Key Clinical Message
Severe cold agglutinin disease with hemodynamic compromise requires rapid stabilization of the autoimmune hemolytic anemia as a bridge to the immunosuppressive effect of rituximab. Herein, we describe eculizumab treatment of severe complement-mediated hemolysis in a patient whose hemodynamic status deteriorated in spite of supportive blood transfusions and therapeutic plasma exchange.
Keywords: Anti-Pr, cold autoimmune hemolytic anemia, eculizumab, rituximab
A previously healthy 58-year-old man was brought to the emergency room with fatigue and severe dyspnea. Preliminary investigations showed a hemoglobin level of 21 g/L with MCV 154.0 fL, an indeterminate platelet count, creatinine 219 μmol/L, and venous blood gas showing a pH of 7.19. He was intubated and transferred to the ICU as a result of increasing respiratory distress and acute kidney injury requiring renal replacement therapy.
The combination of severe anemia, acute kidney injury, and indeterminate platelet count suggested a preliminary differential diagnosis of a thrombotic microangiopathy, autoimmune hemolytic anemia with immune thrombocytopenia, or disseminated intravascular coagulation (DIC) secondary to sepsis.
After transfer to the ICU, blood transfusion and therapeutic plasma exchange were initiated while collateral history was obtained. The patient had a history of immune thrombocytopenia previously treated with prednisone that subsequently relapsed and required splenectomy and IVIg. He had no history of any diagnosed hematological malignancy or any previous blood transfusions. The symptoms of fatigue and dyspnea developed within 1 week prior to being brought to hospital. He had not had any recent fevers or sick contacts. There was no history of diarrhea or melena stools. His only medications were amlodipine and ezetimibe. On physical examination, it was found that the patient had jaundice. There was no evidence of petechiae, and digital rectal examination was negative for any blood. Investigations showed a significant degree of red blood cell agglutination on blood film, a positive Coombs test for C3, haptoglobin <0.07 g/L, indirect bilirubin 83.1 μmol/L, and serum LD of 1776 U/L (normal <225 U/L). A cold agglutinin screen was positive with a thermal amplitude of 37°C (98.6°F). The antibody titer could not be determined because of the strong agglutination reaction, but anti-Pr specificity was later noted. CT scans and bone marrow biopsy were performed to rule out lymphoma or other malignancy associated with the cold agglutinin, and were negative for any abnormality. Autoimmune work-up was negative.
The severity of the RBC agglutination made the initial identification of an antibody difficult. After several attempts, a cold agglutinin antibody of anti-Pr specificity was identified. Along with the anti-I and anti-i IgM antibodies, the anti-Pr IgM antibody is one of the antibodies that may mediate RBC cold agglutination, with the latter being much more rare 1.
As the patient's hemoglobin remained critically low and consistently below 5 g/L in spite of a session of therapeutic plasma exchange and the maintenance of body temperature over 37°C along with transfusion of 8 units of packed red blood cells over the course of 48 h, the decision was made to initiate eculizumab 600 mg IV every 7 days for 4 weeks followed by a single dose of 900 mg IV. One day after initiation of eculizumab, he was started on rituximab 375 mg/m2 weekly for 4 weeks. The result was a stabilization of the hemoglobin value and decrease in hemolytic markers within 1 week (Figure 1). Concurrent with this, he was treated with cyclophosphamide 250 mg IV 5 days later followed by three additional doses of 500 mg IV given weekly.
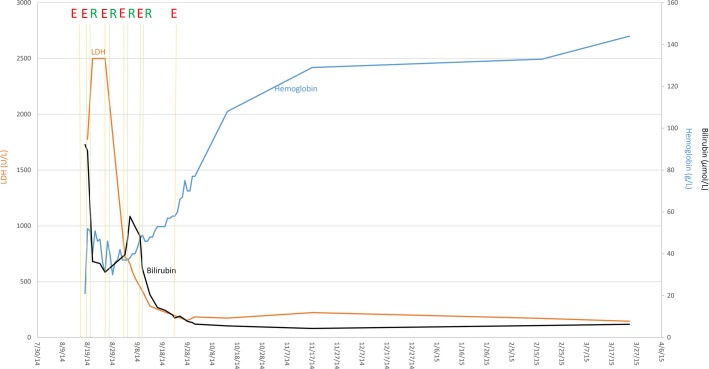
Figure 1.
Response of intravascular hemolysis to eculizumab (E) as a bridge to immunosuppressive therapy with rituximab (R) in a patient with severe cold agglutinin disease. The blue line represents the patient's measured hemoglobin, the black line total bilirubin, and orange line serum LDH. Gold lines indicate the dates when eculizumab was administered. Green lines refer to the administration of rituximab. Durable hemoglobin response was maintained with cyclophosphamide after intravascular hemolysis was corrected.
Although rituximab has been shown in case reports to have excellent efficacy as an immunosuppressive therapy in B-cell-mediated autoimmune hemolytic anemia, its effect in stabilizing hemolysis may take several weeks 2. The decision to use eculizumab as an attempt to provide a rapid stabilization of the complement-mediated intravascular hemolysis was made because frequent supportive blood transfusions were inadequate in maintaining the hemoglobin within a life-sustaining range.
The patient's hemoglobin continued to show improvement after completion of the eculizumab and rituximab treatments with a progressive rise and undetectable cold agglutinin titer noted 5 weeks after initiation of therapy. He was subsequently extubated and transferred out of the ICU in good condition. After completion of eculizumab and rituximab treatment, the patient completed a 2-week course of cyclosporine and has been maintained on tapering doses of oral cyclophosphamide with no further evidence of intravascular hemolysis noted on follow-up.
Commentary
Cold agglutinin (CA) disease is a form of autoimmune hemolytic anemia which is characterized by IgM-mediated RBC agglutination and complement activation leading to intravascular hemolysis. It manifests clinically with anemia, dyspnea, and hemoglobinuria, and unlike in warm antibody-mediated autoimmune hemolytic anemia, treatment with corticosteroids is commonly unsuccessful 3,4.
Chronic primary cold agglutinin disease accounts for about 15% of all cases of autoimmune hemolytic anemia and is more often associated with a monoclonal IgM CA with kappa restriction 4. This attests to the etiology of this disorder being a clonal B cell most commonly associated with lymphoma 5. The degree of hemolysis may be more severe than in acute CA disease, especially when it is exacerbated by an acute onset of trauma or febrile illness associated with an increased production of complement C3 and C4 protein 4. In addition to the intravascular MAC-mediated hemolysis, primary CA disease also has a significant amount of extravascular hemolysis resulting from the coating of erythrocytes with C3b from the earlier steps of complement activation 4. The decision to treat CA disease is dependent on the severity of the anemia, transfusion dependence, and the presence of other sequelae of intra- or extravascular hemolysis 4.
The pharmacologic treatment of primary CA involves therapy targeting the B-cell clone. Rituximab as a single agent has been shown to be effective. In an open uncontrolled phase 2 study of rituximab in CA disease, rituximab monotherapy achieved a partial response, defined as a stable increase in hemoglobin levels, a reduction in serum IgM levels by 50%, and clinical improvement in 19 of 27 patients with a median response duration of 11 months but with a median time to response of 1.5 months 3. There is some evidence for the combination of rituximab with fludarabine to improve response rate and duration of remission of CA disease, although there have been no randomized controlled trial done to prove this. Furthermore, the combination was shown to have an increased number of grade 3–4 hematological toxicity in a prospective uncontrolled trial of fludarabine and rituximab in primary CA disease 4.
Eculizumab is a long-acting humanized monoclonal antibody targeting the terminal pathway of complement activation. Its mechanism of action is to bind the C5 complement protein and prevent its cleavage into active C5a and C5b fragments, thereby inhibiting the formation of the membrane attack complex (MAC) 6,7. It has been approved in paroxysmal nocturnal hemoglobinuria where its use was associated with a sustained reduction of intravascular hemolysis as well as transfusion independence in 50% of patients as observed in a randomized clinical trial 6. It has also received FDA approval for use in atypical HUS 6. Although there have been case reports of its efficacy in CA disease, a randomized clinical trial is yet to be done.
When used in CA disease, eculizumab has shown in case reports to significantly reduce the degree of MAC-induced hemolysis 4,8. Based on its mechanism of action whereby it targets the terminal complement cascade, eculizumab could stabilize the degree of complement-mediated hemolysis within hours to days. It would not, however, prevent the formation of C3b that occurs at an earlier stage of complement activation 9. Given the substantial degree of extravascular C3b-mediated hemolysis observed in primary CA disease, the efficacy of eculizumab may be theoretically augmented with agents such as rituximab that reduce the amount of C3b-coated red blood cells by preventing the formation of the complement-fixing CA antibody. Concurrently, eculizumab has a role in the rapid stabilization of intravascular hemolysis while the slower, long-term effect of immunosuppressive therapy takes place.
To the best of our knowledge, we present the first case report of the use of eculizumab as a bridge to immunosuppresive therapy in severe cold agglutinin disease of anti-Pr specificity. The patient's hemodynamic compromise in this case necessitated the use of the rapid effect of eculizumab on stabilizing complement-mediated intravascular hemolysis while awaiting for response from the immunosuppressive therapy. The long-term response to treatment was sustained in this patient with undetectable cold agglutinin titer and normalization of all blood counts at his 10-month follow-up.
Conflict of Interest
None declared.
References
- Ruch J, McMahon B, Ramsey G. Kwaan HC. Catastrophic multiple organ ischemia due to an anti-Pr cold agglutinin developing in a patient with mixed cryoglobulinemia after treatment with rituximab. Am. J. Hematol. 2009;84:120–122. doi: 10.1002/ajh.21330. [DOI] [PubMed] [Google Scholar]
- Berentsen S, Ulvestad E, Gjertsen BT, Hjorth-Hansen H, Langholm R, Knutsen H. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103:2925–2928. doi: 10.1182/blood-2003-10-3597. , et al. [DOI] [PubMed] [Google Scholar]
- Lechner K. Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831–1838. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- Berentsen S. Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107–115. doi: 10.1016/j.blre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Berentsen S, Ulvestad E, Langholm R, Beiske K, Hjorth-Hansen H, Ghanima W. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91:460–466. , et al. [PubMed] [Google Scholar]
- McKeage K. Eculizumab: a review of its use in paroxysmal nocturnal hemoglobinuria. Drugs. 2011;71:2327–2345. doi: 10.2165/11208300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Berentsen S. Complement, cold agglutinins, and therapy. Blood. 2014;123:4010–4012. doi: 10.1182/blood-2014-04-568733. [DOI] [PubMed] [Google Scholar]
- Roth A, Huttmann A, Rother RP, Duhrsen U. Philipp T. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2009;113:3885–3886. doi: 10.1182/blood-2009-01-196329. [DOI] [PubMed] [Google Scholar]
- Schrezenmeier H. Hochsmann B. Drugs that inhibit complement. Transfus. Apheres. Sci. 2012;46:87–92. doi: 10.1016/j.transci.2011.11.012. [DOI] [PubMed] [Google Scholar]