Abstract
Background:
Aseptic meningitis is the most common type of meningitis and is characterized by meningeal inflammation that is not linked to identifiable bacterial pathogens in cerebrospinal fluid (CSF).
Objectives:
This study aimed to evaluate the frequency of aseptic meningitis caused by herpesviruses, namely herpes simplex types I and II (HSV-1, HSV-2), Epstein-Barr virus (EBV), cytomegalovirus (CMV) and varicella-zoster virus (VZV).
Patients and Methods:
A total of 196 CSF samples were collected from patients with suspected meningitis. All samples were smear- and culture-negative for bacterial pathogens. The biochemical and cytological findings of CSF samples were also recorded. DNA was extracted from samples and PCR with specific primers was carried out to detect viruses.
Results:
The 196 samples derived from 100 (52%) men and 96 (48%) women ranging in age from one day to 86 years with an average age of 32.3 ± 25.3 years. Of them, 8 (4.08%) samples yielded positive results, including 5 (2.55%) cases of VZV infection and 3 (1.53%) cases of HSV-1 infection. No cases of HSV-2, CMV or EBV infection were detected. CSF protein and glucose levels among positive cases were all in the normal range.
Conclusions:
The results indicate a considerable rate of herpesvirus infection in patients with aseptic meningitis, and that VZV is the most common herpesvirus to cause infection followed by HSV-1. Our results also showed that a moderate increase in the WBC count and predominance of lymphocytes can be valuable clues in diagnosing viral meningitis. Given the different approaches of drug therapy in bacterial and viral meningitis, use of molecular methods is necessary in hospitals to rapidly discriminate between them.
Keywords: Aseptic Meningitis, Herpesvirus, Polymerase Chain Reaction, Iran
1. Background
Meningitis is among the most common acute diseases affecting the central nervous system (CNS) (1). It is a severe and potentially fatal infection of the CNS and can be caused by bacteria, viruses, fungi, or parasites. Aseptic meningitis is the most prevalent type of meningitis and is characterized by meningeal inflammation that is not associated with identifiable bacterial pathogens in CSF (1, 2). Most patients with aseptic meningitis present with abrupt onset of fever, headache, stiff neck, lethargy, and anorexia, and they may also experience vomiting, diarrhea, sore throat, and rash. However, it is indistinguishable from bacterial meningitis when considering only clinical signs and symptoms. Furthermore, CNS infections in neonates may not be accompanied by obvious signs of meningeal inflammation (1-3). Aseptic meningitis is mostly caused by viral agents including those from the Herpesviridae family (4, 5).
The herpesviruses that most commonly infect the CNS are the herpes simplex viruses (HSVs) type 1 and 2 (HSV-1 and HSV-2), which are the common cause of acute sporadic encephalitis in adults and children over 6 months of age (6). CNS diseases caused by these viruses include meningitis, encephalitis, and myelitis (7). There are two age-related peaks for HSV infections: people over 50 years of age and under 20 years of age. HSV-2 has more often been linked to recurrent aseptic meningitis than HSV-1 and causes neurological complications more often than most other viruses (6, 8, 9). HSV-2 meningitis has also been recognized as a significant cause of morbidity and mortality in immunocompromised patients (10).
Other herpesviruses associated with meningitis and meningoencephalitis include Epstein-Barr virus (EBV), cytomegalovirus (CMV), and varicella-zoster virus (VZV). People who are infected with CMV shed the virus in the urine, saliva, semen, and to a lesser extent, in other body fluids. Transmission can also occur from an infected mother to her fetus or newborn and by blood transfusion and organ transplantation (11). CNS infections with these viruses are mostly seen in immunocompromised people, and CMV in particular has been linked to chronic meningoencephalitis in human immunodeficiency virus (HIV) infection (12). VZV is an exclusively human neurotrophic alpha-herpesvirus (13). Primary infection manifests as varicella (chickenpox), after which the virus becomes latent in dorsal root ganglia. With advancing age or immunosuppression, cell-mediated immunity to VZV declines and virus reactivation causes herpes zoster (shingles) which can be complicated by central and peripheral nervous system involvement. CMV and VZV may also cause myelitis or, occasionally, ventriculitis, and VZV has been associated with a large-vessel cerebral vasculitis causing strokes, particularly in the elderly (14). The detection of viral infection of CNS is not routine in many medical laboratories in Iran. Consequently, the diagnosis of viral meningitis may be missed and the epidemiology of these infections remains unclear in many parts of Iran, including Kermanshah.
2. Objectives
This study aimed to determine the frequency of viral meningitis caused by HSV-1, HSV-2, CMV, EBV, and VZV in patients with suspected aseptic meningitis in the city of Kermanshah in western Iran.
3. Patients and Methods
3.1. Patients’ Samples
The CSF samples of 223 patients were collected from March 2012 to December 2012 in Imam Reza Hospital in Kermanshah, Iran. Bacterial pathogens were found in 27 samples using bacteriological culture methods and PCR testing. The remaining samples (196) were negative for bacterial pathogens and were thus selected for detection of herpesvirus infection. All samples were kept in a freezer at -70°C until testing was completed. Patient characteristics were collected from their hospital files. Data from biochemical findings of CSF samples including glucose and protein levels, as well as cytological findings including white blood cell count and WBC differential were also collected.
3.2. Study Approval
This study was subject to approval by the Ethics Committee of the Kermanshah University of Medical Sciences and was registered (Number 91279).
3.3. DNA Extraction
We used 100 μL CSF samples for DNA extraction by CinnaPure DNA viral kit (SinaClon, Iran) according to manufacturer’s instructions. Extracted DNA was kept at -20°C until PCR testing.
3.4. PCR Reaction
PCR primers were used to amplify conserved regions of HSV-1, HSV-2, CMV, EBV, and VZV (Table 1) (15). Primers were synthesized by SinaClon (Tehran, Iran). PCR reaction for viruses was prepared in a final volume of 25 μL using 12.5 μL of Master Mix Ampliqon III (Ampliqon, Denmark), 1 μL of primers, 6.5 μL deionized distilled water and 4 μL of DNA template. The Thermocycler was BioRad C1000 (USA). PCR products were detected by agarose gel (1%) electrophoresis and gels were visualized using GelDoc (BioRad, USA) after staining with ethidium bromide.
Table 1. Primers Used and Their Characteristics.
| Virus | Primer Sequence (5’ - 3’) | Genome Region Amplified | Amplicon, bp |
|---|---|---|---|
| VZV | ORF 8 | 275 | |
| Forward | CACACGATAATGCCTGATCGG | ||
| Reverse | TGCTGATATTTCCACGGTACAGC | ||
| HSV-1 | RL 2 | 147 | |
| Forward | TGGGACACATGCCTTCTTGG | ||
| Reverse | CCCTTAGTCAGACTCTGTTACTTACCC | ||
| HSV-2 | UL 28 | 227 | |
| Forward | GTACAGACCTTCGGAGG | ||
| Reverse | CGCTTCATCATGGGC | ||
| EBV | Exon 4/5 terminal protein RNA | 182 | |
| Forward | AACATTGGCAGCAGGTAAGC | ||
| Reverse | ACTTACCAAGTGTCCATAGGAGC | ||
| CMV | IRL 11 | 256 | |
| Forward | GTACACGCACGCTGGTTACC | ||
| Reverse | GTAGAAAGCCTCGACATCGC |
3.5. Positive Controls
The approved DNA of herpesviruses was kindly provided by the Pasteur Institute (Tehran, Iran) and used as positive controls for PCR. Finally, a number of PCR products were sequenced and the sequence data were analyzed using Bioinformatics software (BLAST) to confirm viral DNA.
3.6. Statistical Analysis
All data were inserted into an Excel file and analyzed using SPSS software version 18.
4. Results
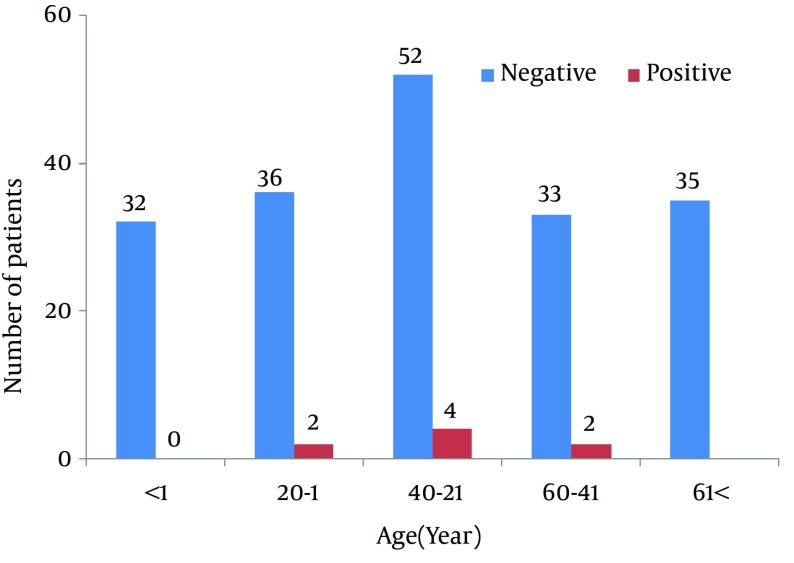
One hundred and ninety-six CSF samples were tested, deriving from 100 (52%) men and 96 (48%) women ranging in ages from one day to 86 years with an average age of 32.3 ± 25.3 years (Figure 1). Of the samples, 8 (4.08%) yielded results positive for herpesviruses, including 5 (2.55%) cases of VZV infection and 3 (1.53%) cases of HSV-1 infection (Figure 2). No cases of HSV-2, CMV or EBV infection were detected.
Figure 1. The Age Distribution of Patients and Positive Cases.
Figure 2. Electrophoresis of PCR Products.
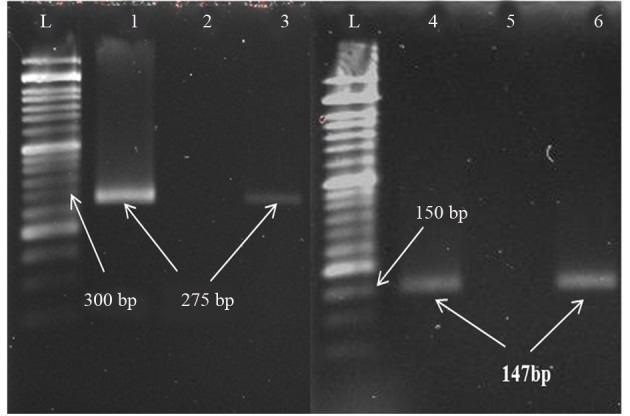
L: Ladder 50 bp (Cat.NO.PR 901633); 1: Positive control VZV (275 bp); 2; Negative control; 3: Positive sample VZV (275 bp); 4: Positive control HSV-1 (147 bp); 5: Negative control; 6: Positive sample HSV-1 (147 bp).
The 8 cases of viral meningitis consisted of four males and four females ranging in age from 5 to 46 years with an average age of 28.2 years (Table 2). The increased number of white blood cells in positive cases compared to negative cases was statistically significant with 85% lymphocytes in the WBC differential. There were no significant changes in CSF protein or glucose in positive samples in comparison to negative samples (Table 3). Co-infection of herpesviruses was not found among positive cases.
Table 2. The Comparison of Characteristics in Positive and Negative Cases.
| PCR Results | Number of Samples | Age, y | Protein, mg/dL | Glucose, mg/dL | WBC count (Average) |
|---|---|---|---|---|---|
| Negative | 188 | 32.21 | 27.8 | 63.16 | 3 |
| Positive | 8 | 28.25 | 27.87 | 51.5 | 151.2 |
| P value | - | 0.24 | 0.973 | 0.124 | 0.001 |
Table 3. The Characteristics of Positive Cases a.
| Virus/Gender | Age, y | Protein, mg/dL | Glucose, mg/dL | WBC, μL | WBC Differential Count | |
|---|---|---|---|---|---|---|
| Seg. b | Lym. b | |||||
| HSV-1 | ||||||
| Female | 21 | 25 | 23 | 60 | 10 | 90 |
| Female | 18 | 32 | 39 | 310 | 5 | 95 |
| Male | 42 | 22 | 86 | 240 | 30 | 70 |
| VZV | ||||||
| Female | 31 | 35 | 49 | 400 | 10 | 90 |
| Female | 23 | 14 | 60 | 70 | 10 | 90 |
| Male | 46 | 30 | 55 | 400 | 10 | 90 |
| Male | 40 | 35 | 60 | 400 | 10 | 90 |
| Male | 5 | 30 | 40 | 50 | 35 | 65 |
a Abbreviations: Seg. = segmented cell; Lym. = lymphocyte.
b Values unit is %.
5. Discussion
This is the first study from western Iran to provide a better view of herpesvirus infection among patients of a wide age range with suspected aseptic meningitis. It is important to differentiate viral meningitis from bacterial meningitis in order to better manage the disease and to understand the local epidemiology of microbial agents. Herpesvirus infections can cause serious diseases of the central nervous system and need to be distinguished from bacterial infections (16).
Our results showed a considerable rate of herpesvirus infection among patients with suspected aseptic meningitis, suggesting the need for molecular tests to diagnose viral meningitis. The majority of infected patients were young and middle-aged that may indicate the primary infection or secondary reactivation of viral infections. Viral infection among cases of aseptic meningitis has been studied in many areas in the world and various rates of infection have been reported. For instance, in a study in Finland on 213 suspected CNS infections in patients aged 4 - 16 years, 7 (3.2%) cases of VZV infection were found. Similar to our results, it was the most common herpesvirus infection detected (17). As our results indicated, it seems that the rates of HSV-2, EBV, and CMV meningitis are lower than the rates of HSV-1 and VZV meningitis.
In a comprehensive study in England on 2,233 CSF samples with suspected aseptic meningitis, researchers found HSV-1 in 0.89% of samples, followed by VZV in 0.71%, EBV in 0.49%, HSV-2 in 0.4%, and CMV in 0.13%. This data correlates with our results regarding higher rates of HSV-1 and VZV infections and lower rates of other herpesviruses (18). In another study in England on 1,683 CSF samples, HSV-2 was the most commonly detected DNA virus with 33 positive cases, followed by VZV with 29 cases, and HSV-1 with 25 cases (19). A study of 86 CSF samples in Greece indicated HSV-1 infection in three patients (3.5%) with encephalitis and VZV in four patients (4.6%) with meningitis. Furthermore, in a study in Tehran on 132 children with meningoencephalitis, HSV-1 was found in 5.3% and CMV in 1.5% (20). Since this study involved children only, and not a wide range of ages, results may differ slightly from ours. Our results showed no herpesvirus co-infection in the central nervous system. This is also consistent with the results of other reports, indicating that detection of more than one virus in any clinical specimen is uncommon (18).
On the other hand, some characteristics of CSF have been used to differentiate between bacterial and viral meningitis (21). The results of our study are consistent with the common knowledge that levels of CSF protein and glucose in viral meningitis do not significantly differ from levels in uninfected people. However, there was an increase in the WBC differential with a significant predominance of lymphocytes (P < 0.001). This data is in agreement with the fact that unlike changes in CSF from bacterial meningitis, in viral meningitis lymphocytosis may provide a clue for diagnosis, a finding similar to the results reported by another Iranian paper (22). Although in our study a small number of the samples were positive, most of these infections occurred between May and July, a timeframe similar to those given in other reports indicating a higher occurrence of viral meningitis during spring and summer (22-24).
In conclusion, our results indicate a considerable prevalence of herpesvirus infections in patients with suspected aseptic meningitis, with VZV being the most commonly detected herpesvirus followed by HSV-1, in particularly in young and middle-aged people. An increased WBC count and predominance of lymphocytes can be valuable clues for herpesvirus meningitis. Use of molecular testing methods should be required in medical diagnostic laboratories since they can differentiate viral from bacterial meningitis and help to shorten hospital stays for patients and avoid unnecessary use of antibiotics.
Acknowledgments
Dr. Azadmanesh from Pasteur Institute of Iran kindly provided positive controls for the viruses. We also appreciate the co-operation of all medical microbiology laboratory personnel in the Faculty of Medicine Kermanshah University of Medical sciences.
Footnotes
Authors’ Contributions:Alisha Akya developed the original idea, designed the research, provided materials, supervised the work, analyzed the data and wrote the final manuscript; Kamal Ahmadi, Shahram Zehtabian, Afsaneh Salimi, Azam Elahi and Sayed Hamid Madani carried out sampling, lab works, statistical analysis, data collection and drafting.
Funding/Support:This study was supported by Kermanshah University of Medical Sciences.
References
- 1.Irani DN. Aseptic meningitis and viral myelitis. Neurol Clin. 2008;26(3):635–55. doi: 10.1016/j.ncl.2008.03.003. vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalwai A, Ahmad S, Al-Nakib W. Echoviruses are a major cause of aseptic meningitis in infants and young children in Kuwait. Virol J. 2010;7:236. doi: 10.1186/1743-422X-7-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R. Aseptic meningitis: diagnosis and management. Indian J Pediatr. 2005;72(1):57–63. doi: 10.1007/BF02760582. [DOI] [PubMed] [Google Scholar]
- 4.Bastos MS, Lessa N, Naveca FG, Monte RL, Braga WS, Figueiredo LT, et al. Detection of Herpesvirus, Enterovirus, and Arbovirus infection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J Med Virol. 2014;86(9):1522–7. doi: 10.1002/jmv.23953. [DOI] [PubMed] [Google Scholar]
- 5.Nourbakhsh S, Tonkaboni H, Asghaee M, Hosseini F, Vahed L, Tabatabaee A. Detection of Herpes Virus Infection Frequency in Aseptic Meningitis in Children Admitted to Rasoul-e-Akram & Mofid Hospitals. Razi J Med Sci. 2004;11(42):659–65. [Google Scholar]
- 6.Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret's. Herpes. 2004;11 Suppl 2:57A–64A. [PubMed] [Google Scholar]
- 7.Big C, Reineck LA, Aronoff DM. Viral infections of the central nervous system: a case-based review. Clin Med Res. 2009;7(4):142–6. doi: 10.3121/cmr.2009.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingues RB, Tsanaclis AM, Pannuti CS, Mayo MS, Lakeman FD. Evaluation of the range of clinical presentations of herpes simplex encephalitis by using polymerase chain reaction assay of cerebrospinal fluid samples. Clin Infect Dis. 1997;25(1):86–91. doi: 10.1086/514494. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrom T, Vahlne A, Alestig K, Jeansson S, Forsgren M, Lycke E. Primary and recurrent herpes simplex virus type 2-induced meningitis. J Infect Dis. 1990;162(2):322–30. doi: 10.1093/infdis/162.2.322. [DOI] [PubMed] [Google Scholar]
- 10.Mommeja-Marin H, Lafaurie M, Scieux C, Galicier L, Oksenhendler E, Molina JM. Herpes simplex virus type 2 as a cause of severe meningitis in immunocompromised adults. Clin Infect Dis. 2003;37(11):1527–33. doi: 10.1086/379520. [DOI] [PubMed] [Google Scholar]
- 11.Prince AM, Szmuness W, Millian SJ, David DS. A serologic study of cytomegalovirus infections associated with blood transfusions. N Engl J Med. 1971;284(20):1125–31. doi: 10.1056/NEJM197105202842004. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick DR. Viral meningitis. Br Med Bull. 2005;75-76:1–14. doi: 10.1093/bmb/ldh057. [DOI] [PubMed] [Google Scholar]
- 13.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203(3):316–23. doi: 10.1093/infdis/jiq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito S, Bosis S, Pinzani R, Morlacchi L, Senatore L, Principi N. A case of meningitis due to varicella zoster virus reactivation in an immunocompetent child. Ital J Pediatr. 2013;39:72. doi: 10.1186/1824-7288-39-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markoulatos P, Georgopoulou A, Siafakas N, Plakokefalos E, Tzanakaki G, Kourea-Kremastinou J. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J Clin Microbiol. 2001;39(12):4426–32. doi: 10.1128/JCM.39.12.4426-4432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitley RJ. Viral encephalitis. N Engl J Med. 1990;323(4):242–50. doi: 10.1056/NEJM199007263230406. [DOI] [PubMed] [Google Scholar]
- 17.Huttunen P, Lappalainen M, Salo E, Lonnqvist T, Jokela P, Hyypia T, et al. Differential diagnosis of acute central nervous system infections in children using modern microbiological methods. Acta Paediatr. 2009;98(8):1300–6. doi: 10.1111/j.1651-2227.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 18.Read SJ, Jeffery KJ, Bangham CR. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997;35(3):691–6. doi: 10.1128/jcm.35.3.691-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Read SJ, Kurtz JB. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J Clin Microbiol. 1999;37(5):1352–5. doi: 10.1128/jcm.37.5.1352-1355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noorbakhsh S, Tabatabaei A, Parvaresh M, Tonekaboni H. Determination of CMV infection in CSF of children with meningoencephalitis: PCR method. Tehran Univ Med Sci. 2009;66(10) [Google Scholar]
- 21.Zimmerli W. How to differentiate bacterial from viral meningitis. Intensive Care Med. 2005;31(12):1608–10. doi: 10.1007/s00134-005-2810-2. [DOI] [PubMed] [Google Scholar]
- 22.Sasan MS, Alborzi A, Ziyaeyan M. Epidemiology of Aseptic Meningitis in Infants and Children (Shiraz-Iran). Arch Clin Infect Dis. 2012;7(4):116–8. [Google Scholar]
- 23.Hosseininasab A, Alborzi A, Ziyaeyan M, Jamalidoust M, Moeini M, Pouladfar G, et al. Viral etiology of aseptic meningitis among children in southern Iran. J Med Virol. 2011;83(5):884–8. doi: 10.1002/jmv.22056. [DOI] [PubMed] [Google Scholar]
- 24.Michos AG, Syriopoulou VP, Hadjichristodoulou C, Daikos GL, Lagona E, Douridas P, et al. Aseptic meningitis in children: analysis of 506 cases. PLoS One. 2007;2(7):e22639. doi: 10.1371/journal.pone.0000674. [DOI] [PMC free article] [PubMed] [Google Scholar]