Abstract
Orexin (hypocretin) and melanin-concentrating hormone (MCH) neurons are unique to the lateral hypothalamic (LH) region, but project throughout the brain. These cell groups have been implicated in a variety of functions, including reward learning, responses to stimulants, and the modulation of attention, arousal and the sleep/wakefulness cycle. Here, we examined roles for LH in two aspects of attention in associative learning shown previously to depend on intact function in major targets of orexin and MCH neurons. In experiments 1 and 2, unilateral orexin-saporin lesions of LH impaired the acquisition of conditioned orienting responses (ORs) and bilaterally suppressed FOS expression in the amygdala central nucleus (CeA) normally observed in response to food cues that provoke conditioned ORs. Those cues also induced greater FOS expression than control cues in LH orexin neurons, but not in MCH neurons. In experiment 3, unilateral orexin-saporin lesions of LH eliminated the cue associability enhancements normally produced by the surprising omission of an expected event. The magnitude of that impairment was positively correlated with the amount of LH damage and with the loss of orexin neurons in particular, but not with the loss of MCH neurons. We suggest that the effects of the LH orexin-saporin lesions were mediated by their effect on information processing in the CeA, known to be critical to both behavioral phenomena examined here. The results imply close relations between LH motivational amplification functions and attention, and may inform our understanding of disorders in which motivational and attentional impairments co-occur.
Keywords: amygdala, attention, central nucleus, lateral hypothalamus, melanin-concentrating hormone, orexin
Introduction
Orexin (hypocretin) and melanin-concentrating hormone (MCH) neurons are unique to the lateral hypothalamic (LH) region, but project throughout the brain (Peyron et al., 1998; Hassani et al., 2009). Since their discovery, behavioral functions attributed to LH (and especially to those cell groups) have expanded well beyond the traditional regulation of feeding and body weight (Sakurai et al., 1998; Pissios et al., 2006). For example, MCH neurons have been implicated in responses to stimulants (Whiddon & Palmiter, 2013), and learning of conditioned reinforcement (Sherwood et al., 2012) and passive avoidance (Adamantidis et al., 2005). Similarly, orexin neurons have been implicated in food and drug reward learning (Aston-Jones et al., 2010), addiction (de Lecea et al., 2006) and the cholinergic modulation of attention (Fadel & Burk, 2010). Furthermore, both sets of neurons play important roles in sleep/wakefulness cycles and arousal (Sakurai, 2007; Monti et al., 2013).
Here we examined roles for LH orexin and MCH neurons in two aspects of attention in associative learning that depend on the function of the amygdala central nucleus (CeA). The CeA is a prime target of projections from LH, and especially orexin neurons (Peyron et al., 1998; Fadel & Deutch, 2002; Ceriello et al., 2003), and is also a major innervator of orexin- and MCH-rich LH subregions (Petrovich et al., 2000). Previous research showed that both the conditioning of orienting responses (ORs), and the surprise-induced enhancement of associability (the rate with which a cue forms new associations) depend on the integrity of circuits that include the CeA (reviewed by Holland & Maddux, 2010). Thus, on the basis of CeA–LH connectivity, LH might also be expected to play a significant role in these attentional functions.
Pairings of a conditioned stimulus (CS) and a food unconditioned stimulus (US) can produce not only the acquisition of US-appropriate conditioned responses (CRs), but also the potentiation of attentional ORs specific to the CS, such as approach to visual CSs. Rats with compromised CeA function fail to display conditioned ORs, but show intact unconditioned ORs to novel CSs and normal acquisition of food-appropriate CRs (Gallagher et al., 1990; McDannald et al., 2004). Experiment 1 examined the role of LH–CeA connectivity in conditioned orienting using a disconnection lesion strategy, and experiment 2 examined the effects of unilateral orexin-saporin lesions of LH on conditioned ORs, and on FOS expression in CeA and in various neuron types in LH.
Experiment 3 examined the role of LH in a more subtle aspect of attention in associative learning. According to several models of associative learning (Pearce & Hall, 1980) and much experimental evidence (Holland & Maddux, 2010), the violation of expectancies (prediction error) increases attention to contemporaneous cues, such that those cues will be more rapidly associated with new events in the future. Experiment 3 examined the effects of unilateral orexin-saporin lesions of LH and the role of LH–CeA connectivity in the enhancement of associability by surprise, using a serial prediction task previously shown to require intact CeA function (Holland & Gallagher, 1993, 2006).
Materials and methods
Animals
Experimentally naive male Long–Evans rats (Charles River Laboratories, Raleigh, NC, USA), weighing approximately 300–325 g on arrival, were individually caged and maintained on a 12-h light/dark cycle (lights on at 07:00 h). Behavioral training sessions were conducted during the light portion of that cycle. After surgical recovery, rats were reduced to 85% of their free-feeding weight by restricting their access to food, and were maintained at this weight during all procedures. They were allowed ad libitum access to water throughout the experiment. There were 24 rats in experiment 1, 32 rats in experiment 2, and 76 rats in experiment 3. The care and experimental treatment of rats was conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Johns Hopkins University Animal Care and Use Committee.
Apparatus
The behavioral training apparatus consisted of 12 individual chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls, clear acrylic sides and top, and a floor made of 0.48-cm stainless steel rods spaced 1.9 cm apart. A dimly illuminated food cup was recessed in the center of the front wall. An infrared photocell placed just inside the food cup was polled (1 kHz) by computer circuitry to record the time each rat spent with its head in the food cup. Each chamber was enclosed in a sound-resistant shell. A speaker was mounted on the inside wall of the inner shell, 10 cm above the experimental chamber and even with the front wall of the chamber. A 6-W lamp was mounted behind a jeweled lens on the front wall, 10 cm above the food cup; illumination of this lamp served as the ‘light’ stimulus. Ventilation fans provided masking noise (70 dB). Constant dim illumination was provided by a 6-W lamp behind a dense red lens mounted next to the speaker. An infrared activity monitor (Coulbourn H24-61) was mounted on the top of each chamber. Finally, a video camera was mounted on the inside wall of each shell; video images from each chamber were digitized, merged and stored on video tape for subsequent analysis.
Surgical procedures
Rats in all experiments received neurotoxic or sham lesions of CeA, LH or both regions. Experiment 1 evaluated the role of LH–CeA connectivity in conditioned ORs. All rats received an orexin-saporin lesion of LH in one hemisphere, and an ibotenic acid lesion of CeA in either the contralateral (Contra) or ipsilateral (Ipsi) hemispheres. Because both CeA→LH and LH→CeA projections are nearly exclusively ipsilateral (Petrovich et al., 2001; Yoshida et al. 2006), destruction of CeA and LH in contralateral hemispheres minimizes communication between those two regions, but leaves the function of each region intact in one hemisphere. Thus, any function that requires convergence of CeA and LH information would be disrupted by contralateral lesions, but functions involving communication between CeA and other regions would be left intact in one hemisphere. Previous research showed that an intact CeA in one hemisphere was sufficient for normal attentional function in the tasks investigated here: unilateral lesions of CeA alone had no effect on either conditioned ORs or surprise-induced enhancements of associability (Han et al., 1997, 1999). Control rats with unilateral lesions of both structures ipsilaterally had the same amount of damage to each structure as contralaterally lesioned rats, but LH–CeA communication would be intact in the unlesioned hemisphere. In experiment 2, rats received a unilateral orexin-saporin lesion, a sham lesion or no lesion of LH. In experiment 3, some rats received orexin-saporin lesion of LH in one hemisphere and an ibotenic acid lesion of CeA in the Contra hemisphere, some received a unilateral orexin-saporin lesion of LH and a unilateral sham lesion of CeA (Uni-LH), some rats received a unilateral ibotenic acid lesion of CeA and a unilateral sham lesion of LH (Uni-CeA), and some rats received unilateral sham lesions of both regions (sham). All lesions in all experiments were counterbalanced across the hemisphere. Unoperated rats in experiment 2 were assigned a ‘lesion’ hemisphere randomly, for analytic purposes.
Rats were anesthetized with isoflurane gas, and stereotaxic surgery was conducted under aseptic conditions. LH lesions were made using 0.5 μL of 1 μg/μL Orexin B-saporin conjugate (Advanced Targeting Systems, San Diego, CA, USA) in phosphate-buffered saline (PBS) solution, infused with a Hamilton 2.0-μL syringe over a 6-min period at the stereotaxic coordinates 2.9 mm posterior, 1.4 mm lateral and 8.6 mm ventral from bregma. CeA lesions were made using 0.25 μL of 10 μg/μL ibotenic acid (Sigma, St Louis, MO, USA) in PBS solution, infused with a Hamilton 2.0-μL syringe over a 3-min period, at stereotaxic coordinates 2.3 mm posterior to bregma and 4.3 mm right or left of the midline, with infusions at a depth of 8.0 mm from the skull surface. Before they were removed, the injectors remained in place for 3 min after CeA infusions and 10 min after LH infusions. Most sham-lesioned rats received injections of the PBS vehicle, with corresponding amounts, times and coordinates, although in experiment 2, eight of the sham-lesioned rats (four in each training condition) received no injections. After surgery, each rat received a single 0.015-mL subcutaneous injection of 0.4 mg/mL buprenorphine hydrochloride (Sigma) for amelioration of pain, and was allowed to recover from surgery for 7–10 days before beginning behavioral training.
Behavioral training procedures
Experiments 1 and 2
All rats first received one 64-min session to train them to approach and eat from the food cup upon food delivery. This session included 16 deliveries of two 45-mg grain pellets (Test Diets, Richmond, Indiana, USA), the food reinforcer used throughout these experiments. Next, they received a session to evaluate unconditioned ORs to the light and tone cues to be used in conditioning. This 64-min session included eight non-reinforced presentations each of a 10-s intermittent (3 Hz) illumination of the light stimulus and a steady 10-s presentation of a 78-dB, 1500-Hz tone. Then, the rats received 10 training sessions designed to establish both conditioned ORs and food cup entry CRs to the light for rats in the paired (P) group, but not in an unpaired (U) group. Each of these sessions included eight reinforced presentations (CS+) of the light and eight non-reinforced presentations (CS−) of the tone in group P, or eight reinforced presentations of the tone CS+ and eight non-reinforced presentations of the light CS− in group U. Rats trained in group U served as a behavioral control for conditioning to the light CS+. The rats in experiment 2 received an additional final test session to evaluate FOS expression induced by the light stimulus. In this test, each rat received four non-reinforced presentations of the 10-s light in a 16-min test session. Rats were killed and their brains prepared for immunocytochemistry 90 min after the start of this session.
Experiment 3
In experiment 3, we examined lesion effects on the enhancement of cue associability by the surprising omission of an expected stimulus. After the rats were trained to eat food pellets from the recessed food cups as in experiments 1 and 2, we exposed them to a three-stage serial prediction task (Table 1; Wilson et al., 1992), using the same visual and auditory stimuli used in experiments 1 and 2. In an initial ‘expectancy’ phase, the rats first received consistent serial light→tone pairings to establish the light as a highly valid predictor of the tone. In each 64-min session of this phase, eight of the 16 trials comprised a 10-s light → 10-s tone compound reinforced with food, and the other eight trials were non-reinforced presentations of the same light→tone compound. Trial order in each session was randomly determined. After 10 sessions of expectancy training, rats were allocated into shift or consistent groups, and given two ‘surprise’ phase sessions. For rats in the consistent condition, these sessions were identical to those in the expectancy phase, but for rats in the shift condition the tone was omitted on the eight non-reinforced trials in each session. Finally, in each of the five sessions in the test phase, all rats received 16 presentations of the light CS alone followed immediately by food reinforcement. More learning of food cup CRs to the light CS in the test was taken as evidence of enhanced associability of that CS.
Table 1.
Outline of behavioral training procedures of experiment 3
| Training group | Expectation phase | Surprise phase | Test phase |
|---|---|---|---|
| Shift | light→tone→Food | light→tone→food | light→food |
| light→tone→nothing | light→nothing | ||
| Consistent | light→tone→Food | light→tone→food | light→food |
| light→tone→nothing | light→tone→nothing |
Automated behavioral measures
Food cup CRs were recorded as the percentage of time each rat spent with its head in the recessed food cup, as indicated by the food cup photocells. We reported this measure during pre-CS periods and during the last half of each 10-s interval of cue presentations. Previous data show that conditioned food cup CRs occur chiefly in this interval, immediately prior to food delivery (Holland, 1977). We reported activity counts/min indicated by the activity monitors during the pre-CS intervals as a measure of overall activity levels.
Behavioral observation procedures
ORs, which are cue-specific responses produced both prior to and after conditioning, were assessed from videotapes. Observations of each rat’s behavior were made at 1.25-s intervals, paced by auditory signals recorded on the tapes. Observations were made during the 5-s period rearing, which was defined as standing on the hind legs with both front legs off the floor, but not grooming. To assess the objectivity of behavioral scoring, many of the video tapes were scored by multiple observers, who agreed on 95% of over 4000 joint observations. The number of observations of each behavior was divided by the total number of observations to form the measure ‘percentage behavior’. Because the number of observations in each CS interval was constant, this measure is an absolute frequency measure, which does not depend on overall levels of behavior. In previous studies (Holland, 1977), ORs occurred primarily during the first 5-s periods of CS presentations, so we only reported ORs occurring in that interval and during pre-CS periods of equivalent duration. Note that because rear ORs and food cup CRs occur at different times during CS presentations, there is little opportunity for competition between them.
Histological procedures
All rats were anesthetized and perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed, post-fixed and cryoprotected overnight in 4% paraformaldehyde in 0.1 M PB containing 12% sucrose, frozen with powdered dry ice, and stored at −80 °C. A freezing microtome was used to take 40-μm sections from each brain. In experiments 1 and 3, of every six consecutive sections, the first was mounted and Nissl-stained to evaluate CeA and LH lesions; the second, third and fourth sections were processed for immunocytochemistry for orexin, MCH, and orexin + MCH, respectively; and the sixth was discarded. In experiment 2, of every six consecutive sections, the first was mounted and Nissl-stained to evaluate LH lesions; and the second–sixth sections were processed for FOS, orexin + FOS, MCH + FOS, orexin, and MCH, respectively.
Immunocytochemistry procedures
Standard immunohistochemical protocols were used (Lee et al., 2005). The primary antibodies used were rabbit FOS antibody SC-32 (1 : 10,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit orexin antibody AB3704 (1 : 1000 dilution; Millipore, Billerica, MA, USA) and rabbit MCH antibody H-070-47 (1 : 1000–4000 dilutions; Phoenix Pharmaceuticals, Burlingame, CA, USA); and the secondary antibody was biotinylated goat anti-rabbit IgG (1 : 250 dilution; Vector, Burlingame, CA, USA). Sections were incubated in avidin-biotin peroxidase conjugate (Vector), and reacted using diaminobenzidine (DAB; brown) or DAB-NiCl2 (black) to visualize cells immunoreactive for FOS, orexin and MCH (FOS+, ORX+ and MCH+, respectively). Sections were mounted on slides, dehydrated in ascending concentrations of alcohol, and coverslipped with Permount.
Lesion evaluation
CeA and LH lesions were first evaluated from photographs of Nissl-stained sections at four coronal planes of CeA and LH. Outlines of the lesion extents were drawn on digital images from Swanson (1998) using Adobe Photoshop 11.0.2. Calculation of percentage damage was performed within Photoshop by comparing the area of the intersection of lesion and region extent with the area within the region’s borders. We included the entire CeA within our calculations but, for LH, which has considerable rostral-caudal extent, we limited our calculations to the region between plates 24 and 34 of Swanson (1998), which includes most orexin and MCH neurons and most projections from CeA (Petrovich et al., 2001; Swanson et al., 2005).
In experiment 1, sections stained for orexin, MCH and orexin + MCH were evaluated qualitatively, to supplement the LH lesion characterizations made from Nissl-stained sections. In experiment 2, to provide quantitative analyses of neuronal damage, ORX+ and MCH+ neurons were counted by experimenters blind to the training and lesion conditions, although lesion condition was usually obvious. Photomicrographs were taken from four LH planes that included the largest concentration of ORX+ and MCH+ neurons (plates 28–31; Swanson, 1998), using an Olympus BH2 microscope with a 10 × objective and a MicroPublisher RTV camera (Q-Imaging, Burnaby, B.C., Canada). For each plane, the images from six of these photos in each hemisphere, encompassing the entire LH region, were merged in Photoshop to form four large-scale but high-resolution images for each hemisphere in each rat, on which a grid of counting squares was superimposed. Cells were counted using non-biased counting algorithms patterned after those implemented by commercial cell-counting software systems. We also counted FOS+ cells in experiment 2, using these same methods. Because the counts of single-labeled ORX+ and MCH+ cells were similar in single- and double-labeled (with FOS+) sections, we reported only the MCH+ and ORX+ counts from double-labeled sections in experiment 2. In experiment 3, we used these same methods to count ORX+ and MCH+ neurons in sections single-stained for orexin or MCH. The purpose of these counts was to correlate test behavior of rats in the shift condition (which revealed effects of surprise on learning, and which was affected by the LH lesions) with integrity of ORX+ and MCH+ neurons in LH. Counts were made only in brains of LH-lesioned rats in the shift training condition.
Analysis of FOS expression in CeA and LH
In experiment 2, FOS expression in CeA was analysed by an experimenter who was blind to the training and LH lesion conditions. Medial and lateral subnuclei of CeA were defined according to Swanson’s (1998) brain atlas, in four sections (plates 24, 26, 27 and 28). Images of the FOS-stained sections and the adjacent thionin-stained sections were acquired as described for LH sections. Borders of the medial and lateral CeA were then drawn on the images of FOS sections, guided by the thionin-stained sections, using Adobe Photoshop. Separate images were obtained for right and left hemisphere regions. Using an image analysis system (NIH Image 1.63), a threshold for background density was set for each medial and lateral CeA region on the FOS sections, and FOS+ cells with a density that was at least 2 SDs above the background threshold were counted using the software. Total counts were then divided by the area sampled to yield FOS counts/unit area.
Comparable methods were used to assess overall LH FOS from single-label FOS+ sections. However, the primary analysis of FOS expression in LH was based on sections stained for both orexin and FOS, and sections stained for both MCH and FOS. While using the unbiased stereological methods described previously for counting ORX+ and MCH+ neurons, we also counted ORX + FOS, MCH + FOS, and single-labeled FOS+ cells. Although we tabulated cell counts for lateral, medial and perifornical subregions of LH (Petrovich et al., 2012), we observed comparable effects in all three of these subregions, so we presented only total LH cell counts.
Data analysis
Cell counts and behavioral measures were analysed with mixed analyses of variance (ANOVAs), which applied the Greenhouse–Geisser correction to compensate for any violations of sphericity assumptions. Post hoc comparisons used Tukey’s honestly significant difference (HSD) procedure for unequal ns. A value of P < 0.05 was used as the criterion for statistical significance throughout.
Results
Experiment 1 lesion evaluation
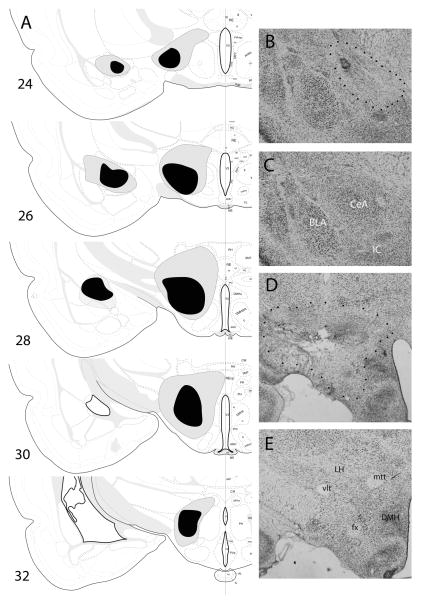
In experiment 1, three rats with unacceptable lesions were excluded, leaving seven rats with ipsilateral CeA–LH lesions and seven rats with contralateral lesions in Group P, and three rats with ipsilateral lesions and four rats with contralateral lesions in Group U. CeA lesions were generally discrete, with relatively defined borders, and uniform and nearly complete neuronal loss within those borders. The area within those borders encompassed 60.5 ± 8.9% of CeA in rats with ipsilateral lesions and 63.7 ± 9.9% of CeA among rats with contralateral lesions. LH damage was more variable in size, shape and uniformity of neuronal damage within the drawn lesion borders. The area within those borders comprised 77.9 ± 13.5% and 81.0 ± 14.0% of the target LH regions in those same groups of rats, respectively. Although most of the lesions were confined to LH, the largest accepted lesions included damage to small portions of the arcuate and dorsomedial hypothalamic nuclei, the zona incerta and subincertal nucleus, and portions of the ventromedial thalamus. The lesions were of comparable sizes in both training conditions (P > 0.810). Figure 1A–E shows drawings of CeA and LH lesions (based on Nissl-stained sections), and representative Nissl-stained sections of lesioned and unlesioned hemispheres.
Fig. 1.
(A) Drawings of the largest (gray) and smallest (black) accepted lesions of amygdala central nucleus (CeA) and lateral hypothalamus (LH), based on Nissl-stained sections, in experiment 1. All lesions are shown in the same hemisphere, regardless of whether they were made in the right or left hemisphere, and whether the CeA and LH lesions were made ipsilaterally or contralaterally. (B–E) Sample Nissl-stained sections of lesioned (B and D; lesions indicated by dotted borders) and unlesioned (C and E) CeA (B and C) and LH (D and E). The sections all come from a single rat, which received contralateral lesions of CeA and LH. Images in (C and D) were reversed left-to-right to portray all sections in a common orientation, matching (A). BLA, basolateral amygdala; DMH, dorsomedial hypothalamus; fx, fornix; IC, intercalated nuclei; mtt, mammillothalamic tract; vlt, ventrolateral hypothalamic tract. Drawings of brain sections in (A) are from Swanson (1998); used by permission of Elsevier.
Qualitative examination of orexin−, MCH−, and orexin + MCH-stained sections suggested that the orexin-saporin lesions destroyed a larger proportion of ORX+ neurons than MCH+ neurons, but also a large proportion of the neurons that were neither ORX+ nor MCH+. This relative lack of specificity of the lesions is not surprising because many neuron types within LH express orexin 2 receptors, to which the orexin-saporin binds (Backberg et al., 2002; Burdakov et al., 2003). In addition, we observed very few cells that double-labeled for both MCH and orexin, consistent with the distinctiveness of these two cell types and manufacturer’s reports of low cross-labeling for the antibodies used here. In experiment 2, we evaluated loss of these neuronal subtypes quantitatively.
Experiment 1 behavior
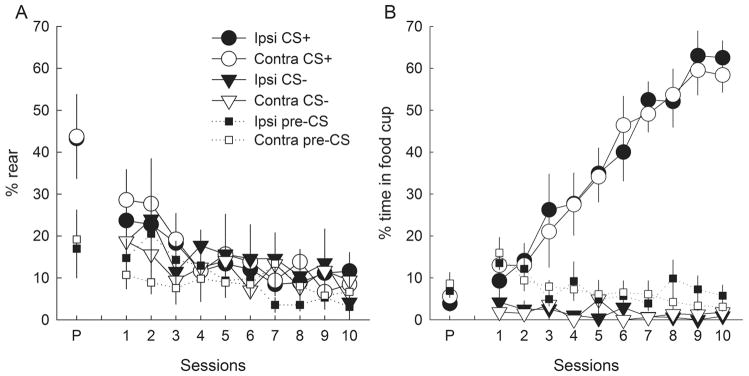
The performance of rats with contralateral CeA–LH lesions did not differ from that of rats with ipsilateral control lesions of those two regions, for either ORs (Fig. 2A) or CRs (Fig. 2B). However, unlike normal rats or rats with unilateral CeA lesions in previous experiments (see also sham-lesioned rats in experiment 2; Fig. 5), there was no evidence for acquisition of conditioned ORs in either lesion condition. Instead, after moderate (and typical) levels of unconditioned ORs in the pre-test, ORs declined over the course of training, and were no greater in rats for which the light was reinforced than in rats for which the light was non-reinforced. A lesion (contralateral or ipsilateral) × treatment (P or U) × session ANOVA of ORs showed a significant (declining) effect of session (F9,153 = 4.10, P < 0.001), but no other significant main effect or interaction (P > 0.617). By contrast, in both lesion conditions, the rats’ acquisition of conditioned food cup behavior closely resembled that of normal rats or CeA-lesioned rats in previous experiments; the effects of treatment (F1,17 = 85.87, P < 0.001), session (F9,153 = 19.58, P < 0.001) and their interaction (F9,153 = 23.60, P < 0.001) were all significant, whereas neither lesion nor any of its interactions was significant (P > 0.866). Pre-CS (baseline) behavior decreased over sessions, but was unaffected by the lesions. ANOVAs showed significant effects of sessions (F9,153 > 2.39, P < 0.015), but no other significant main effects or interactions (P > 0.227).
Fig. 2.
Performance (mean ± SEM) of (A) orienting responses (ORs; rear) and (B) food cup entry responses during the pre-test (P) and conditioning (1–10) sessions of experiment 1. Contra (open symbols) and Ipsi (filled symbols) refer to rats with contralateral or ipsilateral placement of unilateral lesions of the amygdala central nucleus (CeA) and lateral hypothalamus (LH). CS+ (circles) refers to responding during the light conditioned stimulus that was paired with food in group P (paired), and CS− (triangles) refers to responding during the light that was not paired with food in group U (unpaired). Pre-CS (squares) refers to responding prior to CS presentations. The rats failed to acquire conditioned ORs, but rapidly acquired conditioned food cup responses.
Fig. 5.
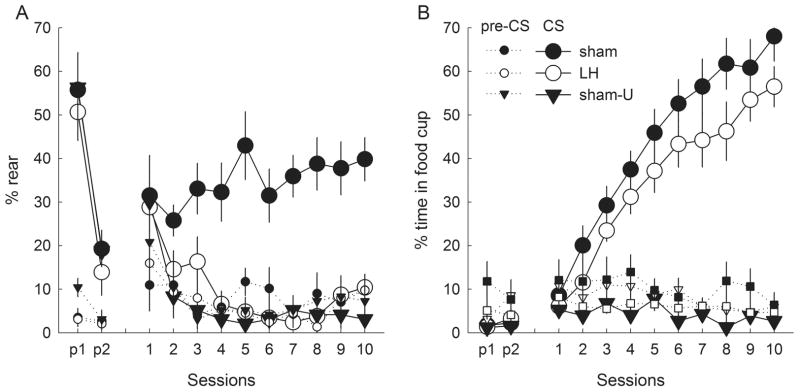
Performance (mean ± SEM) of (A) orienting responses (ORs; rear) and (B) food cup entry responses during the first and second halves of the pre-test session (p1 and p2, respectively) and the 10 conditioning sessions of experiment 2. Rats received either unilateral orexin-saporin lesions of lateral hypothalamus (LH; open symbols) or sham lesions of that region (filled symbols). The rats in groups sham and LH received light–food pairings, whereas the light was unpaired with food in group sham-U. Responding during conditioned stimulus (CS) presentations (solid lines) and prior to CS presentations (pre-CS, dotted lines) are shown separately. Rats with lesions of LH failed to acquire conditioned ORs, but were unimpaired in their acquisition of food cup responses and the initial performance of unconditioned ORs.
Thus, the combination of a unilateral CeA lesion with a unilateral LH lesion, regardless of whether the lesions were ipsilateral or contralateral to each other, appeared to prevent acquisition of conditioned ORs. Because in previous experiments, unilateral CeA lesions alone had no effect on conditioned ORs, one would expect contralateral CeA–LH lesions to have greater effects than ipsilateral lesions, if CeA and LH acted in a serial circuit. The simplest account for these results is that unilateral LH lesions alone prevent the acquisition or performance of conditioned ORs. Experiment 2 was designed to provide clear evidence of this impaired conditioned orienting with appropriate controls, and to determine if unilateral LH lesions disrupt CeA processing. We compared the performance of rats with unilateral orexin-saporin lesions of LH with that of rats with sham lesions of LH, and by examined FOS expression in CeA and various neuron types in LH, induced by test presentations of the visual stimulus alone.
Experiment 2 lesion evaluation
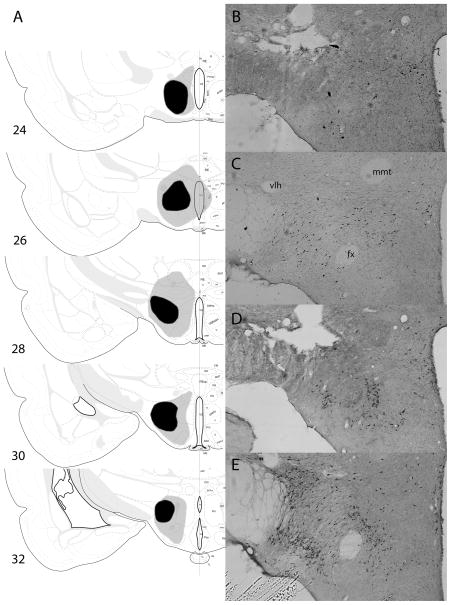
In experiment 2, three rats with unacceptable lesions were excluded, leaving 12 rats with sham lesions (group sham) and nine rats with unilateral LH lesions (group LH) trained with light–food pairings, and six sham-lesioned rats (group sham-U) and two rats with unilateral LH lesions trained with the light and food unpaired. Because of the small number of lesioned rats in the unpaired control condition, those rats’ data were not included in our analyses. However, their data was well within the distribution of rats in group sham-U. Damage to LH in the lesioned rats, based on drawings of the lesion borders from Nissl-stained sections (Fig. 3A), was 84.5 ± 9.6% on the lesioned side and 2.9 ± 3.2% on the unlesioned side (the lesion damage occasionally extended across the midline).
Fig. 3.
(A) Drawings of the largest (gray) and smallest (black) accepted lesions of lateral hypothalamus (LH), based on Nissl-stained sections, in experiment 2. All lesions are shown as if they were made in the same hemisphere. (B–E) Sample lesioned (B and D) and unlesioned (C and E) sections, stained for orexin (B and C) or melanin-concentrating hormone (MCH; D and E). The images all came from the same rat, with those shown in (C and E) reversed left-to-right to portray all sections in the same orientation, matching (A). The images in (B and C) are from the same section, and the images in (D and E) are from the same section, adjacent to the section displayed in (B and D). fx, fornix; mmt, mammillothalamic tract; vlt, ventrolateral hypothalamic tract. Drawings of brain sections in (A) are from Swanson (1998); used by permission of Elsevier.
As in experiment 1, the LH lesions destroyed many neurons that were neither ORX+ nor MCH+. Lesion-produced damage to orexin and MCH neurons specifically was evaluated by counting ORX+ and MCH+ neurons in ORX + FOS and MCH + FOS double-labeled sections, respectively. Figure 3B–E shows representative sham- and lesioned sections stained for orexin or MCH, and Fig. 4A and B shows the results of the counting procedures. The simplest description of these results is that the orexin-saporin lesions selectively reduced the numbers of ORX+ cells compared with MCH+ cells. Rats with unilateral lesions showed significantly lower ORX+ cell counts in the lesioned hemisphere than in the unlesioned hemisphere or in sham-lesioned rats. By contrast, MCH+ cell counts in the lesioned hemisphere were not significantly lower than MCH+ cell counts in the sham-lesioned rats. This relatively selective sparing of MCH+ cells contrasts with Ocampo-Garces et al.’s (2011) observation of a significant positive correlation between losses in numbers of ORX+ and MCH+ neurons after orexin-saporin lesions. Our observation of greater sparing of MCH+ neurons reflected both sparing of zona incerta, which includes a large subpopulation of MCH neurons but few if any orexin neurons (Swanson et al., 2005), and greater sparing of MCH neurons in perifornical regions in which large numbers of both MCH and orexin neurons are found. Surprisingly, lesioned rats showed significantly ‘greater’ numbers of MCH+ cells in the hemisphere contralateral to the orexin-saporin lesion than sham-lesioned rats.
Fig. 4.
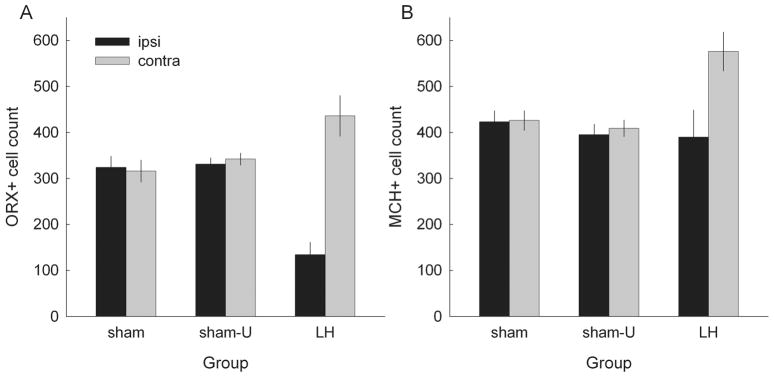
Mean ± SEM counts of orexin-positive (A) and melanin-concentrating hormone (MCH)-positive (B) cells in experiment 2, in rats with orexin-saporin lesions of lateral hypothalamus (LH) or sham lesions of that region. Rats in groups sham and LH received light–food pairings, and rats in group sham-U received those stimuli unpaired. Bars labeled Ipsi refer to counts in the lesioned or sham-lesioned hemisphere, and bars labeled Contra refer to counts in the undisturbed hemisphere.
Separate group (LH, sham or sham-U) × hemisphere ANOVAs of ORX+ and MCH+ cell counts each showed significant main effects of hemisphere (F1,23 > 22.57, P < 0.001) and group × hemisphere interactions (F2,23 > 18.58, P < 0.001). Post hoc Tukey HSD comparisons showed that there were significantly fewer ORX+ cells in the lesioned hemisphere of rats in group LH than in their non-lesioned hemisphere or in either hemisphere of rats in either Groups sham or sham-U (P < 0.001). The apparently greater numbers of ORX+ neurons contralateral to the lesion than in sham-lesioned rats was only of marginal significance (P > 0.063). For MCH+ cells, the lesions produced no significant damage compared with sham-lesioned rats (P > 0.746). However, counts were significantly greater in the hemisphere contralateral to the lesion than in either the lesioned hemisphere or in the sham-lesioned rats (P < 0.001), suggesting a detectable level of compensatory plasticity (e.g. in MCH immunoresponsivity) after contralateral LH damage. Finally, a post hoc contrast within an additional ANOVA that included both ORX+ and MCH+ cell counts showed that the difference between cell counts in the lesioned and unlesioned hemispheres was significantly greater for ORX+ cells than for MCH+ cells (P = 0.029).
To more fully characterize the nature of LH damage, we also examined the correlations among the numbers of remaining ORX+ cells, MCH+ cells, and overall sparing in the lesioned LH (as evaluated from Nissl sections, and calculated as 100 minus the % damage). The correlation between the number of remaining ORX+ cells and overall sparing was r = 0.65 (P = 0.042), whereas the correlation between the number of remaining MCH+ cells and overall sparing was r = 0.23 (P = 0.523). Finally, the correlation between the numbers of remaining ORX+ and MCH+ cells was r = 0.36 (P = 0.303). Thus, these lesions are more conservatively described as lesions of LH rather than exclusively of orexin neurons, because the loss of ORX+ neurons was correlated with the loss of other cell types (but not MCH+ neurons) within LH.
Experiment 2 behavior
As in experiment 1, rats with unilateral LH lesions showed no evidence for the acquisition of conditioned ORs (Fig. 5A), despite normal unconditioned ORs in the pre-test and early conditioning sessions, and normal acquisition of conditioned food cup behavior (Fig. 5B). A group × session ANOVA of ORs over the 10 conditioning sessions showed significant main effects of group (F2,24 = 24.55, P < 0.001), session (F9,216 = 3.52, P < 0.001) and their interaction (F18,216 = 2.67, P < 0.001). Critically, a post hoc HSD test showed that ORs were significantly more frequent in group sham than in either group sham-U (P < 0.001), showing that the ORs depended on light–food pairing, or group LH (P < 0.001), showing that the ORs were impaired by LH lesions. Groups LH and sham-U did not differ (P = 0.843). However, for ORs in the light pre-test session, a group × block (first or second half of the pre-test session) ANOVA showed only a significant effect of block (F1,24 = 53.20, P < 0.001); other P > 0.709. ANOVA of food cup CRs during the conditioning phase showed significant effects of group (F2,24 = 30.51, P < 0.001), session (F9,216 = 29.01, P < 0.001) and their interaction (F18,216 = 7.55, P < 0.001). A post hoc HSD test showed that food cup CRs were less frequent in group sham-U than in either group LH or sham (P < 0.001), which did not differ significantly from each other (P = 0.188). As in experiment 1, pre-CS behavior during conditioning did not differ across groups: ANOVAs showed no effects of group or group × session interactions (P > 0.523).
The normal levels of conditioned food cup behavior and unconditioned ORs observed in LH-lesioned rats indicated that the effect of the lesions on conditioned ORs did not reflect deficits in motivation, or ability to perform the OR. Furthermore, the LH lesion did not affect the general activity of the rats in the absence of the visual CS. A group × session ANOVA of baseline activity counts from the infrared activity monitor (not shown) showed no significant effect of group or group × session interactions (P > 0.403). Thus, the effects of the LH lesion were confined to the acquisition of conditioned ORs.
Behavior in the FOS test (Table 2) was similar to that at the end of training. One-way (groups) ANOVAs followed by HSD tests showed that ORs were more frequent during the light CS in group sham than in either group sham-U or group LH (P < 0.001), whereas food cup CRs were more frequent (P < 0.015) in both groups sham and LH (which did not differ, P = 0.875) than in group sham-U. Baseline activity did not differ across groups (F2,24 = 0.059, P = 0.943).
Table 2.
Test performance in experiment 2
| Rear OR | Food cup CR | Activity | |||
|---|---|---|---|---|---|
| Group | Pre-light | During light | Pre-light | During light | Pre-light |
| Sham (12) | 10.7 ± 1.7 | 45.3 ± 3.0 | 7.9 ± 3.1 | 38.9 ± 6.7 | 42.6 ± 9.3 |
| LH (9) | 9.0 ± 1.8 | 11.1 ± 2.9 | 4.7 ± 3.2 | 34.5 ± 6.2 | 38.0 ± 11.2 |
| Sham-U (6) | 6.3 ± 2.3 | 7.3 ± 4.1 | 5.0 ± 2.8 | 1.0 ± 0.6 | 41.0 ± 7.2 |
CR, food cup conditioned response; LH, unilateral lesion of lateral hypothalamus; OR, rear orienting response.
The numbers in parentheses are the number of rats tested in each condition. Group entries (mean ± SEM) for rear ORs are percentage behavior, for food CRs, percentage time in the food cup, and for activity, counts per min.
Finally, we intended to relate the levels of rearing to the counts of ORX+ and MCH+ neurons in group LH. However, because in most lesioned rats the number of conditioned ORs was zero or near zero, there was insufficient variance in the amount of rearing to permit calculating correlation coefficients.
Experiment 2 FOS expression in CeA
The major purpose of experiment 2 was to relate unilateral LH lesion-produced deficits in ORs to brain activity in CeA and different neuronal populations in LH. As expected, in sham-lesioned rats, FOS expression in CeA (Fig. 6) was greater in response to test presentations of the previously-reinforced light stimulus (CS+, group sham) than in response to the previously-non-reinforced light (CS−, group sham-U). More important, FOS expression after CS+ testing was greater in rats with sham lesions of LH than in rats with unilateral LH lesions. Remarkably, that reduction in CeA FOS was equivalent in the hemispheres ipsilateral and contralateral to the LH lesion. Furthermore, the effects of the LH lesions on FOS expression after CS+ presentation were comparable in lateral and medial subregions of CeA (mean ± SEM counts/area for medial CeA were 22.24 ± 2.03 for shams and 13.54 ± 1.68 for lesioned rats, and for lateral CeA were 25.03 ± 2.78 for shams and 12.94 ± 1.41 for lesioned rats). Notably, although some investigators have found orexin innervation of CeA chiefly to its lateral subdivision (Ciriello et al., 2003; Yoshida et al., 2006), others have described greater LH innervation of medial CeA (Fadel & Deutch, 2002), and Baldo et al. (2003) characterized this innervation as centered in lateral CeA, on its border with medial CeA.
Fig. 6.
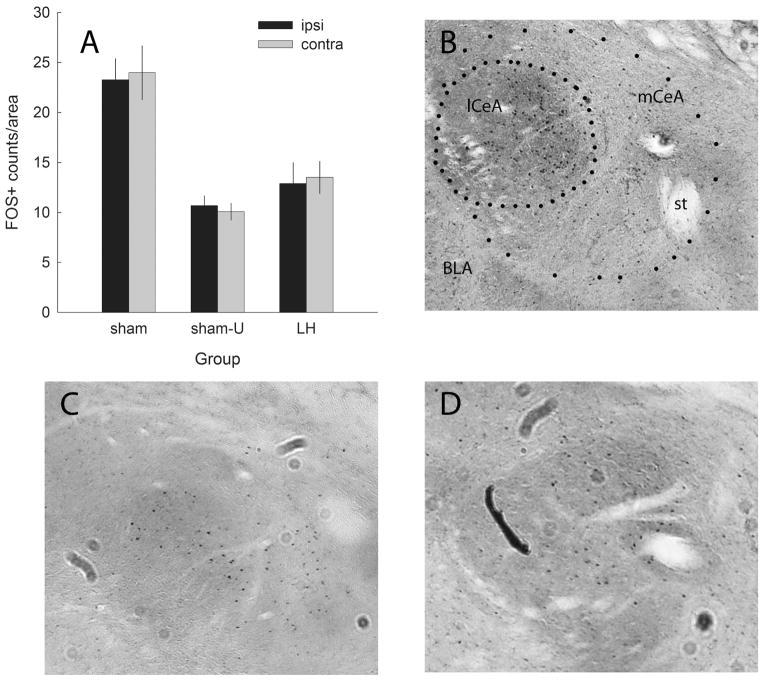
FOS expression in amygdala central nucleus (CeA) after presentations of the light conditioned stimulus (CS) in the test session of experiment 2. (A) Mean ± SEM counts of FOS+ cells (adjusted for area) in the hemispheres ipsilateral (black bars) and contralateral (gray bars) to the lateral hypothalamic (LH) orexin-saporin or sham lesion. For groups sham (n = 12) and LH (n = 9), the light had been previously paired with food; and for group sham-U (n = 6), the light had been unpaired with food. (B–D) Sample FOS-stained sections of a sham-lesioned rat tested with CS+, a LH-lesioned rat tested with CS+, and a sham-lesioned rat tested with CS−. Approximate areas of lateral (lCeA) and medial (mCeA) CeA are enclosed in dotted lines. BLA, basolateral amygdala; st, stria terminalis.
These assertions were supported by a group × hemisphere (ipsilateral or contralateral to the LH lesion) × subregion (medial or lateral) ANOVA of the FOS counts/area. Only the main effect of group was significant (F2,23 = 17.27, P < 0.001; other P > 0.499). An HSD test showed that for each CeA subregion and for CeA as a whole, FOS counts/area were significantly greater in group sham than in either group sham-U or group LH (P < 0.012), which did not differ from each other (P > 0.893).
Finally, in group LH, overall CeA FOS expression was significantly positively correlated with the number of remaining ORX+ neurons in the lesioned hemisphere (r = 0.70, P = 0.036), but not with the number of remaining MCH+ neurons (r = 0.08, P = 0.830).
Experiment 2 FOS expression in LH
FOS expression in LH was assessed from ORX + FOS and MCH + FOS double-labeled sections (see Materials and methods). Figure 7 shows representative sections, and Fig. 8 shows cell counts. Although unilateral orexin-saporin lesions of LH did not significantly reduce the number of MCH+ neurons, they produced substantial unilateral loss of both ORX+ neurons and neurons that were neither ORX+ nor MCH+. These losses were accompanied by losses in LH FOS expression in response to presentations of the light CS+. Furthermore, in lesioned rats, surviving ORX+ neurons were less likely to express FOS than ORX+ neurons in sham-lesioned rats.
Fig. 7.
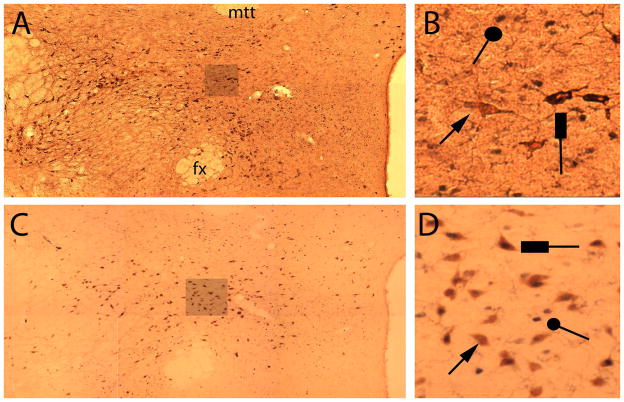
Sample photomicrographs of the hypothalamic region (approximately plate 30; Swanson, 1998) in one, unlesioned hemisphere, showing double-labeling for FOS+ and ORX+ (A and B) or MCH+ (C and D) cells in experiment 2. (A and C) each show montages of six individual high-power images from the same section (one of four from which counts were taken for that hemisphere for that rat); and (B and D) show enlarged segments from those montages, on which example cells staining positive for ORX- or MCH-only (arrows), FOS-only (circles), or both FOS and ORX or MCH (rectangles) are indicated. fx, fornix; mtt, mammillothalamic tract.
Fig. 8.
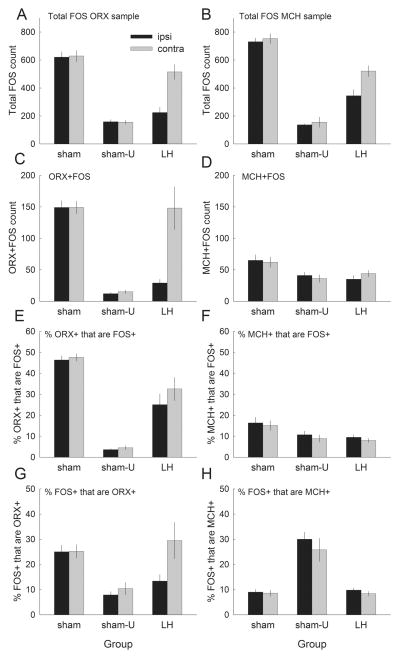
Summary hypothalamic cell counts in experiment 2. (A and B) Counts of cells in the lateral hypothalamus (LH) that stained positive for FOS (either single-labeled or double-labeled) from sections also stained for ORX or MCH, respectively. (C and D) Counts of cells that double-labeled for FOS and either ORX or MCH, respectively. (E) The percentages of all cells that labeled for ORX that also labeled for FOS; (F) the percentages of all cells that labeled for MCH that also labeled for FOS. (G and H) The percentages of all FOS-positive cells that also labeled for ORX or MCH, respectively. The bars indicate mean ± SEM counts in sham-lesioned rats that were tested with a light previously paired with food (group sham, n = 12) or with a light previously unpaired with food (group sham-U, n = 6), or in rats with unilateral orexin-saporin lesions of LH (group LH, n = 8). The black bars (Ipsi) show counts in the hemisphere in which the orexin-saporin or sham lesion of LH was made; and gray bars (Contra) show counts in the other hemisphere.
FOS+ cell counts were subjected to group × hemisphere ANOVAs, followed by post hoc HSD tests. Greater cell counts after exposure to the reinforced light (group sham) than to the non-reinforced light (group sham-U) revealed the effects of conditioning on FOS expression, and lower counts in the lesioned hemisphere in group LH than in the unlesioned hemisphere, or in group sham, indicated lesion efficacy.
Figure 8A and B shows total FOS+ cell counts, which were higher after CS+ than after CS− presentations, and were lower in lesioned rats. For both ORX + FOS (left panels) and MCH + FOS (right panels) samples, the effects of group (F2,23 > 45.83, P < 0.001), hemisphere (F1,23 > 10.49, P < 0.004) and their interaction (F2,23 > 5.47, P < 0.012) were all significant. FOS+ counts were significantly greater in group sham than in group sham-U (P < 0.001). For both samples, in group LH there were significantly fewer FOS+ cells on the lesioned side than on the non-lesioned side (P < 0.001), and on either side in group sham (P < 0.001). In the MCH + FOS sample, FOS+ cell counts in group LH were also lower in the contralateral hemisphere than in either hemisphere of rats in group sham (P < 0.001).
Figure 8C and D shows counts for double-labeled ORX + FOS and MCH + FOS cells, respectively. ANOVAs of ORX + FOS and MCH + FOS cell counts both showed significant main effects of group (F2,23 > 3.80, P < 0.033). ORX + FOS cell counts also showed significant effects of hemisphere (F1,23 = 10.75, P = 0.003) and group × hemisphere interaction (F2,23 = 10.51, P < .001). The numbers of both ORX + FOS and MCH + FOS cells were greater in group sham than in group sham-U (P < 0.001), indicating that FOS expression in both of these neuron types was enhanced by prior conditioning of the light CS. Similarly, the lesion reduced the numbers of both ORX + FOS and MCH + FOS+ neurons relative to the numbers in group sham (P < 0.001). However, although the number of ORX + FOS cells was significantly lower in the lesioned than in the unlesioned hemisphere in group LH (P < 0.001), that difference was of only marginal significance (P = 0.077) for MCH + FOS cells.
It is notable that in group sham, there were substantially more ORX + FOS cells than MCH + FOS cells, despite the overall greater numbers of MCH+ than ORX+ cells (Fig. 4). To compensate for the different numbers of ORX+ and MCH+ cells overall, and in the hemispheres of lesioned and non-lesioned rats, we calculated the percentage of ORX+ or MCH+ cells that were also FOS+ (Fig. 8E and F, respectively). A group × hemisphere × cell type (ORX+ or MCH+) ANOVA of these percentages showed significant effects only of group (F2,23 = 53.07, P < 0.001) and cell type (the double-labeling percentages were higher for ORX+ cells, F1,23 = 68.76, P < 0.001), and their interaction (F2,23 = 34.00, P < 0.001). Significantly greater percentages of ORX+ neurons also labeled for FOS in group sham than in group LH, and both of those groups showed more double-labeling than in group sham-U (P < 0.001). Thus, in group LH, surviving ORX+ neurons were less likely to express FOS after CS+ exposure than ORX+ neurons in sham-lesioned rats. This result may reflect impaired function in surviving ORX+ neurons, or loss of input from elements of networks within which ORX+ neurons participate, including other ORX+ neurons.
By contrast, the percentages of MCH+ neurons that also labeled for FOS did not differ among the groups (P > 0.165).
Figure 8G and H shows the percentages of FOS+ cells that were also ORX+ or MCH+, respectively. For ORX+, the effects of group (F2,23 = 6.78, P = 0.005), hemisphere (F1,23 = 5.76, P = 0.025) and their interaction (F2,14 = 3.91, P = 0.035) were all significant. These percentages were greater in group sham and the unlesioned hemisphere of group LH than in group sham-U (P < 0.036). By contrast, for MCH+, ANOVA revealed only a significant effect of group (F2,23 = 38.31, P < 0.001), and the percentage of FOS+ neurons that were MCH+ was significantly higher in group sham-U than that in the other two groups (P < 0.001), which did not differ (P > 0.967).
Finally, we estimated the number of FOS+ cells that were neither MCH+ nor ORX+ (‘FOS-only’), by subtracting the number of MCH + FOS and ORX + FOS cells from the total FOS+ counts in both ORX+ and MCH+ samples. (Single-labeled FOS+ cells in ORX + FOS samples would include MCH+ cells, and single-labeled FOS+ cells in MCH + FOS samples would include ORX+ cells.) Among lesioned rats, we found 252 ± 34 FOS-only cells in the lesioned hemisphere and 421 ± 38 on the contralateral side. Among rats in group sham, we found 571 ± 26 and 585 ± 33 FOS-only cells in the two sides; and in group sham-U, 121 ± 9 and 130 ± 20 cells. ANOVA showed significant effects of group (F2,23 = 69.73, P < 0.001), hemisphere (F1,23 = 10.50, P = 0.003) and their interaction (F2,23 = 7.46, P = 0.004). A Tukey HSD test showed higher counts in each of the hemispheres of the rats in group sham than in either group LH or group sham-U (P < 0.003), and higher counts in the unlesioned hemisphere of rats in group LH than in their lesioned hemisphere or in group sham-U (P < 0.001).
Summary of experiment 2 lesion, FOS expression and behavior relations
Unilateral orexin-saporin lesions of LH had little effect on MCH neurons, but unilaterally reduced the numbers of orexin neurons and their expression of FOS in response to a previously-reinforced light CS+. Most important, the unilateral orexin-saporin lesions both prevented the acquisition of conditioned ORs to the reinforced light, and substantially (and equivalently) reduced FOS expression in response to the reinforced light in both hemispheres of CeA. Thus, a unilateral lesion of LH impaired processing of a visual CS+ equally in both hemispheres of CeA.
Lesion evaluation in experiment 3
In experiment 3, 13 rats with unacceptable lesions of CeA, LH or both regions were excluded, leaving 16 (eight shift, eight consistent) rats with contralateral LH/CeA lesions, 22 (10 shift, 12 consistent) rats with unilateral LH-only lesions, 13 rats (seven shift, six consistent) with unilateral CeA-only lesions, and 12 rats (seven shift, five consistent) with sham lesions of both regions. The CeA and LH lesions were both similar to those in the previous experiments, with the average CeA damage 61.5 ± 5.9% and average LH damage 77.2 ± 3.3%. Neither LH nor CeA damage differed as a function of behavioral treatment group (P > 0.651). Rats with sham lesions showed no evidence of damage, except along the injector tracks. Figure 9A shows drawings of CeA and LH lesions, based on Nissl-stained sections.
Fig. 9.
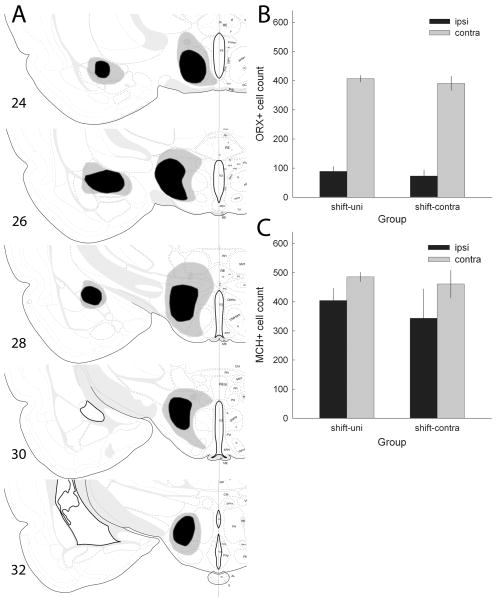
(A) Drawings of the largest (gray) and smallest (black) accepted lesions of amygdala central nucleus (CeA) and lateral hypothalamus (LH), based on Nissl-stained sections, in experiment 3. All lesions are shown in the same hemisphere, regardless of whether they were made in the right or left hemisphere. (B and C) Mean ± SEM counts of ORX+ and MCH+, respectively, cells in the hypothalamus of rats that were trained in the shift condition (Table 1). The rats in the shift-Contra group received an orexin-saporin lesion of the LH in one hemisphere and an ibotenic acid lesion of the CeA in the other, whereas rats in the shift-Uni group received only a unilateral LH lesion. The black bars (Ipsi) refer to counts in the lesioned hemisphere and the gray bars (Contra) refer to counts in the undisturbed hemisphere. Drawings of brain sections in (A) are from Swanson (1998); used by permission of Elsevier.
As noted in the Materials and methods, we also counted ORX+ and MCH+ neurons in the brains of rats in the shift-Uni and shift-Contra groups, to examine the correlation between expected behavioral deficits in those groups and neuron loss. Figure 9B and C shows the numbers of ORX+ and MCH+ neurons, respectively, in the lesioned and unlesioned hemispheres of those rats. As in experiment 2, there were fewer of both neuron types in the lesioned hemisphere, but the loss was greater for ORX+ than for MCH+ cells. Group (shift-Uni LH or shift-Contra) × lesion (lesioned or unlesioned hemisphere) ANOVAs for each of ORX+ and MCH+ cells showed significant main effects of lesion (F1,16 > 10.75, P < 0.005), but no main effects of group or group × lesion interactions (P > 0.453) for either cell type. An additional ANOVA that included both MCH+ and ORX+ neuron counts showed a significant main effect of neuron type (F1,16 = 29.88, P < 0.001), and a significant neuron type × lesion interaction (F1,16 = 34.91, P < 0.001), confirming the larger number of MCH+ than ORX+ neurons and the greater loss of ORX+ than MCH+ cells on the lesioned side.
In the lesioned hemispheres of these rats, the number of ORX+ neurons (r = 0.54, P = 0.019) and to some extent the number of MCH+ neurons (r = 0.47, P = 0.051) were correlated with the total sparing (100 minus % damage estimated from Nissl-stained sections) of LH neurons, which included many neurons that were neither ORX+ nor MCH+. The numbers of ORX+ and MCH+ neurons in the lesioned hemispheres of these brains were not significantly correlated (r = 0.23, P = 0.364).
Experiment 3 behavior
In the expectancy phase, all rats acquired considerable conditioned food cup responding to the tone, and showed little food cup responding to the light. By the last two sessions of that phase, responding in the pre-CS period averaged 12.9 ± 2.1%, responding to the light averaged 15.7 ± 1.2%, and responding to the tone averaged 68.7 ± 4.3%. Separate behavioral training condition (shift vs consistent) × lesion ANOVAs performed on the data from each of these measurement epochs showed no significant effects or interactions (P > 0.214). Thus, rats in all groups entered the surprise phase with similar levels of responding. In the surprise phase, responding was maintained at similar levels (13.6 ± 2.4%, 16.4 ± 2.1% and 65.0 ± 5.7%). Performance during the light CS on the final surprise session is shown in the left-most points in Fig. 10A and B. ANOVAs identical to those conducted on the expectancy phase data showed no significant effects or interactions (P > 0.320).
Fig. 10.
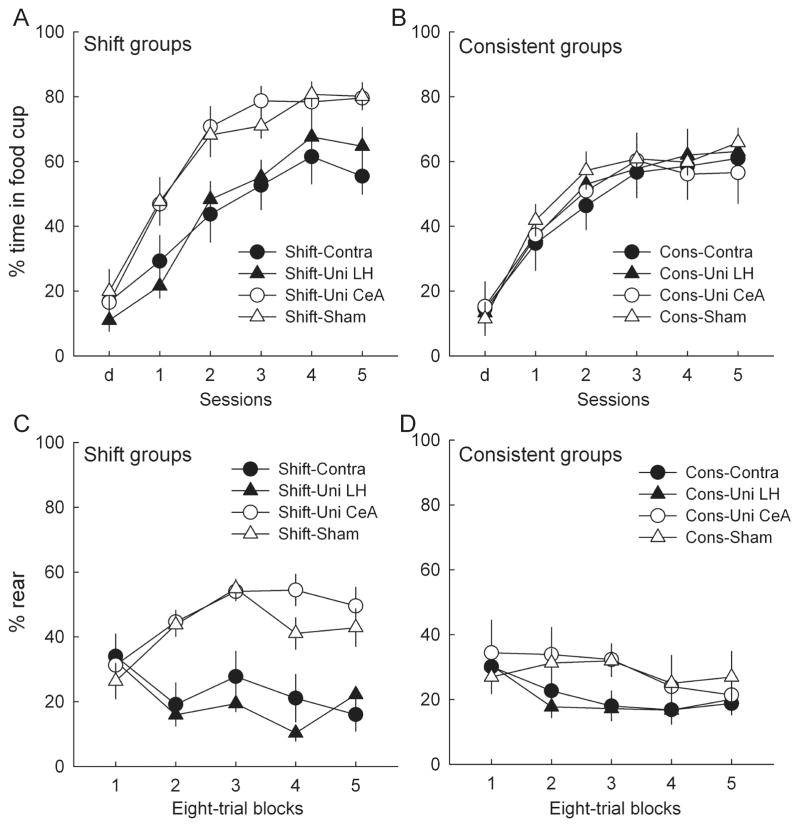
Acquisition performance in the surprise and test phases of experiment 3. Surprise-induced enhancements in associability are indicated by more learning of a light–food association in rats trained in the phase 2 shift condition (A and C; Table 1) than in rats trained in the phase 2 consistent condition (B and D). (A and B) Food cup responding to the light conditioned stimulus (CS) on the second of two surprise sessions (labeled d) and the five test sessions. Of the rats trained in the shift condition (A), those with unilateral lesions of the amygdala central nucleus (Uni-CeA) or with sham lesions showed more responding in the test sessions than rats with orexin-saporin lesions of the lateral hypothalamus (LH), either alone (Uni-LH) or contralateral (Contra) to a unilateral CeA lesion, and more responding than any rats in the consistent condition (B). LH-lesioned rats in the consistent condition were unimpaired relative to rats without LH lesions (B). (C and D) Conditioned orienting (rear) responding in the test sessions. As with food cup responding, rats without LH lesions showed more learning after the shift treatment (C) than after the consistent treatment (D). As in experiments 1 and 2, rats with LH lesions failed to show acquisition of conditioned orienting responses (ORs) to the light paired with food in the test sessions, regardless of training condition. Entries are mean ± SEM.
Figure 10A and B shows the primary data of experiment 3, the acquisition of food cup responding to the light during the test phase. We hypothesized that unilateral lesions of LH would disrupt performance in the shift groups, by eliminating the enhancements of the light’s associability usually found after surprise phase omission of the tone. By contrast, we expected no such differences among consistent groups, none of which would have benefited from increased associability of the light CS, because in those groups the previously-established light→tone expectancies were maintained throughout the surprise phase. Consistent with those predictions, a lesion × session ANOVA of test performance among the shift groups showed a significant main effect of lesion (F3,28 = 3.71, P = 0.023), and planned comparisons showed significantly greater responding in each of groups sham and Uni-CeA than in each of groups Uni-LH and Contra (P < 0.031). By contrast, a comparable ANOVA of responding in the consistent groups showed the main effect of lesion and each of the individual comparisons described for the shift groups to be non-significant (P > 0.631). Finally, a separate contrast of performance in the shift groups with that in the consistent groups showed that responding was significantly greater in the shift groups for rats in groups sham and Uni-CeA (F1,55 = 4.29, P = 0.043), but not in groups Uni-LH and Contra (F1,55 = 0.03, P = 0.854). ANOVA of pre-CS food cup responding showed only an effect of session (responding declined from 18.1 ± 1.5% to 12.4 ± 1.5% over the five test sessions: F4,220 = 7.56, P < 0.001) and a significant lesion × session interaction (F12,220 = 2.25, P = 0.011). Except for apparently higher levels of pre-CS responding early in testing in sham-lesioned rats, the variation among lesion groups over sessions did not appear to be systematic.
Figure 10C and D shows the performance of rear ORs to the light in the test phase. All groups showed comparable rearing in the first block of trials, likely reflecting a reemergence of the unconditioned OR often observed when training contingencies are altered (Kaye & Pearce, 1984). Consistent with the results of experiments 1 and 2, rats with unilateral lesions of LH failed to acquire conditioned ORs. Contrasts of responding in those groups with responding in the rats without LH lesions (groups sham and Uni-CeA) showed a lesion deficit in both the shift condition (P < 0.001) and the consistent condition (P = 0.019). Nevertheless, consistent with the food cup measure shown in Fig. 10A and B, rats in groups sham and Uni-CeA showed more rearing in the shift condition than in the consistent condition (P < 0.006), whereas rats in groups Uni-LH and Contra did not (P > 0.699).
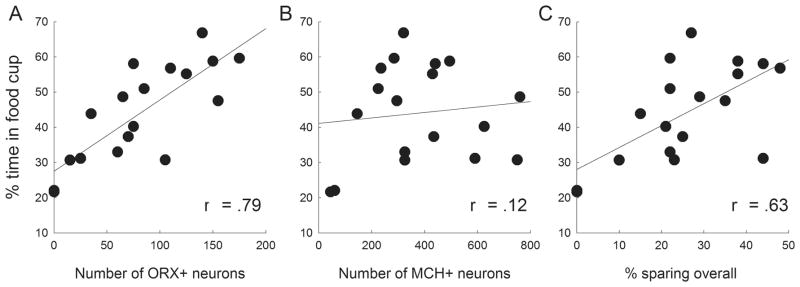
Finally, Fig. 11 shows the relations between mean food cup responding in the test phase and various measures of damage among LH-lesioned rats in the shift condition. There was a significant positive correlation between the number of ORX+ neurons spared by the LH lesions and food cup responding in the test phase (Fig. 11A; r = 0.79, P < 0.001), but not between the number of spared MCH+ neurons and food cup responding (Fig. 11B; r = 0.12, P = 0.641). Furthermore, these correlations differed significantly from each other (Meng et al., 1992; z = 2.60, P = 0.005), suggesting a role for ORX+, but not MCH+, neurons in the surprise-induced enhancement of associability. Nevertheless, test responding was also correlated with the amount of LH tissue sparing overall (Fig. 11C; r = 0.63, P = 0.005), as might be expected given that sparing of ORX+ neurons was correlated with sparing of all LH neurons.
Fig. 11.
Correlations between learned food cup responding in test and lesion damage in the lesioned rats in the shift condition of experiment 3. Food cup responding was significantly positively correlated with the number of remaining ORX+ neurons (A) and overall sparing of lateral hypothalamic (LH) tissue (C), but not with the number of remaining MCH+ neurons (B).
Discussion
Our results suggest a role for LH, and especially orexin neurons, in modulating two amygdala-dependent behaviors that are often attributed to learned attentional processes: the acquisition of conditioned ORs and the surprise-induced enhancement of cue associability. The LH lesion effects we observed are unlikely to be related to motor consequences of orexin-saporin LH lesions that many investigators have described (Grossberg et al., 2011; Zhang et al., 2011; Olarte-Sanchez, 2012), or the general disruption of learning, feeding, motivation and even survival often found with widespread, bilateral damage to LH (Teitelbaum & Epstein, 1962). Our lesioned rats showed levels of general activity similar to those of sham-lesioned rats, no deficits in the display of unconditioned ORs, and no deficits in the acquisition of food cup responses under conditions in which associability enhancements were not expected (that is, in experiments 1 and 2, and in the consistent condition of experiment 3). Nevertheless, the effects of unilateral LH lesions on attentional processing in particular were complete, returning rats to control levels in all three experiments.
Nature of LH lesions
We cannot unequivocally attribute these lesion effects to damage to orexin neurons because our lesions also damaged many neurons that were not orexin-immunoreactive. In both experiments 2 and 3, the amount of damage to ORX+ neurons was significantly correlated with the amount of damage to LH overall and, in experiment 3, associability impairment was significantly correlated with both damage to ORX+ neurons alone and overall LH damage. Indeed, in experiment 2, a majority of the FOS+ neurons were not ORX+. LH includes neurons of a number of phenotypes known to have widespread modulatory effects (Gerashchenko & Shiromani, 2004). However, given the relative selectivity of orexin-saporin to neurons that express the orexin 2 receptor and the interconnectivity of various types of LH neurons (Louis, et al., 2010), it seems reasonable to describe our lesions as targeting an LH ‘orexin system’. Moreover, two aspects of our data suggest a substantial role for orexinergic neurons specifically in these attentional functions. First, in all of our experiments, the orexin-saporin lesions appeared to disproportionally spare MCH+ neurons, to the point that no damage was detectable when compared with a sham lesion in experiment 2. Although MCH neurons share many (but not all) targets of orexin neurons, there is ample evidence that their connectivity and function both within and outside LH differ considerably from those of orexin neurons (Huang et al., 2006; Pasumarthi et al., 2006; Hassani et al., 2009; Louis et al., 2010; Bittencourt, 2011; Blouin et al., 2013). Second, in experiment 2, a quarter of the neurons in LH that expressed FOS in response to CS+ were ORX+, and nearly half of all ORX+ neurons expressed FOS. A similar pattern of FOS expression in LH was observed by Petrovich et al. (2012) in food-sated rats, who found that an auditory cue that had previously been paired with food while the rats were food-deprived induced substantial FOS expression in ORX+ neurons, but not MCH+ neurons.
Implications of unilateral lesion effects
The effects of unilateral LH lesions on these CeA-dependent attentional phenomena require consideration, because LH projections to and from CeA (and most other brain regions) are overwhelmingly ipsilateral (Saper et al., 1978; Petrovich et al., 2001; Yoshida et al., 2006). Furthermore, previous studies revealed no effects of unilateral disruption of CeA function on either phenomenon, unless additional lesions of other critical circuit elements were placed in the contralateral hemisphere, thereby interrupting the circuits bilaterally (Han et al., 1997, 1999; Lee et al., 2005, 2008). If unilateral LH lesions interfered with CeA processing only in the ipsilateral hemisphere, as one would predict from their direct connections, we would not have expected to observe deficits in ORs from such lesions. Thus, the observation in experiment 2 that rats with unilateral LH lesions displayed large and equivalent impairments in cue-induced FOS expression in both hemispheres of CeA is of special interest. First, it is consistent with the equivalent effects of ipsilateral, contralateral and unilateral LH lesions in these experiments. Second, it suggests that the behavioral effects of LH lesions are mediated by their effects on CeA activity. Both ORs and surprise effects are accompanied by bilateral enhancement of CeA FOS expression when rats are presented with OR-generating CSs (Lee et al., 2005, 2010) or surprising omission of expected events (Bucci & McLeod, 2007; Lee et al., 2010). Hence, manipulations that reduce the enhancement of CeA FOS expression bilaterally might be expected to produce deficits in those behavioral phenomena.
The question of how unilateral LH dysfunction produces bilateral CeA impairments remains. Importantly, our unilateral LH lesion effects are not readily attributable to unintentional damage to the contralateral LH. First, both qualitative examinations and quantitative neuron counts showed only minor (if any) loss of neurons in LH contralateral to the lesion. Second, in experiment 2, both total FOS expression in LH and FOS expression in orexin neurons specifically were substantially higher contralateral to the lesion and similar to levels found in the brains of sham-lesioned rats. Of course, we cannot rule out more subtle alterations of contralateral LH functions, but it is notable that LH does not show significant interhemispheric connections (Saper et al., 1978), by which irregular function in one hemisphere might readily disrupt function in the other.
Based on anatomical considerations, we considered three potential routes by which a unilateral LH lesion might influence CeA bilaterally. The first involves the ventral tegmental area (VTA), which receives LH orexin projections (Moorman & Aston-Jones, 2010; but see Balcita-Pedicino & Sesack, 2007) and projects to CeA. Interestingly, Lee et al. (2011) found that unilateral lesions of VTA, like unilateral LH lesions, produced deficits in the acquisition of conditioned ORs. Invoking a large literature that indicates bilateral influences on electrical brain stimulation reward functions (Waraczynski, 2006), Lee et al. (2011) suggested that these effects of unilateral VTA lesions might be mediated by the VTA decussation (Fereira et al., 2008) or bilateral afferents to VTA from the pedunculopontine tegmental area, which has reciprocal connections with CeA. However, unlike with LH lesions, unilateral lesions of CeA contralateral to VTA lesions ‘rescued’ ORs from the impairments produced by unilateral VTA lesions (Lee et al., 2011). Furthermore, also unlike unilateral LH lesions, unilateral VTA lesions suppressed CeA FOS expression in response to a food cue only in the hemisphere ipsilateral to the VTA lesion (Lee et al., 2011). Thus, this route seems unlikely to mediate the influence of LH on conditioned ORs.
A related, but less direct route involves the lateral habenula, which has been reported to receive major projections from both ipsilateral and contralateral LH (Poller et al., 2013; but see Herkenhan & Nauta, 1977). Importantly, these projections appear to densely target the habenula neurons (Poller et al., 2013) thought to be critically involved in suppression of VTA dopamine release (Matsumoto & Hikosaka, 2007, 2009). These habenula neurons inhibit VTA activity both through direct projections to the VTA and indirectly via the rostromedial tegmental nucleus (Jhou et al., 2009a, b). Although the neuronal interactions within this multi-synaptic circuit are complex, they provide a route by which unilateral lesions of LH might produce bilateral dysfunction of VTA signaling, which could in turn lead to bilateral disruption of CeA activity (Lee et al., 2011), as observed in experiment 2.
A third route by which unilateral damage to LH might affect CeA function bilaterally is via the insular cortex. This region receives both ipsilateral and contralateral input from LH, has strong interhemispheric connections, and projects to CeA (Saper, 1982; Rempel-Clower & Barbas, 1998). Thus, unilateral disruption of function in LH might have bilateral impact on CeA function, as we observed. However, at present we have no evidence bearing on the involvement of this region in conditioned ORs or other attentional phenomena in rats.
Alternative targets of LH orexin system action
Although we emphasize the importance of functional relations between LH and CeA, it remains possible that the involvement of LH in these attentional phenomena and its effect on CeA FOS expression are independent. For example, in experiment 2, unilateral LH lesions might have suppressed the performance of conditioned ORs independently of CeA function, and the reduction of CeA FOS expression was a ‘consequence’ of that reduction in ORs. From this perspective, FOS expression in CeA after CS presentations ‘reports’ performance of an OR generated elsewhere in the brain, rather than reflects CeA processing responsible for that OR. However, McDannald et al. (2004) found that transient pharmacological suppression of CeA prevented the acquisition, but not the subsequent performance, of conditioned ORs, implying a causal relation between CeA activity and OR learning, not between OR performance and CeA activity. Thus, we think this particular conjecture about the potential independence of the effects of LH lesions on CeA FOS expression and on the two attentional phenomena we examined is unlikely.
Nevertheless, other dissociations among these effects may be more plausible. Notably, LH projects widely in the brain and could influence these functions by many routes. For example, LH and the basal forebrain substantia innominata (SI) are richly interconnected (Mogenson et al., 1985; Fadel et al., 2005), and many researchers have asserted that LH orexin neurons play an important role in modulating basal forebrain cholinergic system activity (Frederick-Duus et al., 2008; Arrigoni et al., 2010; Fadel & Burke, 2010). Activity of cholinergic neurons in SI modulates a wide range of attentional phenomena (Everitt & Robbins, 1997; Sarter & Bruno, 1997), including the surprise-induced enhancement of cue associability (Chiba et al., 1995; Holland & Gallagher, 2006; Maddux et al., 2007), which we examined in experiment 3. Hence, interactions between LH and SI may play an important role in attentional processing in associative learning, independent of contributions of CeA.
However, it is notable that disruptions of SI function do not affect conditioned ORs, as in experiments 1 and 2 (Chiba et al., 1995; Han et al., 1999). By contrast, CeA function is critical to both the acquisition of conditioned ORs (Gallagher et al., 1990; McDannald et al., 2004) and the processing of surprise in the serial prediction task used in experiment 3 (Holland & Gallagher, 1993, 2006). A parsimonious inference, consistent with our observation of detrimental effects of LH lesions on CeA FOS expression as well as on performance in both of these tasks, is that these attentional functions involve interactions between LH and CeA.
Modulatory functions of LH orexin neurons
We follow others in suggesting that LH orexin neurons may act by amplifying the actions of other brain systems (Horvath et al., 1999; Tao et al., 2006; Gompf & Aston-Jones, 2008). For example, medial prefrontal cortical dopamine efflux (Vittez & Berridge, 2006), behavioral sensitization to cocaine (Borgland et al., 2006; Bonci & Borgland, 2009), and responses to palatable food (Zheng et al., 2007) are all enhanced by orexin signaling in the VTA. Likewise, Ho & Berridge (2013) reported enhanced hedonic ‘liking’ responses to sweet tastes after infusion of orexin into the ventral pallidum. LH may similarly amplify the action of CeA in the acquisition of conditioned ORs, and in the processing of surprise after the omission of expected events. The mechanism of such amplification is unknown, but it is notable that Bisetti et al. (2006) reported that in a slice preparation, orexin administration excited potentially plasticity-related ‘low-threshold burst neurons’ in medial CeA, augmenting the normal amygdala response to stimulation.
In this context, it is important to note that previous studies showed that whereas CeA function is critical for acquiring conditioned ORs and for adjusting cue associability at the time of surprise, it is not needed for either the display of previously-acquired ORs, or the expression of enhanced cue associability in faster learning at the time of test in the serial prediction task. As noted previously, once conditioned ORs are acquired, neither CeA lesions nor pharmacological disruptions of CeA function affects their expression (McDannald et al., 2004). Similarly, in the serial prediction task used in experiment 3, although pharmacological disruption of CeA activity at the time of surprise eliminates surprise-induced enhancements of cue associability, that same disruption conducted only at the time of test does not affect the display of such enhancements produced while CeA function was intact at the time of surprise (Holland & Gallagher, 2006). Thus, LH likely amplifies these critical plasticity-related processes in CeA. Examination of the effects of transient inactivation of LH at the times of acquisition or performance could determine its specific role in attentional processing.
Relation to broader role of LH orexin neurons
The original discovery and study of orexin (de Lecea et al., 1998; Sakurai et al., 1998) focused on its potential roles in the modulation of feeding. Although it is clear that orexin neurons are responsive to both internal signals of energy state (Adamantidis & de Lecea, 2009; Tsujino & Sakurai, 2013) and external food-related cues (especially in overriding the effects of satiety; Hirasawa et al., 2007; Petrovich et al., 2012), most subsequent research explored the role of orexin neurons in general arousal, the sleep/wakefulness cycle and other rhythmic activity (Siegel, 2004; Gompf & Aston-Jones, 2008; Tsujino & Sakurai, 2013).
Nevertheless, several investigators have noted that in addition to these general arousal effects, orexin neurons also affect attention more specifically (Lambe et al., 2005; Govindaiah & Cox, 2006; Kirouac et al., 2006; Huang et al., 2007; Fadel & Burk, 2010) in vigilance, sustained and divided attention tasks. Similarly, orexin neurons have been assigned critical roles in the modulation of learning and memory (Akbari et al., 2006; Soya et al., 2013), especially the acquisition of incentive properties by cues that predict reward (Harris et al., 2005; Harris & Aston-Jones, 2006; Cason et al., 2010). Consistent with views that a key role of orexin neurons may be in integrating all these functions (Scammell & Saper, 2005; Frederick-Duus et al., 2007), the present research showed that orexin neurons may also be critical to enhancing attention in associative learning about cues that predict reward.
Understanding the action of orexin and other populations of LH neurons may be important for our understanding of a variety of psychological disorders. For example, narcolepsy is strongly associated with orexin loss or dysfunction (Peyron et al., 2000; Siegel, 2004; Blouin et al., 2005; Burgess & Scammell, 2012), and researchers have noted relations between orexin function and the amygdala in this disorder (Blouin et al., 2013) and its animal models (Burgess et al., 2013). Similarly, many investigators have pointed to parallels between reward learning with natural and drug rewards, and have suggested roles for orexin neurons in drug seeking, drug abuse and addiction (Scammel & Saper, 2005; Carr & Kalivas, 2006; Harris & Aston Jones, 2006; Boutrel & de Lecea, 2008; Aston-Jones et al., 2009; Pasumarti & Fadel, 2010), and in withdrawal from drug use (Sharf et al., 2008). Indeed, Cason et al. (2010) suggested those parallels extend to overeating, ‘food addictions’ and obesity. Furthermore, some investigators have suggested relations between abnormal activation of orexin systems and mood disorders (Nestler & Carlezon, 2006), and schizophrenia (Deutch & Bubser, 2007; Lambe et al., 2007; Borgland & Labouebe, 2010). The present study, which links the function of LH and orexin systems to acquired attentional processing via regulation of the amygdala, may help illuminate commonalities among these disorders.
Acknowledgments
This research was funded by NIH grant MH53667. A preliminary analysis of a portion of the data of experiment 3 was presented at the 2008 annual meeting of the Society for Neuroscience.
Abbreviations
- CeA
amygdala central nucleus
- Contra
contralateral
- CR
conditioned response
- CS
conditioned stimulus
- CS+
conditioned stimulus paired with food
- CS−
conditioned stimulus not paired with food
- DAB
diaminobenzidine
- FOS+
immunoreactive for FOS
- HSD
honestly significant difference
- Ipsi
ipsilateral
- LH
lateral hypothalamic region
- MCH
melanin-concentrating hormone
- MCH+
immunoreactive for melanin-concentrating hormone
- MCH + FOS
immunoreactive for both melanin-concentrating hormone and FOS
- OR
orienting response
- ORX+
immunoreactive for orexin
- ORX + FOS
immunoreactive for orexin and FOS
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- SI
substantia innominata
- Uni
unilateral
- US
unconditioned stimulus
- VTA
ventral tegmental area
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis A, Thomas E, Foidart A, Tyhon A, Coumans B, Minet A, Tirelli E, Seutin V, Grisat T, Lakaye B. Disrupting the melanin-concentrating hormone receptor 1 in mice leads to cognitive deficits and alterations of NMDA receptor function. Eur J Neurosci. 2005;21:2837–2844. doi: 10.1111/j.1460-9568.2005.04100.x. [DOI] [PubMed] [Google Scholar]
- Akbari E, Naghdi N, Motamedi F. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav Brain Res. 2006;173:47–52. doi: 10.1016/j.bbr.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurons. Acta Physiol. 2010;198:223–235. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic oriexin neurons in reward processing and addiction. Neuropharmacol. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not be exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC. Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen Comp Endo. 2011;172:185–197. doi: 10.1016/j.ygcen.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Muhlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neurosci. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurol. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000175219.01544.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin AM, et al. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat Com. 2013;4:1547. doi: 10.1038/ncomms2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Borgland SL. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacol. 2009;56(Suppl 1):107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Labouebe G. Orexin/hypocretin in psychiatric disorders: present state of knowledge and future potential. Neuropsychopharmacol. 2010;35:353–354. doi: 10.1038/npp.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, De Lecea L. Addiction and arousal: The hypocretin connection. Physiol Behav. 2008;93:947–951. doi: 10.1016/j.physbeh.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, MacLeod JE. Changes in neural activity associated with a surprising change in the predictive validity of a conditioned stimulus. Eur J Neurosci. 2007;26:2669–2676. doi: 10.1111/j.1460-9568.2007.05902.x. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium-calcium exchanger. J Neurosci. 2003;23:4951–4957. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE. Amygdala lesions reduce cataplexy in orexin knock-out mice. J Neurosci. 2013;33:9734–9742. doi: 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR, Scammell TE. Narcolepsy: Neural mechanisms of sleepiness and catalepsy. J Neurosci. 2012;36:12305–12311. doi: 10.1523/JNEUROSCI.2630-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Kalivas PW. Orexin: a gatekeeper of addiction. Nat Med. 2006;12:274–276. doi: 10.1038/nm0306-274. [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, Rosas-Arellano MP, Soplano-Flores LP, de Oliveira CVR. Identification of neurons containing orexin-B (hypocretin-2) immunoreactivity in limbic structures. Brain Res. 2003;967:123–131. doi: 10.1016/s0006-8993(02)04233-6. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R. Addiction and arousal. J Neurosci. 2006;26:10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff T, Peyron C, Gao XB, Foye PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–27. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Bubser M. The orexins/hypocretins and schizophrenia. Schiz Bull. 2007;33:1277–1283. doi: 10.1093/schbul/sbm096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Ann Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neurosci. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fadel J, Frederick-Duus D. Orexin/hypocretin modulation of the basal forebrain cholinergic system. Pharmacol Biochem Behav. 2008;90:152–162. doi: 10.1016/j.pbb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neurosci. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Ferreira JGP, Del-Fava F, Hasue RH, Shammah-Lagnado J. Organization of ventral tegmental area projections to the ventral tegmental area-nigral complex in the rat. Neurosci. 2008;153:196–213. doi: 10.1016/j.neuroscience.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Frederick-Duus D, Guyton MF, Fadel J. Food-elicited increases in cortical acetylcholine release require orexin transmission. Neurosci. 2007;149:499–507. doi: 10.1016/j.neuroscience.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Different neural phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29:41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- Gompf HS, Aston-Jones G. Role of orexin input in diurnal rhythm of locus coeruleus impulse activity. Brain Res. 2008;1224:43–52. doi: 10.1016/j.brainres.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL. Modulation of thalamic neuron excitability by orexins. Neuropharmacol. 2006;51:414–425. doi: 10.1016/j.neuropharm.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Grossberg AJ, Zhu XX, Leinninger GM, Levasseur PR, Braun TP, Myers MG, Marks DL. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. 2011;31:11376–11386. doi: 10.1523/JNEUROSCI.2311-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behav Neurosci. 1999;113:143–151. doi: 10.1037//0735-7044.113.1.143. [DOI] [PubMed] [Google Scholar]
- Han JS, McMahan RW, Holland PC, Gallagher M. The role of an amygdalo-nigrostriatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nat. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Nat Acad Sci USA. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJH. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Parsons MP, Alberto CO. Interaction between orexins and the mesolimbic sytem for overriding satiety. Rev Neurosci. 2007;18:383–393. doi: 10.1515/revneuro.2007.18.5.383. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic “liking” for sweetness. Neuropsychopharmacol. 2013;38:1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Proc. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav Neurosci. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci. 2006;26:3791–3797. 4715. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Maddux J-M. Brain systems of attention in associative learning. In: Mitchell CJ, LePelley ME, editors. Attention and learning. Oxford: Oxford University Press; 2010. pp. 305–349. [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Killduff TS, van den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Huang H, Ghosh P, van den Pol AN. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, selectively encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:788–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, DeGarmo B, Zahm DS. The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye H, Pearce JM. The strength of the orienting response during Pavlovian conditioning. J Exp Psychol Anim Beh Proc. 1984;10:90–109. [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2006;1059:179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Liu RJ, Aghajanian GK. Schizophrenia, hypocretin (orexin), and the thalamocortical activating system. Schiz Bull. 2007;33:1284–1290. doi: 10.1093/schbul/sbm088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: Correlation with improved attention in rat. J Neurosci. 2005;25:5225–5229. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Gallagher M, Holland PC. The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learn Mem. 2010;17:531–538. doi: 10.1101/lm.1889510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Wheeler DS, Holland PC. Interactions between amygdala central nucleus and the ventral tegmental area in the acquisition of conditioned cue-directed behavior in rats. Eur J Neurosci. 2011;33:1876–1884. doi: 10.1111/j.1460-9568.2011.07680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, Gallagher M, Holland PC. Temporally-limited role of substantia nigra-central amygdala connections in surprise-induced enhancement of learning. Eur J Neurosci. 2008;27:3043–3049. doi: 10.1111/j.1460-9568.2008.06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GW, Leinninger GM, Rhodes CJ, Myers MG. Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci. 2010;30:11278–11287. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JM, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: Effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behav Neurosci. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nat. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2007;248:475–517. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannald MA, Kerfoot E, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting. Eur J Neurosci. 2004;20:240–248. doi: 10.1111/j.0953-816X.2004.03458.x. [DOI] [PubMed] [Google Scholar]
- Meng X, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychol Bull. 1992;111:172–175. [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Evidence that projections from substantia innominata to zona incerta and mesencephalic locomotor region contribute to locomotor activity. Brain Res. 1985;334:65–76. doi: 10.1016/0006-8993(85)90568-2. [DOI] [PubMed] [Google Scholar]
- Monti JM, Torterolo P, Lagos P. Melanin-concentrating hormone control of sleep-wake behavior. Sleep Med Rev. 2013;17:292–298. doi: 10.1016/j.smrv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: Diurnal influences. J Neurosci. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Ocampo-Garces A, Ibanez F, Perdomo G, Torrelaba F. Orexin-B-saporin lesions in the lateral hypothalamus enhance photic masking of rapid eye movement sleep in the albino rat. J Sleep Res. 2011;20:3–11. doi: 10.1111/j.1365-2869.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- Olarte-Sanchez CM, Toreres LV, Body S, Cassaday HJ, Bradshaw CM, Szabadi E. Effect of orexin-B-saporin-induced lesions of the lateral hypothalamus on performance on a progressive ratio schedule. J Psychopharmacol. 2012;26:871–886. doi: 10.1177/0269881111409607. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Stimulation of lateral hypothalamic glutamate and acetylcholine efflux by nicotine: implications for mechanisms of nicotine-induced activation of orexin neurons. J Neurochem. 2010;113:1023–1035. doi: 10.1111/j.1471-4159.2010.06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur J Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ. Selective FOS induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates eating in sated rats. Neurosci. 2012;224:70–80. doi: 10.1016/j.neuroscience.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: the multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- Poller WJ, Madai VI, Bernard R, Laube G, Veh RW. A glutamatergic projection from the lateral hypothalamus targets VTS-projecting neurons in the lateral habenula of the rat. Brain Res. 2013;1507:45–60. doi: 10.1016/j.brainres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1978;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Saper CB. Orexin, drugs, and motivated behaviors. Nat Neurosci. 2005;10:1286–1288. doi: 10.1038/nn1005-1286. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, DiLeone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Wosiski-Kuhn M, Nguyen T, Holland PC, Lakaye B, Adamantidis A, Johnson AW. The role of melanin concentrating hormone in conditioned reward learning. Eur J Neurosci. 2012;36:3126–3133. doi: 10.1111/j.1460-9568.2012.08207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): Role in normal behavior and neuropathology. Ann Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawam M, Miedam M, Sakurai T. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J Neurosci. 2013;33:14549–14557. doi: 10.1523/JNEUROSCI.1130-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the rat brain. 2. Elsevier; Amsterdam: 1998. [Google Scholar]
- Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Let. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, McKenna JT, Thakkar MM, Winston S, Strecker RE, McCarley RW. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neurosci. 2006;141:1101–1105. doi: 10.1016/j.neuroscience.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:1–13. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittez NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacol. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Waraczynski MA. The central extended amygdala network as a proposed circuit underlying reward valuation. Neurosci Biobehav Rev. 2006;30:472–496. doi: 10.1016/j.neubiorev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. J Neurosci. 2013;33:2009–2016. doi: 10.1523/JNEUROSCI.3921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PN, Boumphrey P, Pearce JM. Restoration of the orienting response to a light by a change in its predictive accuracy. Quart J Exper Psychol. 1992;44B:17–36. [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li B, Yu L, He YC, Li H-Z, Zhu JN, Wang JJ. A role for orexin in central vestibular motor control. Neuron. 2011;69:793–404. doi: 10.1016/j.neuron.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson lM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]