Abstract
Background
Adolescent major depressive disorder (MDD) is a life-threatening brain disease with limited interventions. Treatment resistance is common, and the illness burden is disproportionately borne by females. 31-Phosphorus magnetic resonance spectroscopy (31P MRS) is a translational method for in vivo measurement of brain energy metabolites.
Methods
We recruited 5 female adolescents who had been on fluoxetine (Prozac®) for ≥8 weeks, but continued meet diagnostic criteria for MDD with a Children’s Depression Rating Scale-Revised (CDRS-R) raw score ≥40. Treatment response was measured with the CDRS-R. 31P MRS brain scans were performed at baseline, and repeated following adjunctive creatine 4 g daily for 8 weeks. For comparison, 10 healthy female adolescents underwent identical brain scans performed 8 weeks apart.
Results
The mean CDRS-R score declined from 69 to 30.6, a decrease of 56%. Participants experienced no Serious Adverse Events, suicide attempts, hospitalizations or intentional self-harm. There were no unresolved treatment-emergent adverse effects or laboratory abnormalities. MDD participants’ baseline CDRS-R score was correlated with baseline pH (p=0.04), and was negatively correlated with beta-nucleoside triphosphate (β-NTP) concentration (p=0.03). Compared to healthy controls, creatine-treated adolescents demonstrated a significant increase in brain Phosphocreatine (PCr) concentration (p=0.02) on follow-up 31P MRS brain scans.
Limitations
Lack of placebo control; and small sample size.
Conclusions
Further study of creatine as an adjunctive treatment for adolescents with SSRI-resistant MDD is warranted.
Keywords: Female, Adolescent, Depression, Creatine, Magnetic resonance spectroscopy, Combination drug therapy
1. Introduction
Adolescent major depressive disorder (MDD) is associated with significant disability and mortality (Birmaher et al., 2007). Up to 8% of adolescents have MDD at any point in time (SAMHSA, 2008), and MDD eventually effects nearly 1 in 4 American children (Lewinsohn et al., 1993). In the 13–17 age group, mood disorder is the leading cause of hospitalization (Owens et al., 2003), and juvenile MDD predicts depression and psychosocial impairment in adulthood (Fombonne et al., 2001; Pine et al., 1999; Weissman et al., 1999). Pediatric depression also imposes substantial economic costs on society (Lynch and Clarke, 2006). Thus, novel interventions for MDD in the critical adolescent stage of development are urgently needed.
Female adolescents are affected by MDD at twice the rate of males (Merikangas et al., 2010), and this gender disparity continues throughout women’s reproductive years (Blazer et al., 1994; Kessler et al., 2003; Nolen-Hoeksema, 1990; Robins and Regier, 1991). The sex-based imbalance is one of the most replicated findings in epidemiology, and is robust to international sampling across continents and cultures (Maier et al., 1999; Seedat et al., 2009; Weissman et al., 1996). Despite the evidence for sex-based differences in MDD pathophysiology (Hyde et al., 2008; Young and Korszun, 2010) and treatment response (Bigos et al., 2009; Dalla et al., 2010), few somatic treatment studies have focused on adolescent females.
Selective serotonin reuptake inhibitors (SSRIs) are first-line medications for adolescent MDD (Birmaher et al., 2007; Ma et al., 2005), and are annually prescribed to 3.9% of American teenagers (Vitiello et al., 2006). However, at least 40% of adolescents with MDD receiving an SSRI show an inadequate response treatment (Brent et al., 2008; Kennard et al., 2009). The response rate in pediatric MDD trials favors SSRIs over placebo by just 10.8% (Usala et al., 2008); even this small margin may be due to publication bias (Whittington et al., 2004). In addition concerns regarding efficacy, meta-analyses and systematic reviews suggest that when prescribed to young people, SSRIs increase the risk for suicidality (Dubicka et al., 2006; Hammad et al., 2006; Hetrick et al., 2007), and attempted or completed suicide (Barbui et al., 2009). Clearly, safe and novel treatments for adolescent MDD are urgently needed.
Converging lines of evidence suggest that mood disorders are associated with changes in cerebral energy metabolism (Kato, 2007; Moretti et al., 2003; Rezin et al., 2009; Shao et al., 2008), that normalize with resolution of a mood episode (Iosifescu et al., 2008; Iosifescu and Renshaw, 2003). 31-Phosphorus magnetic resonance spectroscopy (31P MRS) is the only method for in vivo measurement of these changes (Renshaw et al., 2001). The 31P MRS literature in pediatric MDD is expanding (Kondo et al., 2011), and 31P MRS studies in adults show a distinctive pattern of energy-related metabolites in MDD: decreased beta-nucleoside triphosphate (β-NTP; largely adenosine triphosphate, or ATP) and increased phosphocreatine (PCr) (Iosifescu et al., 2008; Moore et al., 1997; Volz et al., 1998). This pattern is more common in females (Renshaw et al., 2001), and is associated with increased likelihood of responding to both an SSRI in treatment naïve MDD (Renshaw et al., 2001), and thyroid augmentation in treatment-resistant MDD (Iosifescu et al., 2008). In healthy adults, administration of the dietary supplement creatine induces this pattern in brain chemistry (Lyoo et al., 2003), suggesting the possibility of utilizing creatine to modify brain energy metabolism.
Creatine is an organic acid occurring naturally in vertebrates, where it plays a role in energy homeostasis in tissues with variable energy demands, principally skeletal muscle and brain. Through the reversible creatine kinase reaction, creatine raises cellular PCr levels, which increases capacity for cellular ATP resynthesis (Jost et al., 2002).
Data from preclinical animal studies suggest that creatine may have sex-specific antidepressant properties that favor females. The Porsolt Forced Swim Test (FST) is a widely-used experimental model of depression (Porsolt et al., 1977). Creatine supplementation confers a longer latency to immobility in the FST in female Sprague–Dawley rats, but not males (Allen et al., 2010b). Creatine’s sex-specific antidepressant effect in rats is independent of SSRI administration (Allen et al., 2010a).
To date there are no studies of adjunctive creatine in female adolescents with SSRI-resistant MDD. Given the gender-specific effect of creatine in animal studies (Allen et al., 2010a, 2010b), clinical trials in male adolescents must be deferred on until creatine is shown to be safe in adult males with SSRI-refractory MDD. We conducted an open-label study of adjunctive creatine for female adolescents with MDD who had failed an adequate trial of fluoxetine (Prozac®). In addition to standardized clinical assessments, participants underwent 31P MRS brain scans at baseline, and after 8 weeks of creatine augmentation. Healthy female controls were recruited for comparison 31P MRS brain scans.
2. Methods
The University of Utah Institutional Review Board (IRB) approved the study. Informed consent included both written parental consent and written participant assent. An external Data Safety and Monitoring Board with authority to halt the study monitored patient safety outcomes.
Participants were recruited through referrals and IRB-approved advertising. Consecutive patients who met inclusion criteria were enrolled. Inclusion criteria were: females 13–18 years of age with a primary diagnosis of MDD; current fluoxetine treatment for ≥8 weeks with ≥4 weeks at a dose of ≥40 mg/day (if doses higher than 20 mg/day were not tolerated, participants could meet inclusion criteria by taking fluoxetine 20 mg/day for ≥8 weeks); and a current Children’s Depression Rating Scale-Revised (CDRS-R) (Poznanski and Mokros, 1996) raw score ≥40. No restrictions were placed on concomitant medications or psychotherapy. Study exclusion criteria were: renal disease; proteinuria; contraindication to magnetic resonance scanning (e.g. ferromagnetic implant); primary diagnosis other than MDD; psychotic symptoms; positive pregnancy test; active use of alcohol or illicit drugs; or mental retardation. Diagnoses were established with the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997). A complete blood count, metabolic panel, lipid profile, TSH and urinalysis were obtained at baseline. Laboratory studies were repeated at the conclusion of treatment, to prospectively identify abnormalities associated with creatine administration.
Participants with MDD were treated with fixed-dose Creapure® brand of creatine (AlzChem LLC, Trostberg, Germany) 4 g by mouth daily for 8 weeks. At each visit vital signs and adverse were recorded, and the following rating scales were administered: CDRS-R, the Clinical Global Impressions scale (Guy, 1976) and the Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner, 2010). The primary outcome was the change from baseline in CDRS-R raw score.
31P MRS brain scans were acquired with a Siemens 3 Tesla MRI scanner (Siemens AG, Munich, Germany) that is approved for clinical use. A two-dimensional chemical shift imaging free induction decay (2D CSI FID) pulse sequence with an Fourier voxel resolution of 25×25×25 mm3, Field of View (FOV) = 200 × 200 × 25 mm3, TR/TE = 3000/2.3 ms, vector size=1024, bandwidth=2500 Hz, data collection time=11.2 min and the number of averages=24 was implemented to collect 2D CSI FID data, using a 31P/1H dual-tuned coil (Clinical MR Solutions LLC, Brookfield, Wisconsin). The high-resolution localization images of CSI data were acquired using an inversion recovery magnetization prepared rapid gradient echo (MPRAGE) pulse sequence with isotropic 1 mm3 resolution. The imaging parameters were as follows: TR/TE=2000/3.37 ms, FOV=256×192× 144 mm3, and matrix size=256×192×144, total acquisition time=4.8 min. Participants were instructed that they could discontinue scanning at any time if they experienced discomfort (e.g. claustrophobic anxiety).
31P MRS data were analyzed using the jMRUI software package (jMRUI version 4.0, European Community). A Hamming filter was applied to reduce signal contamination from neighboring voxels, prior to the 2D Fast Fourier Transform (FFT) on the raw data. Nine voxels from a 25 mm slice located at the corpus callosum, anterior commissure and posterior commissure were summed following 2D FFT. Each voxel FID was apodized with a 10 Hz exponential line broadening before zero filling and FFT. Zero-order and first-order phase correction was performed in all spectrums. Signal amplitudes for individual metabolites were calculated with the Advanced Method for Accurate, Robust and Efficient Spectral fitting of MRS data (AMARES), an algorithm in the jMRUI application.
3. Results
3.1. Clinical measures
Summary results for MDD participants are presented in Table 1. All were Caucasian females. No participant initiated or terminated psychosocial treatment or psychotropic medication during the study, and fluoxetine doses were held constant. Five participants completed 8 weeks of adjunctive creatine and the 31P MRS scans. No participant withdrew from the study.
Table 1.
Summary of results for open-label adjunctive creatine treatment for 8 weeks.
| # | Age sex | Diagnoses | Duration of MDD episode (weeks) | CDRS-R baseline | CDRS-R final | Adverse events | C-SSRS baseline | C-SSRS during treatment with creatine (weeks) | Concomitant medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 F | MDD social phobia | 32 | 82 | 24 | Tremor† (Multi-year history of tremor prior to study) | –Active suicidal ideation with plan and intent 5 months prior to study entry –Wish to be dead 2 weeks prior to study drug |
None during treatment | Fluoxetine 40 mg Aripiprazole 2.5 mg Clonidine 0.1 mg Clonazepam 0.5 mg |
| 2 | 15 F | MDD dysthymic disorder social phobia | 24 | 76 | 44 | Suicidal ideation† Sinus congestion† Dyspepsia* |
–Suicide Attempt-Overdose 11 months prior to study entry –Wish to be dead 2 weeks prior to study drug |
Active suicidal ideation (1–2) Resolved at week 3 No recurrence |
Fluoxetine 40 mg Ethinyl estradiol/levonorgestrel 20 μg/0.1 mg |
| 3 | 18 F | MDD social phobia | 190 | 59 | 29 | Suicidal ideation† H1N1 influenza† Headache* Nausea/Vomiting* |
–Suicide attempt-overdose 5 years prior to study entry –Wish to be dead 1 week prior to study drug |
Wish to be dead (1) Resolved at week 2 No recurrence |
Fluoxetine 40 mg Norgestimate/ethinyl estradiol 0.25 mg/0.035 mg |
| 4 | 14 F | MDD | 18 | 66 | 23 | Suicidal ideation† Bruising† Headache* Acne† URI† |
–Two suicide attempts-overdose 22 months prior to study entry 5 months prior to study entry –Wish to be dead 1 week prior to study drug |
Wish to be dead (1–5) Resolved at week 6 No recurrence |
Fluoxetine 20 mg |
| 5 | 15 F | MDD generalized anxiety disorder | 92 | 62 | 33 | URI† Epistaxis† Headache* |
–No lifetime suicidal ideation or suicide attempts | None during treatment | Fluoxetine 40 mg |
CDRS-R: Children’s Depression Rating Scale-Revised Raw Score.
C-SSRS: Columbia Suicide Severity Rating Scale.
MDD: major depressive disorder.
URI: upper respiratory infection.
Adverse event possibly or probably related to study drug.
Adverse event unrelated to study drug.
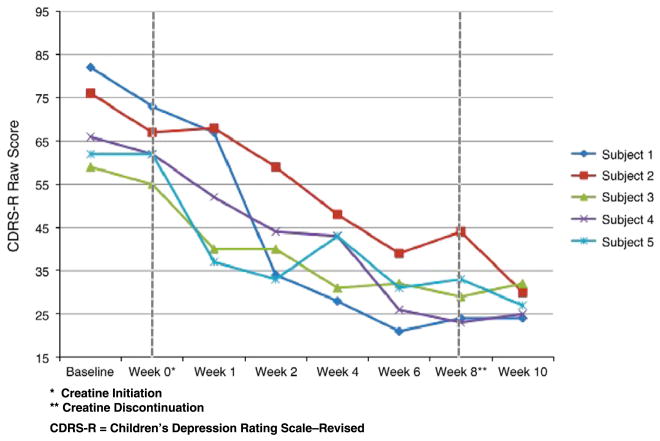
The mean CDRS-R raw score at baseline was 69 (SD 9.69). After 8 weeks of adjunctive creatine the mean CDRS-R score was 30.6 (SD 8.50), an average decrease of 38.4 (56%). The participants’ CDRS-R raw scores during 8 weeks of treatment and the 2-week follow-up period are depicted graphically in Fig. 1. After discontinuation of adjunctive creatine, treatment gains were maintained. In fact, the mean CDRS-R raw score two weeks after the end of treatment (Week 10) was lower than at the conclusion of treatment.
Fig. 1.
CDRS-R scores during adjunctive creatine treatment.
3.2. 31P MRS neuroimaging results
MDD participants’ 31P MRS scans were performed prior to the first dose of creatine, and repeated following 8 weeks of treatment. We also recruited ten healthy control adolescents, six of whom returned 8 weeks later for a follow-up scan. Statistical analyses were performed using JMP 8 (SAS Inc., Cary, North Carolina USA). Neuroimaging analyses were considered exploratory, and no correction for multiple testing was applied.
Table 2 displays repeated measures of 31P MRS metabolites in MDD participants and controls. Following 8 weeks of treatment with creatine, depressed adolescents demonstrated a significant increase in PCr (p=0.02; paired t-test; 2-tailed) compared to controls. There was no change in creatine-treated participants’ mean β-NTP, pH or PCr/β-NTP concentrations.
Table 2.
Baseline and week 8 31P-MRS results for female adolescents with MDD vs. healthy controls.
| Phosphorus metabolite | Major depressive disorder (n=5)
|
Healthy controls (n=6)
|
||||
|---|---|---|---|---|---|---|
| Mean baseline (SD) | Mean 8 weeks (SD) | p-value | Mean baseline (SD) | Mean 8 weeks (SD) | p-value | |
| PCr/TP | 0.1513 (0.0137) | 0.1610 (0.0165) | 0.020 | 0.1556 (0.0086) | 0.1558 (0.0154) | 0.969 |
| β-NTP/TP | 0.1254 (0.0087) | 0.1280 (0.0125) | 0.729 | 0.1203 (0.0048) | 0.1253 (0.0109) | 0.329 |
| pH | 7.059 (0.1383) | 7.0712 (0.0359) | 0.648 | 7.0526 (0.0290) | 7.0294 (0.0143) | 0.054 |
| PCr/β-NTP | 1.221 (0.0528) | 1.2700 (0.1380) | 0.528 | 1.2962 (0.1005) | 1.2584 (0.2172) | 0.645 |
Paired t-test, 2-tailed.
PCr: Phosphocreatine.
β-NTP: beta-Nucleoside Triphosphate.
TP: Total Phosphorus Resonance.
P MRS: 31-Phosphorus Magnetic Resonance Spectroscopy.
SD: Standard Deviation.
Using the technique of a previous report (Renshaw et al., 2001), correlations between baseline depression rating scale scores and baseline neurometabolite levels were assessed using Spearman rank correlation and generalized two-tailed least squares modeling methods. CDRS-R baseline score was correlated with baseline pH (correlation=0.8919; 95% CI 0.045–0.993; p=0.04). CDRS-R baseline score was negatively correlated with β-NTP concentration (Spearman’s p=−0.90; p=0.03). (Data not shown.)
3.3. Adverse events
Adverse events, summarized in Table 1, were self-limited with no unresolved treatment-emergent side effects. There was no attempted suicide, self-injurious behavior or psychiatric hospitalization during the study. There were no significant changes in vital signs or laboratory tests; no participant developed proteinuria or an abnormal serum creatinine.
3.4. Suicidality
All but one participant endorsed a history of suicidality, highlighting the severity of MDD in this population. Three participants had attempted suicide, and four participants reported suicidal ideation during the 2 weeks prior to their initial visit. During the treatment phase of the study, two participants reported no suicidality. Three participants reported suicidal ideation lasting from 2 to 6 weeks during treatment. In all cases, suicidal ideation resolved during the study and did not recur during the treatment phase, or at the Week 10 follow-up visit. The participant who reported suicidal ideation during the first 6 weeks of treatment endorsed chronic suicidal ideation for the 22 months prior to study entry.
4. Discussion
The authors report the results of an open-label study of adjunctive creatine for female adolescents with SSRI-resistant MDD. The aims of the study were twofold: 1) to obtain pilot data for creatine augmentation in this population; and 2) to demonstrate the feasibility of pre- and post-treatment 31P MRS brain scans for in vivo measurement of bioenergetic neurometabolites. Eight weeks of creatine augmentation was associated with a mean decrease in CDRS-R score from 69 to 30.6. Because brain tissue is unavailable for pathologic sampling, the lack of non-invasive assessment tools is a critical barrier to research in the neurobiology of mood disorders (Drevets et al., 2008). Cerebral 31P MRS is the only method capable of in vivo measurement of energy-related metabolites such as PCr and β-NTP, proved to be acceptable to participants and was well-tolerated.
Multiple lines of evidence implicate mitochondrial dysfunction in the pathophysiology of mood disorders. In adults with primary mitochondrial disorders, the rate of MDD is 54% (Fattal et al., 2007), and altered brain energy metabolism has been proposed as the etiology of MDD in pediatric mitochondrial illness (Koene et al., 2009). Mice with neuronal mitochondrial DNA mutations display a depressive phenotype (Kasahara et al., 2006). Support also comes from molecular biology research: in a post-mortem study of MDD and suicide, altered expression of genes involved in ATP synthesis and utilization was found in prefrontal cortex (Klempan et al., 2009). The Genetics of Recurrent Early-Onset Depression (GenRED) project found that matrilineal relatives, i.e. those with the same mitochondrial genome as the proband, were more likely to suffer from a mood disorder than non-matrilineal relatives (Bergemann and Boles, 2010).
We found that brain PCr concentration was increased after 8 weeks in creatine-treated participants compared with untreated controls. During neuronal activation, PCr is rapidly depleted in order to maintain ATP levels at a constant (Rango et al., 1997; Sappey-Marinier et al., 1992). Creatine supplementation reduces levels of cerebral oxygenated hemoglobin during mental tasks, indicating increased oxygen utilization (Watanabe et al., 2002). Creatine also improves working memory and intelligence testing results (p<0.0001) in healthy subjects (Rae et al., 2003). Previous work from our group showed that creatine increases brain PCr concentration in healthy volunteers (Lyoo et al., 2003), and that pre-treatment PCr is a robust predictor of treatment response in MDD (Iosifescu et al., 2008). The creatine kinase reaction utilizes PCr to synthesize ATP at 12 times the rate of oxidative phosphorylation, and over 70 times faster than de novo synthesis (Wallimann et al., 1992).
This study is limited by the lack of a placebo control. The placebo response rate in SSRI-resistant adolescent MDD is unknown, but there are two reasons to speculate that the rate could be lower than the placebo response in treatment-naïve MDD: first, it is thought that the placebo response in MDD is inversely proportional to the severity of depression (Fournier et al., 2010). A second reason is that by definition, SSRI-resistant adolescents have already had the opportunity to exhibit the placebo response. In the TORDIA trial, the medication response rate was 40.5% (Brent et al., 2008). If the difference between active drug and placebo response rates in SSRI-resistant adolescent MDD is similar to that found in treatment-naïve patients, we would expect a placebo response of ~29.7% (Brent et al., 2008; Usala et al., 2008). In our open-label study, 3 of 5 participants (60%) experienced a reduction in CDRS-R score of ≥50%. For the Pearson chi-square statistic from a multinomial sample under general conditions (Agresti, 1989), with alpha set at 0.05, a sample size of n=86 (43 subjects per group) would provide a power of 0.803 to detect the between-group difference. This provides an estimate of the sample size required for a placebo-controlled study of creatine’s efficacy in this population.
Our study was limited to female participants, and offers an opportunity to point to the disproportionate burden-of-illness imposed on girls and women by MDD. By the middle teenage years, females experience double the rate of depressive disorders found in males (Garrison et al., 1997; Wade et al., 2002). This 2:1 gender disparity continues throughout women’s reproductive years (Kessler et al., 1993). In addition, initial episodes of depression are longer and symptoms more severe in girls compared to boys (McCauley et al., 1993). Girls with MDD also have a longer risk of recurrence, compared with women who experience their first depressive episode in adulthood (Kovacs, 1996, 1997). Depression is the leading cause of disability among females between the ages of 15 and 44 (Murray and Lopez, 1996), implying that adolescent ‘window of vulnerability’ to depression (Andersen and Teicher, 2008; Hankin et al., 1998) represents a singular opportunity to reduce the burden of illness in MDD.
Polypharmacy is commonplace in the treatment of pediatric MDD (McIntyre and Jerrell, 2009). In fact, multiclass psychotropic treatment occurs in 32.2% of children’s physician office visits in which psychotropic medicine is prescribed (Comer et al., 2010). Yet the medical knowledge that is available to guide augmentation psychopharmacology in adolescent MDD is sparse. The updated 2007 Texas Children’s Medication Algorithm for juvenile MDD states that the best augmentation agent for SSRI partial- or non-responders has not been determined (Hughes et al., 2007). Even in adult MDD, there is little medical scientific evidence to support augmentation with lithium, thyroid hormone, buspirone, stimulants or pindolol (Connolly and Thase, 2011). Given the reality of pediatric polypharmacy and the sizeable proportion of SSRI non-responders, augmentation studies of adolescent MDD are important research priorities (Szigethy, 2011).
An unexpected finding from this study is the resolution of suicidal ideation associated with adjunctive creatine. The related problems of adolescent MDD and suicidality are pressing public health concerns. Data from the Youth Risk Behavior Surveillance System show that 26.1% of U.S. high school students report feeling “so sad or hopeless almost every day for 2 or more weeks in a row” during the past year that they stopped some of their usual activities, 13.8% report having “seriously considered attempting suicide,” and 6.3% endorse having made one or more actual suicide attempts (Eaton et al., 2010). These data are made more striking by the fact that respondents are asked to report on the previous 12 months only — not on their lifetime history. Another study found that 21.9% of adolescents with MDD report having made a suicide attempt (Kessler and Walters, 1998). Further, treatment with antidepressant medication is associated with both suicide attempts and suicide deaths in patients 6–18 years of age with severe depression (Olfson et al., 2006).
At a time when ethicists have argued that antidepressant prescription to pediatric patients should be “severely restricted” (Shearer and Bermingham, 2008), interventions with potential to reduce depressive symptoms and suicidality in adolescent MDD are urgently required. Long studied as an ergogenic supplement for athletic skeletal muscle performance, it is now clear that creatine plays a vital role in brain function (Brosnan and Brosnan, 2007). Based on these results and evidence from multiple disciplines implicating mitochondrial dysfunction in depression, further study of adjunctive creatine for adolescent females with SSRI-resistant MDD is warranted.
Acknowledgments
Role of funding source
Funding for this study was provided by a Funding Incentive Seed Grant from the University of Utah Research Foundation (UURF) to Dr. Kondo and Dr. Renshaw; and by the Utah Science Technology and Research Initiative (USTAR). The funding agencies had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the manuscript for publication.
Dr. Heinz Ridder and Ms. Susanne Hayes of AlzChem LLC provided Creapure® brand of creatine for the study. The Columbia-Suicide Severity Rating Scale was utilized with the permission of Dr. Kelly Posner. The MRUI software package was provided by participants in the EU Network programs: Human Capital and Mobility, CHRX-CT94-0432, and Training and Mobility of Researchers, ERB-FMRX-CT970160.
Footnotes
Conflict of interest
Dr. Renshaw serves as a consultant to Kyowa Hakko, Novartis and Roche. He has received research support from GlaxoSmithKline and Roche. Dr. Renshaw and Dr. Kondo are inventors on a patent application that has been assigned to the University of Utah, and describes the use of creatine as a treatment for depressive disorders. The application was filed after the subjects described in this report completed the research protocol and all aspects of study participation. All other authors declare that they have no conflicts of interest.
References
- Agresti A. Tutorial on modeling ordered categorical response data. Psychol Bull. 1989;105:290–301. doi: 10.1037/0033-2909.105.2.290. [DOI] [PubMed] [Google Scholar]
- Allen PJ, D’Anci KE, Darrell C, Galinko L, Salvatore J, Kanarek RB, Renshaw PF. Creatine supplementation offsets depressive behavior in female rats independent of fluoxetine. Society for Neuroscience 40th Annual Meeting; San Diego, CA. 2010a. [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology. 2010b;35:534–546. doi: 10.1038/npp.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Barbui C, Esposito E, Cipriani A. Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies. CMAJ. 2009;180:291–297. doi: 10.1503/cmaj.081514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergemann ER, Boles RG. Maternal inheritance in recurrent early-onset depression. Psychiatr Genet. 2010;20:31–34. doi: 10.1097/YPG.0b013e3283351153. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Stankevich BA, Bies RR. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: an updated review. Gend Med. 2009;6:522–543. doi: 10.1016/j.genm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent D, Bernet W, Bukstein O, Walter H, Benson RS, Chrisman A, Farchione T, Greenhill L, Hamilton J, Keable H, Kinlan J, Schoettle U, Stock S, Ptakowski KK, Medicus J. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. doi: 10.1146/annurev.nutr.27.061406.093621. [DOI] [PubMed] [Google Scholar]
- Comer JS, Olfson M, Mojtabai R. National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996–2007. J Am Acad Child Adolesc Psychiatry. 2010;49:1001–1010. doi: 10.1016/j.jaac.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71:43–64. doi: 10.2165/11587620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106:226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubicka B, Hadley S, Roberts C. Suicidal behaviour in youths with depression treated with new-generation antidepressants: meta-analysis. Br J Psychiatry. 2006;189:393–398. doi: 10.1192/bjp.bp.105.011833. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Lim C, Whittle L, Brener ND, Wechsler H. Youth risk behavior surveillance—United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- Fattal O, Link J, Quinn K, Cohen BH, Franco K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. 2007;12:429–438. doi: 10.1017/s1092852900015303. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Wostear G, Cooper V, Harrington R, Rutter M. The Maudsley long-term follow-up of child and adolescent depression. 2 Suicidality, criminality and social dysfunction in adulthood. Br J Psychiatry. 2001;179:218–223. doi: 10.1192/bjp.179.3.218. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison CZ, Waller JL, Cuffe SP, McKeown RE, Addy CL, Jackson KL. Incidence of major depressive disorder and dysthymia in young adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36:458–465. doi: 10.1097/00004583-199704000-00007. [DOI] [PubMed] [Google Scholar]
- Guy W. National Institute of Mental Health, E.C.D.E., Psychopharmacology Research Branch, editor. ECDEU Assessment Manual for Psychopharmacology, Revised. U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch. Division of Extramural Research Programs; Rockville, MD: 1976. pp. 217–222. [Google Scholar]
- Hammad TA, Laughren TP, Racoosin JA. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hetrick S, Merry S, McKenzie J, Sindahl P, Proctor M. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2007:CD004851. doi: 10.1002/14651858.CD004851.pub2. [DOI] [PubMed] [Google Scholar]
- Hughes CW, Emslie GJ, Crismon ML, Posner K, Birmaher B, Ryan N, Jensen P, Curry J, Vitiello B, Lopez M, Shon SP, Pliszka SR, Trivedi MH. Texas children’s medication algorithm project: update from Texas consensus conference panel on medication treatment of childhood major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 2008;115:291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Renshaw PE. 31P-magnetic resonance spectroscopy and thyroid hormones in major depressive disorder: toward a bioenergetic mechanism in depression? Harv Rev Psychiatry. 2003;11:51–63. doi: 10.1080/10673220303959. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Jost CR, Van Der Zee CE, In’ t Zandt HJ, Oerlemans F, Verheij M, Streijger F, Fransen J, Heerschap A, Cools AR, Wieringa B. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci. 2002;15:1692–1706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, Kato T. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry. 2006;11(577–593):523. doi: 10.1038/sj.mp.4001824. [DOI] [PubMed] [Google Scholar]
- Kato T. Mitochondrial dysfunction as the molecular basis of bipolar disorder: therapeutic implications. CNS Drugs. 2007;21:1–11. doi: 10.2165/00023210-200721010-00001. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kennard BD, Silva SG, Tonev S, Rohde P, Hughes JL, Vitiello B, Kratochvil CJ, Curry JF, Emslie GJ, Reinecke M, March J. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:186–195. doi: 10.1097/CHI.0b013e31819176f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Walters EE. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depress Anxiety. 1998;7:3–14. doi: 10.1002/(sici)1520-6394(1998)7:1<3::aid-da2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- Koene S, Kozicz TL, Rodenburg RJ, Verhaak CM, de Vries MC, Wortmann S, van de Heuvel L, Smeitink JA, Morava E. Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J Affect Disord. 2009;114:327–332. doi: 10.1016/j.jad.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, Delmastro KK, Shi X, Renshaw PF. Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat. 2011;2011:650450. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Presentation and course of major depressive disorder during childhood and later years of the life span. J Am Acad Child Adolesc Psychiatry. 1996;35:705–715. doi: 10.1097/00004583-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Emanuel Miller memorial lecture 1994. Depressive disorders in childhood: an impressionistic landscape. J Child Psychol Psychiatry. 1997;38:287–298. doi: 10.1111/j.1469-7610.1997.tb01513.x. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. J Abnorm Psychol. 1993;102:133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lynch FL, Clarke GN. Estimating the economic burden of depression in children and adolescents. Am J Prev Med. 2006;31:S143–S151. doi: 10.1016/j.amepre.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, Cohen BM, Renshaw PF. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003;123:87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- Ma J, Lee KV, Stafford RS. Depression treatment during outpatient visits by U.S. children and adolescents. J Adolesc Health. 2005;37:434–442. doi: 10.1016/j.jadohealth.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Maier W, Gansicke M, Gater R, Rezaki M, Tiemens B, Urzua RF. Gender differences in the prevalence of depression: a survey in primary care. J Affect Disord. 1999;53:241–252. doi: 10.1016/s0165-0327(98)00131-1. [DOI] [PubMed] [Google Scholar]
- McCauley E, Myers K, Mitchell J, Calderon R, Schloredt K, Treder R. Depression in young people: initial presentation and clinical course. J Am Acad Child Adolesc Psychiatry. 1993;32:714–722. doi: 10.1097/00004583-199307000-00003. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Jerrell JM. Polypharmacy in children and adolescents treated for major depressive disorder: a claims database study. J Clin Psychiatry. 2009;70:240–246. doi: 10.4088/jcp.08m04212. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–118. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- Moretti A, Gorini A, Villa RF. Affective disorders, antidepressant drugs and brain metabolism. Mol Psychiatry. 2003;8:773–785. doi: 10.1038/sj.mp.4001353. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. The Global Burden of Disease. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- Nolen-Hoeksema S. Sex Differences in Depression. Stanford University Press; Palo Alto, CA: 1990. [Google Scholar]
- Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: a case-control study. Arch Gen Psychiatry. 2006;63:865–872. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- Owens PL, Thompson J, Elixhauser A, Ryan K. Care of Children and Adolescents in U.S. Hospitals. Agency for Healthcare Research and Quality; Rockville, MD: 2003. [Google Scholar]
- Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? Am J Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Posner K. Columbia-Suicide Severity Rating Scale (C-SSRS) Center for Suicide Risk Assessment. Columbia University Medical Center; New York: 2010. [Google Scholar]
- Poznanski EO, Mokros HB. Children’s Depression Rating Scale, Revised (CDRS-R) Manual. Western Psychological Services; Los Angeles, CA: 1996. [Google Scholar]
- Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proc Biol Sci. 2003;270:2147–2150. doi: 10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rango M, Castelli A, Scarlato G. Energetics of 3.5 s neural activation in humans: a 31P MR spectroscopy study. Magn Reson Med. 1997;38:878–883. doi: 10.1002/mrm.1910380605. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, de Frederick BB, Fava M, Hennen J, Cohen BM. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–2055. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction in psychiatric disorders. Neurochem Res. 2009;34:1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Robins LN, Regier DA. Psychiatric Disorders in America: the Epidemiologic Catchment Area Study. Free Press, Collier Macmillan; Canada: Maxwell Macmillan International; New York Toronto: 1991. [Google Scholar]
- SAMHSA. The NSDUH Report. Substance Abuse and Mental Health Services Administration; Washington, D.C: 2008. Major Depressive Episode among Youths Aged 12 to 17 in the United States: 2004 to 2006; p. 4. [Google Scholar]
- Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992;12:584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Williams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66:785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Bunney WE, Vawter MP. Mitochondrial involvement in psychiatric disorders. Ann Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer MC, Bermingham SL. The ethics of paediatric antidepressant use: erring on the side of caution. J Med Ethics. 2008;34:710–714. doi: 10.1136/jme.2007.023119. [DOI] [PubMed] [Google Scholar]
- Szigethy E. Research forum: perspectives on current pediatric psychopharmacology research needs. AACAP News. 2011;42:34–36. [Google Scholar]
- Usala T, Clavenna A, Zuddas A, Bonati M. Randomised controlled trials of selective serotonin reuptake inhibitors in treating depression in children and adolescents: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2008;18:62–73. doi: 10.1016/j.euroneuro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Zuvekas SH, Norquist GS. National estimates of antidepressant medication use among U.S. children, 1997–2002. J Am Acad Child Adolesc Psychiatry. 2006;45:271–279. doi: 10.1097/01.chi.0000192249.61271.81. [DOI] [PubMed] [Google Scholar]
- Volz HP, Rzanny R, Riehemann S, May S, Hegewald H, Preussler B, Hubner G, Kaiser WA, Sauer H. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:289–295. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- Wade TJ, Cairney J, Pevalin DJ. Emergence of gender differences in depression during adolescence: national panel results from three countries. J Am Acad Child Adolesc Psychiatry. 2002;41:190–198. doi: 10.1097/00004583-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res. 2002;42:279–285. doi: 10.1016/s0168-0102(02)00007-x. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Klier CM, Ryan ND, Dahl RE, Wickramaratne P. Depressed adolescents grown up. JAMA. 1999;281:1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363:1341–1345. doi: 10.1016/S0140-6736(04)16043-1. [DOI] [PubMed] [Google Scholar]
- Young E, Korszun A. Sex, trauma, stress hormones and depression. Mol Psychiatry. 2010;15:23–28. doi: 10.1038/mp.2009.94. [DOI] [PubMed] [Google Scholar]