Abstract
Malignant glioma comprises the majority of primary brain tumors. Coincidently, most of those malignancies express an inducible tryptophan catabolic enzyme, indoleamine 2,3 dioxygenase 1 (IDO1). While IDO1 is not normally expressed at appreciable levels in the adult central nervous system, it's rapidly induced and/or upregulated upon inflammatory stimulus. The primary function of IDO1 is associated with conversion of the essential amino acid, tryptophan, into downstream catabolites known as kynure-nines. The depletion of tryptophan and/or accumulation of kynurenine has been shown to induce T cell deactivation, apoptosis and/or the induction of immunosuppressive programming via the expression of FoxP3. This understanding has informed immunotherapeutic design for the strategic development of targeted molecular therapeutics that inhibit IDO1 activity. Here, we review the current knowledge of IDO1 in brain tumors, pre-clinical studies targeting this enzymatic pathway, alternative tryptophan catabolic mediators that compensate for IDO1 loss and/or inhibition, as well as proposed clinical strategies and questions that are critical to address for increasing future immunotherapeutic effectiveness in patients with incurable brain cancer.
Keywords: Immunosuppression, Tryptophan, Kynurenine, Glioblastoma, IDO2, TDO
Introduction
The term, “brain tumors” refers to a group of heterogeneous neoplasms that differ in biology, etiology and disease progression between individual subtypes. Glioma, which accounts for 70 % of malignant adult primary brain tumors are stratified into low grade (II) and high-grade (grades III–IV), thought to originate from neural stem cells, progenitor cells or from de-differentiated mature glial cells. Though all glioma patients are treated with equal seriousness, grade IV astrocytoma, otherwise known as glioblastoma multiforme (GBM), is the most common and aggressive form with a mean onset age of 55 years. Resection is the first line of treatment, followed by radio-therapy (RT) and temozolomide (TMZ) administration. Yet, overall survival for patients with GBM remains at ~14.6 months post-diagnosis [1].
Brain tumors actively dampen the immune response by expanding and/or recruiting immunosuppressive regulatory T cells (Treg; CD3+CD4+CD25+FoxP3+). Although the exact factors required for Treg accumulation in GBM has yet to be described, CCL17 and CCL22 have been suggested to play a role [2, 3]. In vitro, Treg-produced TGF-β, IL-10, perforin and Granzyme B, as well as direct cell–cell contact via CTLA-4 and B7-H4 have been demonstrated to mediate the suppression of effector T cells, antigen presenting cells (APC) and natural killer (NK) cells. Most likely, it is the collective action of these mechanisms that convey Treg with the ability to pathologically contribute to brain tumor progression.
In addition to the professional T cell effectors of immunosuppression, several molecules have also been demonstrated to contribute in a similar fashion. Cytotoxic T lymphocyte antigen-4 (CTLA-4) serves as a critical immunoregulatory inhibitor at the level of initial T cell activation, in secondary lymphoid organs, as well as in tumor-infiltrating tissues. CTLA-4 competes with CD28 for binding to the co-stimulatory molecules, CD80 and CD86, on APC. CTLA-4:CD80/CD86 ligation inhibits T-cell activation by dephosphorylating the CD3ζ chain through the recruitment of SHP2 and PP2A phosphatases. CTLA-4 neutralizing antibodies have shown exciting pre-clinical promise, both with regard to reactivating the anti-brain tumor immune response, as well as increasing overall survival in animal models [4].
An alternative immunosuppressive pathway includes the PD-1 receptor and its ligands, PD-L1/2, which enforce and maintain T cell anergy. PD-L1 is expressed by GBM [5] and GBM-associated macrophages [6]. Several pharmaceutical entities are actively developing PD-1 (Merck; Bristol-Myers Squibb; Curetech) and PD-L1 (Medimmune; Roche) neutralizing antibodies. Two of these antibodies have achieved FDA designations. Among these, the humanized PD-1 mAbs, nivolumab and lambrolizumab, from Bristol-Myers Squibb and Merck, respectively, were recently demonstrated to possess safety and clinical efficacy in patients with end-stage melanoma [7]. Coincidently, a phase I–II trial evaluating the effectiveness of PD-1 blockade with CT-011 (pidilizumab) in patients with recurrent high-grade glioma is ongoing (NCT01952769).
More recently, linkage analysis between brain tumor metabolism and immunoresistance has highlighted a targetable pathway that promotes immunosuppression. Indoleamine 2,3 dioxygenase 1 (IDO1) is an inducible and rate limiting enzyme of tryptophan catabolism that, has emerged as one such candidate. Although not normally expressed and/or found at very low levels in the brain, IDO1 is rapidly increased upon inflammatory stimulus. As such, IDO1 is expressed in 96 % of malignant glioma of which, mRNA and protein expression levels correlate with overall patient survival [8, 9]. The selective nature of IDO1 expression in malignant glioma provides a higher potential for targeting specificity, of which, several pharmaceutical companies have designed high quality inhibitors against, including INCB24360 (Eli Lilly, Indianapolis, IN) and NLG919 (NewLink Genetics, Ames, IA).
IDO1 and tumor immunobiology
A relationship between cancer and elevated tryptophan catabolism was recognized in the early 1950s by analyzing the urine of bladder cancer patients [10]. Elevated urinary tryptophan catabolites were also found in breast cancer, prostate cancer, Hodgkin's lymphoma and leukemia [11–14]. Several studies suggested that IDO1 overexpression was associated with poor prognosis. Accordingly, IDO1 mRNA expression was positively associated with paclitaxel resistance of surgically-resected serous ovarian tumor specimens from patients with stage III disease. Additionally, its expression in tumor sections, detected by immunohistochemical (IHC) staining inversely correlated with patient survival in stage III and IV cancer [15]. Independently, high IDO1 expression in colorectal cancer was associated with a significant reduction of CD3+ infiltrating T cells and an increased frequency of liver metastases, when compared with tissue samples of low IDO1 expression [16].
In 1998, Munn et al. demonstrated that female mice pregnant with allogenic pups and treated with an IDO1-targeted inhibitor resulted in maternal immune-mediated rejection [17]. Later studies of experimental autoimmune encephalomyelitis (EAE) demonstrated that IDO1- generated tryptophan catabolites and their derivatives, shifted a primarily Th1-mediated disease into a Th2 non-pathological condition [18].
The role of IDO1 in tumor immune-mediated evasion was first introduced in 2002 when Friberg et al. found that Lewis lung carcinoma (LLC) cells stimulated a more robust allogeneic T cell response when cultured in the presence of an IDO1 inhibitor [19]. Importantly, the systemic administration of the same inhibitor delayed LLC tumor growth, in vivo. Shortly after this landmark discovery, Uyttenhove et al. demonstrated that P815B cells transfected to express IDO1, resisted immune-mediated rejection post-tumor cell implantation, while simultaneous administration with an IDO1 inhibitor led to an efficient rejection of the P815B tumors [20].
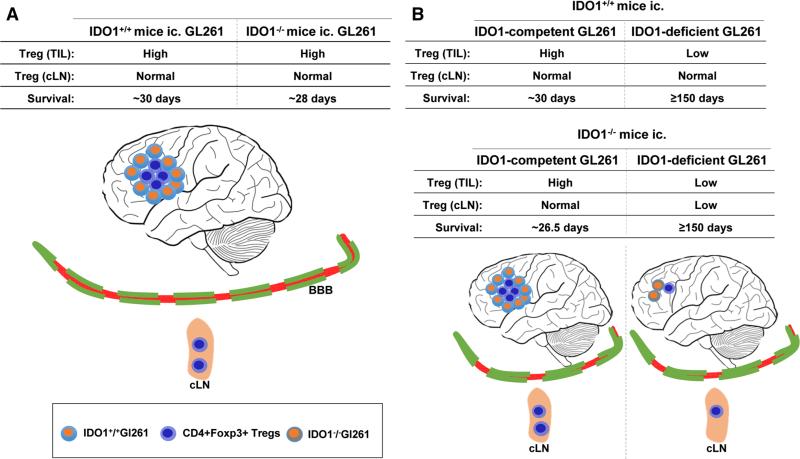
More recently, an association between IDO1 and Treg was elucidated. Nakamura et al. first studied the correlation of IDO1 and Treg abundance in the development and progression of uterine cervical cancer. IDO1 expression by tumor cells and infiltrating Treg were in close juxtaposition in the cervix with hallmarks of intraepithelial neoplasia [21]. Following this work, studies from two independent groups demonstrated that IDO1-expressing dendritic cells (DC) regulated the proliferation and activation of Treg [22, 23]. More recently, we found that IDO1 performed an immunosuppressive function in an immunocompetent model of malignant glioma. Using the syngeneic GL261 cell-based intracranial model, it was demonstrated that tumor derived-, rather than peripheral DC derived-IDO1, was required for Treg recruitment and suppression of the anti-brain tumor immune response. This was surprising given the data from peripheral tumor models demonstrating the requirement of DCs to express IDO1 for perpetuating maladaptive antitumor immune-mediated effects. Accordingly, the genetic silencing of IDO1 in GL261 cell-based brain tumors led to a substantial decrease in Treg recruitment and long-term survival (Fig. 1). Paradoxically, we recently found that the monotherapeutic inhibition of IDO1 via l-1MT failed to elicit a significant tumor rejection response [24]. Independent work confirmed our observation and further demonstrated that the combinatorial therapy of dl-1MT with RT and TMZ led to a synergistic survival benefit greater than the combination of RT and TMZ, alone [25]. These pre-clinical data highlight the complex nature of targeting IDO1 in brain tumors, but suggest that finding the appropriate combinatorial therapeutic strategy leads to an effective survival benefit.
Fig. 1.
Glioma-derived IDO1 plays a critical role in immune-mediated suppression in an experimental orthotopic brain tumor model. The in vivo role of IDO1 was investigated using the intracranially-injected GL261 model of malignant glioma. a The peripheral (i.e. any non-tumor cell) absence of IDO1 neither affected Treg levels in the brain tumor nor overall survival. b The genetic absence of brain tumor-derived IDO1 significantly decreased Treg levels in the brain tumor and increased overall survival. When brain tumors were deficient for IDO1, but competent in the periphery, Treg levels were normal in the draining cervical lymph nodes (cLN). In contrast, when mice were co-deficient for IDO1, both in the brain tumor and periphery, Treg levels were significantly decreased in the cLN, suggesting a level of communication between the central (tumor) and peripheral (cLN) compartments related to the expression of IDO1
Clinically, upregulated IDO1 mRNA in patient glioma has been shown to be associated with decreased overall survival (OS), indicated by 24.9 ± 2.76, 34 ± 2.71 and 44.3 ± 6.21 month survival in patients with upregulated-, intermediate-, and downregulated-IDO1 expression, respectively [9]. This study's findings were complemented by an independent analysis at the protein level, correlating upregulated IDO1 expression to an earlier average time of death, when compared with intermediately (P < 0.05)- and downregulated (P < 0.005)- expressing glioma [8]. IDO1 was found in a high frequency of glioma (72 of 75 specimens) with stronger expression more likely to be observed in high-grade- when compared to low-grade-glioma. Notably, IDO1 expression was also increased in the 6 cases of secondary glioblastoma, when compared to the initial low-grade counterparts. Most importantly, GBM patients stratified by strong versus weak IDO1 expression were found to possess significantly worse overall survival rates (P = 0.04) when IDO1 expression levels were high. Collectively, these clinical data confirm that upregulated IDO1 expression predicts a poor prognosis in glioma patients and that this trend predominates in patients with high-grade glioma.
Tryptophan catabolism
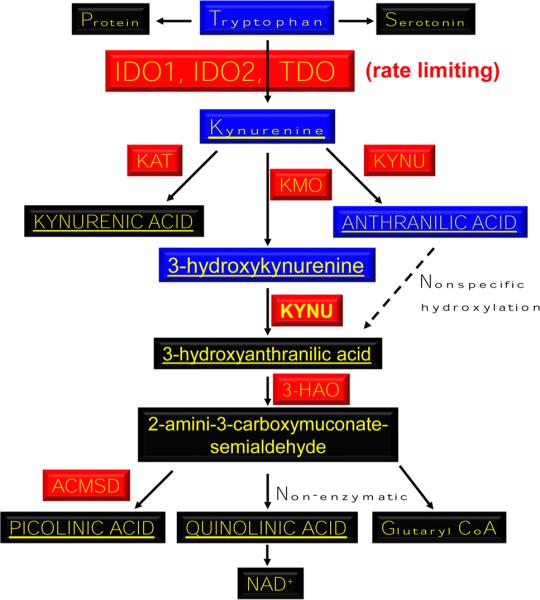
The first and rate-limiting step required for conversion of tryptophan into kynurenine (Fig. 2) is mediated by oxidation of the 2,3-double bond of tryptophan to form N-formylkynurenine, which is almost immediately converted to l-kynurenine (non-enzymatically). IDO1 is a monomeric heme-containing protein encoded by human chromosome 8p12. Recently, the gene homolog, IDO2, was discovered and characterized on the same chromosome [26] equipped with a similar tryptophan converting capability [27–29]. Sequence analysis indicated that, for humans and mice, IDO1 and IDO2 proteins possess 43 % homology and that the residues required for tryptophan catalytic activity are highly conserved [29]. It's important to note, however, that IDO1 possesses a higher affinity for l-tryptophan, when compared to IDO2 [30]. However, the role of the latter enzyme in brain tumors has yet to be comprehensively addressed, in vivo.
Fig. 2.
IDO1/IDO2-mediated tryptophan catabolism. The essential amino acid, tryptophan, is converted to the immunosuppressive catabolite, kynurenine, via IDO1 and IDO2. Kynurenine is further catabolized to other downstream substrates. KAT Kynurenine amino-transferase (I, II, III), KMO Kynurenine 3-monooxygenase, KYNU Kynureninase, 3-HAO 3-Hydroxyanthranilate 3,4-dioxygenase, ACMSD 2-Amino-3-carboxymuconate smialdehyde carboxylase. Red enzymes. Blue crosses the blood brain barrier (BBB). Underlined immunosuppressive
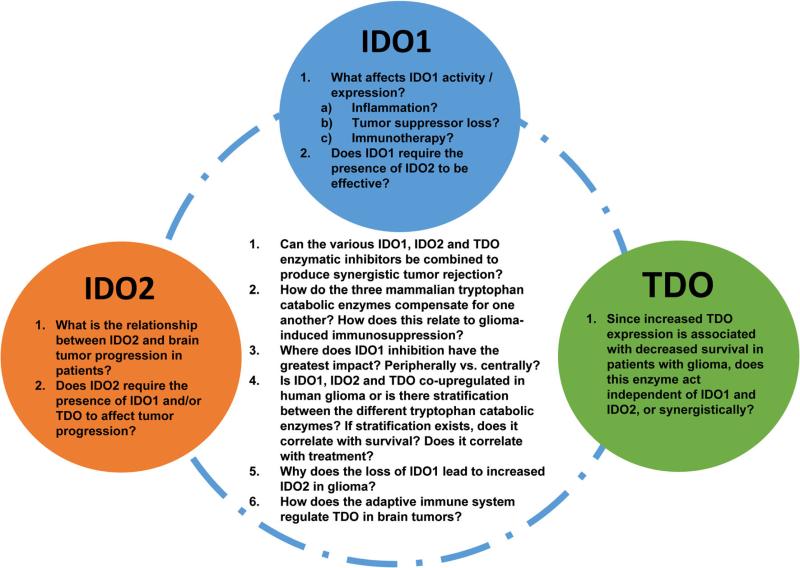
A third tryptophan catabolic enzyme, tryptophan dioxygenase (TDO), is also capable of cleaving tryptophan into kynurenine and is an interesting enzyme given that it functionally exists as a homotetrameric protein. In contrast to the, ‘need as required’, inducibility of IDO1, TDO is constitutively expressed in the liver and thought to serve as the primary mediator of systemic kynurenine levels [31]. Relevantly, upregulated TDO mRNA expression, like IDO1, has previously been correlated with overall survival in patients with glioma [32, 33]. Collectively, these data highlight the multiple enzymes that can lead to the immunosuppressive catabolite, kynurenine, and raise questions regarding future tryptophan catabolic inhibitory strategies (Fig. 3).
Fig. 3.
Critical questions addressing compensatory tryptophan catabolic pathways that decrease the effectiveness of immunotherapy against brain tumors. The complexity of the three tryptophan catalytic enzymes originates from a common functional redundancy with independent tissue-driven regulators. Intrinsic to each enzyme are independent questions that address the induction, maintenance and modification of expression (along the circle), while queries that encompass dependent pathways are also provided (in the circle). Notably, how these enzymes compensate for one another when pharmacologically targeted versus genetically inhibited is a currently unexplored topic that is potentially relevant for significantly increasing brain tumor immunotherapeutic efficacy. Also, based on our previous study demonstrating the substantial impact of adaptive immune deficiency on the expression of tryptophan catabolic enzymes in brain tumors [24], highlights the lack of insight into molecular mechanisms addressing this regulation
The capability of tryptophan passing the plasma membrane via the large amino acid transporter raises the possibility that a ‘tryptophan sink’ can be formed in a microenvironment concentrated for IDO1 expressing cells [34]. Since the affinity of tRNA synthetase for tryptophan is higher than that of IDO1 in most cells [35–37], this ‘tryptophan sink’ has a minimal effect on the proliferation of most cells. Accordingly, in the context of brain tumors, it's tempting to speculate that the high expression of IDO1 might not convey an inhibitory effect on tumor cells, but rather, focus the impact on immune cells. This is based on several lines of evidence suggesting that T cells undergo a rapid and substantial growth arrest under such conditions due to a tryptophan-sensitive checkpoint which inhibits the cell cycle in the G1 phase [34]. Assuming that this latter mechanism holds true, in vivo, it likely contributes to the dominant tolerance of tumors, transplants and the allogeneic fetus [17, 20, 38]. Additionally, IDO1 activity leads to the induction of GCN2, a kinase activated by uncharged tRNA at the ribosome that initiates an integrated stress response via phosphorylation of the a-subunit of eukaryotic translation initiation factor 2 (eIF2a); ultimately resulting in the suppression of effector T cell proliferation [39]. The GCN2 pathway has also been shown to play a critical role in IDO1-mediated Treg activation in association with other immunosuppressive signals including CTLA-4 [23]. Notably, IDO2 also activates this pathway, but with independent regulatory consequences [28]. Tryptophan depletion also triggers the mTOR signaling pathway resulting in autophagy, which is reversed by tryptophan and/or d-1MT, a tryptophan mimetic [40].
The inaccessibility of tryptophan as a driver of immunosuppression is complemented by a related pathway that relies on the presence and accumulation of biologically active tryptophan catabolites in the form of kynurenines. Early mechanistic studies of IDO1 indicated that some tryptophan catabolites possessed the ability to induce apoptosis in CD4+ T cells. Terness et al. [41] demonstrated that l-kynurenine, 3-hydroxykynurenine and 3-hydroxyanthranilic acid suppressed T cell proliferation commensurate with the induction of apoptosis. This finding was independently confirmed by Fallarino et al. [42], demonstrating in vitro that, kynurenines induce the selective apoptosis of murine thymocytes and Th1-, but not Th2-cells. Furthermore, Mezrich et al. [43] recently showed that the interaction of l-kynurenine with the aryl hydrocarbon receptor (AHR) leads to an AHR-dependent induction of Treg. Notably, TGF-β was a requirement for this effect and highlights its role in regulating AHR expression in DC [43]. A subsequent study confirmed this relationship, finding a deficiency in Treg differentiation when mice lacking AHR were cultured in the presence of kynurenine [44].
IDO1 as a signaling molecule
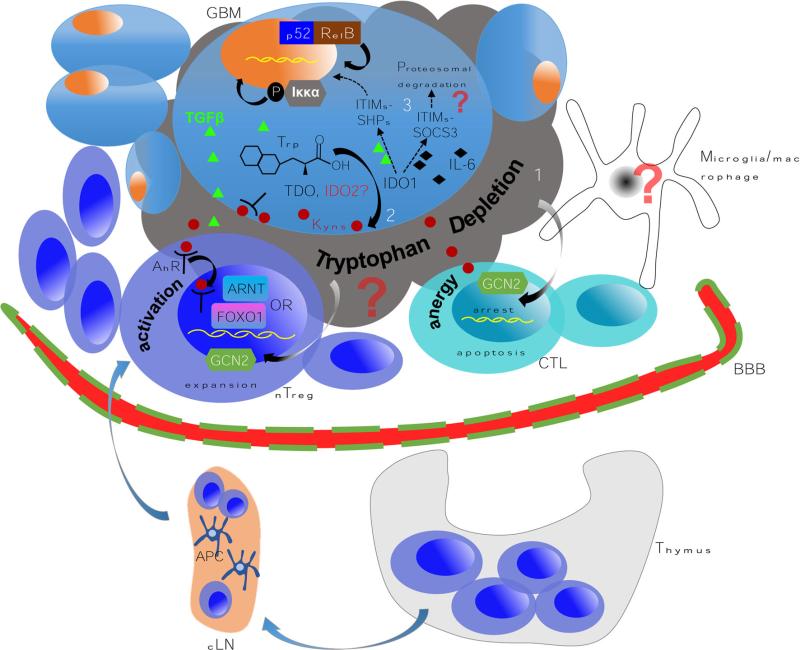
Though the presence and absence of tryptophan related mediators likely contribute to anti-brain tumor immunity, recent work has identified an unappreciated aspect of IDO1 that may reinforce immunosuppression, independent of its enzymatic function. Elegant work from Ursula Groh-mann's laboratory deciphered the mechanisms of differential programing by T cells upon stimulation from either immunogenic or tolerant ligands [45], finding that the suppressor of cytokine signaling 3 (SOCS3), a critical modulator of the immune response, is selectively induced by CD28-Ig/IL-6 [46], binding to IDO1 and targeting the complex for ubiquitination and proteasomal degradation. The structural basis for the IDO1/SOCS3 interaction was located in the SOCS3 Src homology 2 (SH2) domain and tyrosine residues within the putative immunoreceptor tyrosine-based inhibitory motifs (ITIM) intrinsic to IDO1. In their subsequent work [47], they demonstrated that TGF-β activates the phosphatases, SHP-1 and SHP-2, in plasmacytoid DC (pDC). Importantly, the activated phosphatases interacted with tyrosine residues of the IDO1 ITIMs. In contrast to the impact of TGF-β on IDO1 signaling, no such activation and interaction was observed when pDC were treated with IFN-γ, highlighting the contextual nature of this signal transduction-mediated mechanism. These data delineate a new paradigm highlighting the immuno-regulatory function of IDO1, independent of tryptophan catabolism and dependent on cytokine context (Fig. 4). However, the simplicity of these in vitro conditions do not reflect the complexity of a ‘cytokine storm’ that would reflect the microenvironment of a brain tumor. To address this, future studies are aimed at understanding the impact of IDO1 signaling, in situ, in the presence of potent enzymatic inhibition.
Fig. 4.
A paradigm for testing the multiple independent mechanisms that reinforce IDO1-mediated immunosuppression in malignant glioma. 1 The high expression of IDO1 leads to a commensurately high rate of tryptophan conversion and depletion. This induces cell cycle arrest and/or anergy in the effector (anti-glioma) T cell compartment via the eIF2α kinase GCN2-dependent pathway [39]. Simultaneously, the same mechanism activates Treg to become fully functional/mature in association with CTLA4:CD80/CD86 co-inhibition [23]. Although both microglia and macrophages can express IDO1, their contribution to this mechanism has yet to be established. 2 A complementary pathway mediated by IDO1 produces kynurenine that interacts with the aryl hydrocarbon receptor (AhR) leading to the expansion of glioma-resident Treg. Recent work has highlighted multiple interactions between the Ahr:Kyn. complex that may be required for Treg responsiveness. AhR interacts with the aryl hydrocarbon receptor nuclear translocator (ARNT) to mediate specific transcriptional programming [58] that may provide synergistic or independent impact to another recently identified heterodimeric partner, FOXO1 [59]. 3 The final known pathway that IDO1 utilizes to reinforce immunosuppression is via the two intrinsic immunoreceptor tyrosine-based inhibitory motifs (ITIMs) [47]. Phosphorylation of ITIMs in IDO1 triggers a non-canonical NF-κB pathway, leading to phosphorylation of IKKα and nuclear translocation of the NF-κB subunits, p52 and RelB; reinforcing immunotolerance and TGF-β production. While it is still unclear whether all three of these mechanisms are actively involved in glioma, current studies in our laboratory aim to determine the various contributions
IDO1 and combinatorial immunotherapy
Chemical therapeutics have been developed to inhibit IDO1 catalysis with a majority belonging to the tryptophan derivative and β- carboline classes of molecules. Among these small chemicals,l-1MT has long been known to be a competitive inhibitor [48], while phase I clinical trials utilizing d-1MT have been deployed for the treatment of metastatic and/or refractory solid tumors (NCT00567931). In vitro enzymatic assays have confirmed that IDO1 has a tenfold higher affinity for the l- rather than d-stereoisomer of 1MT, indicating that d-1MT is less efficient in neutralizing the enzymatic activity of IDO1 [49]. Therefore, it is somewhat unexpected that, when testing the 1MT stereo-isomers as an adjuvant to radiochemotherapy, it was revealed that l-1MT is more effective for abrogating the inhibition of T cell proliferation in mixed lymphocyte reactions, as well as decreasing the growth of tumors in mice transplanted melanoma and breast cancer [50]. This unexpected outcome is complemented by the finding that the treatment effect of d-1MT is IDO1 dependent, while IDO2 is effectively inhibited by l-1MT [51–53].
The confusion arising from the use of 1MT in models of cancer has prompted the search for more selective IDO inhibitors that distinguish IDO1 versus IDO2. Thus far, two chemical compounds, INCB024360 and Amg-1, are more selective for IDO1 than IDO2 [54, 55]. However, a search for efficient IDO2 inhibitors are underway. Bakmiwewa et al. [56] identified several IDO2 specific inhibitors including tenatoprazole, with an IC50 value of 1.8 μM and no inhibition for IDO1 or TDO at a concentration of 100 μM. Specific targeting of the third tryptophan catabolic enzyme, TDO, has been challenging. However, LM10, a recently identified candidate has shown to be efficient for TDO inhibition, but not IDO1 [32] in vivo. Ultimately, future searches should be aimed at developing molecules that maximally inhibit enzymatic inhibition against all three tryptophan catabolic enzymes.
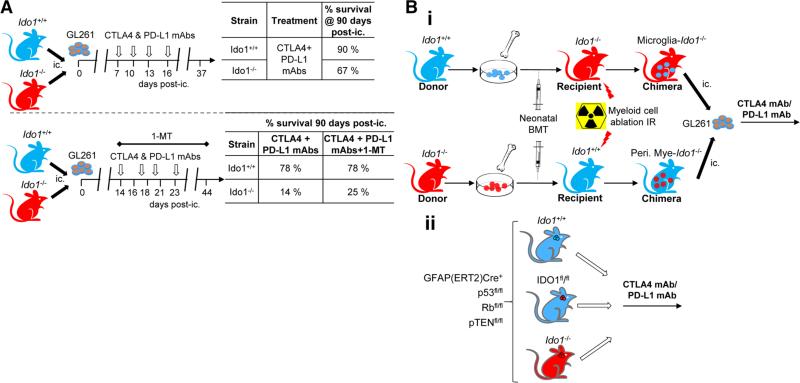
Recently, we tested IDO blockade in combination with neutralization of other immunosuppressive pathways in the context of brain tumors. This was predicated on the hypothesis that therapeutic targeting against IDO, alone, would not lead to an effective anti-tumor immune response required for the rejection of large well-established glioma. Our criteria for ‘large well-established’ brain tumors was based on the 14 day post-intracranial time point using the GL261 mouse model. Whereas single agent blockade of IDO via 1MT led to a relatively small increase in overall survival, immunotherapy targeting IDO, CTLA-4 and PD-L1, simultaneously, demonstrated an ~80 % overall durable survival benefit [24] (Fig. 5a). Interestingly, the dual treatment with CTLA-4 and PD-L1 mAbs resulted in a similar survival benefit when compared to the same strategy plus 1MT, suggesting that IDO blockade may be both a downstream and redundant pathway built into CTLA-4 and/or PD-L1 neutralization. Also notably was that, only the triple, but not dual therapy resulted in a significant decrease in Treg levels suggesting that, although there was no survival difference, the additional inhibition of IDO was favorable for simultaneously decreasing immunosuppression. Paradoxically, brain tumor-bearing mice genetically-deficient for IDO1 showed a substantially decreased dual and triple immunotherapeutic effectiveness, when compared to mice fully competent for IDO1, a finding in direct contrast to what has been reported in the peripheral tumor literature [57]. This latter observation highlights one important consideration for developing effective brain tumor immunotherapeutic strategies; pan-inhibition of immunosuppressive pathways has different effects against tumors that arise, peripherally, when compared to the brain.
Fig. 5.
Role of IDO1 in GBM immunotherapy. a Efficacy of immune checkpoint blockade with or without 1MT was evaluated in peripheral IDO1-competent and -deficient mice using the orthotopic GL261 mouse brain tumor model. Unexpectedly, mice required peripheral IDO1 to maximize immune checkpoint-mediated immunotherapeutic survival benefits. b A hypothetical series of experiments that address the question, ‘which cells require IDO1 to mediate maximally-effective immune checkpoint therapy’? i. A schema that proposes the creation of bone marrow chimeric mice to address which cells require IDO1, including CNS-resident microglia- versus peripheral myeloid (i.e. macrophages/dendritic cells) cells, in the context of immune checkpoint blockade therapy against brain tumors. ii. A separate schema proposes the creation of testing immune checkpoint blockade therapy in a tamoxifen-induced mouse model of malignant glioma [GFAP(ERT2)Cre+/−p53 fl/flRbfl/flpTENfl/fl] [60] crossed with floxed IDO1 (knocking out IDO1 selectively in the astrocytoma)-, as well as global IDO1 knockout (genetically-deficient for IDO1 in all cells)-mice. The latter models are especially relevant, since tumor induction occurs in the absence of inflammation (in contrast to the orthotopic GL261 model) and therefore analogous to the human counterpart
Since peripheral IDO1-deficiency decreased the effectiveness of CTLA-4/PD-L1-mediated immunotherapy against brain tumors, similar deficiencies may also ablate the responsiveness to this treatment in GBM patients. To understand ‘where’ IDO1 is required for therapeutic effectiveness to be maximal, we are in the process of generating and/or analyzing bone marrow chimeric- and high fidelity transgenic-models of GBM that are selectively deficient for IDO1 (Fig. 5b). Ultimately, defining where IDO1 is required, pro-actively, may allow future clinicians to enroll the patient cohort that will benefit from this strategy, optimally, while sparing those who will not.
Conclusion
Based on recent clinical trials demonstrating promise for patients with end-stage melanoma treated with immuno-therapy, we now live in an era of effectively applied cancer immunology. However, understanding how IDO1 fits into the greater picture has yet to be fully realized. In GBM, almost all tumors express some degree of IDO1. Here, we have introduced the concept that the multi-versatility of IDO1, acting both as an enzyme and signal transducer, make it a complicated molecule to target. Future therapeutics should take this pleiotropic action into consideration, while simultaneously assessing the potential for combination with additional immunotherapeutic modalities. Interrogating these interactions is a worthy goal to ensure that patients with malignant brain tumors enjoy the same survival success that those individuals with melanoma now cherish.
Acknowledgments
This work was supported by an American Brain Tumor Association Discovery Grant (D.A.W.), as well as NIH grants NIH F32 NS073366 (D.A.W.), NIH K99 NS082381 (D.A.W.), NIH R00 NS082381 (D.A.W.) R01 CA138587 (M.S.L.), NIH R01 CA122930 (M.S.L.), NIH U01 NS069997 (M.S.L.).
Footnotes
Conflict of interest The authors declare that no competing interests exist.
Contributor Information
Lijie Zhai, Department of Neurological Surgery, Northwestern University Feinberg School of Medicine, 300 East Superior Street, Tarry Building 2-703, Chicago, IL 60611, USA.
Kristen L. Lauing, Department of Neurological Surgery, Northwestern University Feinberg School of Medicine, 300 East Superior Street, Tarry Building 2-703, Chicago, IL 60611, USA
Alan L. Chang, The Brain Tumor Center, The University of Chicago Pritzker School of Medicine, Chicago, IL 60637, USA
Mahua Dey, The Brain Tumor Center, The University of Chicago Pritzker School of Medicine, Chicago, IL 60637, USA.
Jun Qian, Department of Neurological Surgery, Northwestern University Feinberg School of Medicine, 300 East Superior Street, Tarry Building 2-703, Chicago, IL 60611, USA.
Yu Cheng, Shanghai East Hospital, The Institute for Biomedical Engineering and Nano Science, Tongji University School of Medicine, Shanghai, China.
Maciej S. Lesniak, The Brain Tumor Center, The University of Chicago Pritzker School of Medicine, Chicago, IL 60637, USA
Derek A. Wainwright, Department of Neurological Surgery, Northwestern University Feinberg School of Medicine, 300 East Superior Street, Tarry Building 2-703, Chicago, IL 60611, USA
References
- 1.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 3.Mizukami Y, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122(10):2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 4.Fecci PE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 5.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 6.Bloch O, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PD-1 inhibitor becomes “breakthrough therapy”. Cancer Discov. 2013;3(7):OF14. doi: 10.1158/2159-8290.CD-NB2013-074. [DOI] [PubMed] [Google Scholar]
- 8.Mitsuka K, et al. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72(6):1031–1039. doi: 10.1227/NEU.0b013e31828cf945. doi:10.1227/NEU.0b013e31828cf945. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright DA, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012;18(22):6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyland E. The estimation of tryptophan metabolites in the urine of patients with cancer of the bladder. Biochem J. 1995;60:v. Annual General Meeting. [PubMed] [Google Scholar]
- 11.Wolf H, Madsen PO, Price JM. Studies on the metabolism of tryptophan in patients with benign prostatic hypertrophy or cancer of the prostate. J Urol. 1968;100(4):537–543. doi: 10.1016/s0022-5347(17)62566-7. [DOI] [PubMed] [Google Scholar]
- 12.Rose DP. Tryptophan metabolism in carcinoma of the breast. Lancet. 1967;1(7484):239–241. doi: 10.1016/s0140-6736(67)91301-3. [DOI] [PubMed] [Google Scholar]
- 13.Ivanova VD. Disorders of tryptophan metabolism in leukaemia. Acta Unio Int Contra Cancrum. 1964;20:1085–1086. [PubMed] [Google Scholar]
- 14.Ambanelli U, Rubino A. Some aspects of tryptophan–nicotinic acid chain in Hodgkin's disease. Relative roles of tryptophan loading and vitamin supplementation on urinary excretion of metabolites. Haematol Lat. 1962;5:49–73. [PubMed] [Google Scholar]
- 15.Okamoto A, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11(16):6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 16.Brandacher G, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12(4):1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 17.Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 18.Platten M, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310(5749):850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 19.Friberg M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101(2):151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 20.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, et al. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98(6):874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung DJ, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114(3):555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Investig. 2007;117(9):2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wainwright DA, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, et al. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer. 2014;2:21. doi: 10.1186/2051-1426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray MF. The human indoleamine 2,3-dioxygenase gene and related human genes. Curr Drug Metab. 2007;8(3):197–200. doi: 10.2174/138920007780362509. [DOI] [PubMed] [Google Scholar]
- 27.Yuasa H, et al. Evolution of vertebrate indoleamine 2,3-dioxygenases. J Mol Evol. 2007;65(6):705–714. doi: 10.1007/s00239-007-9049-1. [DOI] [PubMed] [Google Scholar]
- 28.Metz R, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound d-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 29.Ball HJ, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Yuasa HJ, et al. Characterization and evolution of vertebrate indoleamine 2, 3-dioxygenases IDOs from monotremes and marsupials. Comp Biochem Physiol B. 2009;153(2):137–144. [PubMed] [Google Scholar]
- 31.Kanai M, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilotte L, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci. 2012;109(7):2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opitz CA, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 34.Munn DH, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189(9):1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Praetorius-Ibba M, et al. Ancient adaptation of the active site of tryptophanyl-tRNA synthetase for tryptophan binding. Biochemistry. 2000;39(43):13136–13143. doi: 10.1021/bi001512t. [DOI] [PubMed] [Google Scholar]
- 36.Kudo Y, Boyd CA. Characterisation of l-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J Physiol. 2001;531(Pt 2):405–416. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo Y, Boyd CA. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochim Biophys Acta. 2000;1500(1):119–124. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 38.Alexander AM, et al. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes. 2002;51(2):356–365. doi: 10.2337/diabetes.51.2.356. [DOI] [PubMed] [Google Scholar]
- 39.Munn DH, et al. GCN2 kinase in T Cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Metz R, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by d-1-methyl-tryptophan. Oncoimmunology. 2012;1(9):1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terness P, et al. Inhibition of allogeneic T Cell proliferation by indoleamine 2,3-dioxygenase–expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196(4):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fallarino F, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 43.Mezrich JD, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen NT, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci. 2010;107(46):19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orabona C, et al. SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc Natl Acad Sci. 2008;105(52):20828–20833. doi: 10.1073/pnas.0810278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orabona C, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5(11):1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 47.Pallotta MT, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 48.Cady SG, Sono M. 1-Methyl-dl-tryptophan, beta-(3-benzofuranyl)-dl-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-dl-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291(2):326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 49.Peterson ACM, Migawa MT, Martin MJ, Hamaker LK, Czerwinski KM, Zhang W, Arend RA, Fisette PL, Okazi Y, Will JA, Brown RR, Cook JM. Evaluation of functionalized tryptophan derivatives and related compounds as competitive inhibitors of indoleamine 2,3-dioxygenase. Med Chem Res. 1994;3:531–544. [Google Scholar]
- 50.Hou DY, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 51.Austin CJ, et al. Biochemical characteristics and inhibitor selectivity of mouse indoleamine 2,3-dioxygenase-2. Amino Acids. 2010;39(2):565–578. doi: 10.1007/s00726-010-0475-9. [DOI] [PubMed] [Google Scholar]
- 52.Qian F, et al. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol Immunother. 2012;61(11):2013–2020. doi: 10.1007/s00262-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuasa HJ, et al. 1-l-methyltryptophan is a more effective inhibitor of vertebrate IDO2 enzymes than 1-d-methyltryptophan. Comp Biochem Physiol B. 2010;157(1):10–15. doi: 10.1016/j.cbpb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Liu X, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115(17):3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 55.Meininger D, et al. Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta. 2011;1814(12):1947–1954. doi: 10.1016/j.bbapap.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 56.Bakmiwewa SM, et al. Identification of selective inhibitors of indoleamine 2,3-dioxygenase 2. Bioorg Med Chem Lett. 2012;22(24):7641–7646. doi: 10.1016/j.bmcl.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Holmgaard RB, et al. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210(7):1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beischlag TV, et al. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kerdiles YM, et al. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33(6):890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chow LM, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19(3):305–316. doi: 10.1016/j.ccr.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]