NaI, CuI, N,N'-dimethylethylenediamine, dioxane, 110 °C, 65 h; (b) Ethylene glycol, TsOH, toluene, 110 °C, 18 h; (c) (i) n-BuLi, THF, −78 °C, 1 h, (ii) (n-Bu)3SnCl, −78 °C; (d) (i) I2, DCM, 0 °C, (ii) 2 M HCl (aq), acetone, 0.5 h; (e) (i) Br2, NaOH (aq), dioxane, 70 °C, 1 h, (ii) conc HCl (aq); (f) (i) n-BuLi, THF, −78 °C, 1 h, (ii) MeSSMe, −78 °C, then rt; (g) HCl/acetone, 1 h, rt; (h) (i) Br2, NaOH, dioxane, 40 °C, 1 h, (ii) conc HCl.
Scheme 2.
Reagents and conditions: (a) ICl, AcOH, 118 °C, 18 h; (b) NaI, NaClO, NaOH, MeOH, 0 °C then rt, 1 h; (c) AcCl, AlCl3, CS2, 5 °C then rt, 18 h.
Attention was then turned to the synthesis of a structurally diverse series of 3-carboxyphenanthrenes bearing hydrophobic substituents at the 9-position as analogues of compounds 1-3. Amongst the initial group of compounds generated was thioether 12 which was synthesized using an identical strategy to that described for 1 with the exception that after being lithiated, acetal 7 was quenched with dimethyl disulfide (Scheme 1). De-protection subsequently afforded acetyl 11 which was then readily converted to carboxylic acid 12 using a haloform reaction.
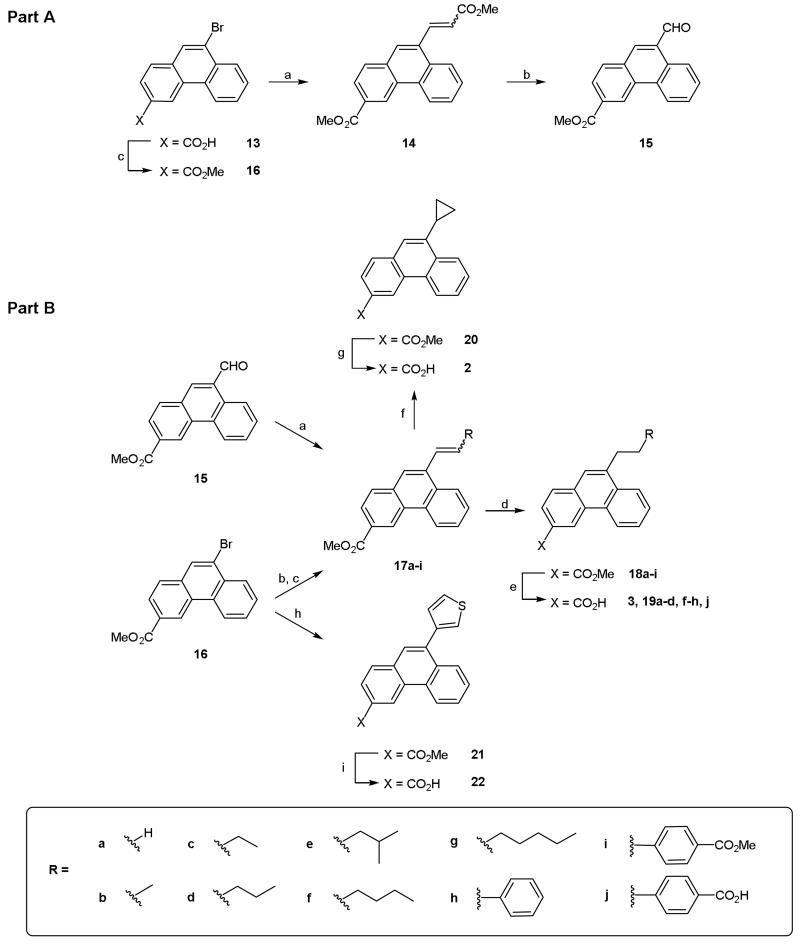
In addition to thioethers, compounds bearing alkyl substituents at the 9-position were synthesized. Initial attempts to generate these derivatives by reacting alkyl aldehydes with lithiated acetal 7 gave only a complex mixture of products. Consequently, an alternative route was devised to allow a range of alkyl chains to be introduced using common intermediates, which could be prepared both quickly and in high yield. With this in mind, the 9-formyl (15) and 9-bromo (16) substituted phenanthrenes were chosen as both functional groups could be easily manipulated using either Wittig or palladium coupling chemistry to afford a large variety of 9-alkylphenanthrenes from commercially available reagents. Both 15 and 16 were conveniently prepared from 9-bromo acid 13 (Scheme 3A).7 Heck coupling of 13 with methyl acrylate followed by esterification with methyl iodide afforded di-ester 14. Oxidation of alkene 14 using osmium tetroxide and cleavage of the resultant 1,2-diol with sodium periodate afforded the 9-formyl derivative 15. Methyl ester 16 was generated in good yield by Fisher esterification of acid 13.4 Conducting either Wittig or Heck chemistry on 15 and 16 proceeded smoothly and led to the synthesis of alkene intermediates 17a-i which, in the majority of cases, were hydrogenated immediately to their corresponding alkyl counterparts 18a-i (Scheme 3B). Base hydrolysis subsequently afforded the desired 9-alkyl-3-carboxyphenanthrenes (3, 19a-d, f-h, j). The 9-cyclopropyl derivative 2 was synthesized from vinyl 17a in 2-steps. Firstly, the cyclopropyl ring was formed via a Simmons-Smith reaction to give 20. Base hydrolysis of the ester subsequently afforded the desired acid (2). Initially, alkene 17a was prepared from 15 via Wittig chemistry. Although this route proved successful we found that the compound was more conveniently prepared via Stille coupling between 16 and (tri-n-butyl)vinyl tin (Scheme 3B).
Scheme 3.
Reagents and conditions: Part A (a) (i) Methyl acrylate, P(o-tolyl)3, TEA, Pd(OAc)2, DMF, 100 °C, 18 h, (ii) MeI, K2CO3, DMF, rt, 18 h; (b) (i) OsO4, TMAO, t-BuOH/H2O, rt, 2 days, (ii) NaIO4; (c) MeOH, H2SO4, reflux, 48 h. Part B (a) RCH2PPh3X, KHMDS, THF, 4 h, rt; (b) alkene, P(o-tolyl)3, TEA, Pd(OAc)2, DMF, 100 °C, 18 h; (c) (n-Bu)3SnCH=CH2, Pd(PPh3)4, toluene, reflux, 4 h; (d) H2, 10% Pd/C, EtOAc, rt, 18 h; (e) (i) NaOH or KOH (aq), THF, reflux or dioxane, 75 °C, (ii) 1 M HCl (aq); (f) 17a, CH2I2, ZnEt2, DCM, 0 °C, 18 h; (g) (i) LiOH (aq), dioxane, rt, 18 h, (ii) 1 M HCl (aq); (h) 3-thienylboronic acid, K2CO3, Pd(dppf)Cl2.DCM, DME, 80 °C, 24 h; (i) (i) NaOH (aq), dioxane, 75 °C, (ii) 1 M HCl (aq), (iii) crystallisation (AcOH).
To investigate the introduction of a heteroaromatic moiety, Suzuki coupling was employed to react 16 and 3-thienyl boronic acid (Scheme 3B). Unfortunately, this reaction did not go to completion and led to a mixture of product and starting material (21 and 16) being isolated (~75:25 by 1H-NMR). Despite investigation of different solvent systems it was not possible to separate the individual esters by silica gel chromatography. Consequently, the mixture was taken forward and hydrolyzed using base. By conducting multiple re-crystallizations from glacial acetic acid we were able to separate the mixture of acids and obtain a pure sample of 22 (Scheme 3B).
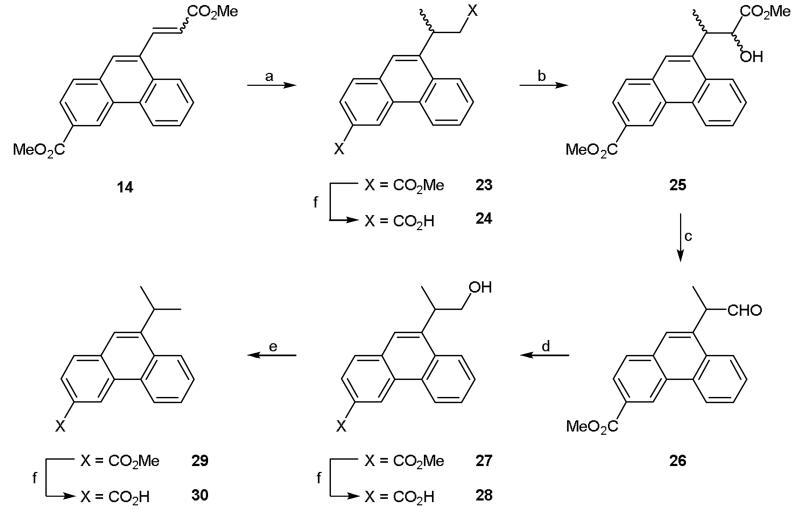
Synthesis of the branched 9-isopropyl derivative (30) required a different strategy to that described above (Scheme 4). This strategy had the added advantage of generating two intermediates (24 and 28) that could be pharmacologically characterized. Starting from diester 14, 1,4-conjugate addition of methyl magnesium chloride afforded 23 in reasonable yield. Whilst a small amount of this compound was hydrolyzed with base to di-acid 24, the majority was reacted with 2-tosyl-3-phenyloxaziridine8 to generate alcohol 25 (Scheme 4). Reduction of the alkyl ester with lithium borohydride and cleavage of the resultant 1,2-diol with sodium periodate led to the synthesis of aldehyde 26. Reduction of the aldehyde with sodium borohydride gave alcohol 27 in good yield. A small amount of this ester was hydrolyzed to the corresponding acid, 28, using base (Scheme 4). Alcohol 27 was then converted to the corresponding mesylate by reaction with methanesulfonyl chloride. The mesylate was then in turn converted to the corresponding iodo derivative via a Finkelstein reaction. Subsequent hydrogenation led to dehalogenation and yielded the 9-isopropyl derivative 29 which was readily hydrolyzed to the desired acid 30 (Scheme 4).
Scheme 4.
Reagents and conditions: (a) CuI, NaI, MeMgCl, TMSCl, DCM/Me2S, −78 °C then rt, 3h; (b) KHMDS, THF, 2-tosyl-3-phenyloxaziridine, −78 °C then rt, 2h; (c) (i) LiBH4, THF, 0 °C, 30 min then rt, 4h, (ii) t-BuOH/H2O (4:1), NaIO4, rt, 30 min; (d) NaBH4, THF, rt, 4h; (e) (i) MsCl, TEA, 0 °C, 1 h then rt, 3h, (ii) NaI, acetone, reflux, 24 h, (iii) H2, TEA, 10% Pd/C, rt, 18 h; (f) (i) NaOH (aq), dioxane, 75 °C, (ii) 1 M HCl (aq).
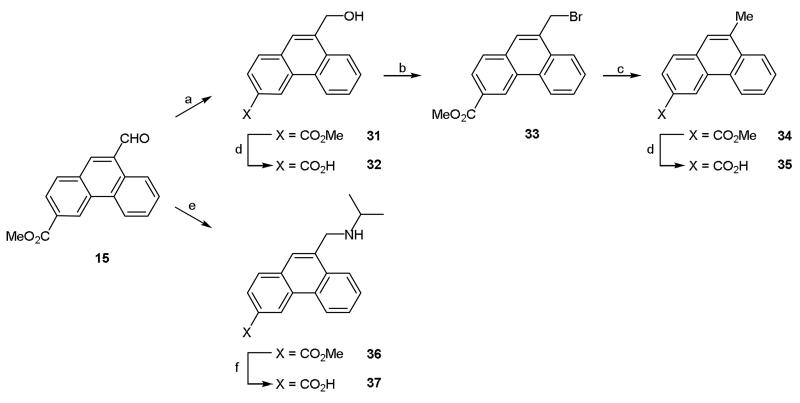
In addition to its use in the previously described Wittig chemistry, aldehyde 15 was utilized as a starting point for the synthesis of the 9-methyl derivative 35 (Scheme 5). Reduction of the aldehyde using sodium borohydride afforded 9-hydroxymethyl derivative 31. Although a small portion of this compound was hydrolyzed to yield acid 32 for pharmacological characterization, the majority was taken forward and reacted with phosphorus tribromide to afford 9-bromomethyl 33. Hydrogenation subsequently afforded 9-methyl derivative 34 which was readily hydrolyzed to the corresponding acid 35 (Scheme 5).
Scheme 5.
Reagents and conditions: (a) NaBH4, THF, rt, 4 h; (b) PBr3, DCM, 0 °C then rt, 2 h; (c) H2, 10% Pd/C, rt, 18 h; (d) (i) NaOH (aq), dioxane, 75 °C, (ii) 1 M HCl (aq); (e) isopropylamine, NaBH(OAc)3, DCE, rt, 40 h; (f) (i) NaOH (aq), dioxane, 75 °C, 4 h, (ii) 1 M HCl (aq), (iii) ion exchange chromatography.
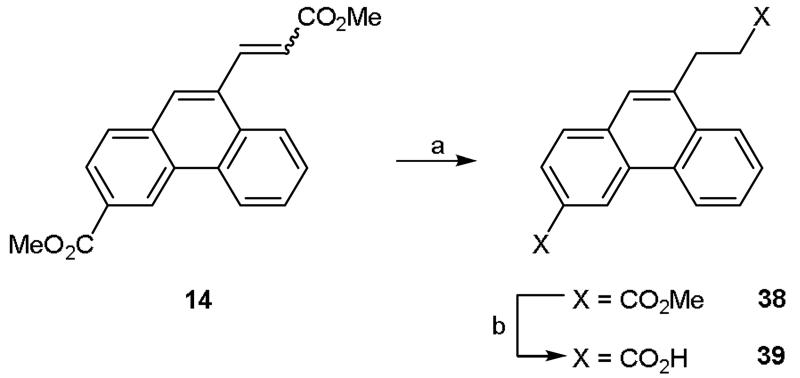
Whilst the introduction of hydrophobic substituents was our primary focus, we decided to synthesize some compounds with more polar groups at the 9-position in order to gather additional data on the requirements for biological activity. For example, aldehyde 15 was reacted with isopropylamine via reductive amination to afford ester 36 which was subsequently hydrolyzed to acid 37 (Scheme 5). Similarly, alkene 14 was hydrogenated to afford alkyl di-ester 38 which was then hydrolyzed to di-acid 39 (Scheme 6).
Scheme 6.
Reagents and conditions: (a) H2, 10% Pd/C, EtOAc, rt, 18 h; (b) (i) NaOH (aq), THF, reflux, 4 h, (ii) 1 M HCl (aq).
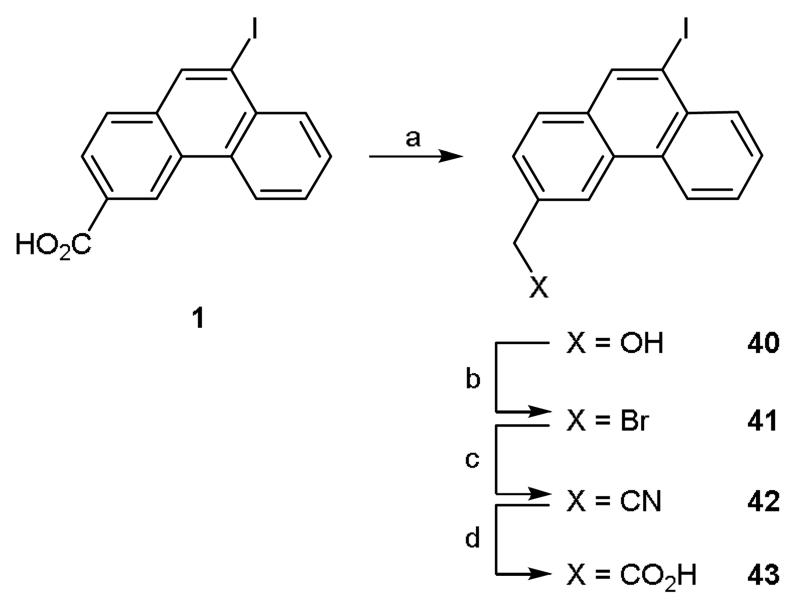
To identify the optimal 3-position substituent for biological activity, the 3-carboxy group in 1 was subjected to chemical modification (Scheme 7). Interestingly, attempts to reduce this moiety using lithium aluminium hydride led not only to reduction of the desired group but also de-halogenation. Consequently, a pathway was devised in which the acid chloride of 1 was generated via reaction with thionyl chloride and then reduced under mild conditions using sodium borohydride (Scheme 7). This route was successful and led to the synthesis of 3-hydroxymethyl 40 in good yield. Reaction of 40 with phosphorus tribromide afforded 3-bromomethyl 41 which was in turn converted to the corresponding nitrile 42 by reaction with sodium cyanide under phase transfer conditions. Hydrolysis of the nitrile under acidic conditions yielded the 3-acetic acid derivative 43 (Scheme 7).9
Scheme 7.
Reagents and conditions: (a) (i) SOCl2, dioxane, reflux, 12 h, (ii) NaBH4, THF, 0 °C, 0.5 h then rt, 12 h; (b) PBr3, DCM, 0 °C then rt, 1 h; (c) NaCN, TBAB, H2O/DCM (1:1), rt, 5 days; (d) AcOH, H2SO4, H2O, 118 °C, 3 h.
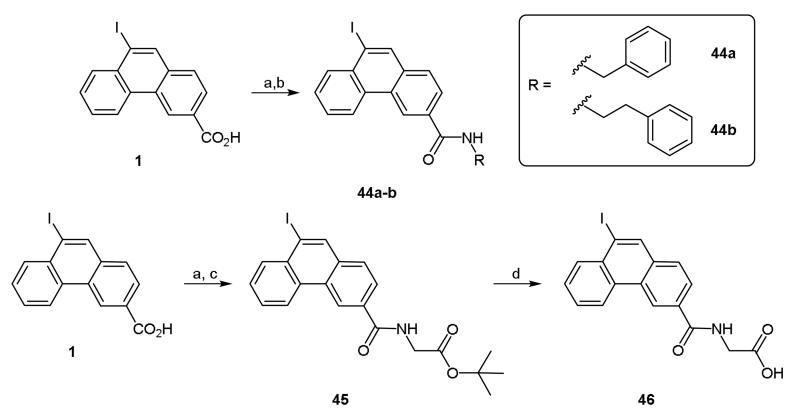
In a further modification to the 3-position, the acid chloride of 1 was reacted with benzylamine, phenethylamine and the t-butyl ester of glycine to afford amides 44a-b and 45 (Scheme 8). De-protection of the t-butyl ester to afford acid 46 was achieved readily and in good yield by reaction with TFA (Scheme 8).
Scheme 8.
Reagents and conditions: (a) SOCl2, C6H6, 80 °C, 12 h; (b) R-NH2, TEA, dioxane, rt, 3 h; (c) H-Gly-OtBu.HCl, TEA, dioxane, rt, 3 h; (d) TFA, DCM, rt, 18 h.
A previously described electrophysiological assay on GluN1 and GluN2A-D subunits individually expressed in Xenopus oocytes3 was used to pharmacologically characterize a selection of the synthesized phenanthrenes. The compounds were tested at a concentration of 100 μM for their effects on GluN1/GluN2A-D receptor responses and percentage antagonism or potentiation of responses to glutamate (10 μM) and glycine (10 μM) was determined (Table 1). Whilst only preliminary, these data suggest that: (a) an alkyl substituent at the 9-position promotes NMDA receptor potentiating activity, (b) as the length and/or size of the alkyl chain increases so does NMDA receptor potentiation (compare activity of 35 vs 2, 3, 19b, 19d & 19f), (c) introduction of a polar group into the alkyl side chain promotes NMDA receptor antagonism over potentiation (37 & 39), (d) the 9-iodo group can be replaced by a 3-thienyl ring without adversely effecting activity (compare activity of 1 vs 22), and (e) moving the carboxyl group away from the phenanthrene ring is beneficial for NMDA receptor antagonism (compare activity of 1 vs 43).
Table 1.
Activity of selected 3,9-disubstituted phenanthrene derivatives at recombinant NMDA receptor subtypesa
| NMDAR (n≥4)b | ||||
|---|---|---|---|---|
| Compoundc | GluN2A | GluN2B | GluN2C | GluN2D |
| 1 | 8.6 ± 4.8 | 0.9 ± 0.1 | −34.1 ± 8.3 | −52.3 ± 3.0 |
| 2 | 36.0 ± 7.4 | 51.2 ± 13.2 | −7.3 ± 0.4 | 5.6 ± 4.7 |
| 3 | 31.5 ± 10.0 | 34.0 ± 8.5 | 21.8 ± 8.1 | 24.3 ± 3.6 |
| 35 | −4.8 ± 4.6 | −3.2 ± 0.3 | −15.1 ± 0.3 | −4.1 ± 0.9 |
| 19b | 6.6 ± 1.2 | 30.0 ± 1.8 | 5.2 ± 4.0 | 7.8 ± 1.8 |
| 19d | 42.6 ± 9.6 | 42.1 ± 14.3 | 26.4 ± 5.4d | 20.3 ± 5.3 |
| 19f | 28.2 ± 11.4 | 20.5 ± 7.5 | 19.6 ± 3.0 | 24.6 ± 7.3 |
| 22 | 10.7 ± 6.4 | 3.9 ± 12.6 | −52.7 ± 9.1 | −45.2 ± 8.7 |
| 37 | −21.9 ± 9.2 | −0.3 ± 1.8 | −13.0 ± 2.5 | −2.6 ± 0.8 |
| 39 | −48.1 ± 6.5 | −51.1 ± 3.4 | −17.8 ± 2.7 | −15.1 ± 0.7 |
| 43 | −23.5 ± 3.9 | −30.9 ± 3.8 | −46.7 ± 4.3 | −66.6 ± 4.1 |
ND = not determined; All compounds tested at a concentration of 100 μM
% inhibition (negative number) or potentiation (positive number) of the responses of recombinant rat NMDA receptors (GluN1 expressed with the indicated GluN2 subunit) expressed in Xenopus oocytes (means ± s.e.m.).
All of the compounds were made up as stocks solutions in DMSO and were soluble up to a concentration of 100 μM in the buffer used in these assays.
19d inhibited 22% in one experiment, this value was not included in the average shown.
In conclusion, we have developed an alternative and robust synthetic pathway to 9-iodophenanthrene-3-carboxylic acid (1), a novel allosteric NMDA receptor modulator. Starting from a few common intermediates, we have synthesized a series of novel phenanthrene derivatives with a variety of substituents at the 3- and 9-positions of the phenanthrene ring. It is hoped that these compounds will lead to a better understanding of the structural requirements for allosteric modulation of the NMDA receptor. The preliminary pharmacological data described here suggests that the new compounds have interesting profiles of activity on NMDA receptor subtypes. Further pharmacological characterization of these newly synthesized compounds is currently on-going and will be reported in due course.
Reagents were purchased from commercial suppliers and purified by standard techniques when necessary. All anhydrous solvents were obtained from either Acros or Sigma-Aldrich and used without further drying. All anhydrous reactions were conducted under an inert atmosphere. Melting points were determined using an Electrothermal IA9100 capillary apparatus and are uncorrected. 1H-NMR spectra were measured on either a Jeol spectrometer at 270.18 MHz, a Jeol JNM-LA300 spectrometer at 300.53 MHz, a Jeol JNM-ECP400 spectrometer at 400.18 MHz, or a Varian 400MR spectrometer at 399.77 MHz. 13C-NMR spectra were recorded on either a Jeol JNM-LA300 spectrometer at 75.57 MHz, a Jeol JNM-ECP400 spectrometer at 100.63 MHz, or a Varian 400MR spectrometer at 100.52 MHz. Chemical shifts (δ) are reported in parts per million (ppm) with 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt in D2O, or tetramethylsilane in CDCl3 or DMSO-d6 used as internal standards. Mass spectrometry was performed in the mass spectroscopy laboratories of the Department of Chemistry, University of Bristol, UK. Elemental analyses were performed in the microanalytical laboratories of the Department of Chemistry, University of Bristol, UK. The purity of all novel compounds was determined by combustion analysis, which confirmed that they were ≥ 95% pure. Thin layer chromatography was performed on Merck silica gel 60 F254 plastic sheets. Flash chromatography was performed on Merck silica gel 60 (220-440 mesh) from Fisher.
2-(9-Bromophenanthren-3-yl)-2-methyl-[1,3]dioxolane (7)
A stirred solution of 44 (20.9 g, 70 mmol), ethylene glycol (8.68 g, 0.14 mol) and TsOH.H2O (0.67 g, 3.5 mmol) in toluene (200 mL) was heated at reflux with a Dean-Stark trap in place overnight. After being allowed to cool to room temperature the reaction mixture was washed with saturated aqueous NaHCO3 (50 mL) and H2O (50 mL). The organic layer was then isolated, dried over MgSO4 and concentrated in vacuo to ~ 50 mL. At this point, the product precipitated out of solution as a white solid and was filtered off. Further concentration of the mother liquor to ~ 10 mL led to the precipitation of a second crop of product which was again collected by filtration. The mother liquor was then concentrated in vacuo and the remaining residue purified by flash chromatography (2% EtOAc in hexane) to give 7 as a white solid (23.4 g, 97%);
1H NMR (300 MHz, CDCl3): δ = 1.79 (s, 3H), 3.79-3.90 (m, 2H), 4.07-4.18 (m, 2H), 7.64-7.86 (m, 4H), 8.10-8.23 (m, 1H), 8.34-8.43 (m, 1H), 8.69-8.79 (m, 2H).
HRMS-CI: m/z [M + H]+ calcd for C18H15O2Br: 343.0334; found 343.0338.
Tributyl-[3-(2-methyl-[1,3]dioxolan-2-yl)phenanthren-9-yl]stannane (8)
To a solution of 7 (22.2 g, 65 mmol) in anhydrous THF (350 mL) at −78 °C was added carefully and dropwise a 2.5 M solution of n-BuLi in hexane (31 mL, 78 mmol). The resultant mixture was allowed to stir for 1 h at −78 °C before being quenched with n-Bu3SnCl (23 mL, 84.5 mmol). After complete addition, the solution was allowed to warm to room temperature. The reaction mixture was then diluted with diethyl ether (500 mL) and the organic layer isolated, washed with water (150 mL), dried over MgSO4 and concentrated in vacuo. The resultant residue was purified by flash chromatography (2% EtOAc in hexane) to afford 8 (31.9 g, 89%) which was utilized in the next step without further analysis.
3-Acetyl-9-iodophenanthrene (9)
8 (31.9 g, 57.7 mmol) was dissolved in DCM (150 mL) and a saturated iodine solution in DCM added slowly at 0 °C until the colour of the last drop of iodine did not disappear within 30 secs. The organic solution was then washed with saturated NaHSO3 (50 mL), water (50 mL), dried over MgSO4 and concentrated in vacuo. The resultant residue was dissolved in acetone (200 mL) and aq 2 M HCl (4 mL) added dropwise. The ketone precipitated out of solution almost immediately and after stirring for 30 min 9 was collected by filtration as a white solid (17.0 g, 85%); mp: 149-151 °C (lit. 148-150 °C)7;
1H NMR (300 MHz, CDCl3): δ = 2.78 (s, 3H), 7.67-7.75 (m, 2H), 7.78 (d, J = 8.4 Hz, 1H), 8.10 (dd, J = 8.4 & 1.8 Hz, 1H), 8.20-8.24 (m, 1H), 8.44 (s, 1H), 8.66-8.71 (m, 1H), 9.24 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 26.7, 102.4, 122.7, 123.8, 126.1, 127.9, 128.1, 128.4, 129.8, 130.7, 132.4, 133.5, 135.1, 135.3, 137.9, 197.8.
9-Iodophenanthrene-3-carboxylic acid (1)
Synthesized from 9 as described previously.7
2-Methyl-2-(9-methylsulfanylphenanthren-3-yl)-[1,3]dioxolane (10)
To a stirring solution of 7 (1.72 g, 5.00 mmol) in anhydrous THF (50 mL) at −78 °C was added dropwise a 2.5 M solution of n-BuLi (2.4 mL, 6.00 mmol). After complete addition the solution was allowed to stir for 1 h before being quenched by the dropwise addition of dimethyl disulfide (0.59 mL, 6.50 mmol). The reaction mixture was then allowed to warm to room temperature before being diluted with diethyl ether (50 mL). The organic layer was isolated, washed with H2O (50 mL), dried over MgSO4 and concentrated in vacuo. The resulting residue was purified by flash chromatography (2% EtOAc in hexane) to afford 10 which was utilized in the next step without further analysis.
1-[9-(Methylsulfanyl)phenanthren-3-yl]ethanone (11)
Concentrated HCl (0.4 mL) was added dropwise to a stirred solution of 10 in acetone (100 mL). The resultant solution was allowed to stir for 1 h during which time a precipitate formed. This solid was filtered off and washed with cold acetone (20 mL). Re-crystallization from acetone afforded 11 as an off-white solid (954 mg, 72%); mp: 135-137 °C;
1H NMR (400 MHz, CDCl3): δ = (s, 3H), 2.78 (s, 3H), 7.50 (s, 1H), 7.67-7.78 (m, 2H), 7.83 (d, J = 8.4 Hz, 1H), 8.12 (dd, J = 8.4 & 1.6 Hz, 1H), 8.31-8.35 (m, 1H), 8.77-8.81 (m, 1H), 9.25 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 15.3, 26.9, 120.8, 123.2, 124.0, 124.8, 126.1, 127.4, 127.7, 127.7, 127.7 128.1, 130.5, 134.1, 134.9, 138.7, 198.0.
HRMS-EI: m/z [M]+ calcd for C17H14OS: 266.0765; found: 266.0766.
Anal. calcd for C17H14OS: C, 76.66, H, 5.30; found: C, 76.60, H, 5.51.
(9-Methylsulfanyl)phenanthrene-3-carboxylic acid (12)
A stirred suspension of 11 (400 mg, 1.50 mmol) in dioxane (50 mL) was heated at 40 °C until complete dissolution of the solid. At the same time, a solution of sodium hypobromite was prepared by the dropwise addition of bromine (0.38 mL, 7.50 mmol) to an ice-cooled solution of sodium hydroxide (1.05 g, 26.3 mmol dissolved in 50 mL of H2O). The sodium hypobromite solution was then added dropwise to the dioxane solution (complete addition took around 10 min) and stirring continued until TLC indicated complete conversion. The mixture was then allowed to cool to room temperature and a saturated sodium sulphite solution (10 mL) added to quench any excess hypobromite. The dioxane was removed in vacuo and the resultant suspension topped up with H2O and acidified to pH 1 using conc HCl. Subsequent filtration yielded a yellow solid which was washed copiously with water (100 mL) and then dried over P2O5. Re-crystallization from a mixture of toluene and ethanol afforded 12 as a light yellow solid (104 mg, 26%); mp: 242-246 °C;
1H NMR (400 MHz, DMSO-d6): δ =2.72 (s, 3H), 7.72-7.83 (m, 3H), 8.02 (d, J = 8.0 Hz, 1H), 8.12 (dd, J = 8.0 & 1.2 Hz, 1H), 8.19-8.25 (m, 1H), 8.88 (d, J = 8.0 Hz, 1H), 9.29 (s, 1H), 13.09 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ =14.1, 120.3, 123.4, 123.9, 124.4, 127.0, 127.0, 127.6, 127.6, 127.9, 129.4, 129.4, 134.1, 137.4, 167.4.
HRMS-ESI: m/z [M − H]− calcd for C16H12O2S: 267.0485; found 267.0489.
Anal. calcd for C16H12O2S·0.25H2O: C, 70.44, H, 4.62; found: C, 70.43, H, 4.53.
Methyl 9-(3-methoxy-3-oxoprop-1-en-1-yl)phenanthrene-3-carboxylate (14)
A flask was charged with 13 (30.1 g, 0.1 mol), palladium acetate (0.24 g, 0.1 mmol) and tri-o-tolylphosphine (1.28 g, 0.4 mmol). The flask was then briefly evacuated and backfilled with argon three times. A degassed solution of triethylamine (40 mL, 0.26 mol) and methyl acrylate (12 mL, 0.13 mol) in DMF (300 mL) was then cannulated into the flask and the resultant mixture heated at 100 °C for 18 h. After being allowed to cool to room temperature any remaining volatile compounds were removed in vacuo. Na2CO3 (10.6 g, 0.1 mol) was then added followed by methyl iodide (12.5 mL, 0.2 mol) and the reaction mixture stirred at room temperature overnight. The mixture was then diluted with diethyl ether (500 mL) and the organic layer isolated, washed with water (2 × 200 mL) and dried over MgSO4. Concentration in vacuo gave 14 as a pale yellow solid (29.5 g, 92%) and a 1:1 mixture of cis and trans isomers; mp: 186-188 °C;
1H NMR (300 MHz, CDCl3): δ = 3.85 (s, 3H), 3.88 (s, 3H), 3.94 (s, 3H), 4.02 (s, 3H), 4.17 (d, J = 6.0 Hz, 1H), 5.45 (d, J = 6.0 Hz, 1H), 6.59 (d, J = 15.0 Hz, 1H), 7.38-7.51 (m, 4H), 7.65-7.75 (m, 2H), 7.84-7.89 (m, 2H), 7.99 (d, J = 7.8 Hz, 1H), 8.06 (d, J = 7.8 Hz, 1H), 8.14-8.17 (m, 2H), 8.46 (d, J = 15.0 Hz, 1H), 8.52 (d, J = 9.3 Hz, 1H), 8.74-8.78 (m, 1H), 9.12 (s, 1H), 9.33 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 52.0, 52.3, 52.5, 52.6, 122.1, 123.3, 123.5,124.0, 124.3, 124.6, 124.9, 125.1, 125.7, 126.6, 127.0, 127.6, 127.8, 128.4, 128.7, 129.0, 129.3, 130.2, 130.5, 130.7, 130.8, 133.4, 133.7, 133.9, 135.9, 142.2, 167.1, 167.2, 167.3, 172.7.
HRMS-CI: m/z [M + H]+ calcd for C20H16O4: 321.1127; found, 321.1125.
Methyl 9-formylphenanthrene-3-carboxylate (15)
A solution of 14 in DCM (6.4 g, 20 mmol in 30 mL) was diluted with t-BuOH (150 mL) and water (50 mL) with vigorous stirring. TMAO (2.45 g, 22 mmol), OsO4 (0.5 g, 0.2 mmol) and tartaric acid (4.2 g, 20 mmol) were added and the reaction monitored by TLC. Once all the starting material had been consumed NaIO4 (21.3 g, 0.1 mol) was added. The aldehyde precipitated out of solution almost immediately. After stirring for an additional 20 min the solvent (mainly t-BuOH) was removed in vacuo, and 15 collected by filtration as a pale yellow solid (5.17 g, 98%); mp: 180-182 °C;
1H NMR (300 MHz, CDCl3): δ = 4.04 (s, 3H), 7.71-7.80 (m, 2H), 8.03 (d, J = 8.8 Hz, 1H), 8.20-8.24 (m, 2H), 8.74-8.77 (m, 1H), 9.29-9.34 (m, 2H), 10.38 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 52.7, 123.0, 125.2, 126.1, 127.2, 128.2, 128.4, 128.8, 130.5, 130.5, 132.2, 132.4, 132.8, 139.7, 166.9, 193.5.
MS (CI+): m/z (%) = 265 (100) [M + H]+.
Anal. calcd for C17H12O3: C, 77.26, 4.58; found, C, 77.22, H, 4.49.
Methyl 9-bromophenanthrene-3-carboxylate (16)
A flask containing 137 (10.0 g, 33.2 mmol) was briefly evacuated and backfilled with argon. Anhydrous methanol (300 mL) was then cannulated into the flask followed by a catalytic amount of concentrated H2SO4 (3 mL). The resultant mixture was heated under reflux for 48 h then allowed to cool to room temperature before being concentrated in vacuo. The resultant dark orange solid was dissolved in DCM (250 mL) and washed with a saturated aqueous NaHCO3 solution (3 × 50 mL), water (50 mL), and brine (50 mL). The organic layer was dried over MgSO4 and concentrated in vacuo to afford 16 as an orange solid (9.06 g, 87%); mp: 151-153 °C (lit. 155-155.5 °C)4,
1H NMR (400 MHz, CDCl3): δ = 4.03 (s, 3H), 7.69-7.77 (m, 2H), 7.78 (d, J = 8.4 Hz, 1H), 8.07 (s, 1H), 8.17 (dd, J = 8.4 & 1.6 Hz, 1H), 8.33-8.37 (m, 1H), 8.71-8.75 (m, 1H), 9.32 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 52.4, 123.0, 124.6, 125.2, 127.1, 127.8, 128.0, 128.0, 128.2, 128.2, 129.1, 129.9, 130.5, 131.3, 134.7, 167.0.
General Procedure A (Wittig Reaction)
To a stirred suspension of the appropriate triphenylphosphonium salt (3.6 mmol) in THF (20 mL) was added dropwise potassium bis(trimethylsilyl)amide (0.5 M solution in toluene, 7.2 mL, 3.6 mmol). The resultant mixture was allowed to stir for 30 min before being added dropwise to a stirred solution of 15 (793 mg, 3 mmol) in THF (20 mL). After complete addition, the mixture was allowed to stir at room temperature for ~ 4 h before the reaction was quenched with a saturated NH4Cl solution (10 mL). The mixture was then diluted with diethyl ether (25 mL) and the organic layer isolated and dried over MgSO4. Concentration in vacuo yielded the crude product which was re-dissolved in diethyl ether (30 mL) and passed through a short silica plug. Concentration in vacuo subsequently afforded the alkene phenanthrenes (17a-17c) which were utilised immediately in the next step.
Methyl 9-vinylphenanthrene-3-carboxylate (17a)
Following general procedure A, methyltriphenylphosphonium iodide (1.46 g) afforded 17a as a light yellow oil (677 mg, 86%).
Methyl 9-prop-1-en-1-ylphenanthrene-3-carboxylate (17b)
Following general procedure A, ethyltriphenylphosphonium bromide (1.34 g) afforded 17b as a light yellow oil (729 mg, 88%).
Methyl 9-but-1-en-1-ylphenanthrene-3-carboxylate (17c)
Following general procedure A, propyltriphenylphosphonium bromide (1.38 g) afforded 17c as a light yellow oil (793 mg, 91%).
General Procedure B (Heck Reaction)
A flask was charged with 16 (1.00 g, 3.17 mmol), palladium acetate (7.2 mg, 1 mol%), tri-o-tolylphosphine (39 mg, 4 mol%), and (if a solid) the appropriate alkene (3.96 mmol). The flask was then briefly evacuated and backfilled with argon three times. Degassed anhydrous DMF (25 mL) was then added followed by (if a liquid) the appropriate alkene (3.96 mmol) and triethylamine (1.11 mL, 7.93 mmol). The resultant mixture was heated at 100 °C overnight. After being allowed to cool to room temperature the reaction mixture was filtered through a celite pad to remove any precipitated Pd(0) and then poured into a stirred solution of EtOAc (100 mL), water (100 mL) and aqueous 1 M HCl (10 mL). The organic layer was subsequently isolated and the aqueous phase further extracted with EtOAc (2 × 30 mL). The organic extracts were pooled, washed with water (5 × 100 mL), brine (100 mL) and dried over MgSO4. Concentration in vacuo afforded the crude alkene phenanthrenes (17d-17i) which were utilised immediately in the next step.
Methyl 9-pent-1-en-1-ylphenanthrene-3-carboxylate (17d)
Following general procedure B, 1-pentene (0.43 mL) afforded 17d as a dark orange oil (850 mg, 88%).
9-(4-Methylpent-1-en-1-yl)phenanthrene-3-carboxylate (17e)
Following general procedure B, 4-methyl-1-pentene (0.50 mL) afforded 17e as a dark orange oil (924 mg, 91%).
Methyl 9-hex-1-en-1-ylphenanthrene-3-carboxylate (17f)
Following general procedure B, 1-hexene (0.49 mL) afforded 17f as a dark orange oil (950 mg, 94%).
Methyl 9-hept-1-en-1-ylphenanthrene-3-carboxylate (17g)
Following general procedure B, 1-heptene (0.56 mL) afforded 17g as a dark orange oil (760 mg, 72%).
Methyl 9-(2-phenylethenyl)phenanthrene-3-carboxylate (17h)
Following general procedure B, styrene (0.45 ml) afforded 17h as a yellow/brown solid (911 mg, 85%).
Methyl 9-[2-(4-methoxycarbonyl)phenylethenyl]phenanthrene-3-carboxylate (17i)
Following general procedure B, methyl 4-vinylbenzoate (642 mg) afforded 17i as a yellow solid (1.02 g, 81%).
General Procedure C (Hydrogenation)
The appropriate alkene phenanthrene was dissolved in ethyl acetate (100 mL) and the resultant solution hydrogenated under 3 bar of hydrogen in the presence of 10 wt % palladium on activated carbon (50 mg) for 18 h. The reaction mixture was then filtered through a celite pad before being concentrated in vacuo. Purification of the resultant residue by flash chromatography (5 → 10% EtOAc in hexane) afforded the individual alkyl phenanthrenes.
Methyl 9-ethylphenanthrene-3-carboxylate (18a)
Following general procedure C, 17a (677 mg, 2.58 mmol) afforded 18a (613 mg, 90%) as a white solid; mp: 82-83 °C;
1H NMR (400 MHz, CDCl3): δ = 1.46 (t, J = 7.6 Hz, 3H), 3.17 (q, J = 7.6 Hz, 2H), 4.02 (s, 3H), 7.61 (s, 1H), 7.64-7.73 (m, 2H), 7.85 (d, J = 8.4 Hz, 1H), 8.11-8.15 (m, 1H), 8.17 (dd, J = 8.4 & 1.6 Hz, 1H), 8.81-8.85 (m, 1H), 9.39 (s, 1H).
13C NMR (100 MHz, CDCl3): δ =14.3, 26.3, 52.2, 123.4, 124.4, 124.4, 125.0, 126.5, 126.7, 127.0, 127.1, 128.1, 129.0, 130.8, 131.4, 134.9, 141.2, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C18H16O2: 265.1229; found 265.1223.
Methyl 9-n-propylphenanthrene-3-carboxylate (18b)
Following general procedure C, 17b (729 mg, 2.62 mmol) afforded 18b (685 mg, 94%) as a clear oil;
1H NMR (300 MHz, CDCl3): δ = 1.09 (t, J = 7.8 Hz, 3H), 1.81 (m, J = 7.8 Hz, 2H), 2.98 (t, J = 7.8 Hz, 2H), 3.23 (s, 3H), 7.43 (s, 1H), 7.57-7.66 (m, 2H), 7.71 (d, J = 9.0 Hz, 1H), 8.01-8.05 (m, 1H), 8.14 (d, J = 9.0 Hz, 1H), 8.72-8.75 (m, 1H), 9.32 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 14.4, 22.9, 31.8, 52.2, 123.4, 124.5, 125.0, 125.4, 126.5,126.7, 127.0, 127.1, 128.1, 129.0, 130.9, 131.5, 134.8, 139.6, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C19H18O2: 279.1380; found 279.1373.
Methyl 9-n-butylphenanthrene-3-carboxylate (18c)
Following general procedure C, 17c (793 mg, 2.71 mmol) afforded 18c (729 mg, 92%) as a clear oil;
1H NMR (400 MHz, CDCl3): δ = 1.04 (t, J = 7.8 Hz, 3H), 1.50-1.59 (m, 2H), 1.79-1.87 (m, 2H), 3.12 (t, J =7.8 Hz, 2H), 4.05 (s, 3H), 7.58-7.59 (m, 1H), 7.67-7.74 (m, 2H), 7.82-7.86 (m, 1H), 8.12-8.15 (m, 1H), 8.20 (d, J = 8.0 Hz, 1H), 8.82-8.85 (m, 1H), 9.41 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 14.2, 23.1, 32.4, 33.4, 52.3, 123.5, 124.7, 125.1, 125.5, 126.6, 126.8, 127.1, 127.2, 128.2, 129.1, 131.0, 131.6, 135.0, 140.1, 167.6.
HRMS-CI: m/z [M + H]+ calcd for C20H20O2: 293.1542; found 293.1553.
Methyl 9-n-pentylphenanthrene-3-carboxylate (18d)
Following general procedure C, 17d (850 mg, 2.79 mmol) afforded 18d (815 mg, 95%) as a viscous yellow oil;
1H NMR (400 MHz, CDCl3): δ = 0.94 (t, J = 6.8 Hz, 3H), 1.36-1.53 (m, 4H), 1.77-1.88 (m, 2H), 3.13 (t, J = 8.0 Hz, 2H), 4.02 (s, 3H), 7.61 (s, 1H), 7.64-7.75 (m, 2H), 7.86 (d, J = 8.0 Hz, 1H), 8.12-8.15 (m, 1H), 8.18 (dd, J = 8.0 & 1.6 Hz, 1H), 8.82-8.86 (m, 1H), 9.41 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 14.1, 22.6, 29.9, 32.1, 33.6, 52.2, 123.4, 124.6, 125.1, 125.4, 126.5, 126.7, 127.0, 127.0, 128.1, 129.0, 130.9, 131.4, 134.8, 140.0, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C21H22O2: 307.1698; found 307.1696.
Methyl 9-(4-methylpent-1-yl)phenanthrene-3-carboxylate (18e)
Following general procedure C, 17e (924 mg, 2.90 mmol) afforded 18e (886 mg, 95%) as a viscous yellow oil;
1H NMR (400 MHz, CDCl3): δ = 0.92 (d, J = 6.4 Hz, 6H), 1.36-1.44 (m, 2H), 1.58-1.70 (m, 1H), 1.78-1.87 (m, 2H), 3.11 (t, J = 7.6 Hz, 2H), 4.02 (s, 3H), 7.61 (s, 1H), 7.65-7.74 (m, 2H), 7.88-7.84 (d, J = 8.4 Hz, 1H), 8.11-8.15 (m, 1H), 8.18 (dd, J = 8.4 & 1.6 Hz, 1H), 8.82-8.86 (m, 1H), 9.41 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 22.6, 28.0, 28.1, 33.9, 39.2, 52.5, 123.4, 124.6, 125.1, 125.4, 126.5, 126.7, 127.0, 127.1, 128.1, 129.0, 130.9, 131.4, 134.9, 140.1, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C22H24O2: 321.1855; found 321.1855.
Methyl 9-n-hexylphenanthrene-3-carboxylate (18f)
Following general procedure C, 17f (950 mg, 2.98 mmol) afforded 18f (899 mg, 94%) as a viscous yellow oil;
1H NMR (400 MHz, CDCl3): δ = 0.91 (t, J = 7.2 Hz, 3H), 1.30-1.42 (m, 4H), 1.43-1.55 (m, 2H), 1.77-1.87 (m, 2H), 3.12 (t, J = 7.6 Hz, 2H), 4.02 (s, 3H), 7.61 (s, 1H), 7.65-7.74 (m, 2H), 7.86 (d, J = 8.4 Hz, 1H), 8.11-8.15 (m, 1H), 8.18 (dd, J = 8.4 & 1.6 Hz, 1H), 8.82-8.86 (m, 1H), 9.40 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 14.1, 22.7, 29.6, 30.2, 31.8, 33.6, 52.2, 123.4, 124.6, 125.1, 125.4, 126.5, 126.7, 127.0, 127.0, 128.1, 129.0, 130.9, 131.4, 134.9, 140.1, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C22H24O2: 321.1855; found 321.1849.
Methyl 9-n-heptylphenanthrene-3-carboxylate (18g)
Following general procedure C, 17g (760 mg, 2.29 mmol) afforded 18g (500 mg, 65%) as a viscous pale yellow oil;
1H NMR (400 MHz, CDCl3): δ = 0.90 (t, J = 7.2 Hz, 3H), 1.24-1.54 (m, 8H), 1.83 (p, J = 7.6 Hz, 2H), 3.13 (t, J = 7.6 Hz, 2H), 4.02 (s, 3H), 7.61 (s, 1H), 7.65-7.74 (m, 2H), 7.86 (d, J = 8.4 Hz, 1H), 8.11-8.15 (m, 1H), 8.18 (dd, J = 8.4 & 1.6 Hz, 1H), 8.82-8.86 (m, 1H), 9.41 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 14.1, 22.7, 29.2, 29.8, 30.2, 31.9, 33.6, 52.2, 123.4, 124.6, 125.1, 125.4, 126.5, 126.7, 127.0, 127.1, 128.1, 129.0, 131.0, 131.4, 134.9, 140.1, 167.5.
HRMS-CI: m/z [M + Na]+ calcd for C23H26O2: 357.1831; found 357.1822.
Methyl 9-phenethylphenanthrene-3-carboxylate (18h)
Following general procedure C, 17h (911 mg, 2.69 mmol) afforded 18h as a viscous clear oil (599 mg, 64%);
1H NMR (400 MHz, CDCl3): δ = 3.11-3.18 (m, 2H), 3.42-3.48 (m, 2H), 4.03 (s, 3H), 7.21-7.26 (m, 1H), 7.27-7.38 (m, 4H), 7.60 (s, 1H), 7.68-7.77 (m, 2H), 7.84 (d, J = 8.8 Hz, 1H), 8.18-8.22 (m, 1H), 8.19 (dd, J = 8.8 & 1.6 Hz, 1H), 8.85-8.89 (m, 1H), 9.42 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 35.5, 36.4, 52.3, 123.5, 124.4, 125.1, 125.7, 126.2, 126.6, 126.8, 127.2, 127.3, 128.2, 128.4, 128.5, 129.1, 130.9, 131.2, 134.7, 138.8, 141.7, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C24H20O2: 341.1542; found 341.1543.
Methyl 9-[2-(4-methoxycarbonylphenyl)ethyl]-phenanthrene-3-carboxylate (18i)
Following general procedure C, 17i (1.02 g, 2.57 mmol) afforded 18i as a viscous clear oil (625 mg, 61%);
1H NMR (400 MHz, CDCl3): δ = 3.15-3.21 (m, 2H), 3.40-3.47 (m, 2H), 3.91 (s, 3H), 4.02 (s, 3H), 7.31 (d, J = 8.4 Hz, 2H), 7.53 (s, 1H), 7.67-7.76 (m, 2H), 7.80 (d, J = 8.0 Hz, 1H), 7.98 (d, J = 8.4Hz, 2H), 8.13-8.19 (m, 2H), 8.83-8.88 (m, 1H), 9.40 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 35.0, 36.4, 52.0, 52.3, 123.6, 124.2, 125.1, 125.8, 126.7, 126.9, 127.3, 127.4, 128.2, 128.5, 129.2, 129.9, 131.0, 131.1, 134.6, 138.2, 147.1, 167.1, 167.4.
HRMS-CI: m/z [M]+ calcd for C26H22O4: 398.1518; found 398.1511.
General Procedure D (Ester Hydrolysis)
The appropriate ester was dissolved in a mixture of either THF or dioxane (100 mL) and H2O (20 mL). A NaOH or KOH solution (3 eqvs dissolved in 20 mL H2O) was then added dropwise and the resulting solution heated either at reflux (THF) or 75 °C (dioxane) until TLC indicated complete hydrolysis. The reaction mixture was then allowed to cool to room temperature and the organic solvent removed in vacuo. The resulting aqueous suspension was topped up with water, extracted with diethyl ether (30 mL) and acidified to pH 1 using aq 1 M HCl. The solid that precipitated out of solution at this stage was filtered off, washed copiously with H2O and then dried over P2O5 to afford the desired acid. Several compounds required purification and were re-crystallised from an appropriate solvent.
9-Ethylphenanthrene-3-carboxylic acid (19a)
Following general procedure D, 18a (550 mg, 2.08 mmol), NaOH (250 mg, 6.24 mmol) and dioxane afforded 19a as a white solid (495 mg, 95%); mp: 245-249 °C (dec);
1H NMR (400 MHz, DMSO-d6): δ = 1.39 (t, J = 7.6 Hz, 3H), 3.15 (q, J = 7.6 Hz, 2H), 7.72-7.79 (m, 3H), 8.02 (d, J = 8.4 Hz, 1H), 8.12 (dd, J = 8.4 & 1.6 Hz, 1H), 8.17-8.22 (m, 1H), 8.86-8.91 (m, 1H), 9.33 (s, 1H), 13.10 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 14.8, 26.0, 123.8, 124.7, 124.8, 124.9, 127.0, 127.6, 127.9, 128.6, 128.8, 130.5, 131.2, 134.7, 141.2, 168.0.
HRMS-ESI: m/z [M − H]− calcd for C17H14O2: 249.0921; found 249.0929.
Anal. calcd for C17H14O2: C, 81.58, H, 5.64; found, C, 81.75, H, 5.91.
9-n-Propylphenanthrene-3-carboxylic acid (19b)
Following general procedure D, 18b (525 mg, 1.89 mmol), NaOH (227 mg, 5.67 mmol) and dioxane afforded 19b as a white solid (465 mg, 93%); mp: 234-238 °C;
1H NMR (400 MHz, DMSO-d6): δ = 1.02 (t, J = 7.6 Hz, 3H), 1.77 (m, J = 7.6 Hz, 2H), 3.09 (t, J = 7.6 Hz, 2H), 7.71-7.78 (m, 3H), 8.00 (d, J = 8.4 Hz, 1H), 8.11 (dd, J = 8.4 & 1.6 Hz, 1H), 8.16-8.21 (m, 1H), 8.85-8.90 (m, 1H), 9.32 (s, 1H), 13.12 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 14.0, 22.9, 34.6, 123.3, 124.3, 124.6, 125.3, 126.5, 127.1, 127.3, 128.1, 128.2, 128.3, 130.1, 130.8, 134.1, 139.2, 167.5.
HRMS-ESI: m/z [M − H]− calcd for C18H16O2: 263.1078; found 263.1085.
Anal. calcd for C18H16O2: C, 81.79, H, 6.10; found, C, 82.05, H, 6.39.
9-n-Butylphenanthrene-3-carboxylic acid (19c)
Following general procedure D, 18c (650 mg, 2.22 mmol), NaOH (266 mg, 6.66 mmol) and dioxane afforded 19c as a white solid which was re-crystallised from a mixture of toluene and ethanol (248 mg, 40%); mp: 208-211 °C;
1H NMR (400 MHz, DMSO-d6): δ = 0.95 (t, J = 7.6 Hz, 3H), 1.45 (m, J = 7.6 Hz, 2H), 1.73 (p, J = 7.6 Hz, 2H), 3.12 (t, J = 7.2 Hz, 2H), 7.71-7.79 (m, 3H), 8.01 (d, J = 8.0 Hz, 1H), 8.11 (dd, J = 8.0 & 1.2 Hz, 1H), 8.16-8.21 (m, 1H), 8.85-8.90 (m, 1H), 9.32 (s, 1H), 13.08 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 13.8, 22.2, 31.9, 32.3, 123.3, 124.3, 124.6, 125.2, 126.5, 127.1, 127.3, 128.0, 128.2, 128.3, 130.1, 130.8, 134.1, 139.4, 167.5.
HRMS-ESI: m/z [M − H]− calcd for C19H18O2: 277.1234; found 277.1232.
Anal. calcd for C19H18O2: C, 81.99, H, 6.52; found, C, 81.85, H, 6.41.
9-n-Pentylphenanthrene-3-carboxylic acid (19d)
Following general procedure D, 18d (573 mg, 1.88 mmol), NaOH (226 mg, 5.64 mmol) and dioxane afforded 19d as a white solid which was re-crystallised from toluene (141 mg, 26%); mp: 194-197 °C;
1H NMR (400 MHz, DMSO-d6): δ = 0.87 (t, J = 7.2 Hz, 3H), 1.29-1.47 (m, 4H), 1.74 (p, J = 7.2 Hz, 2H), 3.10 (t, J = 7.2 Hz, 2H), 7.70-7.79 (m, 3H), 8.00 (d, J = 8.4 Hz, 1H), 8.11 (dd, J = 8.4 & 1.6 Hz, 1H), 8.15-8.20 (m, 1H), 8.84-8.92 (m, 1H), 9.33 (s, 1H), 13.13 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 14.5, 22.6, 30.0, 31.9, 33.2, 123.9, 124.9, 125.1, 125.8, 127.2, 127.6, 127.9, 128.6, 128.8, 128.9, 130.7, 131.4, 134.7, 140.0, 168.2.
MS (ESI−): m/z (%) = 291 (100) [M−H]−, 247 (46).
Anal. calcd for C20H20O2: C, 82.16, H, 6.89; found, C, 82.00, H, 6.82.
9-(4-Methylpent-1-yl)phenanthrene-3-carboxylic acid (3)
Following general procedure D, 18e (675 mg, 2.11 mmol), NaOH (253 mg, 6.33 mmol) and dioxane afforded 3 as a white solid which was re-crystallised from toluene (264 mg, 41%); mp: 193-196 °C;
1H NMR (400 MHz, DMSO-d6): δ = 0.82 (d, J = 6.4 Hz, 6H), 1.24-1.33 (m, 2H), 1.48-1.60 (m, 1H), 1.64-1.74 (m, 2H), 3.03 (t, J = 8.0 Hz, 2H), 7.66-7.77 (m, 3H), 7.97 (d, J = 8.4 Hz, 1H), 8.09-8.16 (m, 2H), 8.82-8.89 (m, 1H), 9.32 (s, 1H), 13.13 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 22.4, 27.3, 27.6, 32.8, 38.4, 123.2, 124.3, 124.5, 125.1, 126.5, 127.0, 127.3, 128.0, 128.2, 128.3, 130.1, 130.7, 134.1, 139.4, 167.5.
MS (ESI−): m/z (%) = 305 (100) [M−H]−, 261 (25).
Anal. calcd for C21H22O2: C, 82.32, H, 7.24; found, C, 82.30, H, 7.17.
9-n-Hexylphenanthrene-3-carboxylic acid (19f)
Following general procedure D, 18f (679 mg, 2.12 mmol), NaOH (254 mg, 6.36 mmol) and dioxane afforded 19f as a white solid which was re-crystallised from toluene (221 mg, 34%); mp: 196-199 °C;
1H NMR (400 MHz, DMSO-d6): δ = 0.85 (t, J = 7.2 Hz, 3H), 1.22-1.37 (m, 4H), 1.43 (p, J = 7.2 Hz, 2H), 1.73 (p, J = 7.2 Hz, 2H), 3.10 (t, J = 7.2 Hz, 2H), 7.71-7.79 (m, 3H), 8.00 (d, J = 8.4 Hz, 1H), 8.11 (dd, J = 8.4 & 1.6 Hz, 1H), 8.14-8.20 (m, 1H), 8.84-8.91 (m, 1H), 9.32 (s, 1H), 13.12 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 13.9, 22.1, 28.8, 29.7, 31.1, 32.7, 123.3, 124.3, 124.6, 125.2, 126.6, 127.1, 127.3, 128.1, 128.2, 128.3, 130.1, 130.8, 134.1, 139.4, 167.6.
MS (ESI−): m/z (%) = 305 (100) [M−H]−, 261 (20).
Anal. calcd for C21H22O2: C, 82.32, H, 7.24; found, C, 82.01, H, 7.08.
9-n-Heptylphenanthrene-3-carboxylic acid (19g)
Following general procedure D, 18g (450 mg, 1.35 mmol), NaOH (162 mg, 4.05 mmol) and dioxane afforded 19g as a white solid which was re-crystallised from toluene (317 mg, 73%); mp: 185-188 °C;
1H NMR (400 MHz, DMSO-d6): δ = 0.83 (t, J = 7.2 Hz, 3H), 1.18-1.45 (m, 8H), 1.72 (p, J = 7.6 Hz, 2H), 3.08 (t, J = 7.6 Hz, 2H), 7.70-7.78 (m, 3H), 7.99 (d, J = 8.0 Hz, 1H), 8.11 (dd, J = 8.0 & 1.2 Hz, 1H), 8.14-8.19 (m, 1H), 8.85-8.90 (m, 1H), 9.32 (s, 1H), 13.09 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 13.9, 22.0, 28.5, 29.1, 29.8, 31.2, 32.6, 123.3, 124.2, 124.6, 125.2, 126.6, 127.0, 127.3, 128.2, 128.3, 130.1, 130.8, 134.1, 139.4, 167.6.
HRMS-ESI: m/z [M − H]− calcd for C22H24O2: ; found .
Anal. calcd for C22H24O2: C, 82.46, H, 7.55; found, C, 82.12, H, 7.50.
9-Phenethylphenanthrene-3-carboxylic acid (19h)
Following general procedure D, 18h (512 mg, 1.50 mmol), NaOH (180 mg, 4.50 mmol) and dioxane afforded 19h as a white solid which was re-crystallised from a mixture of toluene and ethanol (105 mg, 22%); mp: 236-237 °C;
1H NMR (400 MHz, DMSO-d6): δ = 3.07 (t, J = 8.0 Hz, 2H), 3.41 (t, J = 8.0 Hz, 2H), 7.18-7.24 (m, 1H), 7.27-7.37 (m, 4H), 7.73-7.81 (m, 3H), 7.98 (d, J = 8.4 Hz, 1H), 8.11 (dd, J = 8.4 & 1.6 Hz, 1H), 8.26-8.32 (m, 1H), 8.86-8.93 (m, 1H), 9.34 (s, 1H) 13.10 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 34.6, 35.7, 123.3, 124.3, 124.5, 125.4, 125.9, 126.6, 127.1, 127.5, 128.0, 128.1, 128.2, 128.3, 128.3, 130.1, 130.7, 134.0, 138.5, 141.4, 167.5.
MS (ESI−): m/z (%) = 325 (100) [M−H]−.
Anal. calcd for C23H18O2: C, 84.64, H, 5.56; found, C, 84.53, H, 5.58.
9-[2-(4-Carboxyphenyl)ethyl]phenanthrene-3-carboxylic acid (19j)
Following general procedure D, 18i (400 mg, 1.00 mmol), KOH (337 mg, 6.00 mmol) and THF afforded 19j as an off-white solid (216 mg, 58%); mp: >250 °C;
1H NMR (400 MHz, DMSO-d6): δ = 3.15 (t, J = 8.0 Hz, 2H), 3.45 (t, J = 8.0 Hz, 2H), 7.47 (d, J = 8.4 Hz, 2H), 7.75-7.81 (m, 3H), 7.88 (d, J = 8.4 Hz, 2H), 7.98 (d, J = 8.0 Hz, 1H), 8.11 (dd, J = 8.0 & 1.6 Hz, 1H), 8.27-8.32 (m, 1H), 8.87-8.93 (m, 1H), 9.34 (s, 1H), 12.97 (br s, 1H), 13.15 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 34.1, 35.6, 123.3, 124.3, 124.6, 125.5, 126.6, 127.2, 127.5, 128.2, 128.3, 128.4, 128.5, 128.6, 129.4, 130.1, 130.6, 134.0, 138.2, 146.7, 167.2, 167.5.
HRMS-ESI: m/z [M − H]− calcd for C24H18O4: 369.1132; found 369.1142.
Anal. calcd for C24H18O4·0.63H2O: C, 75.51, H, 5.09; found, C, 75.82, H, 5.48.
Methyl 9-vinylphenanthrene-3-carboxylate (17a)
A flask containing 16 (1.00 g, 3.17 mmol) was evacuated and backfilled with argon three times. Anhydrous toluene (50 mL) was cannulated into the flask and the resultant solution de-gassed with argon for approx. 30 mins. Pd(PPh3)4 (109.9 mg, 3 mol%) was then added and the mixture de-gassed for a further 10 mins before vinyl(tri-n-butyl)tin (1.12 mL, 3.80 mmol) was added. The resultant mixture was refluxed for 4 h before being allowed to cool to room temperature and filtered through celite to remove any precipitated Pd(0). The filtrate was then poured into a stirring mixture of EtOAc and saturated aq NH4Cl (50 mL each). The organic layer was isolated and washed with aq 1 M KF (2 × 50 mL) to remove any tin byproducts. The white solid (Bu3SnF) which precipitated from solution after the first wash was removed via filtration through celite. The organic layer was then isolated, washed with water (50 mL), brine (50 mL), dried over MgSO4 and concentrated in vacuo to afford a dark orange oil. Purification by flash chromatography (5% EtOAc in hexane) afforded a pale yellow oil which partially solidified on standing (550 mg, 66%);
1H NMR (400 MHz, CDCl3): δ = 4.03 (s, 3H), 5.59 (dd, J = 10.8 & 1.6 Hz, 1H), 5.91 (dd, J = 17.2 & 1.6 Hz, 1H), 7.48 (ddd, J = 17.2, 10.8 & 0.8 Hz, 1H), 7.65-7.77 (m, 2H), 7.86 (s, 1H), 7.93 (d, J = 8.4 Hz, 1H), 8.16-8.22 (m, 2H), 8.81-8.86 (m, 1H), 9.41 (d, J = 0.8 Hz, 1H).
13C NMR (100 MHz, CDCl3): δ = 52.3, 118.7, 123.4, 124.1, 124.9, 125.2, 126.8, 127.3, 127.3, 127.8, 128.9, 129.8, 130.7, 130.8, 134.8, 134.9. 137.4, 167.5.
HRMS-ES: m/z [M]+ calcd for C18H14O2: 262.0994; found 262.0999.
Methyl 9-cyclopropylphenanthrene-3-carboxylate (20)
Diiodomethane (1.82 g, 6.8 mmol) was dissolved in anhydrous DCM (10 mL) and ZnEt2 (1.0 M solution in hexane, 3.4 mL, 3.4 mmol) added to this solution at 0 °C followed by a solution of 17a in DCM (450 mg, 1.7 mmol in 10 mL). The reaction mixture was then stirred vigorously overnight before being quenched with aq 1 M HCl. The mixture was extracted with diethyl ether (50 mL) and the organic layer isolated, dried over MgSO4 and concentrated in vacuo. The resultant residue was purified by flash chromatography (5% EtOAc in hexane) to afford 20 as a viscous clear oil (400 mg, 85%);
1H NMR (300 MHz, CDCl3): δ = 0.83-0.89 (m, 2H), 1.10-1.16 (m, 2H), 2.31-2.40 (m, 1H), 4.02 (s, 3H), 7.53 (s, 1H), 7.67-7.76 (m, 2H), 7.82 (d, J = 9.0 Hz, 1H), 8.16 (d, J = 9.0 Hz, 1H), 8.49-8.53 (m, 1H), 8.79-8.82 (m, 1H), 9.38 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 6.5, 6.5, 14.1, 52.3, 123.2, 123.9, 125.1, 125.3, 126.6, 127.0, 127.1, 127.3, 128.3, 129.1, 130.7, 132.9, 135.0, 140.4, 167.6.
HRMS-CI: m/z [M + H]+ calcd for C19H16O2: 277.1229; found, 277.1231.
9-Cyclopropylphenanthrene-3-carboxylic acid (2)
20 (350 mg, 1.27 mmol) was dissolved in dioxane (10 mL) and saturated aqueous LiOH added dropwise until the reaction mixture became a slurry (~ 1 mL). The mixture was stirred at room temperature overnight, then extracted with diethyl ether (20 mL) before being acidified to pH 1 with aq 1 M HCl. The acid precipitated out of solution and was subsequently collected by filtration, washed with water and dried over P2O5 to afford 2 as a white solid (310 mg, 93%); mp: >250 °C;
1H NMR (300 MHz, DMSO-d6): δ = 0.81-0.85 (m, 2H), 1.10-1.15 (m, 2H), 2.41-2.49 (m, 1H), 7.69 (s, 1H), 7.77-7.80 (m, 2H), 8.02 (d, J = 8.8 Hz, 1H), 8.10 (d, J = 8.8 Hz, 1H), 8.52-8.54 (m, 1H), 8.85-8.88 (m, 1H), 9.31 (s, 1H).
13C NMR (75 MHz, DMSO-d6): δ = 7.0, 7.0, 13.9, 123.4, 123.5, 124.7, 125.5, 127.0, 127.8, 127.9, 128.6, 128.7, 129.0, 130.3, 132.6, 134.7, 140.4, 168.0.
MS (ESI−): m/z (%) = 261 (100) [M − H]−.
Anal. calcd for C18H14O2: C, 82.42, H, 5.38; found, C, 82.84, H, 5.47.
Methyl 9-(thiophen-3-yl)phenanthrene-3-carboxylate (21)
A flame dried flask was successively charged with 16 (1.00 g, 3.17 mmol), 3-thienylboronic acid (573 mg, 4.48 mmol), K2CO3 (1.31 g, 9.51 mmol) and Pd(dppf)Cl2.DCM (261 mg, 0.32 mmol). After each addition, the flask was briefly evacuated and backfilled with argon. Degassed anhydrous DME (75 mL) was then cannulated into the flask and the resultant mixture stirred at 80 °C for 24 h. After being allowed to cool to room temperature the reaction mixture was diluted with EtOAc (100 mL) and H2O (20 mL). The organic layer was isolated and washed with water (2 × 25 mL) and then brine (2 × 25 mL). After drying over MgSO4, concentration in vacuo afforded a dark brown/black residue which was partially purified by flash chromatography (10% EtOAc in hexane) to give a pale yellow solid (618 mg, 61%) which 1H NMR showed was a mixture of 21 and 16 (~ 75:25). The mixture was taken forward to the next step without further purification.
21: 1H NMR (400 MHz, CDCl3): δ = 4.04 (s, 3H), 7.35 (dd, J = 4.8 & 1.6 Hz, 1H), 7.49 (dd, J = 2.8 & 1.6 Hz, 1H), 7.51 (dd, J = 4.8 & 2.8 Hz, 1H), 7.59-7.64 (m, 1H), 7.71-7.76 (m, 1H), 7.77 (s, 1H), 7.91 (d, J = 8.4 Hz, 1H), 8.08 (dd, J = 8.4 & 1.2, 1H), 8.21 (dd, J = 8.4 & 1.6 Hz, 1H), 8.87 (d, J = 8.4 Hz, 1H), 9.45 (s, 1H).
9-(Thiophen-3-yl)phenanthrene-3-carboxylic acid (22)
Following general procedure D, 21 (541 mg, 1.70 mmol), NaOH (204 mg, 5.10 mmol) and dioxane afforded a pale yellow solid which was re-crystallised 4 times from glacial acetic acid to give 22 (117 mg, 23%); mp: >250 °C;
1H NMR (400 MHz, DMSO-d6): δ = 7.42 (dd, J = 4.8 & 1.6 Hz, 1H), 7.68-7.73 (m, 1H), 7.77-7.83 (m, 3H), 7.94 (s, 1H), 8.04 (dd, J = 8.4 & 1.2 Hz, 1H), 8.11 (d, J = 8.4, 1H), 8.16 (dd, J = 8.4 & 1.6 Hz, 1H), 8.95 (d, J = 8.4 Hz, 1H), 9.39 (s, 1H), 13.15 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 123.7, 124.8, 125.3, 126.8, 127.0, 127.3, 128.0, 128.0, 129.1, 129.3, 129.4, 129.8, 130.7, 131.0, 134.2, 135.8, 140.3, 168.0.
HRMS-ESI: m/z [M − H]− calcd for C19H12O2S: 303.0485; found 303.0495.
Anal. calcd for C19H12O2S·0.55H2O: C, 72.61, H, 4.20; found, C, 72.61, H, 4.02.
Methyl 9-(4-methoxy-4-oxobutan-2-yl)phenanthrene-3-carboxylate (23)
To a cold (−78 °C) stirring mixture of CuI (25.2 g, 0.13 mol) and NaI (36 g, 0.24 mol) in Me2S (79 mL) and DCM (72 mL) was added methylmagnesium chloride (3.0 M solution in THF, 41 mL, 0.12 mol) and TMSCl (31 mL, 0.24 mol). The mixture was then stirred for 30 min at −78 °C when a solution of 14 (7.7 g, 24 mmol) in DCM (72 mL) was added. The resultant mixture was stirred at −78 °C for 10 min and then slowly allowed to warm to room temperature. After stirring for 3 h the reaction was quenched with saturated aq NH4Cl. The organic layer was isolated and the aqueous phase extracted with diethyl ether (100 mL). The organics were pooled, dried over MgSO4, and concentrated in vacuo. Purification of the resultant residue by flash column chromatography (5% EtOAc in hexane) afforded 23 (4.83 g, 60%) as a light coloured oil;
1H NMR (300 MHz, CDCl3): δ = 1.42 (d, J = 7.8 Hz, 3H), 2.69 (dd, J = 7.8 & 12.0 Hz, 1H), 2.89 (dd, J = 7.8 & 12.0 Hz, 1H), 3.87 (s, 3H), 3.93 (s, 3H), 4.06 (m, J = 7.8 Hz, 1H), 7.75-7.78 (m, 2H), 7.84 (s, 1H), 7.92 (d, J = 8.7 Hz, 1H), 7.94 (d, J = 8.7 Hz, 1H), 8.00-8.10 (m, 1H), 8.92-8.95 (m, 1H), 9.33 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 21.7, 31.0, 41.6, 54.3, 56.7, 122.8, 124.0, 124.3, 124.7, 127.0, 127.6, 128.0, 128.7, 128.8, 129.1, 130.6, 130.7, 134.1, 143.4, 168.0, 173.8.
HRMS-CI: m/z [M + H]+ calcd for C21H20O4: 337.1440; found 337.1442.
9-(4-Methoxy-4-oxobutan-2-yl)phenanthrene-3-carboxylic acid (24)
Following general procedure D, 23 (508 mg, 1.51 mmol), NaOH (362.4 mg, 9.06 mmol) and dioxane afforded 24 as a white solid (377 mg, 81%); mp: >250 °C;
1H NMR (300 MHz, DMSO-d6): δ = 1.42 (d, J = 7.8 Hz, 3H), 2.68 (dd, J = 7.8 & 12.0 Hz, 1H), 2.87 (dd, J = 7.8 & 12.0 Hz, 1H), 4.07 (m, J = 7.8 Hz, 1H), 7.77-7.80 (m, 2H), 7.86 (s, 1H), 8.06 (d, J = 8.7 Hz, 1H), 8.13 (d, J = 8.7 Hz, 1H), 8.27-8.30 (m, 1H), 8.90-8.93 (m, 1H), 9.33 (s, 1H).
13C NMR (75 MHz, DMSO-d6): δ = 21.7, 31.0, 41.6, 122.8, 124.0, 124.3, 124.7, 127.0, 127.6, 128.0, 128.7, 128.8, 129.1, 130.6, 130.7, 134.5, 143.4, 168.0, 173.8.
HRMS-ESI: m/z [M − H]− calcd for C19H16O4: 307.0976; found 307.0969.
Anal. calcd for C19H16O4: C, 74.01, H, 5.23; found, C, 74.07, H, 5.18.
Methyl 9-(3-hydroxy-2-methoxy-4-oxobutan-2-yl)phenanthrene-3-carboxylate (25)
To a stirring solution of 23 (2.74 g, 8.15 mmol) in anhydrous THF (40 mL) at −78 °C was added dropwise KHMDS (0.5 M solution in toluene, 17.3 mL, 8.65 mmol) followed by a solution of 2-tosyl-3-phenyloxaziridine8 (3.2 g, 12.25 mmol) in THF (20 mL). After complete addition, the mixture was allowed to warm to room temperature and stirred for ~ 2 h. Water (30 mL) and diethyl ether (60 mL) were then added. The organic layer was subsequently isolated and washed with a saturated sodium sulphite solution (20 mL), aq 1 M HCl (20 mL), and brine (20 mL). Concentration in vacuo afforded 25 as an oil which was utilised in the next step without further purification or analysis.
Methyl 9-(1-oxopropan-2-yl)phenanthrene-3-carboxylate (26)
A stirred solution of 25 in anhydrous THF (20 mL) was cooled to 0 °C and LiBH4 (227 mg, 12.3 mmol) added portionwise over a period of 10 min. After complete addition the mixture was stirred for 30 min at 0 °C and then at room temperature until TLC indicated complete conversion. The reaction was then quenched by the addition of aq 1 M HCl (5 mL) and extracted with diethyl ether (2 × 30 mL). The organic layers were pooled, dried over MgSO4 and concentrated in vacuo to obtain the crude 1,2-diol as an orange oil. This intermediate was dissolved in a mixture of t-BuOH and water (30 mL, 4:1) and NaIO4 (5.13 g, 24 mmol) added to the solution. The resultant mixture was stirred at room temperature for 30 min before the reaction was quenched by the addition of water (20 mL). The aqueous mixture was then extracted with diethyl ether (2 × 30 mL) and the organic layers pooled, dried over MgSO4, and concentrated in vacuo. Purification of the resultant residue by flash chromatography (5% EtOAc in hexane) afforded 26 (1.55 g, 65%) as a viscous light orange oil;
1H NMR (400 MHz, CDCl3): δ = 1.67 (d, J = 7.2, 3H), 4.03 (s, 3H), 4.42 (q, J = 7.2 Hz, 1H), 7.57 (s, 1H), 7.68-7.79 (m, 2H), 7.89 (d, J = 8.4 Hz, 1H), 8.06-8.10 (m, 1H), 8.21 (dd, J = 8.4 & 1.6 Hz, 1H), 8.85-8.89 (m, 1H), 9.41 (s, 1H), 9.82 (d, J = 1.6 Hz, 1H).
13C NMR (100 MHz, CDCl3): δ = 14.4, 49.0, 52.3, 123.8, 123.9, 125.0, 126.0, 126.9, 127.3, 127.6, 128.1, 128.6, 129.5, 130.7, 131.2, 134.2, 135.3, 167.2, 200.9.
HRMS-ESI: m/z [M + Na]+ calcd for C19H16O3: 315.0997; found 315.0992.
Methyl 9-(1-hydroxypropan-2-yl)phenanthrene-3-carboxylate (27)
To a stirred solution of 26 (1.0 g, 3.42 mmol) in anhydrous THF (100 mL) was added portionwise NaBH4 (388 mg, 10.26 mmol). i-PrOH (2 mL) was then added and the resultant suspension stirred at room temperature until TLC indicated complete reduction. Excess NaBH4 was then destroyed via the dropwise addition of water. The solvent was then removed in vacuo and the resultant residue dissolved in a mixture of EtOAc and water (50 mL each). The organic layer was isolated and the aqueous layer further extracted with EtOAc (2 × 25 mL). The organic layers were pooled, washed with water (25 mL), brine (25 mL), dried over MgSO4 and concentrated in vacuo. Purification of the resultant residue by flash column chromatography (10% EtOAc in hexane) afforded 27 as a viscous pale yellow oil (985 mg, 98%);
1H NMR (300 MHz, CDCl3): δ = 1.51 (d, J = 6.7 Hz, 3H), 3.85-3.91 (m, 2H), 4.00 (s, 3H), 4.05 (m, 1H), 7.64-7.73 (m, 3H), 7.87 (d, J = 8.8 Hz, 1H), 8.15-8.22 (m, 2H), 8.83 (d, J = 8.8 Hz, 1H), 9.37 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 17.8, 21.1, 52.4, 67.7, 123.4, 123.7, 125.1, 126.7, 127.0, 127.3, 127.6, 128.6, 129.1, 131.1, 131.3, 134.5, 140.8, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C19H18O3: 295.1329; found 295.1320.
9-(1-Hydroxypropan-2-yl)phenanthrene-3-carboxylic acid (28)
Following general procedure D, 27 (301 mg, 1.02 mmol), NaOH (122 mg, 3.06 mmol), and dioxane afforded 28 as a white solid (213 mg, 75%); mp: >250 °C;
1H NMR (400 MHz, DMSO-d6): δ = 1.44 (d, J = 8.0 Hz, 3H), 3.54-3.64 (m, 1H), 3.69-3.78 (m, 1H), 3.79-3.87 (m, 1H), 4.82 (s, 1H), 8.05 (d, J = 8.0 Hz, 1H), 8.12 (d, J = 8.0 Hz, 1H), 8.27-8.34 (m, 1H), 8.86-8.93 (m, 1H), 9.33 (s, 1H), 13.14 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 17.8, 36.5, 66.3, 123.2, 123.4, 124.1, 124.3, 126.5, 127.0, 127.4, 128.1, 128.6, 130.1, 130.9, 134.1, 141.6, 167.6.
HRMS-ESI: m/z [M − H]− calcd for C18H16O3: 279.1027; found 279.1019.
Anal. calcd for C18H16O3: C, 77.12, H, 5.75; found, C, 77.37, H, 5.89.
Methyl 9-(propan-2-yl)phenanthrene-3-carboxylate (29)
To a stirred solution of 27 (505 mg, 1.72 mmol) in DCM (20 mL) at 0 °C was added triethylamine (0.25 mL, 1.80 mmol) followed by methanesulfonyl chloride (0.14 mL, 1.80 mmol). The resultant mixture was stirred at 0 °C for 1 h and then at room temperature for 3 h. After this time, the reaction was diluted with DCM and water (20 mL each) and the organic layer isolated, washed with aq 1 M HCl (10 mL), brine (10 mL), dried over MgSO4 and concentrated in vacuo. The crude mesylate thus obtained was dissolved in acetone (75 mL) and sodium iodide (645 mg, 4.3 mmol) added to the solution. The resultant mixture was refluxed for 24 h before being allowed to cool to room temperature. Filtration then removed any solids with the filter cake being rinsed with acetone. The filtrate and washes were combined and concentrated in vacuo. The resultant residue was dissolved in diethyl ether (40 mL) and washed with water (20 mL), a saturated sodium sulfite solution (15 mL), water (20 mL), and dried over MgSO4. Concentration in vacuo afforded the crude iodo compound which was then dissolved in dioxane (100 mL). Triethylamine (0.25 mL, 1.80 mmol) was added and the resultant solution hydrogenated under 3 bar of hydrogen in the presence of 10 wt % palladium on activated carbon (50 mg) for 18 h. The reaction mixture was then filtered through a celite pad before being concentrated in vacuo. The resultant residue was taken-up in DCM (50 mL) and washed successively with aq 1M HCl, H2O, and brine (25 mL each). Drying over MgSO4 followed by concentration in vacuo yielded a residue which was purified by flash column chromatography (10% EtOAc in hexane) to afford 29 as a pale yellow oil (349 mg, 73%);
1H NMR (300 MHz, CDCl3): δ = 1.49 (d, J = 7.2 Hz, 6H), 3.74 (sep, J = 7.2 Hz, 1H), 3.85 (s, 3H), 7.66-7.72 (m, 3H), 7.86 (d, J = 8.7 Hz, 1H), 8.18-8.20 (m, 2H), 8.82-8.86 (m, 1H), 9.40 (s, 1H).
13C NMR (75 MHz, CDCl3): δ = 23.2, 28.8, 52.2, 121.7, 123.5, 124.1, 124.9, 126.5, 126.6, 127.0, 127.1, 128.3, 128.8, 131.0, 134.9, 145.5, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C19H18O2: 279.1380; found 279.1371.
9-(Propan-2-yl)phenanthrene-3-carboxylic acid (30)
Following general procedure D, 29 (205 mg, 0.74 mmol), NaOH (89 mg, 2.22 mmol) and dioxane afforded 30 as a white solid (166 mg, 85%); mp: 226-230 °C;
1H NMR (400 MHz, DMSO-d6): δ = 1.42 (d, J = 6.8 Hz, 6H), 3.78 (sep, J = 6.8 Hz, 1H), 7.72-7.79 (m, 2H), 7.83 (s, 1H), 8.06 (d, J = 8.4 Hz, 1H), 8.12 (dd, J = 8.4 & 1.6 Hz, 1H), 8.24-8.31 (m, 1H), 8.86-8.92 (m, 1H), 9.33 (s, 1H), 13.06 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 23.0, 28.1, 121.6, 123.4, 124.1, 124.2, 126.5, 127.0, 127.3, 128.1, 128.1, 128.5, 130.1, 130.3, 134.1, 145.0, 167.5.
HRMS-ESI: m/z [M − H]− calcd for C18H16O2: 263.1078; found 263.1070.
Anal. calcd for C18H16O2: C, 81.79, H, 6.10; found, C, 81.65, H, 6.01.
Methyl 9-(hydroxymethyl)phenanthrene-3-carboxylate (31)
To a stirred solution of 15 (1.10 g, 4.16 mmol) in anhydrous THF (150 mL) was added slowly and portionwise NaBH4 (472 mg, 12.48 mmol). i-PrOH (2 mL) was then added and the resultant suspension stirred at room temperature until TLC indicated complete reduction. Excess NaBH4 was destroyed by the dropwise addition of H2O. Concentration in vacuo afforded a solid which was dissolved in a mixture of EtOAc and H2O (50 mL each). The organic layer was isolated and the aqueous layer further extracted with EtOAc (2 × 25 mL). The organic layers where pooled, washed with water (25 mL), brine (25 mL), dried over MgSO4 and concentrated in vacuo to afford 31 as a pale yellow solid (1.05 g, 95%); mp: 179-182 °C;
1H NMR (400 MHz, DMSO-d6): δ = 3.96 (s, 3H), 5.05 (d, J = 5.6 Hz, 2H), 5.52 (t, J = 5.6 Hz, 1H), 7.69-7.81 (m, 2H), 7.96 (s, 1H), 8.07-8.18 (m, 3H), 8.85-8.91 (m, 1H), 9.34 (s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 52.2, 61.2, 123.2, 123.3, 124.3, 124.3, 126.3, 127.2, 127.2, 127.4, 128.7, 128.9, 129.7, 129.8, 134.2, 139.1, 166.4.
HRMS-ESI: m/z [M + Na]+ calcd for C17H14O3: 289.0835; found 289.0832.
Anal. calcd for C17H14O3: C, 76.68, H, 5.30; found, C, 76.35, H, 5.31.
9-(Hydroxymethyl)phenanthrene-3-carboxylic acid (32)
Following general procedure D, 31 (500 mg, 1.88 mmol), NaOH (226 mg, 5.64 mmol) and dioxane afforded 32 as a light yellow solid which was re-crystallised from a mixture of toluene and ethanol (180 mg, 38%); mp: >250 °C;
1H NMR (400 MHz, DMSO-d6): δ = 5.04 (s, 2H), 7.67-7.78 (m, 2H), 7.93 (s, 1H), 8.06 (d, J = 8.4 Hz, 1H), 8.13 (dd, J = 8.4 & 0.8 Hz, 2H), 8.85 (d, J = 8.0 Hz, 1H), 9.32 (s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 61.4, 123.2, 123.6, 124.5, 126.8, 127.4, 127.5, 128.5, 128.8, 128.9, 130.0, 134.1, 138.9, 167.7.
MS (ESI−): m/z (%) = 251 (100) [M − H]−.
Anal. calcd for C16H12O3·0.25H2O: C, 74.84, H, 4.91; found, C, 74.82, H, 4.83.
Methyl 9-(bromomethyl)phenanthrene-3-carboxylate (33)
A flask containing 31 (1.17 g, 4.39 mmol) was briefly evacuated and backfilled with argon. Anhydrous DCM (100 mL) was cannulated into the flask and the resulting solution cooled to 0 °C. Phosphorus tribromide (1.65 mL, 17.56 mmol) was then added dropwise to the stirring solution. After complete addition, the reaction mixture was stirred at 0 °C for 30 min and then at room temperature until TLC confirmed complete conversion. After ~ 2 h the reaction mixture was again cooled to 0 °C and excess PBr3 destroyed by the dropwise addition of a saturated NaHCO3 solution. The organic layer was subsequently isolated, dried over MgSO4 and concentrated in vacuo to afford an off-white solid which was dissolved in diethyl ether (100 mL) and washed successively with H2O (40 mL) and brine (40 mL). Drying over MgSO4 followed by concentration in vacuo yielded 33 as an off-white solid (994 mg, 69%); mp: 135-139 °C;
1H NMR (300 MHz, CDCl3): δ = 4.03 (s, 3H), 5.00 (s, 2H), 7.73-7.78 (m, 2H), 7.86 (s, 1H), 7.90 (d, J = 8.4 Hz, 1H), 8.20 (dd, J = 8.4 & 1.8 Hz, 1H), 8.22-8.27 (m, 1H), 8.80-8.87 (m, 1H), 9.39 (s, 1H).
13C NMR (100 MHz, CDCl3): δ =31.8, 52.4, 123.5, 124.7, 125.1, 126.9, 127.4, 127.5, 128.1, 128.6, 128.8, 129.6, 130.3, 131.2, 134.0, 134.3, 167.2.
MS (CI+): m/z (%) = 328/330 (69/67) [M+], 249 (100).
Anal. Calcd for C17H13O2Br: C, 62.03, H, 3.98; Found, C, 62.31, H, 4.31.
Methyl 9-methylphenanthrene-3-carboxylate (34)
33 (500 mg, 1.52 mmol) and triethylamine (0.21 mL, 1.52 mmol) were dissolved in dioxane (100 mL) and the resultant solution hydrogenated under 3 bar of hydrogen in the presence of 10 wt % palladium on activated carbon (50 mg) for 18 h. The reaction mixture was then filtered through a celite pad before being concentrated in vacuo. The resultant solid was taken-up in DCM (50 mL) and washed successively with aq 1M HCl, H2O, and brine (25 mL each). Drying over MgSO4 followed by concentration in vacuo afforded 34 as an off-white solid (344 mg, 91%); mp: 152-156 °C;
1H NMR (400 MHz, CDCl3): δ = 2.74 (s, 3H), 4.02 (s, 3H), 7.58 (s, 1H), 7.65-7.75 (m, 2H), 7.81 (d, J = 8.4 Hz, 1H), 8.04-8.09 (m, 1H), 8.43 (dd, J = 8.4 & 1.6 Hz, 1H), 8.78-8.83 (m, 1H), 9.38 (s, 1H).
13C NMR (100 MHz, CDCl3): δ =20.2, 52.2, 123.2, 124.7, 125.0, 126.2, 126.5, 126.8, 127.0, 127.0, 127.8, 129.0, 130.5, 132.1, 134.8, 135.5, 167.5.
HRMS-CI: m/z [M + H]+ calcd for C17H14O2: 251.1067; found 251.1059.
9-Methylphenanthrene-3-carboxylic acid (35)
Following general procedure D, 34 (310 mg, 1.24 mmol), NaOH (149 mg, 3.72 mmol) and dioxane afforded 35 as an off-white solid (199 mg, 68%); mp: >250 °C;
1H NMR (400 MHz, DMSO-d6): δ = 2.70 (s, 3H), 7.69-7.78 (m, 3H), 7.95 (d, J = 8.0 Hz, 1H), 8.09 (dd, J = 8.0 & 1.2 Hz, 1H), 8.09-8.12 (m, 1H), 8.82-8.86 (m, 1H), 9.33 (s, 1H), 13.01 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 19.7, 123.1, 124.3, 124.9, 125.9, 126.6, 127.2, 127.4, 128.0, 128.0, 128.3, 130.0, 131.5, 134.2, 135.2, 167.5.
MS (ESI−): m/z (%) = 235 (100) [M − H]−.
Anal. calcd for C16H12O2: C, 81.34, H, 5.12; found, C, 81.08, H, 5.40.
9-(Isopropylaminomethyl)phenanthrene-3-carboxylic acid methyl ester (36)
To a stirred mixture of 15 (750 mg, 2.84 mmol) and isopropylamine (0.41 mL, 4.97 mmol) in anhydrous DCE (100 mL) was added sodium triacetoxyborohydride (843 mg, 3.98 mmol). The resultant suspension was stirred at room temperature for 24 h. At this point TLC indicated incomplete conversion so 12 drops of glacial acetic acid were added to help catalyse the reaction. Stirring was continued for another 24 h when excess sodium triacetoxyborohydride was destroyed via the dropwise addition of a saturated aqueous NaHCO3 solution. EtOAc (40 mL) was added and the organic phase isolated, washed with brine (40 mL), dried over MgSO4 and concentrated in vacuo to yield an orange oil. Purification by flash chromatography (EtOAc followed by 20% MeOH in EtOAc) afforded 36 as a golden coloured oil (779 mg, 89%);
1H NMR (400 MHz, CDCl3): δ = 1.21 (d, J = 6.4 Hz, 6H), 2.98-3.09 (m, 1H), 4.02 (s, 3H), 4.27 (s, 2H), 7.65-7.74 (m, 2H), 7.77 (s, 1H), 7.88 (d, J = 8.4 Hz, 1H), 8.18 (dd, J = 8.4 & 1.6 Hz, 1H), 8.80-8.84 (m, 1H), 9.38 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 23.1, 49.1, 49.6, 52.3, 123.4, 124.3, 125.0, 125.5, 126.6, 126.9, 127.2, 127.5, 128.4, 129.4, 130.9, 130.9, 134.5, 137.3, 167.4.
HRMS-ESI: m/z [M]+ calcd for C20H21NO2: 305.1572; found 305.1598.
9-(Isopropylaminomethyl)phenanthrene-3-carboxylic acid (37)
To a stirring solution of 36 (740 mg, 2.41 mmol) in a mixture of dioxane (80 mL) and H2O (20 mL) was added dropwise a NaOH solution (289 mg, 7.23 mmol dissolved in 20 mL H2O). The resultant mixture was stirred at 75 °C until TLC indicated complete hydrolysis. After 4 h the reaction mixture was allowed to cool to room temperature and the dioxane removed in vacuo. The resultant aqueous solution was topped up with H2O and acidified to pH 3 using aq 1M HCl. No product precipitated from solution so the pH was re-adjusted to 7 using aq 1 M NaOH and the solution concentrated in vacuo to afford a white solid. The crude product was dissolved in a minimum volume of water and then absorbed onto AG-50 resin. The column was first eluted with water until the pH of the aqueous fractions was neutral. The product was then eluted using aq 1 M pyridine. Concentration of the aqueous pyridine fractions in vacuo afforded 37 as a white solid which was azeotroped with water to remove any remaining pyridine and then dried over P2O5 (496 mg, 70%); mp: >250 °C;
1H NMR (400 MHz, D2O/NaOD, pH 11): δ = 0.99 (d, J = 6.4 Hz, 6H), 2.61-2.72 (m, 1H), 3.46 (s, 2H), 6.99 (s, 1H), 7.33-7.39 (m, 1H), 7.42-7.52 (m, 3H), 7.91 (dd, J = 8.0 & 1.2 Hz, 1H), 8.42 (d, J = 8.0 Hz, 1H), 8.88 (s, 1H).
13C NMR (125 MHz, D2O/NaOD, pH 11): δ = 21.2, 47.1, 47.9, 122.8, 123.1, 123.5, 124.5, 126.5, 126.6, 126.7, 127.8, 128.4, 129.5, 129.8, 132.4, 133.6, 134.1, 175.1.
HRMS-ESI: m/z [M − H]− calcd for C19H19NO2: 292.1343; found: 292.1352.
Anal. calcd for C19H19NO2·0.55H2O: C, 73.93, H, 6.76, N, 4.54. found, C, 73.90, H, 6.41, N, 4.45.
Methyl 9-(3-methoxy-3-oxopropyl)phenanthrene-3-carboxylate (38)
14 (1.00g, 3.12 mmol) was dissolved in ethyl acetate (150 mL) with the aid of stirring and heating. The resultant solution was hydrogenated under 3 bar of hydrogen in the presence of 10 wt % palladium on activated carbon (100 mg) for 18 h. The reaction mixture was then filtered through a celite pad before being concentrated in vacuo to afford a viscous pale yellow oil. Purification by flash column chromatography (5 → 30% EtOAc in hexane) afforded 38 as a light coloured oil which solidified on standing (536 mg, 53%); mp: 88-92 °C;
1H NMR (400 MHz, CDCl3): δ = 2.84 (t, J = 8.0 Hz, 2H), 3.48 (t, J = 8.0 Hz, 2H), 3.72 (s, 3H), 4.02 (s, 3H), 7.63 (s, 1H), 7.66-7.76 (m, 2H), 7.85 (d, J = 8.0 Hz, 1H), 8.10 (dd, J = 8.0 & 1.6 Hz, 1H), 8.15-8.21 (m, 1H), 8.80-8.87 (m, 1H), 9.39 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 28.5, 34.3, 51.8, 52.3, 123.6, 124.1, 125.0, 125.7, 126.6, 127.0, 127.3, 127.5, 128.2, 129.2, 130.9, 130.9, 134.6, 137.5, 167.4, 173.3.
HRMS-ESI: m/z [M + Na]+ calcd for C20H18O4: 345.1097; found 345.1095.
Anal. calcd for C20H18O4: C, 74.52, H, 5.63; found, C, 74.34, H, 6.04.
9-(3-Methoxy-3-oxopropyl)phenanthrene-3-carboxylic acid (39)
Following general procedure D, 38 (350 mg, 1.09 mmol), NaOH (262 mg, 6.54 mmol) and THF afforded 39 as an off-white solid (156 mg, 49%); mp: >250 °C;
1H NMR (500 MHz, DMSO-d6): δ = 2.75 (t, J = 7.5 Hz, 2H), 3.39 (t, J = 7.5 Hz, 2H), 7.73-7.81 (m, 3H), 8.01 (d, J = 8.0 Hz, 1H), 8.12 (dd, J = 8.0 & 1.5 Hz, 1H), 8.17-8.22 (m, 1H), 8.87-8.92 (m, 1H), 9.33 (s, 1H), 12.60 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 28.3, 34.4, 123.9, 124.8, 124.8, 125.7, 127.1, 127.7, 128.0, 128.8, 128.9, 128.9, 130.6, 131.0, 134.4, 138.2, 168.0, 174.2.
HRMS-ESI: m/z [M − H]− calcd for C18H14O4: 293.0819; found 293.0821.
Anal. calcd for C18H14O4·0.25H2O: C, 72.35, H, 4.89; found, C, 72.36, H, 5.02.
(9-Iodophenanthren-3-yl)methanol (40)
A stirred suspension of 1 (3.00 g, 8.62 mmol) and thionyl chloride (10 mL) in anhydrous dioxane (150 mL) was heated under reflux for 12 h. The solution was then allowed to cool to room temperature and the solvent removed in vacuo. The product was then dissolved in anhydrous THF and again concentrated in vacuo to remove any traces of thionyl chloride. The crude acid chloride was then dissolved in anhydrous THF (100 mL) and the resulting solution cooled to 0 °C using an ice water bath. Sodium borohydride (571 mg, 15.09 mmol) was then added portionwise over a period of 10 minutes. After complete addition the suspension was stirred at 0 °C for 30 minutes and then at room temperature for 12 h. Excess sodium borohydride was destroyed via the dropwise addition of H2O. The solvent was then removed in vacuo and the resultant solid suspended between EtOAc and H2O (100 mL each). The aqueous layer was further extracted with EtOAc (2 × 50 mL) and the organics pooled, washed with H2O (50 mL), brine (50 mL), and dried over MgSO4. Concentration in vacuo yielded a yellow solid. Purification by flash chromatography (10 → 40% EtOAc in hexane) afforded 40 as a yellow solid (2.45 g, 85%); mp: 164-168 °C;
1H NMR (400 MHz, DMSO-d6): δ = 4.77 (s, 2H), 5.44 (s, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.72-7.81 (m, 2H), 7.92 (d, J = 8.0 Hz, 1H), 8.10-8.17 (m, 1H), 8.59 (s, 1H), 8.75 (s, 1H), 8.78-8.83 (m, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 63.1, 97.9, 120.0, 123.2, 126.3, 127.5, 127.7, 128.1, 129.4, 130.1, 131.4, 131.5, 132.4, 138.0, 142.1.
HRMS-CI: m/z [M+] calcd for C15H11OI: 333.9855; found 333.9862.
Anal. calcd for C15H11OI: C, 53.92, H, 3.32; found, C, 53.63, H, 3.54.
3-(Bromomethyl)-9-iodophenanthrene (41)
A flask containing 40 (2.43 g, 7.27 mmol) was briefly evacuated and backfilled with argon. Anhydrous DCM (200 mL) was cannulated into the flask and the resulting suspension cooled to 0 °C. Phosphorus tribromide (2.73 mL, 29.08 mmol) was then added dropwise to the stirred suspension. After complete addition the solution was stirred at 0 °C for 30 min and then at room temperature until TLC confirmed complete conversion. After 1 h the reaction mixture was again cooled to 0 °C and excess PBr3 destroyed by the dropwise addition of a saturated NaHCO3 solution. The organic layer was subsequently isolated, dried over MgSO4 and concentrated in vacuo to afford a light yellow solid which was dissolved in diethyl ether (100 mL) and washed successively with H2O (40 mL) and brine (40 mL). Drying over MgSO4 followed by concentration in vacuo yielded 41 as a pale yellow solid (1.87 g, 65%); mp: 124-128 °C;
1H NMR (500 MHz, CDCl3): δ = 4.75 (s, 2H), 7.61 (dd, J = 8.0 & 1.5 Hz, 1H), 7.66-7.72 (m, 2H), 7.74 (d, J = 8.0 Hz, 1H), 8.20-8.23 (m, 1H), 8.41 (s, 1H), 8.59-8.62 (m, 1H), 8.63 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 34.1, 99.7, 122.9, 123.3, 127.8, 128.1, 128.3, 128.4, 130.4, 130.5, 132.4, 132.8, 133.5, 136.7, 138.2.
HRMS-CI: m/z [M+] cald for C15H10BrI: 395.9011; found 395.9012.
(9-Iodophenanthren-3-yl)acetonitrile (42)
41 (1.00 g, 2.52 mmol) was dissolved in anhydrous DCM (75 mL) and stirred vigorously with a solution of sodium cyanide (136 mg, 2.77 mmol) and tetra-nbutylammonium bromide (89 mg, 0.28 mmol) in H2O (75 mL). After 5 days TLC indicated complete conversion. The organic layer was subsequently isolated and the aqueous phase extracted with DCM (2 × 50 mL). The organic layers were combined, dried over MgSO4 and concentrated in vacuo to afford a brown oil. Purification by flash chromatography (10 → 20% EtOAc in hexane) yielded 42 as a yellow solid (492 mg, 57%); mp: 141-145 °C;
1H NMR (500 MHz, CDCl3): δ = 4.00 (s, 2H), 7.50 (dd, J = 8.5 & 2.0 Hz, 1H), 7.68-7.74 (m, 2H), 7.77 (d, J = 8.5 Hz, 1H), 8.20-8.25 (m, 1H), 8.41 (s, 1H), 8.59-8.63 (m, 2H).
13C NMR (100 MHz, CDCl3): δ = 24.2, 99.5, 122.1, 122.8, 126.6, 127.8, 128.3, 128.5, 128.7, 130.0, 130.6, 132.4, 132.4, 133.4, 137.9.
HRMS-ESI: m/z [M + Na] + cald for C16H10NI: 365.9743; found 365.9750.
(9-Iodophenanthren-3-yl)acetic acid (43)
A stirred mixture of 42 (471 mg, 1.37 mmol), glacial acetic acid (15 mL), conc H2SO4 (3 mL) and H2O (3 mL) was heated under reflux until TLC indicated complete consumption of the starting material. After 3 h the mixture was allowed to cool to room temperature and then diluted with H2O (100 mL). The aqueous mixture was extracted with diethyl ether (100 mL then 2 × 50 mL) and the organic layers pooled and extracted with aq 1 M NaOH (3 × 50 mL). The alkaline phases were combined and acidified to pH 1 using aq 2 M HCl. The aqueous solution was then extracted with diethyl ether (100 mL then 2 × 50 mL) and the organic layers pooled, dried over Na2SO4 and concentrated in vacuo to afford 43 as a straw coloured solid (355 mg, 72%); mp: 218-222 °C (dec);
1H NMR (400 MHz, DMSO-d6): δ = 3.87 (s, 2H), 7.58 (dd, J = 8.0 & 1.6 Hz, 1H), 7.73-7.80 (m, 2H), 7.91 (d, J = 8.0 Hz, 1H), 8.11-8.16 (m, 1H), 8.59 (s, 1H), 8.73 (s, 1H), 8.78-8.83 (m, 1H), 12.48 (br s, 1H);
13C NMR (100 MHz, DMSO-d6): δ = 40.9, 98.1, 123.3, 123.6, 127.4, 127.7, 128.2, 129.1, 129.5, 129.9, 131.2, 131.4, 132.4, 134.6, 137.9, 172.5.
HRMS-ESI: m/z [M−H]− cald for C16H11O2I: 360.9735; found 360.9731.
Anal. calcd for C16H11O2I: C, 53.06, H, 3.06; found, C, 52.85, H, 3.17.
9-Iodophenanthrene-3-carboxylic acid benzylamide (44a)
A stirred suspension of 1 (1.00 g, 2.87 mmol) and thionyl chloride (5 mL) in anhydrous benzene (45 mL) was heated under reflux for 12 h. The solution was then allowed to cool to room temperature and the solvent removed in vacuo. The product was dissolved in a second aliquot of anhydrous benzene and again concentrated in vacuo to remove any traces of thionyl chloride. The crude acid chloride was then dissolved in anhydrous dioxane (20 mL) and added dropwise to a rapidly stirring solution of benzylamine (0.31 mL, 2.87 mmol) and triethylamine (0.40 mL, 2.87 mmol) in anhydrous dioxane (30 mL). After complete addition the solution was allowed to stir for 3 h at room temperature. The solvent was then removed in vacuo and the resultant solid dissolved in a mixture of DCM (100 mL) and H2O (40 mL). The organic layer was isolated and washed successively with aq 1M HCl (2 × 30 mL), aq 1M NaOH (2 × 30 mL), and H2O (40 mL). Drying over MgSO4 followed by concentration in vacuo afforded 44a as an off-white solid (459.2 mg, 37%); mp: 208-212 °C (dec);
1H NMR (400 MHz, DMSO-d6): δ = 4.61 (d, J = 6.0 Hz, 2H), 7.23-7.24 (m, 1H), 7.33-7.38 (m, 2H), 7.38-7.43 (m, 2H), 7.78-7.87 (m, 2H), 8.05 (d, J = 8.4 Hz, 1H), 8.16-8.20 (m, 1H), 8.17 (dd, J = 8.4 & 1.6 Hz, 1H), 8.68 (s, 1H), 8.91-8.95 (m, 1H), 9.38 (s, 1H), 9.42 (t, J = 6.0 Hz, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 42.8, 101.0, 122.0, 123.6, 126.1, 126.8, 127.3, 127.7, 128.1, 128.3, 128.6, 129.1, 130.2, 131.6, 132.6, 132.7, 134.0, 137.7, 139.5, 165.8.
HRMS-ESI: m/z [M + Na]+ calcd for C22H16NOI: 460.0169; found 460.0164.
Anal. calcd for C22H16NOI: C, 60.43, H, 3.69, N, 3.20; found, C, 60.14, H, 3.81, N, 2.85.
9-Iodophenanthrene-3-carboxylic acid 2-phenethyl amide (44b)
The procedure was identical to that described for 44a with the exception that phenethylamine (0.36 mL, 2.87 mmol) was used. The reaction afforded 44b as light brown solid (430.8 mg, 33%); mp: 194-197 °C (dec);
1H NMR (400 MHz, DMSO-d6): δ = 2.94 (t, J = 7.6 Hz, 2H), 3.56-3.63 (m, 2H), 7.19-7.24 (m, 1H), 7.28-7.34 (m, 4H), 7.79-7.88 (m, 2H), 8.03 (d, J = 8.4 Hz, 1H), 8.09 (dd, J = 8.4 & 1.6 Hz, 1H), 8.16-8.20 (m, 1H), 8.67 (s, 1H), 8.88-8.92 (m, 1H), 8.95 (t, J = 6.0 Hz, 1H), 9.26 (s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 35.1, 41.0, 100.7, 121.8, 123.3, 125.9, 127.5, 128.0, 128.2, 128.4, 128.5, 129.0, 130.2, 131.6, 132.5, 132.9, 133.8, 137.7, 139.4, 165.8.
HRMS-ESI: m/z [M + Na]+ calcd for C23H18NOI: 474.0325; found 474.0318.
Anal. calcd for C23H18NOI·0.39H2O: C, 60.27, H, 4.13, N, 3.06; found, C, 60.27, H, 4.40, N, 2.96.
[(9-Iodophenanthrene-3-carbonyl)amino]acetic acid tert-butyl ester (45)
Initial synthesis and purification of acid chloride was as described for 44a with the exception that 1 (600 mg, 1.72 mmol), thionyl chloride (3 mL) and anhydrous benzene (45 mL) were used. The crude acid chloride was then dissolved in anhydrous dioxane (20 mL) and added dropwise to a rapidly stirring suspension of glycine t-butyl ester hydrochloride (301.7 mg, 1.80 mmol) and triethylamine (0.48 mL, 3.44 mmol) in anhydrous dioxane (30 mL) which had been pre-stirred for 30 mins. After complete addition the mixture was allowed to stir at room temperature for 3 h. The dioxane was then removed in vacuo and the resulting solid dissolved in a mixture of DCM (100 mL) and H2O (30 mL). The organic layer was isolated and washed successively with aq 1 M HCl (2 × 30 mL), aq 1 M NaOH (2 × 30 mL), H2O (30 mL) and brine (30 mL). Subsequent drying over MgSO4 followed by concentration in vacuo afforded 45 as an orange/yellow oil (576.3 mg, 73%);
1H NMR (400 MHz, CDCl3): δ = 1.55 (s, 9H), 4.24 (d, J = 4.8 Hz, 2H), 7.05 (t, J = 4.8 Hz, 1H), 7.66-7.74 (m, 3H), 7.89 (dd, J = 8.4 & 1.6 Hz, 1H), 8.14-8.20 (m, 1H), 8.36 (s, 1H), 8.58-8.64 (m, 1H), 9.08 (s, 1H).
13C NMR (100 MHz, CDCl3): δ = 28.1, 42.7, 82.7, 101.4, 122.6, 123.0, 124.6, 127.8, 128.0, 128.3, 129.9, 130.4, 131.9, 132.2, 133.3, 134.5, 137.8, 167.1, 169.5.
HRMS-ESI: m/z [M + Na]+ calcd for C21H20NO3I: 484.0380; found 484.0371.
[(9-Iodophenanthrene-3-carbonyl)amino]acetic acid (46)
To a stirred solution of 45 (565.9 mg, 1.23 mmol) and m-dimethoxybenzene (0.81 mL, 6.15 mmol) in DCM (30 mL) was added dropwise TFA (4.57 mL, 61.5 mmol). The resulting mixture was stirred at room temperature until TLC confirmed complete deprotection. The solvent was then removed in vacuo and the resulting solid azeotroped with toluene (3 × 30 mL) to remove any traces of TFA. The solid was then suspended in diethyl ether (30 mL) and stirred for 10 mins before being filtered off and washed thoroughly with diethyl ether. Air drying subsequently afforded 46 as a light yellow solid (346.4 mg, 70%); mp: 241-244 °C (dec);
1H NMR (400 MHz, DMSO-d6): δ = 4.06 (d, J = 5.6 Hz, 2H), 7.79-7.88 (m, 2H), 8.05 (d, J = 8.4 Hz, 1H), 8.14 (dd, J = 8.4 & 1.6 Hz, 1H), 8.16-8.20 (m, 1H), 8.69 (s, 1H), 8.90-8.94 (m, 1H), 9.26 (t, J = 5.6 Hz, 1H), 9.36 (s, 1H), 12.71 (br s, 1H).
13C NMR (100 MHz, DMSO-d6): δ = 41.3, 101.2, 122.1, 123.5, 126.0, 127.8, 128.2, 128.7, 129.1, 130.2, 131.7, 132.1, 132.6, 134.1, 137.7, 166.1, 171.3.
HRMS-ESI: m/z [M − H]− calcd for C17H12NO3I: 403.9789; found 403.9803.
Anal. calcd for C17H12NO3I: C, 50.39, H, 2.99, N, 3.46; found, C, 50.73, H, 2.93, N, 3.11.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH060252, MRC programme grant G0601812 and BBSRC grant BB/L001977/1.
Footnotes
Supporting Information for this article is available online at http://www.thieme-connect.com/products/ejournals/journal/10.1055/s-00000084.
References
- (2).Kovacs A, Vasas A, Hohmann J. Phytochemistry. 2008;69:1084. doi: 10.1016/j.phytochem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- (3) (a).Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT. JPET. 2010;337:614. doi: 10.1124/jpet.110.174144. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Monaghan DT, Irvine MW, Costa BM, Fang G, Jane DE. Neurochem Int. 2012;61:581. doi: 10.1016/j.neuint.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Collingridge GL, Volianskis A, Bannister N, France G, Hanna L, Mercier M, Tidball P, Fang G, Irvine MW, Costa BM, Monaghan DT, Bortolotto ZA, Molnar E, Lodge D, Jane DE. Neuropharmacology. 2013;64:13. doi: 10.1016/j.neuropharm.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mosettig E, Van de Kamp J. J. Am. Chem. Soc. 1932;54:3328. [Google Scholar]
- (5).Schultz J, Goldberg MA, Ordas EP, Carsch G. J. Org. Chem. 1946;11:320. doi: 10.1021/jo01174a003. [DOI] [PubMed] [Google Scholar]
- (6).Klapers A, Buchwald SL. J. Am. Chem. Soc. 2002;124:14844. doi: 10.1021/ja028865v. [DOI] [PubMed] [Google Scholar]
- (7).Irvine MW, Costa BM, Dlaboga D, Culley GR, Hulse R, Scholefield CL, Atlason P, Fang G, Eaves R, Morley R, Mayo-Martin MB, Amici M, Bortolotto ZA, Donaldson L, Collingridge GL, Molar E, Monaghan DT, Jane DE. J. Med. Chem. 2012;55:327. doi: 10.1021/jm201230z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ruano JLG, Aleman J, Fajardo C, Parra A. Org. Lett. 2005;7(24):5493. doi: 10.1021/ol052250w. [DOI] [PubMed] [Google Scholar]
- (9).Fernandez F, Gonzalez C, Gomez G, Lopez C, Medina L. Arch. Pharm. 1990;323:239. doi: 10.1002/ardp.19903230412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.