Abstract
Background
Parasite switches to new host species are of fundamental scientific interest and may be considered an important speciation mechanism. For numerous monogenean fish parasites, infecting different hosts is associated with morphological adaptations, in particular of the attachment organ (haptor). However, haptoral morphology in Cichlidogyrus spp. (Monogenea, Dactylogyridea), parasites of African cichlids, has been mainly linked to phylogenetic rather than to host constraints. Here we determined the position of Cichlidogyrus amieti, a parasite of species of Aphyosemion (Cyprinodontiformes, Nothobranchiidae) in the phylogeny of its congeners in order to infer its origin and assess the morphological changes associated with host-switching events.
Methods
The DNA of specimens of C. amieti isolated from Aphyosemion cameronense in Cameroon was sequenced and analyzed together with that of Cichlidogyrus spp. from cichlid hosts. In order to highlight the influence of the lateral transfer of C. amieti on the haptoral sclerotised parts we performed a Principal Component Analysis (PCA) to compare the attachment organ structure of C. amieti to that of congeners infecting cichlids.
Results
Cichlidogyrus amieti was found to be nested within a strongly supported clade of species described from Hemichromis spp. (i.e. C. longicirrus and C. dracolemma). This clade is located at a derived position of the tree, suggesting that C. amieti transferred from cichlids to Cyprinodontiformes and not inversely. The morphological similarity between features of their copulatory organs suggested that C. amieti shares a recent ancestor with C. dracolemma. It also indicates that in this case, these organs do not seem subjected to strong divergent selection pressure. On the other hand, there are substantial differences in haptoral morphology between C. amieti and all of its closely related congeners described from Hemichromis spp..
Conclusions
Our study provides new evidence supporting the hypothesis of the adaptive nature of haptor morphology. It demonstrates this adaptive component for the first time within Cichlidogyrus, the attachment organs of which were usually considered to be mainly phylogenetically constrained.
Keywords: Phylogeny, Lateral transfer, Cichlidogyrus amieti, Aphyosemion, Nothobranchiidae, Cichlidae, Cameroon, Africa
Background
Teleost fishes of the order Cyprinodontiformes, commonly called cyprinodonts, or rivulines, livebearers and killifishes [1–3], are well known ornamental fishes. American representatives like xiphos (Xiphophorus Heckel, 1848) and guppies (Poecilia Bloch & Schneider, 1801) have been adopted as model species featuring in an increasing number of laboratory studies [4–6]. This is also the case for some African representatives, such as species belonging to Nothobranchius Peters, 1868 [7–10]. They are also established models in ecology and evolutionary biology [11–16] and parasitology [17, 18]. Evolutionary-parasitological research on these fishes often deals with monogeneans, a species-rich clade of mostly ectoparasitic flatworms. Fish-monogenean systems are established models to study the evolution of host-parasite interactions (e.g. [19, 20]). A diverse fauna of gyrodactylid monogeneans has been described from cyprinodontiform hosts in both the Neotropics [21] and Africa [22]. The first dactylogyridean monogenean parasites from African cyprinodonts were described by Birgi and Euzet [23] on the gills of some species of Aphyosemion Myers, 1924 sampled in different localities [Kala, Zamakoe and Yaoundé (Central Region)] in Cameroon. Members of this fish genus in general inhabit narrow, shallow and slowly-flowing forest streams [3, 24]. One of these killifish monogenean species, Cichlidogyrus amieti Birgi & Euzet [23], was isolated from the gills of Aphyosemion cameronense (Boulenger, 1903) and Aphyosemion obscurum (Ahl, 1924), two related species [2]. This discovery raised questions regarding the specificity of species belonging to Cichlidogyrus Paperna [25]. Indeed, no representative of Cichlidogyrus had, at that time, ever been collected from a fish not belonging to Cichlidae [26]. Birgi and Euzet [23] therefore hypothesized that the presence of C. amieti on the above mentioned two African cyprinodonts was probably the result of a lateral transfer from cichlid fishes. Switches to new host species represent a substantial risk to, e.g., aquaculture and fisheries [27, 28]. They are also of fundamental scientific interest [20], e.g. in understanding disease transmission [29], host biogeography [30, 31] and the relationship between niche specialization and host range [32]. Several analyses on phylogeny and evolution of host specificity of the monogenean gill parasites of African cichlids have been conducted [33–36]. However, congeners infecting non‐cichlids such as Cichlidogyrus amieti have not yet been included in these analyses. Hence the aspect of host-switching over larger phylogenetic distances was not looked into. Moreover, Pariselle et al. [19, 31] raised the question of the origin of Cichlidogyrus spp. described from cichlid hosts in Africa. Based on fossil, genetic and parasitic evidence, the authors hypothesized that cichlids may have originated from Madagascar [37, 38] after the Gondwanan split and subsequently dispersed over Africa, Central and South America, India and the Middle East across various marine pathways [31, 38–40]. In this case, these teleosts would have encountered salinities that resulted in the loss of their ectoparasitic monogeneans (probably representatives of Malagasy Insulacleidus Rakotofiringa & Euzet [41] or one of their ancestors) which show a poor tolerance to salinity and osmotic variations [31]. It is then likely that cichlids, after reaching the African continent, have been newly colonized by an ancestor species of Cichlidogyrus, presumably transferred from a currently unspecified African fish. From there, the ancestor of Cichlidogyrus evolved and specialized on members of Cichlidae [26], and became host-specific (i.e. oioxenous [42]). As C. amieti is known to infect representatives of Cyprinodontiformes, it could be possible that these fish represent the origin of the first host-switch to cichlids from which the present-day species-rich assemblage of Cichlidogyrus spp. on old world cichlids arose. Indeed, similar radiation episodes following a switch to a new host family have been identified in monogeneans, for example in Gyrodactylus [43]. In gyrodactylids, host-switching is even considered an important speciation mechanism [44]. It has been suggested for a range of monogeneans that colonization of different hosts is associated with morphological adaptations, in particular to the attachment organ ([45] and references therein). However, morphological analysis linked the structure of haptoral hard parts in Cichlidogyrus to phylogenetic rather than to host-related constraints [46]. Parasites belonging to Cichlidogyrus infecting non-cichlid hosts have never been taken into account in this context. Therefore, the influence of phylogenetically distant host-switches on haptoral morphology and speciation of Cichlidogyrus remains to be tested.
This paper therefore aims at determining the position of C. amieti in the phylogenetic tree of Cichlidogyrus spp. using molecular analyses. This will allow testing whether the putative switch between cyprinodonts and cichlids happened early in the history of Cichlidogyrus, seeding its radiation, or whether it rather represents a more recent event. If C. amieti is phylogenetically close to the species that first host-switched onto a cichlid, it should be situated close to the root of the tree of Cichlidogyrus spp. If C. amieti (or its ancestor) originated from a lateral transfer from a cichlid species, it should be closely related to a species of Cichlidogyrus found on that cichlid.
Determining the origin of C. amieti will also allow us to compare it morphologically to its closely related congeners, hence assessing the changes associated with a host-switch between fish families.
Methods
Sample collection and PCR amplification
Specimens of Aphyosemion spp. from some forest streams of the central and southern plateau and the littoral plain of Cameroon were caught using a dipnet of 2 mm x 2 mm mesh size, and immediately transferred into an empty container for freezing or into 96° alcohol for fixation and conservation. In the laboratory, fishes were dissected; gills from both sides were removed, placed in glass Petri dishes and examined under a Wild dissecting microscope. Fish identifications were done following Amiet [2] and Sonnenberg [47]. The studied specimens of Cichlidogyrus amieti were collected from the gills of A. obscurum captured in the locality of Mbalelon (03°33’54”N, 011°22’07”E, 695 m), A. cameronense from the localities of Oman II (03°37’45”N, 011°27’40”E, 720 m), Nkol Ngbwa (02°56’53”N, 011°50’07”E, 693 m) and Nkong (03°32’58”N, 011°25’00”E, 700 m) and A. exiguum from Nkong. They were individually placed in-between slide and coverslip, in a drop of water and examined under a Leica DM2500 microscope equipped with a LEICA DFC425 video camera. Parasite identification was performed using the morphology and size of sclerotized parts of the attachment apparatus (haptor) and that of the genitalia (vagina and male copulatory organs) following the original description of Birgi and Euzet [23]. While some individuals were fixed and mounted in a mixture of glycerin and ammonium picrate [48] for further morphological study, three adult specimens (fixed alive together with the host and preserved in alcohol) were prepared for PCR amplification following the protocol of Marchiori et al. [49], i.e., directly without DNA extraction. Standard PCR was performed with two primers specific to the D1-D2 domain of the large subunit region (LSU) of the 28S ribosomal gene: C1 (forward; 5’-ACCCGCTGAATTTAAGCAT-3’) and D2 (reverse; 5’-TGGTCCGTGTTTCAAGAC-3’) [50]. The amplification protocol began with 2 min at 93 °C for initial denaturation followed by 40 cycles of 30 s at 93 °C, 30 s at 56 °C for annealing, 1 min 30 s at 72 °C for extension, with a final 5 min extension step at 72 °C. The different reagents’ final concentrations were as follows: GoTaq Flexibuffer (Promega) 1x, MgCl2 2.5 mM, PCR nucleotide mix, 0.2 nM of each DNTP, forward and reverse primers 1 μM each, GoTaq (Promega) DNA polymerase 2 U, template DNA 0.2 μg (between 1.6and 3 μl depending on the DNA extract concentration), nuclease-free water to 20 μl. Sequencing was performed using the same primers as in initial PCR amplification: C1 and D2. Purification was performed with an Agencourt® AMPure® PCR purification kit following the manufacturer’s recommendations.
Sequence analyses
Sequences were aligned and improved manually using BioEdit version 5.09 [51]. Additional sequences obtained from GenBank were also included in the analysis (Table 1). Aligned sequences were analysed using Maximum Likelihood (ML), Maximum Parsimony (MP) and Minimum Evolution (ME) using MEGA (Molecular Evolutionary Genetics Analysis) version 5.1 [52]. Prior to analysis, an evolutionary model for ML and ME was selected by MEGA 5.1 using the Bayesian information criterion (BIC) [53]. Models with the lowest BIC scores are considered to describe the substitution pattern the best. Support for inferred clades was obtained in all three methods through non-parametric bootstrap [54] with 2000 replicates.
Table 1.
List of monogenean species used in this study including their host species and accession numbers for the LSU 28S rDNA sequences
| Parasite Species | Host Species | GenBank Accession Number |
|---|---|---|
| Cichlidogyrus aegypticus Ergens, 1981 [73] | Tilapia guineensis (Günther, 1862) | HQ010021 |
| Cichlidogyrus amieti Birgi & Euzet, 1983 [23] | Aphyosemion cameronense (Boulenger, 1903) | KT945076 |
| Cichlidogyrus amphoratus Pariselle & Euzet, 1996 [74] | Tilapia guineensis (Bleeker, 1862) | HE792772 |
| Cichlidogyrus arthracanthus Paperna, 1960 [25] | Tilapia guineensis (Günther, 1862) | HQ010022 |
| Cichlidogyrus cirratus Paperna, 1964 [76] | Oreochromis niloticus (Linnaeus, 1758) | HE792773 |
| Cichlidogyrus cubitus Dossou, 1982 [71] | Tilapia guineensis (Günther, 1862) | HQ010037 |
| Cichlidogyrus digitatus Dossou, 1982 [71] | Tilapia guineensis (Günther, 1862) | HQ010023 |
| Cichlidogyrus douellouae Pariselle, Bilong & Euzet, 2003 [72] | Sarotherodon galilaeus (Linnaeus, 1758) | HE792774 |
| Cichlidogyrus dracolemma Řehulková, Mendlová & Šimková, 2013 [63] | Hemichromis letourneuxi Sauvage, 1880 | HQ010027 |
| Cichlidogyrus ergensi Dossou, 1982 [71] | Tilapia guineensis (Günther, 1862) | HQ010038 |
| Cichlidogyrus falcifer Dossou & Birgi, 1984 [60] | Hemichromis fasciatus Peters, 1857 | HQ010024 |
| Cichlidogyrus halli (Price & Kirk, 1967) [77] | Sarotherodon galilaeus (Linnaeus, 1758) | HQ010025 |
| Cichlidogyrus longicirrus Paperna, 1965 [61] | Hemichromis fasciatus Peters, 1857 | HQ010026 |
| Cichlidogyrus njinei Pariselle, Bilong Bilong & Euzet, 2003 [72] | Sarotherodon galilaeus (Linnaeus, 1758) | HE792775 |
| Cichlidogyrus pouyaudi Pariselle & Euzet, 1994 [70] | Tylochromis intermedius (Boulenger, 1916) | HQ010039 |
| Cichlidogyrus sclerosus Paperna & Thurston, 1969 [75] | Oreochromis niloticus (Linnaeus, 1758) | DQ157660 |
| Cichlidogyrus tiberianus Paperna, 1960 [25] | Tilapia guineensis (Bleeker, 1862) | HE792776 |
| Cichlidogyrus yanni Pariselle & Euzet, 1996 [74] | Tilapia guineensis (Bleeker, 1862) | HE792777 |
| Haliotrema cromileptis Young, 1968 [64] | Epinephelus coioides (Hamilton, 1822) | EU523146.1 |
| Haliotrema johnstoni Bychowsky & Nagibina, 1970 [65] | Upeneus luzonius Jordan & Seale, 1907 | DQ157664.1 |
| Ligophorus chabaudi Euzet & Suriano, 1977 [66] | Mugil cephalus Linnaeus, 1758 | JN996833.1 |
| Ligophorus cephali Rubtsova et al., 2006 [67] | Mugil cephalus Linnaeus, 1758 | JN996830.1 |
| Thaparocleidus asoti (Yamaguti, 1937 [68]) | Parasilurus asotus (Linnaeus, 1758) | DQ157669.1 |
| Tetrancistrum sp. | Siganus fuscescens (Houttuyn, 1782) | AF026114 |
Principal Component Analysis (PCA)
A PCA, using Statistica 9, was performed with “standardised” measurements to avoid morphometrical differences possibly due to developmental stage of the examined parasite or the influence of temperature on the size of the sclerites [55, 56]: i.e. the length of all sclerotized haptoral parts were divided by that of uncinuli pair II (= pair V sensu Mizelle [57]), which is supposed to keep its larval size (see [58]). The following characters were used in this analysis: total length of uncinuli I [I], III [VI], IV [VII], V [IV], VI [III], VII [II]; dorsal transverse bar: total length, maximum width, distance between auricles and auricle length; ventral transverse bar: branch total length and maximum width; total length of (ventral and dorsal) anchor, and the length of their blade, shaft, guard and point. Ten specimens of each of the following species of Cichlidogyrus were considered: C. cf. bychowskii (Markevich [59]) (see remark below) collected on the gills of an Hemichromis bimaculatus Gill, 1862 (MRAC 74155-63 voucher specimen) from the Congo River at Bokalakala (2°08'00"S, 16°22'00"E) in the Democratic Republic of Congo; C. euzeti Dossou & Birgi [60] and C. longicirrus Paperna [61] on H. cf. elongatus from a small stream near Idenao (4°13’24”N, 8°59’18”E) (both) and Soo River on the road between Abang and Adjap (3°19’21”N, 11°28’55”E) and Ossa Lake near Dizangué (3°46’43”N, 10°00’02”E) (respectively) in Cameroon; C. falcifer Dossou & Birgi [60] on H. fasciatus Peters, 1852 from Banjul on the Casamance River in the Gambia (13°26’51”N, 16°35’09”W); C. sanseoi Pariselle & Euzet [62] and C. teugelsi Pariselle & Euzet [62] both on H. fasciatus from a small stream near Kounoukou (4°49'37"N, 6°24'04"W) (misspelled Kounougou in the original description) in Ivory Coast. The voucher specimen of C. amieti we deposited in the invertebrate collection of the Royal Museum for Central Africa (Tervuren, Belgium) (MRAC 37784, host: A. cameronense, locality: Nkol Ngbwa) was used for supplementary observations.
Ethical approval
Fish were handled in respect with the Cameroon National Ethical Committee Reg. Num. FWAIRD 0001954.
Remark
Paperna [61] found and re-described on Hemichromis bimaculatus in southern Ghana, a species of Cichlidogyrus he named C. bychowskii only based on haptoral sclerotized parts morphology. Due to the fact that this was the only species already described on this cichlid, that Paperna did not know the morphology of its copulatory organ (no drawing in the original description and description done in Russian [59]), that the haptoral sclerotized parts are quite similar in all Cichlidogyrus spp. from hosts belonging to Hemichromis, and according to Řehulková et al. [63], we think that Paperna [61] confused the species of Cichlidogyrus living in Africa (Ghana) on H. bimaculatus with C. bychowskii described from a dead fish from the Leningrad aquarium [59]. The latter parasite, which possesses a spirally coiled copulatory organ [63], has never been recovered from H. bimaculatus nor on the closely related H. letourneuxi in the wild in Africa. Then we consider that either Markevich’ identification of the host was wrong, or the parasite he described was laterally transferred from another cichlid host present in that aquarium. Consequently, C. bychowskii, of which neither type nor voucher specimens have been deposited in any museum, should be considered as a numen nudum. In this study the parasite species collected from H. bimaculatus, although morphologically related to C. dracolemma Řehulková et al., [63], does not necessarily belong to the latter parasite species which was described from H. letourneuxi. Pending genetic comparison, we therefore used C. cf. bychowskii to designate the parasites we collected from H. bimaculatus from the Congo River basin.
Results
Eleven species of Aphyosemion Myers, 1924 (Cyprinodontiformes, Nothobranchiidae) were captured: Aphyosemion loennbergii (Boulenger, 1903) (266 specimens), A. koungueense (Sonnenberg, 2007) (5 specimens), A. omega (Sonnenberg, 2007) (85 specimens), A. riggenbachi (Ahl, 1924) (18 specimens), A. ahli Myers, 1933 (86 specimens), A. raddai Scheel, 1975 (83 specimens), A. exiguum (Boulenger, 1911) (100 specimens), A. amoenum Radda & Pürzl, 1976 (71 specimens), A. obscurum (46 specimens), A. cameronense (133 specimens) and A. batesii (Boulenger, 1911) (61 specimens). The parasite Cichlidogyrus amieti was recovered from the gills of only three of them: A. obscurum captured in the locality of Mbalelon (2 worms), A. cameronense from the localities of Oman II (2 worms) and Nkol Ngbwa (23 worms), and A. exiguum from Nkong (3 worms). This is the first record of C. amieti on A. exiguum.
Phylogenetic analysis
A 827 base pair alignment for the 28S rDNA region of the nuclear genome was obtained after trimming the ends of each sequence. The three newly sequenced specimens of C. amieti have the same haplotype (GenBank accession number KT945076). This unique sequence was then aligned and compared to 17 other Cichlidogyrus sequences available in GenBank (Table 1). Sequences from other dactylogyridean representatives, namely Tetrancistrum sp., Haliotrema cromileptis Young [64], H. johnstoni Bychowsky & Nagibina [65], Ligophorus chabaudi Euzet & Suriano [66], L. cephali Rubtsova et al. [67] and Thaparocleidus asoti (Yamaguti [68]) (Table 1), were used to root the tree.
A total of 445 variable sites were identified in the dataset, 327 of which were parsimony informative (i.e. shared by at least two different sequences). The optimal model of sequence evolution was TN93 + G [69]. The G parameter indicates that non-uniformity of evolutionary rates among sites is modeled by using a discrete Gamma distribution. This model was used for the subsequent analysis. The three different methods used gave congruent results summarized in Fig. 1.
Fig. 1.
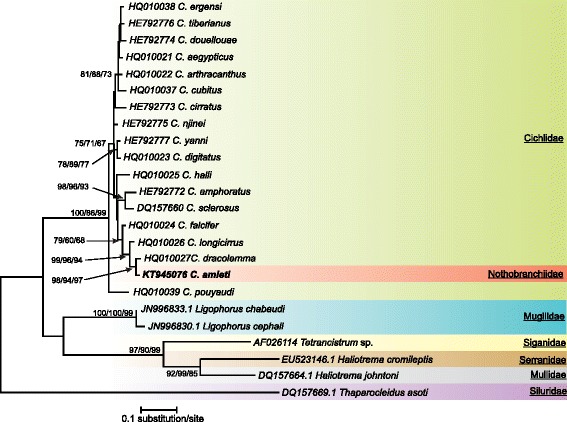
Consensus tree obtained with Maximum Likelihood analysis. Bootstrap values correspond to ME/MP/ML values respectively after 2000 iterations. Only values ≥ 50 have been indicated. Species newly sequenced for this study is in bold. Species belonging to Ligophorus, Tetrancistrum, Haliotrema and Thaparocleidus were used as outgroups. GenBank sequence ID precedes species name
Relative to the outgroup taxa, all the species of Cichlidogyrus appeared grouped in a monophyletic assemblage supported by high bootstrap values (100, 86 and 99 % for ME, MP and ML respectively). Cichlidogyrus pouyaudi Pariselle & Euzet [70] occupied a basal position in this group (bootstrap values, 75, 71 and 67 %) being the sister species of all the other species of Cichlidogyrus as already observed by Mendlová et al. [35].
Four clusters with high bootstrap support were apparent. One cluster was made up of C. ergensi Dossou [71], C. tiberianus Paperna [25], C. douellouae Pariselle, Bilong & Euzet, [72], C. aegypticus Ergens [73], C. arthracanthus Paperna [25] and C. cubitus Dossou [71] (bootstrap values 81, 88 and 73 %). Another cluster was made up of C. yanni Pariselle & Euzet [74] and C. digitatus Dossou [71] (78, 79 and 77 %), a third one of C. amphoratus Pariselle & Euzet [74] and C. sclerosus Paperna & Thurston, [75] (98, 96 and 93 %) and the last one of C. falcifer, C. longicirrus, C. dracolemma and C. amieti. Within this last cluster, C. falcifer was the sister species of C. longicirrus, C. dracolemma and C. amieti (99, 96 and 94 %) while C. longicirrus was sister to C. dracolemma and C. amieti (98, 94 and 97 %). These four clusters were not supported by high bootstrap values. Three other species: C. cirratus Paperna [76], C. njinei Pariselle, Bilong & Euzet [72] and C. halli Price & Kirk [77] did not appear related to any group or species.
Principal Component Analysis (PCA)
The PCA analysis shows a well-defined clustering (64 % of variance on axes 1 and 2) of parasite individuals according to their respective host species (Fig. 2). The specimens of C. cf. bychowskii from H. bimaculatus are closer to those from H. fasciatus s. l. (C. euzeti, C. falcifer, C. longicirrus, C. sanseoi and C. teugelsi) than to the one collected from Aphyosemion cameronense (C. amieti), the latter been set apart regarding the two axes. The most represented variables and their coordinates on axis 1 are DA a (–0.95), DA b (–0.93), VA a (–0.93), VB x (–0.92) and I (–0.87); and VII [II] (–0.82), VI [III] (–0.75), III [VI] (–0.70) on factor axis 2 (Table 2).
Fig. 2.
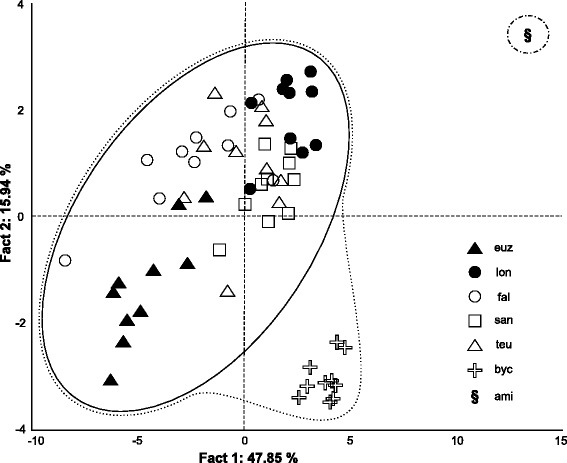
Principal component analysis scatterplot of 10 Cichlidogyrus specimens of each of the following species. (euz) C. euzeti and (lon) C. longicirrus both from Hemichromis cf. fasciatus in Cameroun; (fal) C. falcifer, (san) C. sanseoi and (teu) C. teugelsi all from H. fasciatus in Senegal (fal) or Ivory Coast (san and teu); (byc) C. cf. bychowskii from H. bimaculatus in DRC; (ami) one specimen of C. amieti from Aphyosemion cameronense in Cameroon was used for supplementary observations
Table 2.
Loadings and explained variance of the first two PC of the PCA conducted on the “standardized” size of sclerites
| Fact. 1 | Fact. 2 | |
|---|---|---|
| Variance (%) | 47.85 | 15.94 |
| I (I) | −0.873132 | −0.389889 |
| III (VI) | −0.535295 | −0.709933 |
| IV (VII) | −0.352651 | −0.638280 |
| V (IV) | 0.032798 | −0.186986 |
| VI (III) | −0.242397 | −0.753181 |
| VII (II) | −0.383708 | −0.825743 |
| DB L | −0.805421 | −0.199626 |
| DB y | −0.705113 | −0.021445 |
| DB w | −0.838653 | 0.044545 |
| DB h | −0.797425 | 0.231792 |
| DA a | −0.954211 | 0.212423 |
| b | −0.935548 | 0.165900 |
| c | −0.700350 | 0.015457 |
| d | −0.864610 | 0.324455 |
| e | −0.416138 | 0.547829 |
| VB x | −0.926706 | 0.077698 |
| VB w | −0.790515 | 0.033730 |
| VA a | −0.931174 | −0.004236 |
| b | −0.933118 | 0.063475 |
| c | −0.532477 | −0.117323 |
| d | −0.720919 | 0.190484 |
| e | −0.586523 | 0.488463 |
(I) [I], (III) [VI], (IV) [VII], (V) [IV], (VI) [III], (VII) [II] total length of uncinuli [Mizelle [57] nomenclature]; dorsal transverse bar: (DB L) total length, (DB y) distance between auricles, (DB w) maximum width, (DB h) auricle length; (DA a) total length of dorsal anchor:, (b) blade length, (c) shaft length, (d) guard length, (e) point length; ventral transverse bar: (VB a) branch total length, (VB x) maximum width; (VA a) ventral anchor total length, (b) blade length, (c) shaft length, (d) guard length, (e) point length
Discussion
Origin and host range of Cichlidogyrus amieti
Cichlidogyrus is the most species-rich ectoparasitic dactylogyridean monogenean genus known to parasitize African cichlid fishes. Species are distributed among a wide range of cichlid hosts [33, 58, 78]. The description of C. amieti from the gills of representatives of Cyprinodontiformes by Birgi and Euzet [23] raises the question whether a species from this fish order could have been the source host at the origin of the Cichlidogyrus radiation in cichlids (see theories on cichlid biogeography above). An alternative explanation is lateral parasite transfer from a cichlid to a killifish host.
Our phylogenetic reconstruction indicates that C. amieti is phylogenetically nested within the parasites from species of Hemichromis Peters, 1857 at a derived position of the tree. Although we cannot rule out incomplete taxon coverage of Central West African Cichlidogyrus, the present results suggest that C. amieti results from a recent transfer from cichlids to nothobranchiids. That is in accordance with the Birgi and Euzet [23] hypothesis. Such lateral transfer or host-switch can occur between related host species [31, 33, 79], but even between phylogenetically distant host species, both in artificial and natural conditions [19, 20, 80–84].
Aphyosemion spp. inhabit small forest streams [2, 3] where they live in sympatry with Hemichromis spp.. Bilong Bilong [85], based on morphological features, already hypothesized that C. amieti could derive from Hemichromis’ monogeneans.
Birgi and Euzet [23] reported that C. amieti was restricted to A. cameronense and A. obscurum, two species belonging to the same lineage (i.e. the A. cameronense group), but differing from one another by their biology and the fact that they are never found together in the same biotope [2]. In this study, C. amieti was also collected from A. exiguum, a species that does not belong to the A. cameronense group. This new host record can be explained by the sympatry of A. exiguum and A. cameronense or A. obscurum and by the relative phylogenetic proximity of these fish species (compared to the phylogenetic distance between species of Aphyosemion and Hemichromis).
Influence of host-switching on haptoral and reproductive morphology
While the morphology and size of the sclerotized parts of the haptor and copulatory organs of species of Dactylogyrus Diesing, 1850 [86], Anacanthorus Mizelle & Price, 1965 [87] or other genera are subject to distinct selective constraints [88–90], for Cichlidogyrus spp. these sclerotized parts seem to be mostly shaped by phylogenetic constraints [33, 35, 46]. In this case, the haptoral sclerite morphology is more suitable for inferring phylogenetic relationships, while the morphology of the reproductive organs is more useful for species-level identification, probably because of its faster evolutionary change [33, 35, 46]. In fact, for a given host species, the constraints on the haptoral sclerites aim to harmonize their morphologies (adapted to attach to the specific host’s gills), when those on the reproductive organs aim to make their morphologies mechanically incompatible, so profoundly different (leading to their reproductive isolation) (see Figs. 3 and 4).
Fig. 3.
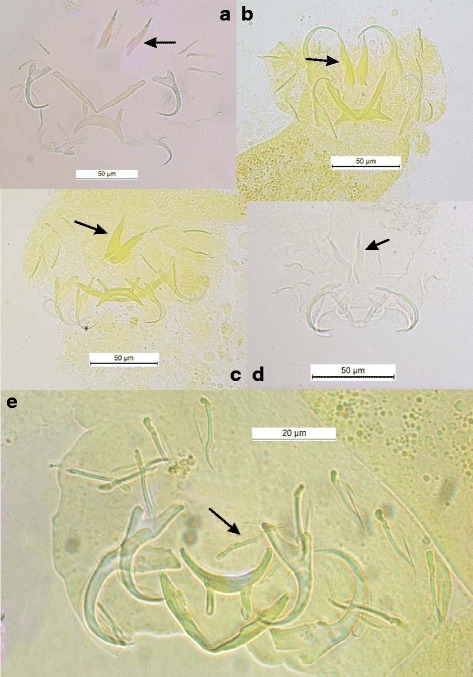
Haptoral sclerotized parts of some Cichlidogyrus spp. parasitizing Hemichromis spp. and C. amieti Birgi & Euzet [23] from Aphyosemion cameronense Boulenger, 1903. (a) C. longicirrus Dossou & Birgi [60]; (b) C. euzeti Dossou & Birgi [60]; (c) C. falcifer Dossou & Birgi [60]; (d) C. cf. bychowskii (Markevich [59]); (e) C. amieti Birgi & Euzet [23]. Arrow indicates uncinuli pair I [I]
Fig. 4.
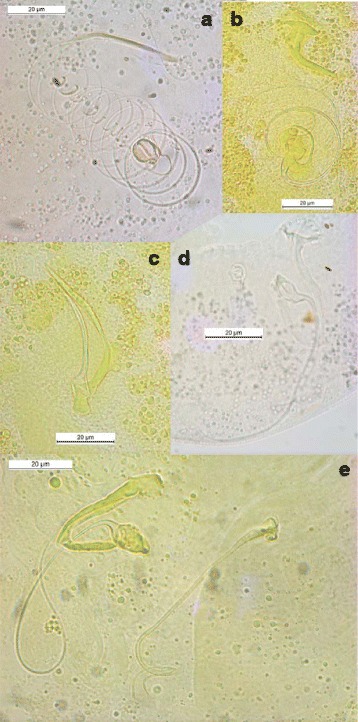
Male copulatory organs of some Cichlidogyrus spp. parasitizing Hemichromis spp. and C. amieti Birgi & Euzet [23] from Aphyosemion cameronense Boulenger, 1903. (a) C. longicirrus Dossou & Birgi [60]; (b) C. euzeti Dossou & Birgi [60]; (c) C. falcifer Dossou & Birgi [60]; (d) C. cf. bychowskii (Markevich [59]); (e) C. amieti Birgi & Euzet [23] (male copulatory organ on the left, vagina on the right)
Working on species of Dactylogyrus, Šimková et al. [91] stated that congeneric monogenean species occupying similar niches tend to have similar attachment organs. This resemblance is due to the fact that they are subject to the same considerable selective pressure imposed by the microhabitat within the host, possibly the gill morphology [92–96]. When these parasites occur on different host species, their attachment organs tend to differ from each other in their morphology and/or size, because different host species may have different gill structures. As pointed out by Šimková et al. [91], the morphology of the haptor is therefore an important adaptation of parasites to their hosts (host specificity) and to specific sites within their hosts (niche preference).
The phylogenetic tree obtained in this study (Fig. 1) suggests that C. amieti clusters within the monophyletic group already proposed by Mendlová et al. [34] and Řehulková et al. [63], made up of C. longicirrus, C. dracolemma and C. falcifer, all of them parasitizing Hemichromis spp.. The Cichlidogyrus spp. that parasitize Hemichromis spp. have a highly homogenous configuration of their haptoral sclerotized parts (group B in Vignon et al. [46]): very large first pair and small pairs III to VII of marginal hooks (= pairs II-III-IV and VI-VII sensu Mizelle [57]) combined with short auricles that are continuous with the dorsal surface of the dorsal transverse bar (Fig. 3a, b, c and d); this morphological relationship is also well supported by the PCA analysis (Fig. 2). In contrast, in C. amieti all marginal hooks are of similar small size including pair I (group A in Vignon et al. [46]) (Fig. 3e); this difference is also highlighted by our PCA results where this species is set apart regarding the two axes from all the other ones parasitizing Hemichromis spp. (Fig. 2). Therefore we hypothesize that, as soon as the ancestor of C. amieti (with group B morphology of its haptoral sclerites) colonized a species of Aphyosemion, selective pressures lead to a substantial morphological change in the haptoral sclerites, the most visible being the drastic reduction of the size of marginal hook pair I (Fig. 3 arrows). Vignon et al. [46], focusing on the same monogenean genus, did not find any evidence of host-related adaptation of the haptor morphology. However, these authors only considered Cichlidogyrus spp. infecting cichlids. The present study, considering also a more distant host-switch, provides new evidence supporting the hypothesis of the adaptive nature of haptor morphology also within Cichlidogyrus in accordance with studies on other monogeneans by Morand et al. [97, 98], Huyse and Volckaert [99] and Bush et al. [100].
Rohde and Hobbs [101] and Šimková et al. [91] showed that congeneric parasite species living in the same niche presented differences in the morphology or size of their reproductive organs, as a result of random differentiation, which made possible their coexistence according to the hypothesis of reinforcement of reproductive barriers by mate discrimination [102–104]. This is the case for Cichlidogyrus spp. harbored by Hemichromis spp., which are well differentiated from each other by the morphology or size of their reproductive organs (Fig. 4a, b, c and d). Regarding the male copulatory organ (MCO) of C. amieti, we notice that it presents a tubular filiform single-looped penis without swollen portion and with a well-developed heel, and a sharply curved accessory piece with rounded ending [23, 58] (Fig. 4e). It resembles C. dracolemma (Fig. 4e) as pointed out by Řehulková et al. [63]. Therefore we may assume that C. dracolemma or a close relative was transferred from a species of Hemichromis to an Aphyosemion. This suggestion is strongly supported by the close phylogenetic relationship between these two parasite species (Fig. 1). Finally, the specialization of these two parasite species on phylogenetically distant hosts (i.e. cichlid and killifish species) prevented their hybridization, thus explaining why their MCO morphologies have not been affected by selective pressure and thus did not substantially diverge.
Conclusion
Phylogenetic analysis suggests that C. amieti results from a recent host-switch from a cichlid species belonging to Hemichromis. The fact that the haptoral hard parts of C. amieti are of a different morphotype than those of its closely related congeners infecting Hemichromis spp., is the first proof, within Cichlidogyrus, of an adaptive component to haptoral morphology influenced by transfer to a new host. Previously, haptoral morphology of Cichlidogyrus was considered to be mainly phylogenetically constrained. The changes in the haptoral elements after the host-switching event are in stark contrast to the similarity in male genital morphology to the parasites of representatives of Hemichromis. As genital differentiation between monogenean species is thought to be linked to reinforcement of parasite genetic isolation within the same host species, we suggest this similarity is a consequence of C. amieti having speciated as a result of host-switching. This study underscores the potential of Cichlidogyrus as a model to test the influence of ecology and evolution on parasite speciation [19, 78]. The fact that the adaptive component of haptoral morphology of Cichlidogyrus was not inferred when including only species infecting cichlids, also demonstrates the importance of including the full phylogenetic or host range of a parasite clade to reconstruct its speciation mechanisms.
Acknowledgements
This work was partially funded (2013) by the representation of IRD (Institut de Recherche pour le Développement) in Cameroon through the project « Programme Pilote Régional: Forêt Tropicale Humide d’Afrique Centrale » (PPR FTH-AC). M.P.M.V. is supported by the Czech Science Foundation, Project no. P505/12/G112 (European Centre of Ichthyoparasitology (ECIP) - Centre of excellence). Sequences were produced through the technical facilities of the Centre Méditerranéen Environnement Biodiversité (CeMEB). Authors want to thank W. Boeger and the two anonymous referees for their input to the revised version of the manuscript. This is publication ISE-M 2015-179 SUD.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: FMM CBB AP JFA. Performed the experiments: FMM CBB AP JFA. Analyzed the data: FMM CBB AP JFA MPMV ARBN. Contributed reagents/materials/analysis tools: FMM AP JFA ARBN. Wrote the paper: FMM CBB AP JFA MPMV. All authors read and approved the final version of the manuscript.
Contributor Information
Françoise D. Messu Mandeng, Email: messumandeng@yahoo.fr
Charles F. Bilong Bilong, Email: bilong_bilong@yahoo.com
Antoine Pariselle, Email: antoine.pariselle@ird.fr.
Maarten P. M. Vanhove, Email: mvanhove@naturalsciences.be
Arnold R. Bitja Nyom, Email: bitja.nyom_arnold@ymail.com
Jean-François Agnèse, Email: agnese@univ-montp2.fr.
References
- 1.Parenti LR. A phylogenetic and biogeographic analysis of cyprinodontiform fishes (Teleostei, Atherinomorpha) Bull. American Mus. Nat. Hist. 1981;168:335–557. [Google Scholar]
- 2.Amiet J-L. Faune du Cameroun. Compiègne: Sciences Nat; 1987. [Google Scholar]
- 3.Stiassny MLJ, Teugels GG, Hopkins CD. Poissons d’eaux douces et saumâtres de basse Guinée, ouest de l’Afrique centrale. Paris: IRD, MnHn, MRAC; 2007. [Google Scholar]
- 4.Basolo AL, Wagner WE., Jr Covariation between predation risk, body size and fin elaboration in the green swordtail, Xiphophorus helleri. Biol. J. Linn. Soc. 2004;83:87–100. doi: 10.1111/j.1095-8312.2004.00369.x. [DOI] [Google Scholar]
- 5.Reznick D, Bryant M, Holmes D. The evolution of senescence and post-reproductive lifespan in Guppies (Poecilia reticulata) PLoS Biol. 2006;4:136–43. doi: 10.1371/journal.pbio.0040136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dargent F, Scott ME, Hendry AP, Fussmann GF. Experimental elimination of parasites in nature leads to the evolution of increased resistance in hosts. Proc. Roy. Soc. B. 2013;280:20132371. doi: 10.1098/rspb.2013.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genade T, Benedetti M, Terzibasi E, Roncaglia P, Valenzano DR, Cattaneo A, Cellerino A, et al. Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell. 2005;4:223–33. doi: 10.1111/j.1474-9726.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 8.Valenzano DR, Terzibasi E, Cattaneo A, Domenici L, Cellerino A. Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell. 2006;5:275–8. doi: 10.1111/j.1474-9726.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- 9.Terzibasi E, Lefrançois C, Domenici P, Hartmann N, Graf M, Cellerino A. Effects of dietary restriction on mortality and age-related phenotypes in the short-lived fish Nothobranchius furzeri. Aging Cell. 2009;8:88–99. doi: 10.1111/j.1474-9726.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 10.Cellerino A, Valenzano DR, Reichard M. From the bush to the bench: the annual Nothobranchius fishes as a new model system in biology. Biol. Rev. 2015;doi: 10.1111/brv.12183. [DOI] [PubMed]
- 11.Reznick DN, Endler JA. The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata) Evolution. 1982;36:125–48. doi: 10.1111/j.1558-5646.1982.tb05021.x. [DOI] [PubMed] [Google Scholar]
- 12.Reznick DN, Shaw FH, Rodd FH, Shaw RG. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–7. doi: 10.1126/science.275.5308.1934. [DOI] [PubMed] [Google Scholar]
- 13.van Oosterhout C, Trigg RE, Carvalho GR, Magurran AE, Hauser L, Shaw PW. Inbreeding depression and genetic load of sexually selected traits: how the guppy lost its spots. J. Evol. Biol. 2003;16:273–81. doi: 10.1046/j.1420-9101.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 14.van Oosterhout C, Joyce DA, Cummings SM. Evolution of MHC class IIB in the genome of wild and ornamental guppies, Poecilia reticulata. Heredity. 2006;97:111–8. doi: 10.1038/sj.hdy.6800843. [DOI] [PubMed] [Google Scholar]
- 15.Bartáková V, Reichard M, Janko K, Polačik M, Blažek R, Reichwald K, Cellerino A, Bryja J, et al. Strong population genetic structuring in an annual fish, Nothobranchius furzeri, suggests multiple savannah refugia in southern Mozambique. BMC Evol. Biol. 2013;13:196. doi: 10.1186/1471-2148-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinceel T, Vanschoenwinkel B, Deckers P, Grégoir A, Ver Eecke T, Brendonck L. Early and late developmental arrest as complementary embryonic bet‐hedging strategies in African killifish. Biol. J. Lin. Soc. 2015;doi: 10.1111/bij.12474.
- 17.Cable J, van Oosterhout C. The role of innate and acquired resistance in two natural populations of guppies (Poecilia reticulata) infected with the ectoparasite Gyrodactylus turnbulli. Biol. J. Linn. Soc. 2007;90:647–55. doi: 10.1111/j.1095-8312.2006.00755.x. [DOI] [Google Scholar]
- 18.King TA, van Oosterhoutj C, Cable J. Experimental infections with the tropical monogenean, Gyrodactylus bullatarudis: potential invader or experimental fluke? Parasitol. Int. 2009;58:249–54. doi: 10.1016/j.parint.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Pariselle A, Morand S, Deveney M, Pouyaud L. Parasite species richness of closely related hosts: historical scenario and “genetic” hypothesis. In: Combes C, Jourdane J, editors. Taxonomy, Ecology and Evolution of Metazoan Parasites. Perpignan: Presses de l’Université de Perpignan; 2003. pp. 147–66. [Google Scholar]
- 20.Vanhove MPM, Huyse T. Host-specificity and species-jumps in fish-parasite systems. In: Morand S, Krasnov B, Littlewood DTJ, editors. Parasite diversity and diversification: evolutionary ecology meets phylogenetics. Cambridge: Cambridge University Press; 2015. pp. 401–19. [Google Scholar]
- 21.Cable J, van Oosterhout C, Barson N, Harris PD. Gyrodactylus pictae n. sp. (Monogenea: Gyrodactylidae) from the Trinidadian swamp guppy Poecilia picta Regan, with a discussion on species of Gyrodactylus von Nordmann, 1832 and their poeciliid hosts. Syst Parasitol. 2005;60:159–64. doi: 10.1007/s11230-004-6348-4. [DOI] [PubMed] [Google Scholar]
- 22.Christison KW, Shinn AP, van As JG. Gyrodactylus thlapi n. sp (Monogenea) from Pseudocrenilabrus philander philander (Weber) (Cichlidae) in the Okavango Delta, Botswana. Syst. Parasitol. 2005;60:165–73. doi: 10.1007/s11230-004-6342-x. [DOI] [PubMed] [Google Scholar]
- 23.Birgi E, Euzet L. Monogènes parasites des poissons des eaux douces du Cameroun. Présence des genres Cichlidogyrus et Dactylogyrus chez Aphyosemion (Cyprinodontidae) Bull. Soc. Zool. Fr. 1983;108:101–6. [Google Scholar]
- 24.Brosset A. Le peuplement des Cyprinodontes du bassin de l'Ivindo. Gabon. Rev. Ecol. (Terre Vie) 1982;36:233–92. [Google Scholar]
- 25.Paperna I. Studies on Monogenetic Trematodes in Israel. 2 Monogenetic Trematodes of Cichlids. Bamidgeh. Bull. Fish Cult. Israel. 1960;12:20–33. [Google Scholar]
- 26.Paperna I. Monogenea of inland water fish in Africa. Ann. Mus. Roy. Afri. Centr. – Sci. Zool. 1979;226:1–131. [Google Scholar]
- 27.Bauer ON. Spread of parasites and diseases of aquatic organism by acclimatization: a short review. J. Fish Biol. 1991;39:679–86. doi: 10.1111/j.1095-8649.1991.tb04398.x. [DOI] [Google Scholar]
- 28.Bakke T, Harris P, Cable J. The biology of gyrodactylid monogeneans: the “Russian-doll Killers”. Adv. Parasitol. 2007;64:161–376. doi: 10.1016/S0065-308X(06)64003-7. [DOI] [PubMed] [Google Scholar]
- 29.Cooper N, Griffin R, Franz M, Omotayo M, Nunn CL. Phylogenetic host specificity and understanding parasite sharing in primates. Ecol. Let. 2012;15:1370–7. doi: 10.1111/j.1461-0248.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- 30.Barson M, Přikrylová I, Vanhove MPM, Huyse T. Parasite hybridization in African Macrogyrodactylus spp. (Monogenea, Platyhelminthes) signals historical host distribution. Parasitology. 2010;137:1585–95. doi: 10.1017/S0031182010000302. [DOI] [PubMed] [Google Scholar]
- 31.Pariselle A, Boeger WA, Snoeks J, Bilong Bilong CF, Morand S, Vanhove MPM. The monogenean parasite fauna of Cichlids: a potential tool for host biogeography. Int. J. Evol. Biol. 2011;doi:10.4061/2011/471480. [DOI] [PMC free article] [PubMed]
- 32.Brant SV, Loker ES. Can specialized pathogens colonize distantly related hosts? Schistosome evolution as a case study. PLoS Pathog. 2005;1:e38. doi: 10.1371/journal.ppat.0010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pouyaud L, Desmarais E, Deveney M, Pariselle A. Phylogenetic relationships among monogenean gill parasites (Dactylogyridea, Ancyrocephalidae) infesting tilapiine hosts (Cichlidae): Systematic and evolutionary implications. Mol. Phyl. Evol. 2006;38:241–9. doi: 10.1016/j.ympev.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Mendlová M, Pariselle A, Vyskočilová M, Šimková A. Molecular phylogeny of monogeneans parasitizing African, freshwater Cichlidae inferred from LSU rDNA sequences. Parasitol. Res. 2010;107:1405–13. doi: 10.1007/s00436-010-2008-6. [DOI] [PubMed] [Google Scholar]
- 35.Mendlová M, Desdevises Y, Civáňová K, Pariselle A, Šimková A. Monogeneans of West African Cichlid Fish: Evolution and Cophylogenetic Interactions. PLoS One. 2012;7:1–17. doi: 10.1371/journal.pone.0037268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendlová M, Šimková A. Evolution of host specificity in monogeneans parasitizing African cichlid fish. Parasit. Vect. 2014;7:2–14. doi: 10.1186/1756-3305-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiassny MLJ. Phylogenetic intrarelationships of the family Cichlidae: an overview. In: Keenleyside MHA, editor. Cichlid fishes: behaviour, ecology and evolution. London: Chapman and Hall; 1991. pp. 1–35. [Google Scholar]
- 38.Murray AM. The fossil record and biogeography of the Cichlidae (Actinopterygii: Labroidei) Biol. J. Linn. Soc. 2001;78:517–32. doi: 10.1111/j.1095-8312.2001.tb01409.x. [DOI] [Google Scholar]
- 39.Vences M, Freyhof J, Sonnenberg R, Kosuch J, Veith M. Reconciling fossils and molecules: Cenozoic divergence of cichlid fishes and the biogeography of Madagascar. J. Biogeogra. 2001;28:1091–9. doi: 10.1046/j.1365-2699.2001.00624.x. [DOI] [Google Scholar]
- 40.Friedman M, Keck BP, Dornburg A, Eyta RI, Martin CH, Hulsey CD, et al. Molecular and fossil evidence place the origin of cichlid fishes long after Gondwanan rifting. Proc. Roy. Soc. B: Biol. Sci. 2013;280:20131733. doi: 10.1098/rspb.2013.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rakotofiringa S, Euzet L. Monogènes parasites de Cichlidae (Teleostei) endémiques de Madagascar. Bull. Soc. Zool. Fr. 1983;108:107–14. [Google Scholar]
- 42.Euzet L, Combes C. Les problèmes de l'espèce chez les animaux parasites. Mém. Soc. Zool. Fr. 1980;40:239–85. [Google Scholar]
- 43.Ziętara MS, Lumme J. Speciation by host-switching and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea, Gyrodactylidae) Evolution. 2002;56:2445–58. doi: 10.1111/j.0014-3820.2002.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 44.Boeger WA, Kritsky DC, Pie MR. Context of diversification of the viviparous Gyrodactylidae (Platyhelminthes, Monogenoidea) Zool. Scr. 2003;32:437–48. doi: 10.1046/j.1463-6409.2003.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bueno-Silva M, Boeger WA, Pie MR. Choice matters: incipient speciation in Gyrodactylus corydori (Monogenoidea: Gyrodactylidae) Int. J. Parasitol. 2011;41:657–67. doi: 10.1016/j.ijpara.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Vignon M, Pariselle A, Vanhove MPM. Modularity in attachment organs of African Cichlidogyrus (Platyhelminthes: Monogenea: Ancyrocephalidae) reflects phylogeny rather than host specificity or geographic distribution. Biol. J. Linn. Soc. 2011;102:694–706. doi: 10.1111/j.1095-8312.2010.01607.x. [DOI] [Google Scholar]
- 47.Sonnenberg R. Description of three new species of the genus Chromaphyosemion Radda, 1971 (Cyprinodontiformes:Nothobranchiidae) from the coastal plains of Cameroon with a preliminary review of the Chromaphyosemion splendopleure complex. Zootaxa. 2007;1591:1–38. [Google Scholar]
- 48.Malmberg G . On the occurence of Gyrodactylus on Swedish fishes. 1956. pp. 19–76. [Google Scholar]
- 49.Marchiori N, Pariselle A, Pereira J, Jr, Agnèse J-F, Durand J-D, Vanhove MPM. A comparative study of Ligophorus uruguayense and Ligophorus saladensis (Monogenea, Ancyrocephalidae) from Mugil liza (Teleostei, Mugilidae) in southern Brazil. Folia Parasit. 2015;62:024. doi: 10.14411/fp.2015.024. [DOI] [PubMed] [Google Scholar]
- 50.Wu XY, Chilton NB, Zhu XQ, Xie MQ, Li AX. Molecular and morphological evidence indicates that Pseudorhabdosynochus lantauensis (Monogenea: Diplectanidae) represents two species. Parasitol. 2005;130:669–77. doi: 10.1017/S0031182004007152. [DOI] [PubMed] [Google Scholar]
- 51.Hall TA. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999;41:95–8. [Google Scholar]
- 52.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6:461–4. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- 54.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 55.Ergens R, Gelnar M. Experimental verification of the effect of temperature on the size of hard parts of opisthaptor of Gyrodactylus katharineri Malmberg, 1964. Folia Parasitol. (Praha). 1985;32:377–80. [Google Scholar]
- 56.Appleby C. Variability of the opisthaptoral hard parts of Gyrodactylus callariatis Malmberg, 1957 (Monogenea: Gyrodactylidae) from Atlantic cod Gadus morhua L. in the Oslo Fjord, Norway. Syst. Parasitol. 1996;33:199–207. doi: 10.1007/BF01531201. [DOI] [Google Scholar]
- 57.Mizelle JD. New species of trematodes from the gills of Illinois fishes. Am Midl Nat. 1936;17:785–806. doi: 10.2307/2420687. [DOI] [Google Scholar]
- 58.Pariselle A, Euzet L. Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa, the Levant and Madagascar. Zoosys. 2009;31:849–98. doi: 10.5252/z2009n4a6. [DOI] [Google Scholar]
- 59.Markevich AP. Parasitic diseases of fish and their control. Publ. Koiz., Leningrad. 1934;3–100. in Russian.
- 60.Dossou C, Birgi E. Monogènes parasites d'Hemichromis fasciatus Peters, 1857 (Teleostei, Cichlidae) Ann Sci Nat Zool. 1984;6:101–9. [Google Scholar]
- 61.Paperna I. Monogenetic Trematodes collected from fresh water fish in southern Ghana. Bamidgeh, Bull. Fish Cult. 1965;17:107–15. [Google Scholar]
- 62.Pariselle A, Euzet L. Two new species of Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae) gill parasites on Hemichromis fasciatus Peters, 1858 in Africa, with remarks on parasite geographical distribution. Parasite. 2004;11:359–64. doi: 10.1051/parasite/2004114359. [DOI] [PubMed] [Google Scholar]
- 63.Řehulková E, Mendlov M, Šimková A. Two new species of Cichlidogyrus (Monogenea: Dactylogyridae) parasitizing the gills of African cichlid fishes (Perciformes) from Senegal: morphometric and molecular characterization. Parasitol. Res. 2013;112:1399–410. doi: 10.1007/s00436-013-3291-9. [DOI] [PubMed] [Google Scholar]
- 64.Young PC. Ten new species of Haliotrema Johnston and Tiegs, 1922 (Monogenoidea: Dactylogyridae) from Australian fishes and a revision of the genus. J. Zool. 1968;154:41–75. doi: 10.1111/j.1469-7998.1968.tb05039.x. [DOI] [Google Scholar]
- 65.Bychowsky BE, Nagibina LF. Ancyrocephalinae (Monogenoidea, Dactylogyridae) from the sea fishes of the family Pomadasyidae. An. Instit. Biolo. Uni. Nacio. Autón. México. Ser. Zool. 1970;41:19–28. [Google Scholar]
- 66.Euzet L, Suriano DM. Ligophorus n. g. (Monogenea, Ancyrocephalidae) parasite des Mugilidae (Téléostéens) en Méditerranée. Bull. Mus. Nat. Hist. Nat. 1977;472:799–822. [Google Scholar]
- 67.Rubtsova NY, Balbuena JA, Sarabeev VL, Blasco-Costa I, Euzet L. Description and morphological variability of a new species of Ligophorus and Ligophorus chabaudi (Monogenea: Dactylogyridae) on Mugil cephalus (Teleostei) from the Mediterranean basin. J. Parasitol. 2006;92:486–95. doi: 10.1645/GE-747R.1. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguti S. Studies on the helminth fauna of Japan. Part 19. Fourteen new ectoparasitic trematodes of fishes. Kyoto, Japan: Published by the author. 1937:1-28.
- 69.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–26. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 70.Pariselle A, Euzet L. Three new species of Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae) parasitic on Tylochromis jentinki (Steindachner, 1895) (Pisces, Cichlidae) in West Africa. Syst. Parasitol. 1994;29:229–34. doi: 10.1007/BF00009678. [DOI] [Google Scholar]
- 71.Dossou C. Parasites de Poissons d'eau douce du Bénin III. Espèces nouvelles du genre Cichlidogyrus (Monogenea) parasites de Cichlidae. Bull IFAN. 1982;44:295–322. [Google Scholar]
- 72.Pariselle A, Bilong Bilong CF, Euzet L. Four new species of Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae), all gill parasites from African mouthbreeder tilapias of the genera Sarotherodon and Oreochromis (Pisces, Cichlidae), with a redescription of C. thurstonae Ergens, 1981. Syst Parasitol. 2003;56:201–10. doi: 10.1023/B:SYPA.0000003807.27452.bd. [DOI] [PubMed] [Google Scholar]
- 73.Ergens R. Nine species of the genus Cichlidogyrus Paperna, 1960 (Monogenea: Ancyrocephalinae) from egyptian fishes. Folia Parasitol. (Praha). 1981;28:205–14. [Google Scholar]
- 74.Pariselle A, Euzet L. Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae): gill parasites from West African Cichlidae of the subgenus Coptodon Regan, 1920 (Pisces), with descriptions of six new species. Syst. Parasitol. 1996;34:109–24. doi: 10.1007/BF00009685. [DOI] [Google Scholar]
- 75.Paperna I, Thurston JP. Monogenetic trematodes collected from cichlid fish in Uganda; including the description of five new species of Cichlidogyrus. Rev. Zool. Bot. Afri. 1969;LXXIX:15–33. [Google Scholar]
- 76.Paperna I. Parasitic helminths of inland-water fishes in Israel. Israel J. Zool. 1964;13:1–26. [Google Scholar]
- 77.Price CE, Kirk RG. First description of a monogenetic trematode from Malawi. Rev. Zool. Bot. Afri. 1967;76:137–44. [Google Scholar]
- 78.Pariselle A, Muterezi Bukinga F, Van Steenberge M, Vanhove MPM. Ancyrocephalidae (Monogenea) of Lake Tanganyika: IV: Cichlidogyrus parasitizing species of Bathybatini (Teleostei, Cichlidae): reduced host-specificity in the deepwater realm? In: Koblmüller S, Albertson RC, Genner MJ, Sefc KM, Takahashi T, editors. Advances in cichlid research: Behavior, ecology and evolutionary biology. Hydrobiologia. 2015;748:99–119. doi: 10.1007/s10750-014-1975-5. [DOI] [Google Scholar]
- 79.Bilong Bilong CF, Birgi E, Euzet L. Enterogyrus barombiensis n. sp. (Monogenea, Ancyrocephalidae) parasite stomacal de trois Cichlidae endémiques du Lac du cratère Barombi Mbo (Cameroun) Ann. Parasitol. Hum Comp. 1991;66:105–8. [Google Scholar]
- 80.Kaneko JJ, II, Yamada R, Brock JA, Nakamura RM. Infection of tilapia, Oreochromis mossambicus (Trewavas), by a marine monogenean, Neobenedenia melleni (MacCallum, 1927) Yamaguti, 1963 in Kaneohe Bay, Hawaii, USA, and its treatment. J. Fish Disea. 1988;11:295–300. doi: 10.1111/j.1365-2761.1988.tb01225.x. [DOI] [Google Scholar]
- 81.Cable J, Scott ECG, Tinsley RC, Harris PD. Behavior favoring transmission in the viviparous monogenean Gyrodactylus turnbulli. J. Parasitol. 2002;88:183–4. doi: 10.1645/0022-3395(2002)088[0183:BFTITV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 82.Huyse T, Audenaert V, Volckaert FAM. Speciation and host-parasite relationships in the parasite genus Gyrodactylus (Monogenea, Platyhelminthes) infecting gobies of the genus Pomatoschistus (Gobiidae, Teleostei) Int. J. Parasitol. 2003;33:1679–89. doi: 10.1016/S0020-7519(03)00253-4. [DOI] [PubMed] [Google Scholar]
- 83.Pariselle A, Bitja Nyom AR, Bilong Bilong CF. Checklist of the ancyrocephalids (Monogenea) parasitizing Tilapia species in Cameroon, with the description of three new species. Zootaxa. 2013;3599:78–86. doi: 10.11646/zootaxa.3599.1.7. [DOI] [PubMed] [Google Scholar]
- 84.Pérez-Ponce de Léon G, Choudhury A. Biogeography of helminth parasites of freshwater fishes in Mexico: the search for patterns and processes. J. Biogeogra. 2005;32:645–59. doi: 10.1111/j.1365-2699.2005.01218.x. [DOI] [Google Scholar]
- 85.Bilong Bilong CF. Les Monogènes parasites des poissons d’eau douce du Cameroun : biodiversité et spécificité; biologie des populations inféodées à Hemichromis fasciatus. Thèse de Doctorat d’État, Université de Yaoundé I, Yaoundé, Cameroun. 1995.
- 86.Diesing KM. Systema helminthum. Vol. 1. Vindobonae: Braumfiller W; 1850. [Google Scholar]
- 87.Mizelle JD, Price CE. Studies on monogenetic Trematodes. XXVIII. Gill parasites of the Piranha with proposal of Anacanthorus gen. n. J Parasitol. 1965;51:30–6. [PubMed]
- 88.Guégan J-F, Lambert A. Twelve new species of dactylogyrids (Platyhelminthes, Monogenea) from West African barbels (Teleostei, Cyprinidae), with some biogeographical implications. Syst. Parasitol. 1990;17:153–81. doi: 10.1007/BF00009552. [DOI] [Google Scholar]
- 89.van Every LR, Kritsky DC. Neotropical Monogenoidea. 18. Anacanthorus Mizelle and Price, 1965 (Dactylogyridae, Anacanthorinae) of Piranha (Characoidea, Serrasalmidae) from the Central Amazon, their phylogeny, and aspects of host–parasite coevolution. J Helminthol. Soc. Washin. 1992;59:52–75. [Google Scholar]
- 90.El Gharbi S, Lambert A, Berrebi P. Le genre Barbus (sous-genre Barbus et Labeobarbus) au Maroc. Génétique et Parasitologie. Cah. Ethol. 1993;13:223–6. [Google Scholar]
- 91.Šimková A, Ondračková M, Gelnar M, Morand S. Morphology and coexistence of congeneric ectoparasite species: reinforcement of reproductive isolation? Biol. J. Linn. Soc. 2002;76:125–35. [Google Scholar]
- 92.Huyse T, Malmberg G. Molecular and morphological comparisons between Gyrodactylus ostendicus n. sp. (Monogenea: Gyrodactylidae) on Pomatoschistus microps (Krøyer) and G. harengi Malmberg, 1957 on Clupea harengus membras L. Syst Parasitol. 2004;58:105–13. doi: 10.1023/B:SYPA.0000029423.68703.43. [DOI] [PubMed] [Google Scholar]
- 93.Rohde K, Rohde PP. The ecological niches of parasites. In: Rohde K, editor. Marine parasitology. Wallingford: CABI Publishing; 2005. pp. 286–93. [Google Scholar]
- 94.Šimková A, Morand S. Co-evolutionary patterns in congeneric monogeneans: a review of Dactylogyrus species and their cyprinid hosts. J. Fish Biol. 2008;73:2210–27. doi: 10.1111/j.1095-8649.2008.02064.x. [DOI] [Google Scholar]
- 95.Mancheva K, Karaivanova E, Atanasov G, Stojanovski S, Nedeva I. Analysis of the influence of the host body size on morphometrical characteristics of Ancylodiscoides siluri and Ancylodiscoides vistulensis. Biotec. Biotechnol. Equip. 2009;23:735–41. doi: 10.1080/13102818.2009.10818529. [DOI] [Google Scholar]
- 96.Poisot T, Desdevises Y. Putative speciation events in Lamellodiscus (Monogenea: Diplectanidae) assessed by a morphometric approach. Biol. J. Linn. Soc. 2010;99:559–69. doi: 10.1111/j.1095-8312.2009.01381.x. [DOI] [Google Scholar]
- 97.Morand S, Hafner MS, Page RDM, Reed DL. Comparative body size relationships in pocket gophers and their chewing lice. Biol. J. Linn. Soc. 2000;70:239–49. doi: 10.1111/j.1095-8312.2000.tb00209.x. [DOI] [Google Scholar]
- 98.Morand S, Šimková A, Matejusová I, Plaisance L, Verneau O, Desdevises Y. Investigating patterns may reveal processes: evolutionary ecology of ectoparasitic monogeneans. Int. J. Parasitol. 2002;32:111–9. doi: 10.1016/S0020-7519(01)00347-2. [DOI] [PubMed] [Google Scholar]
- 99.Huyse T, Volckaert FAM. Identification of a host-associated species complex using molecular and morphometric analyses, with the description of Gyrodactylus rugiensoides n. sp. (Gyrodactylidae, Monogenea) Int. J Parasitol. 2002;32:907–19. doi: 10.1016/S0020-7519(02)00026-7. [DOI] [PubMed] [Google Scholar]
- 100.Bush S, Sohn E, Clayton DH. Ecomorphology of parasite attachment: experiments with feather lice. J. Parasitol. 2006;92:25–31. doi: 10.1645/GE-612R.1. [DOI] [PubMed] [Google Scholar]
- 101.Rohde K, Hobbs R. Species segregation: Competition of reinforcement of reproductive barriers? In: Cremin M, editor. Parasite lives. Papers on parasites, their hosts and their associations to honour JFA Sprent. St. Lucia: University of Queensland Press; 1986. pp. 189–99. [Google Scholar]
- 102.Butlin RK. Reinforcement of premating isolation. In: Otte D, Endler JA, editors. Speciation and its consequences. Sunderland: Sinauer Associates; 1989. pp. 158–79. [Google Scholar]
- 103.Butlin RK. Reinforcement: an idea evolving. TREE. 1995;10:432–4. doi: 10.1016/s0169-5347(00)89173-9. [DOI] [PubMed] [Google Scholar]
- 104.Jiggins CD, Mallet J. Bimodal hybrid zones and speciation. TREE. 2000;15:250–5. doi: 10.1016/s0169-5347(00)01873-5. [DOI] [PubMed] [Google Scholar]


