Key Clinical Message
Scalp hair loss is an underreported adverse event of somatostatin analogs therapy that in severe cases may require treatment withdrawal. It can be related to an acute decrease in GH/IGF-1 levels, but a direct effect cannot be ruled out.
Keywords: alopecia, hair loss, Lanreotide, somatostatin analogs
Dear Sir,
We describe a case of severe nonscarring diffuse scalp hair loss in a 49-year-old female treated with Lanreotide Autogel (LA) because of acromegaly. She noticed a progressive enlargement of the size of her teeth, feet, and fingers in last 9 years, and also complained of paresthesia in her hands, moderate headache, visual impairment, and hyperhidrosis. A bilateral oophorectomy and total hysterectomy were performed when she was 43 years old, because of myomatous disease. Five years before presentation, an impaired fasting glucose level was detected. Her facial deformities and occlusive dental disease were treated with orthognathic surgery 4 years before. No cases of endocrine diseases or alopecia were reported in her family.
Her weight, height, and body mass index were 65 kg, 158 cm and 26 kg/m2, respectively, and she had normal blood pressure. Laboratory tests revealed high IGF-1 (Insulin-like Growth Factor-1): 1419 μg/L (Normal range: 94–252), GH: 29.50 μg/L (0–18), and PRL (Prolactin): 984.25 mUI/L (101.54–493.96) levels, whereas TSH and FSH/LH levels were normal. Pituitary Magnetic Resonance Image (MRI) showed a sellar tumor that eroded the dural membrane, sphenoidal sinus, and para-carotid bone. The echocardiogram, colonoscopy, and visual field tests were normal, but a low-grade apnea–hypopnea syndrome was diagnosed.
An endoscopic transsphenoidal surgery (TS) was performed and histopathologic study confirmed a pituitary adenoma with GH- and PRL-positive immunohistochemistry. Two months later IGF-1 levels were still high (993 μg/L), and no GH suppression, after a 75-gr oral glucose tolerance test (OGTT), was observed (Basal GH: 8.66; GH-Nadir: 6.82 μg/L). LA, at a dose of 120 mg every 56 days, was then prescribed. She reported abdominal discomfort and diarrhea within the first 15 days of treatment, with no other signs or symptoms. An excellent biochemical response was found after 111 days of treatment (IGF-1: 221 μg/L; GH: 0.48 μg/L), but 4 days after the second dose, she noticed a progressive diffuse scalp hair loss (Figs. 2). We ruled out other causes of alopecia: testosterone, DHEAs (Dehydroepiandrosterone sulfate), 17-OH progesterone, and thyroid hormone levels were normal, and no signs of infection were present in the scalp. After examination, we concluded that she had a nonscarring diffuse hair loss, affecting the scalp globally, and more compatible with Telogen effluvium. There was no evident loss of brows, lashes, or body hair. The intensity of hair loss increased, and psychological disturbances related to these new appearance changes obliged us to suspend the treatment, such that the third or subsequent doses were not administered. A new MRI showed remains of adenoma 6 months after the first TS. IGF-1 levels rose again, and hair loss improved considerably 5 months later, without any other treatment than LA withdrawal (Fig. 3). The disease persisted active (IGF-1: 775 μg/L; GH-nadir 1.98 μg/L after a 75-gr OGTT) after a second endoscopic TS, although no remains of adenoma were found in a postsurgical pituitary MRI. Cabergoline in increasing doses was then prescribed, showing a slow decrease in IGF-1 and GH levels. A complete biochemical response was achieved after 18 months of cabergoline therapy (IGF-1: 230 μg/L; GH 1.4 μg/L) and hair loss reappeared coinciding with the increase in cabergoline dose, however, it was not as severe as in case of Lanreotide treatment.
Figure 2.
Hair lost within the first (1), second (2) and third, and fourth week (3) after the beginning of alopecia.
Figure 3.
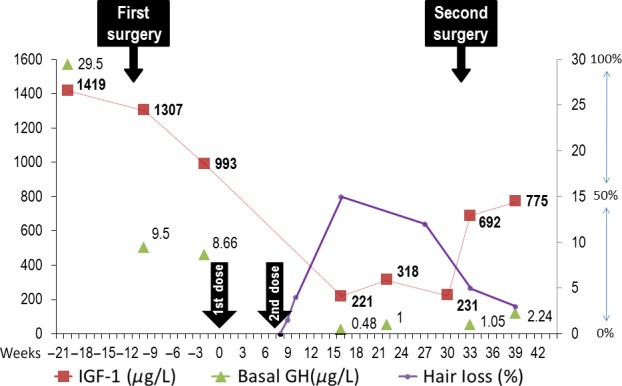
IGF-1 and GH levels before and after Lanreotide Autogel (LA) treatment. Hair loss represented in percentage as referred by the patient. Week “0” and “8” indicates the moment of administration of first and second LA doses (Arrows).
Alopecia has been reported in patients with acromegaly during Pegvisomant treatment 1; after TS 2, and rarely, during somatostatin analogs (SSA) treatment 3. To our knowledge there are 10 reported cases of alopecia in acromegalic patients during Octreotide and 25 cases during Lanreotide treatment.
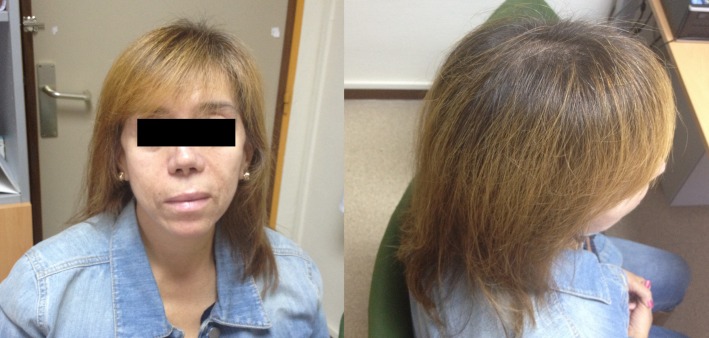
Figure 1.
Photo of the patient after 4 weeks of beginning of the hair loss.
All the patients treated with Octreotide showed a diffuse scalp hair loss and the time to onset ranged from 1 to 9 months since treatment initiation. Hair growth resumed 46 months later in the six patients that stopped Octreotide, but persisted in the three that continued on treatment. One patient showed an improvement of hair loss after switching to Lanreotide 3.
Suliman et al. reported four female patients that developed alopecia during SR-Lanreotide 30 mg every 7–14 days; one patient had to stop Lanreotide because of its severity. Treatment duration did not appear to affect the occurrence or severity of alopecia 4. Shimatsu et al. 5 reported a transient LA withdrawal in five from 32 acromegalic patients, because of alopecia. Only one patient withdrew treatment of the 12 hair loss reported cases by Caron et al. 6 from 90 acromegalic patients treated primarily with LA. In contrast, LA treatment was maintained in the four acromegalic patients with alopecia (out of 95) reported by Salvatori et al. 7.
Despite this not being the first case of hair loss reported with Lanreotide, the exact mechanism is not yet known. Acromegaly is usually associated with hypertrichosis, presumably related to high IGF-1 levels. The exogenous IGF-1 administration increased hair follicle numbers and prolonged the growing phase during the transition from anagen to telogen in mice 8. Yamada et al. 2 have recently reported a high nonsomatostatin-related alopecia prevalence (54%) in acromegalic patients after TS, that was significantly more common in cured patients and in patients with severe postoperative GH/IGF-1 deficiency, indicating that an acute decrease in GH/IGF-1 levels was the main cause of alopecia.
Similar to what Yamada et al. showed, an acute decrease in GH/IGF-1 levels might also cause alopecia during SSA treatment. Moreover, the decrease in the GH/IGF-1 levels during further treatment with SSA must be added to the partial biochemical response after unsuccessful TS, as occurred in our patient. If this was the case, hair loss could be considered an early clinical marker of good response to SSA, and treatment withdrawal might not be the best option in patients with milder forms of alopecia, but dose adjustment with or without combination therapy may be recommended instead.
We cannot rule out a direct effect of Lanreotide, because with cabergoline, we also observed a normalization of IGF-1 levels, and the degree of alopecia that the patient referred was not as severe as with Lanreotide; although the slow decrease in the GH/IGF-1 levels could be other difference.
In order to examine the value of hair loss as a marker of good response to SSA we propose identifying those patients that develop alopecia during SSA therapies – prescribed either primary or after unsuccessful surgery – revise their biochemical, clinical, and tumoral responses to SSA, and compare them to the corresponding responses of patients who did not show hair loss. A detailed evaluation of hair loss severity and its impact on quality of life in patients with acromegaly is needed to individualize its management, without worsening the control of their disease.
Conflict of Interest
None declared.
References
- Ezzat S, Gaspo R, Serri O, Ur E. Chik CL. A Canadian multi-centre, open-label long-term study of Pegvisomant treatment in refractory acromegaly. Clin. Invest. Med. 2009;32:E265. doi: 10.25011/cim.v32i6.10662. [DOI] [PubMed] [Google Scholar]
- Yamada S, Fukuhara N, Nishioka H, Yamaguchi-Okada M, Takeshita A. Takeuchi Y. Scalp hair loss after transsphenoidal adenomectomy in patients with acromegaly. Clin. Endocrinol. 2013;79:386–393. doi: 10.1111/cen.12040. [DOI] [PubMed] [Google Scholar]
- Lami M-C, Hadjadj S. Guillet G. Hair loss in three patients with acromegaly treated with octreotide. Br. J. Dermatol. 2003;149:655–680. doi: 10.1046/j.1365-2133.2003.05478.x. [DOI] [PubMed] [Google Scholar]
- Suliman M, Jenkins R, Ross R, Powell T, Battersby R. Cullen DR. Long-term treatment of acromegaly with the somatostatin analogue SR-lanreotide. J. Endocrinol. Invest. 1999;22:409–418. doi: 10.1007/BF03343583. [DOI] [PubMed] [Google Scholar]
- Shimatsu A, Teramoto A, Hizuka N, Kitai K, Ramis J. Chihara K. Efficacy, safety, and pharmacokinetics of sustained-release lanreotide (lanreotide Autogel) in Japanese patients with acromegaly or pituitary gigantism. Endocr. J. 2013;60:651–663. doi: 10.1507/endocrj.ej12-0417. [DOI] [PubMed] [Google Scholar]
- Caron PJ, Bevan JS, Petersenn S, Flanagan D, Tabarin A, Prévost G. PRIMARYS Investigators tumor shrinkage with Lanreotide Autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trial. J. Clin. Endocrinol. Metab. 2014;99:1282–1290. doi: 10.1210/jc.2013-3318. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori R, Nachtigall LB, Cook DM, Bonert V, Molitch ME, Blethen S. Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naïve patients with acromegaly. Pituitary. 2010;13:115–122. doi: 10.1007/s11102-009-0207-x. , et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang Z, Li Z, Gu L, Wang Y. Sung C. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-β1 in C57BL/6 mice in vivo. Growth Hormon. IGF Res. 2014;24:89–94. doi: 10.1016/j.ghir.2014.03.004. [DOI] [PubMed] [Google Scholar]