Abstract
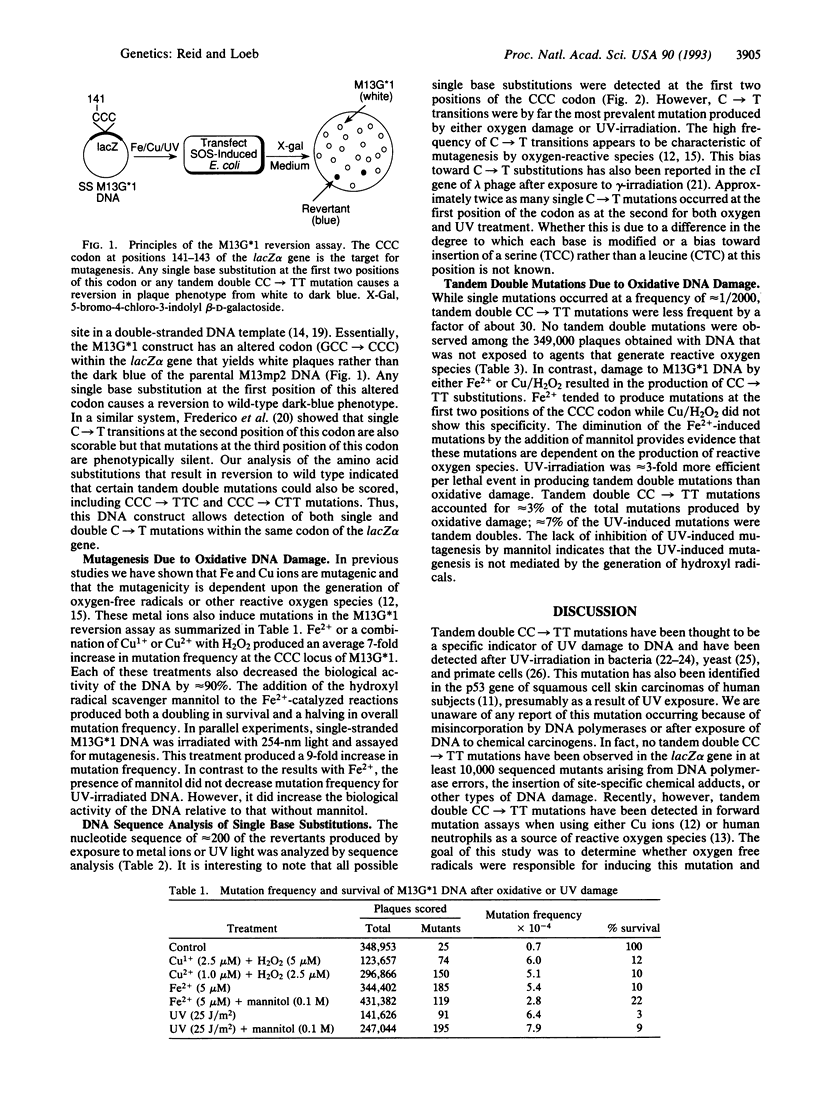
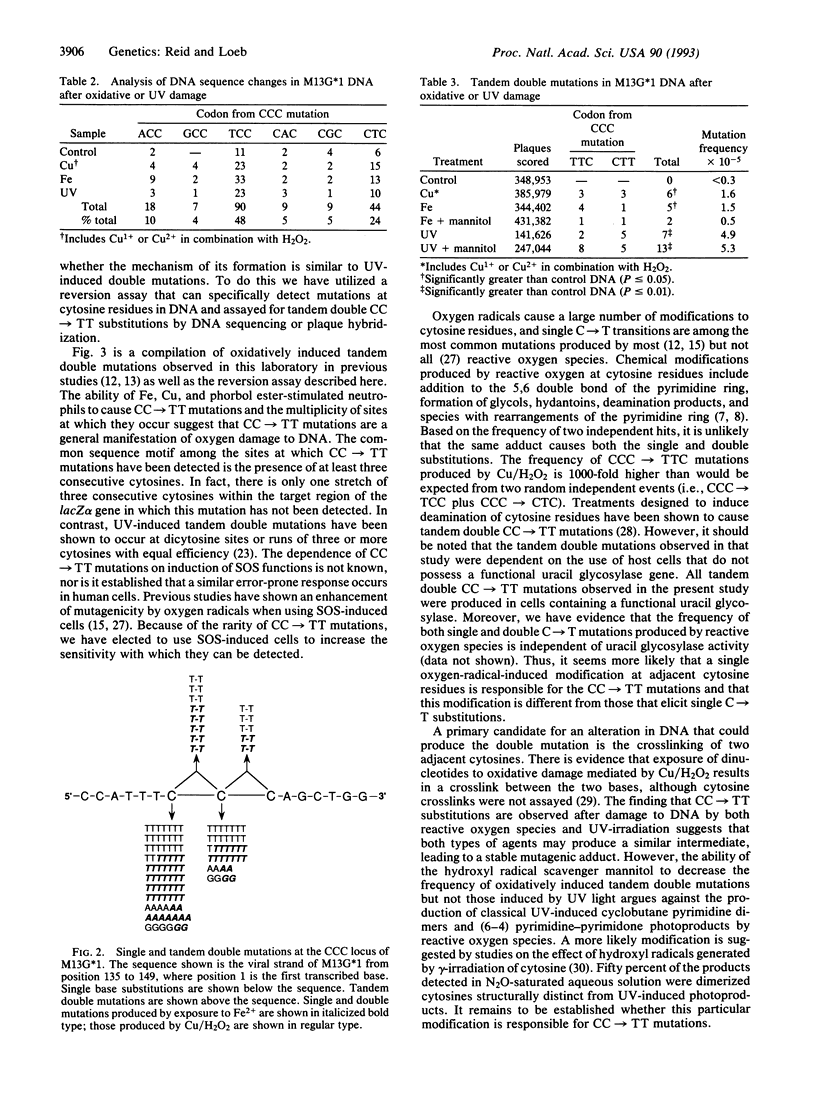
Oxidative damage to DNA is mutagenic and thus may play a role in carcinogenesis. Because of the large number of different DNA lesions formed by oxidative species, no genetic alteration so far identified is exclusively associated with oxygen damage. Tandem double CC-->TT mutations are known to occur via UV damage to DNA and are thought to be a specific indicator of UV exposure. Using a sensitive reversion assay that can detect both single and double mutations within the same codon of the M13-encoded lacZ alpha gene, we show that treatments that produce reactive oxygen species can also produce tandem double CC-->TT mutations. The frequency at which these mutations occur is less than that for single base mutations by a factor of approximately 30. The induction of these mutations is inhibited by treatment that scavenges hydroxyl radicals. This unique mutation provides a marker of oxygen free radical-induced mutagenesis in cells that are not exposed to UV-irradiation and an indicator for assessing the involvement of oxidative damage to DNA in aging and tumor progression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous DNA damage as related to cancer and aging. Mutat Res. 1989 Sep;214(1):41–46. doi: 10.1016/0027-5107(89)90196-6. [DOI] [PubMed] [Google Scholar]
- Armstrong J. D., Kunz B. A. Site and strand specificity of UVB mutagenesis in the SUP4-o gene of yeast. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9005–9009. doi: 10.1073/pnas.87.22.9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael P. L., Shé M. N., Phillips D. H. Detection and characterization by 32P-postlabelling of DNA adducts induced by a Fenton-type oxygen radical-generating system. Carcinogenesis. 1992 Jul;13(7):1127–1135. doi: 10.1093/carcin/13.7.1127. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Cheng K. C., Preston B. D., Cahill D. S., Dosanjh M. K., Singer B., Loeb L. A. The vinyl chloride DNA derivative N2,3-ethenoguanine produces G----A transitions in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9974–9978. doi: 10.1073/pnas.88.22.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizdaroglu M., Simic M. G. Radiation-induced crosslinking of cytosine. Radiat Res. 1984 Oct;100(1):41–46. [PubMed] [Google Scholar]
- Frederico L. A., Kunkel T. A., Shaw B. R. A sensitive genetic assay for the detection of cytosine deamination: determination of rate constants and the activation energy. Biochemistry. 1990 Mar 13;29(10):2532–2537. doi: 10.1021/bi00462a015. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Johnson D. L., Reid T. M., Lee M. S., Romano L. J., King C. M. Mutagenesis by single site-specific arylamine-DNA adducts. Induction of mutations at multiple sites. J Biol Chem. 1989 Nov 25;264(33):20120–20130. [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991 Apr 9;281(1-2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Harris C. C. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res. 1991 Sep 15;51(18 Suppl):5023s–5044s. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Joenje H. Genetic toxicology of oxygen. Mutat Res. 1989 Jul;219(4):193–208. doi: 10.1016/0921-8734(89)90001-5. [DOI] [PubMed] [Google Scholar]
- Kastenbaum M. A., Bowman K. O. Tables for determining the statistical significance of mutation frequencies. Mutat Res. 1970 May;9(5):527–549. doi: 10.1016/0027-5107(70)90038-2. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- McBride T. J., Preston B. D., Loeb L. A. Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry. 1991 Jan 8;30(1):207–213. doi: 10.1021/bi00215a030. [DOI] [PubMed] [Google Scholar]
- McBride T. J., Schneider J. E., Floyd R. A., Loeb L. A. Mutations induced by methylene blue plus light in single-stranded M13mp2. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6866–6870. doi: 10.1073/pnas.89.15.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Albertini A., Hofer M., Combépine C. Construction of plasmids carrying lacI mutations. J Mol Biol. 1985 Mar 5;182(1):65–68. doi: 10.1016/0022-2836(85)90027-0. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Tuteja N., Munson P. J., Hauser J., Kraemer K. H., Dixon K. UV light-induced cyclobutane pyrimidine dimers are mutagenic in mammalian cells. Mol Cell Biol. 1986 Oct;6(10):3349–3356. doi: 10.1128/mcb.6.10.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T. M., Lee M. S., King C. M. Mutagenesis by site-specific arylamine adducts in plasmid DNA: enhancing replication of the adducted strand alters mutation frequency. Biochemistry. 1990 Jul 3;29(26):6153–6161. doi: 10.1021/bi00478a007. [DOI] [PubMed] [Google Scholar]
- Reid T. M., Loeb L. A. Mutagenic specificity of oxygen radicals produced by human leukemia cells. Cancer Res. 1992 Mar 1;52(5):1082–1086. [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L., Glickman B. W. Mechanisms of ultraviolet-induced mutation. Mutational spectra in the Escherichia coli lacI gene for a wild-type and an excision-repair-deficient strain. J Mol Biol. 1987 Nov 20;198(2):187–202. doi: 10.1016/0022-2836(87)90305-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Takahashi T., Kuroishi T., Suyama M., Ariyoshi Y., Takahashi T., Ueda R. p53 mutations in non-small cell lung cancer in Japan: association between mutations and smoking. Cancer Res. 1992 Feb 1;52(3):734–736. [PubMed] [Google Scholar]
- Teebor G. W., Boorstein R. J., Cadet J. The repairability of oxidative free radical mediated damage to DNA: a review. Int J Radiat Biol. 1988 Aug;54(2):131–150. doi: 10.1080/09553008814551591. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stein J., Hutchinson F. Changes in DNA base sequence induced by gamma-ray mutagenesis of lambda phage and prophage. Genetics. 1988 Apr;118(4):551–560. doi: 10.1093/genetics/118.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkeshelashvili L. K., McBride T., Spence K., Loeb L. A. Mutation spectrum of copper-induced DNA damage. J Biol Chem. 1991 Apr 5;266(10):6401–6406. [PubMed] [Google Scholar]
- Totter J. R. Spontaneous cancer and its possible relationship to oxygen metabolism. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1763–1767. doi: 10.1073/pnas.77.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]



