Abstract
Introduction
Hantavirus infections are characterized by both activation and dysfunction of the endothelial cells. The underlying mechanisms of the disease pathogenesis are not fully understood. Here we tested the hypothesis whether the polymorphisms of endothelial nitric oxide synthase, eNOS G894T, and inducible nitric oxide synthase, iNOS G2087A, are associated with the severity of acute Puumala hantavirus (PUUV) infection.
Patients and Methods
Hospitalized patients (n = 172) with serologically verified PUUV infection were examined. Clinical and laboratory variables reflecting disease severity were determined. The polymorphisms of eNOS G894T (Glu298Asp, rs1799983) and iNOS G2087A (Ser608Leu, rs2297518) were genotyped.
Results
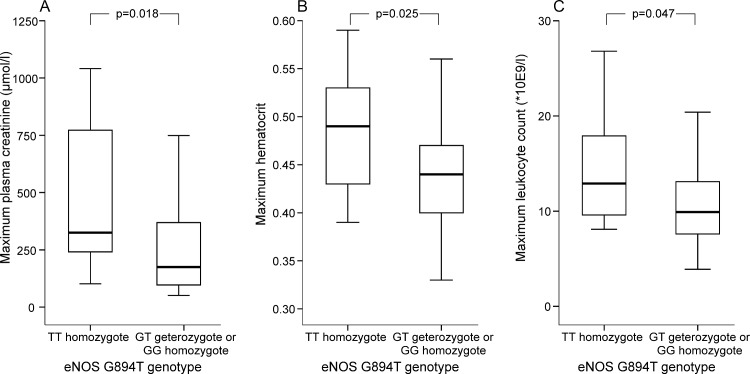
The rare eNOS G894T genotype was associated with the severity of acute kidney injury (AKI). The non-carriers of G-allele (TT-homozygotes) had higher maximum level of serum creatinine than the carriers of G-allele (GT-heterozygotes and GG-homozygotes; median 326, range 102–1041 vs. median 175, range 51–1499 μmol/l; p = 0.018, respectively). The length of hospital stay was longer in the non-carriers of G-allele than in G-allele carriers (median 8, range 3–14 vs. median 6, range 2–15 days; p = 0.032). The rare A-allele carriers (i.e. AA-homozygotes and GA-heterozygotes) of iNOS G2087A had lower minimum systolic and diastolic blood pressure than the non-carriers of A-allele (median 110, range 74–170 vs.116, range 86–162 mmHg, p = 0.019, and median 68, range 40–90 vs. 72, range 48–100 mmHg; p = 0.003, respectively).
Conclusions
Patients with the TT-homozygous genotype of eNOS G894T had more severe PUUV-induced AKI than the other genotypes. The eNOS G894T polymorphism may play role in the endothelial dysfunction observed during acute PUUV infection.
Introduction
Hantaviruses cause two clinical syndromes in humans, the haemorrhagic fever with renal syndrome (HFRS) in Europe and Asia, and hantavirus cardiopulmonary syndrome (HCPS) in the Americas. Puumala hantavirus (PUUV) is the most common hantavirus causing HFRS in Europe [1]. The main characteristics of PUUV-HFRS are increased capillary leakage, thrombocytopenia and acute kidney injury (AKI). Although PUUV-HFRS has a low rate of case fatality (up to 0.4%), significant acute-phase complications as well as long-term hormonal, renal and cardiovascular consequences can occur [1, 2].
The main pathophysiological mechanisms of hantavirus infection include activation of cytokines [3, 4] and cytotoxic CD8+ T-lymphocytes [5], vascular endothelial growth factors [6, 7], and the complement system [8]. Recently discovered biomarkers that reflect PUUV-HFRS disease severity include pentraxin-3, indoleamine 2,3-dioxygenase, plasma cell-free DNA, soluble urokinase-type plasminogen activator and GATA-3 [9]. Host genetic factors also influence the outcome of acute PUUV infection. In the Finnish population, individuals with Human Leukocyte Antigen (HLA) alleles B8, C4A*Q0 and DRB1*0301 are more prone to have a severe form of PUUV infection [10, 11]. Also polymorphisms of the cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1) and IL-1 receptor antagonist impact on the clinical severity of PUUV-HFRS [10, 12]. Likewise, genetic polymorphisms of plasminogen activator inhibitor, the main physiological regulator of fibrinolysis, and platelet glycoprotein 1a, associate with severe PUUV infection [13].
Increased nitric oxide (NO) levels induced by elevated TNF-α concentrations have been suggested to participate in the pathogenesis of hantaviral infections [14, 15, 16]. Elevated concentrations of NO correlate with increased serum creatinine value and hypotension, and inversely correlate with platelet count in patients with acute PUUV infection [15]. According to a Swedish study, NO has antiviral effects on hantaviruses by inhibiting viral replication at the early phase of infection [17]. Endothelial nitric oxide synthase (eNOS), and inducible nitric oxide synthase (iNOS) that can be induced in a variety of cell types, are the key enzymes catalysing NO synthesis [18].
A widely explored polymorphism of the eNOS gene, the G894T (rs1799983) polymorphism encoded by the NOS3 gene in chromosome 7, has been linked to increased risk of coronary artery disease (CAD), myocardial infarction, coronary spasms, hypertension, and ischemic stroke [19–24]. Recent data suggest that the T-allele of G894T polymorphism is also associated with increased susceptibility to and risk of end-stage renal disease (ESRD) [25], and also with earlier onset age of ESRD in males with autosomal dominant polycystic disease [26]. The iNOS, encoded by the NOS2 gene in chromosome 17, is expressed in macrophages, neutrophils and hepatocytes as a host immune response to cytokines. The G2087A (rs2927518) polymorphism of iNOS has been implicated in a variety of diseases, including inflammatory bowel disease, gastric cancer, migraine with aura, septic shock, and non-Hodgkin lymphoma [27–30]. So far there is no evidence whether the NOS polymorphisms influence the clinical course of hantaviral infections.
We aimed to study the influence of the above polymorphisms that have the potential to affect endothelial and vascular function, eNOS G894T (Glu298Asp, rs1799983) and iNOS G2087A (Ser608Leu, rs2297518), on disease severity in patients with acute PUUV infection. We sought to determine whether the genetic variations within the genes of NOS3 and NOS2 could contribute to individual differences in the outcome of acute PUUV infection.
Material and Methods
Ethics statement
The study was carried out at Tampere University Hospital and University of Tampere, School of Medicine. All patients were recruited and enrolled after providing a written informed consent. In addition, informed verbal consent was obtained from the guardians of the minors. Blood samples of the minors were collected before the current Medical Reseach Act was valid in Finland. The Ethics Committee of Tampere University Hospital approved the study protocol and consent procedure according to the ethical principles at the time of the study. The study was conducted according to the principles expressed in the Declaration of Helsinki.
Patients
The study cohort consisted of 172 prospectively collected, consecutive hospitalized patients with serologically confirmed acute PUUV infection. The collection of clinical data including routine laboratory measurements has been described in detail elsewhere [13].
All patients came from the Pirkanmaa area and were hospitalized in Tampere University Hospital, Finland for the median time of six days (range 2–15) during the period from September 1997 to February 2009. The median age of the patients was 40 years (ranging from 15 to 74 years), and 119 were males (69%). The concomitant diseases of the study group were arterial hypertension (n = 12), dyslipidemia (n = 7), CAD (n = 5), bronchial asthma (n = 6), atrial fibrillation (n = 3) and rheumatoid arthritis (n = 3). There were also patients with celiac disease, inflammatory bowel disease, valvular heart disease or neurological disease (n = 2 for each).
Definition of AKI
AKI was defined according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria as an increase in plasma creatinine ≥1.5 times baseline, which was presumed to have occurred within the prior seven days [31]. The upper limits of the reference values for plasma creatinine (women 90 μmol/l and men 100 μmol/l) were taken as baseline levels. Thus, plasma creatinine ≥135 μmol/l in women, and ≥150 μmol/l in men was defined as AKI.
Genotyping
DNA was extracted from whole blood using a commercially available kit (QIAGEN Inc., Hilden, Germany). The gene polymorphisms of eNOS G894T (Glu298Asp, rs1799983) and iNOS G2087A (Ser608Leu, rs2297518) were genotyped with TaqMan® SNP Genotyping Assay (Life Technologies Ltd, Carlsbad, CA, USA) under standard conditions using the ABI Prism 7900HT Sequence Detection System (Taqman, Applied Biosystems, Foster City, CA, USA). Reaction volume used was 5 μl and it was prepared with TaqMan Genotyping MasterMix (Life Technologies Ltd, Carlsbad, CA, USA). Amplification data were analyzed with SDS 2.2 software (Taqman, Applied Biosystems, Foster City, CA, USA). The distributions of all SNPs did not deviate from the Hardy–Weinberg equation. The genotyping was successful in 167 of 172 patients (97%) for eNOS and in 166 patients (96.5%) for iNOS.
Statistical analysis
The highest and lowest values of continuous variables measured during acute PUUV infection were designated as maximum or minimum values. In order to describe the data, medians and ranges were given for skewed continuous variables and numbers for categorical variables. The allele frequencies were calculated, and the patients were grouped into carriers (including both homozygotes and heterozygotes) and non-carriers of specific alleles. Differences in the clinical severity of PUUV infection between groups were tested using Mann-Whitney U-test or Kruskal-Wallis test for numerical data and χ2-test or Fisher`s exact test for categorical data, as appropriate. All p-values were two-tailed, and the statistical significance was considered at 0.05. Statistical analyses were performed using IBM SPSS software version 21.
Results
Clinical and laboratory findings
The clinical and laboratory findings of the 172 patients with acute PUUV infection are shown in Table 1. All patients suffered from serologically verified [32] and clinically typical PUUV infection, and they were examined and hospitalized during the acute phase of illness. Three patients (2%) were in clinical shock on admission to the hospital. AKI was found in 96 (56%) patients. Seven patients (4%) needed hemodialysis treatment during hospitalization. All patients recovered.
Table 1. The clinical and laboratory findings in 172 patients with acute Puumala hantavirus infection.
| Clinical or laboratory variable | Median | Range |
|---|---|---|
| Days from the onset of fever* | 4 | 1–14 |
| Length of hospital stay (days) | 6 | 2–15 |
| Systolic blood pressure, minimum (mmHg) | 113 | 74–170 |
| Diastolic blood pressure, minimum (mmHg) | 70 | 40–100 |
| Change in weight (kg)** | 2.1 | 0–12.0 |
| Hematocrit, maximum | 0.44 | 0.33–0.60 |
| Hematocrit, minimum | 0.36 | 0.25–0.46 |
| Platelet count, minimum (x 109/l) | 62 | 3–238 |
| Leukocyte count, maximum (x 109/l) | 10 | 3.9–31.2 |
| CRP, maximum (mg/ml) | 75 | 11–269 |
| Creatinine, maximum (μmol/l) | 185 | 51–1499 |
| Interleukin-6, maximum (pg/ml) | 14.5 | 1.3–107 |
Abbreviation: CRP, C-reactive protein.
Normal values: CRP < 10 mg/ml, creatinine ≤ 100 μmol/l for males and ≤ 90 μmol/l for females, platelet count 150–360 x 109/l, leukocyte count 3.4–8.2 x 109/l, hematocrit 0.35–0.50 for males and 0.35–0.46 for females.
*Equals to the onset of illness before the first blood test was taken.
**Change in weight during hospital stay reflects the fluid accumulation in the body during the oliguric phase.
Association of eNOS G894T (Glu298Asp, rs1799983) polymorphism with clinical and laboratory findings
The genotype distributions and allele frequencies of eNOS G894T and iNOS G2087A polymorphisms are presented in Table 2. The rare genotype of eNOS G894T gene polymorphism was associated with the severity of AKI. The non-carriers of the G-allele of this eNOS polymorphism (TT-homozygotes, n = 10) had 1.9 times greater maximum level of serum creatinine than the carriers of the common G-allele (median 326, range 102–1041 vs. median 175, range 51–1499 μmol/l; p = 0.018, respectively: Fig 1A). The TT-homozygotes had numerically highest maximum creatinine level of all eNOS G894T genotypes, followed by the GT-heterozygotes and the GG-homozygotes, (median concentrations 326, 196, and 166 μmol/l, respectively, p = 0.061). Three out seven (43%) patients that needed hemodialysis treatment were T-allele carriers, and one of them was a TT-homozygote.
Table 2. The genotype distributions and allele frequencies in 167 patients for eNOS G894T (rs1799983) and in 166 patients for iNOS G2087A (rs2297518) out of 172 patients with acute Puumala hantavirus infection * .
| Genotype % | Allele % | ||||
|---|---|---|---|---|---|
| Polymorphism | homozygote common | heterozygote | homozygote rare | common | rare |
| eNOS(rs1799983) | 59 (GG) | 35 (GT) | 6 (TT) | 76 (G) | 24 (T) |
| iNOS(rs2297518) | 65 (GG) | 33 (GA) | 2 (AA) | 81 (G) | 19 (A) |
Abbreviations: eNOS = endothelial nitride oxide synthase, iNOS = inducible nitride oxide synthase.
*The genotyping was successful in 167 patients for eNOS and in 166 patients for iNOS.
Fig 1. Box plots of the maximum plasma creatinine (A), hematocrit (B) and leukocyte count in TT-homozygotes (n = 10) and GT-heterozygotes or GG-homozygotes (n = 157) of eNOS G894T(rs1799983) polymorphism in 169 patients with PUUV infection.
Box plot illustrates median (thick line inside box), 25th and 75th percentiles (box), and range (whiskers). Extremes and outliers have been omitted from the figure.
The non-carriers of the G-allele had higher maximum blood hematocrit value than the G-allele carriers (median 0.49, range 0.39–0.59 vs. median 0.44, range 0.33–0.60; p = 0.025, respectively; Fig 1B). The non-carriers of the G-allele had also higher maximum blood leukocyte count during acute phase of PUUV infection than the carriers of the common G-allele (GT-heterozygotes and GG-homozygotes, median 12.9, range 8.1–26.8 vs. median 9.9, range 3.9–31.2 *109/l; p = 0.047; Fig 1C).
The length of hospital stay was longer in the non-carriers of the G-allele (TT-homozygotes) when compared with the G-allele carriers (median 8 days, range 3–14 days vs. median 6 days, range 2–15 days; p = 0.032). There were no statistically significant associations with the lowest and highest blood pressure, change in weight, lowest platelet count, maximum C-reactive protein (CRP), maximum plasma IL-6, or the need of hemodialysis treatment, and the polymorphism of eNOS G894T (S1 and S2 Files).
Association of iNOS G2087A (Ser608Leu, rs2297518) polymorphism with clinical and laboratory findings
Carriers of the rare A-allele of the iNOS G2087A gene (AA-homozygotes and GA-heterozygotes, n = 59) had lower minimum systolic blood pressure during PUUV infection when compared with the non-carriers (GG-homozygotes, median 110, range 74–170 vs. 116, range 86–162 mmHg, respectively; p = 0.019). Furthermore, the A-allele carriers had also lower minimum diastolic blood pressure level than the non-carriers A-allele (median 68, range 40–90 vs. 72, range 48–100 mmHg, respectively; p = 0.003).There were no significant associations with change in weight, the lowest platelet count, maximum CRP, maximum plasma IL-6, or the need of hemodialysis treatment, and the iNOS polymorphism studied here (S1 and S3 Files).
Discussion
In this study we found that the TT-genotype of eNOS G894T polymorphism was associated with the severity of PUUV infection. PUUV-infected patients with the TT-homozygous genotype were prone to more severe AKI and longer hospital stay than the GT-heterozygotes or GG-homozygotes. They had also higher maximum leukocyte count and hematocrit values measured in the acute phase of the infection when compared with the other genotypes. The G894T polymorphism of eNOS gene was not associated with the depth of thrombocytopenia, which is in concordance with our previous finding that the severity of AKI does not associate with thrombocytopenia in acute PUUV infection in Finnish patients [33]. In some German studies severe thrombocytopenia has predicted severe AKI in PUUV infection [34, 35, 36]. We don’t know the reason for these divergent results, but genetic factors in different populations may influence.
Furthermore, we also found that the iNOS G2087A gene polymorphism was associated with decreased blood pressure. The carriers of the rare A-allele of iNOS G2087A gene variant were the most susceptible ones to suffer from severe hypotension during the acute phase of infection. However, this iNOS polymorphism was not associated with the other clinical and laboratory markers reflecting disease severity.
NO is important in maintaining vascular homeostasis via relaxation of vascular smooth muscle cells and inhibition of growth, platelet activation and aggregation, as well as leukocyte adhesion to the endothelium [37]. NO is synthesized via a calcium-dependent process in endothelial cells from the amino acid L-arginine by the constitutively expressed eNOS, i.e. NOS3 [38]. The calcium-independent formation of NO via iNOS in macrophages is mainly expressed during inflammation and infection, and is triggered by cytokines [39]. In addition to maintaining vasodilatation of the vasculature and thus controlling blood pressure, eNOS has numerous vasoprotective and anti-atherosclerotic effects [40]. Polymorphism of the NOS3 gene, localized in 7q36 region of chromosome 7 [41], seems to have functional significance. The eNOS polymorphism G894T (Glu298Asp) results in a substitution of glutamate for aspartate at position 298 in eNOS exon 7, and this change has been associated with reduced basal NO production in the forearm of healthy subjects [42].
Increased capillary permeability and vascular leakage explain many clinical features of hantavirus infection, such as hemoconcentration, hypotension, shock and tissue edema. Hantaviruses target the endothelial cells in the small vasculature [1, 9]. The data so far does not suggest that the infection would have direct cellular cytotoxicity to the endothelium, but virus-induced inflammation and host immune responses may contribute to the loss of endothelial barrier function [7, 43]. In this study, the rare G894T TT-homozygous genotype was associated with many of the clinical markers of severe PUUV infection, including hemoconcentration, leukocytosis, longer length of hospital stay, and especially the severity of AKI. As subjects with the common GG-genotype of eNOS G894T polymorphism had the mildest form of acute illness, the presence of this common G-allele might have some protective role during acute PUUV infection.
Effective renal blood flow is decreased in PUUV-induced AKI, but hypotension does not seem to explain the underlying intrarenal functional changes. Renal failure can occur without hypotension, and blood pressure levels do not correlate with the severity of AKI [9]. Renal tubular cells and mesangial cells produce NO, which is a significant regulator and also a protector of renal blood flow, glomerular filtration rate, and tubular function [44]. Interestingly, the increase in glomerular filtration rate and renal plasma flow in response to exogenous L-arginine infusion has been found to be blunted in subjects with the G894T allele of endothelial NOS, suggesting that this polymorphism is a functional variant also in human kidneys [45]. Thus, diminished NO bioavailability due to eNOS G894T polymorphism could predispose to the impairment of vascular and renal function through vasoconstriction.
Polymorphism of iNOS G2087A (Ser608Leu) leads to an amino acid substitution from serine to leucine in the coding region of exon 16 in NOS2 [30]. This gene variant is supposed to promote excessive NO formation and inflammation through increased iNOS activity within the A-allele carriers. In macrophages NO is a mediator of tumoricidal and bactericidal actions [30]. Previous studies have indicated that iNOS plays an important role in the origin of hypotension in septic shock. The A-allele carriage has been associated with increased susceptibility to septic shock [29]. Our finding indicated that the rare A-allele carriers (i.e. GA-heterozygotes and AA-homozygotes) of iNOS G2087A gene variant also suffered from more severe hypotension than the non-carriers of A-allele during acute PUUV infection.
We recently reported two cases of PUUV-HFRS with severe capillary leakage syndrome that were successfully treated with icatibant, a bradykinin B2-receptor antagonist [46, 47]. The activation of the kinin-kallikrein system and the subsequent formation of bradykinin is enhanced in hantavirus-infected endothelial cells [48]. The synthesis of eNOS is activated by bradykinin, which causes blood vessels to dilate via the release of NO and other endothelial autacoids [49]. Interestingly, it has been demonstrated that there is an association between HFRS and acute myocardial infarction and stroke in the acute phase of the disease, which may be partly explained by the increased platelet activation [50, 51]. Many studies have implicated eNOS polymorphism in the development of cardiovascular diseases [52], and the homozygous mutant (TT) genotype of G894T has conferred increased susceptibility to CAD [53, 54]. In the present study, the TT-homozygotes had the most severe AKI as evaluated by maximum creatinine levels, followed by the GT-heterozygotes and the GG-homozygotes. Although the number of the TT-homozygotes in our study here was only 10 patients, the results well correspond to the other findings above that have been associated with the G894T polymorphism of the eNOS gene. Taken together, our findings point to the possibility of impaired constitutive NO synthesis in the pathogenesis of acute hantavirus infection.
In conclusion, this study implies that eNOS G894T polymorphism may influence the clinical course of PUUV infection. This eNOS gene variant, associated with various vascular diseases, may also play some part in the endothelial and kidney dysfunction in the complex pathogenesis of acute PUUV infection. Among PUUV-infected patients, those with the rare TT-genotype of eNOS G894T polymorphism were more susceptible to severe AKI. Moreover, patients with the rare A-allele of iNOS G2087A polymorphism had more severe hypotension during the acute phase of infection. To our knowledge this is the first study to associate eNOS and iNOS polymorphisms and disease severity of HFRS.
Supporting Information
(PDF)
(PDF)
(PDF)
Acknowledgments
The skillful technical assistance of Ms Katriina Ylinikkilä and Eini Eskola is greatly appreciated.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was financially supported by the Competetive State Research Financing of the Expert Responsibility Area of Tampere University Hospital, European Union 7th Framework Program grant number 201668 for the AtheroRemo Project, the grant NIH/NIAID U19 A157319, the Finnish Kidney Foundation, and the Maud Kuistila Foundation and Sigrid Juselius Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The Fimlab Laboratories Ltd., provided support in the form of salaries for authors (ST, PJK), but did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the `authors contributions` section.
References
- 1. Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen A, Mäkelä S, et al. Uncovering the mysteries of hantavirus infection. Nat Rev Microbiol. 2013;11: 539–550. [DOI] [PubMed] [Google Scholar]
- 2. Mäkelä S, Jaatinen P, Miettinen M, Salmi J, Ala-Houhala I, Huhtala H, et al. Hormonal deficiences during and after Puumala hantavirus infection. Eur J Clin Microbiol Infect Dis. 2010;29: 705–713. 10.1007/s10096-010-0918-y [DOI] [PubMed] [Google Scholar]
- 3. Linderholm M, Ahlm C, Settergren B, Waage A, Tärnvik A. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J Infect Dis. 1996;173: 38–43. [DOI] [PubMed] [Google Scholar]
- 4. Mäkelä S, Mustonen J, Ala-Houhala I, Hurme M, Koivisto AM, Vaheri A, et al. Urinary excretion of interleukin-6 correlates with proteinuria in acute Puumala hantavirus-induced nephritis. Am J Kidney Dis. 2004;43: 809–816. [DOI] [PubMed] [Google Scholar]
- 5. Terajima M, Hayasaka D, Maeda K, Ennis FA. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: Do CD8+ T cells trigger capillary leakage in viral hemorrhagic fevers? Immunol Lett. 2007;113: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mackow E, Gavrilovskaya I. Hantavirus regulation of endothelial cell functions. Thromb Haemost. 2009;102: 1030–1041. 10.1160/TH09-09-0640 [DOI] [PubMed] [Google Scholar]
- 7. Gavrilovskaya I, Gorbunova E, Mackow N, Mackow E. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol. 2008;82: 5797–5806. 10.1128/JVI.02397-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sane J, Laine O, Mäkelä S, Paakkala A, Jarva H, Mustonen J, et al. Complement activation in Puumala hantavirus infection correlates with disease severity. Ann Med. 2012;44: 468–475. 10.3109/07853890.2011.573500 [DOI] [PubMed] [Google Scholar]
- 9. Mustonen J, Mäkelä S, Outinen T, Laine O, Jylhävä J, Arstila P, et al. The pathogenesis of nephropathia epidemica: New knowledge and unanswered questions. Antiviral Research. 2013;100: 589–604. 10.1016/j.antiviral.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 10. Mustonen J, Partanen J, Kanerva M, Pietilä K, Vapalahti O, Pasternack A, et al. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 1996;49: 217–221. [DOI] [PubMed] [Google Scholar]
- 11. Mäkelä S, Mustonen J, Ala-Houhala I, Hurme M, Partanen J, Vapalahti O, et al. Human leukocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-alpha(-308) G/A polymorphism. J Infect Dis. 2002;186: 843–846. [DOI] [PubMed] [Google Scholar]
- 12. Mäkelä S, Hurme M, Ala-Houhala I, Mustonen J, Koivisto A-M, Partanen J, et al. Polymorphism of the cytokine genes in hospitalized patients with Puumala hantavirus infection. Nephrol Dial Transplant. 2001;16: 1368–1373. [DOI] [PubMed] [Google Scholar]
- 13. Laine O, Joutsi-Korhonen L, Mäkelä S, Mikkelsson J, Pessi T, Tuomisto S, et al. Polymorphisms of PAI-1 and platelet GP Ia may associate with impairment of renal function and thrombocytopenia in Puumala hantavirus infection. Thromb Res. 2012;129: 611–615. 10.1016/j.thromres.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Linderholm M, Groeneveld PH, Tärnvik A. Increased production of nitric oxide in patients with hemorrhagic fever with renal syndrome—relation to arterial hypotension and tumor necrosis factor. Infection. 1996;24: 337–340. [DOI] [PubMed] [Google Scholar]
- 15. Groeneveld PH, Colson P, Kwappenberg KM, Clement J. Increased production of nitric oxide in patients infected with the European variant of hantavirus. Scand J Infect Dis. 1995;27: 453–456. [DOI] [PubMed] [Google Scholar]
- 16. Davis IC, Zajac AJ, Nolte KB, Botten J, Hjelle B, Matalon S. Elevated generation of reactive oxygen/nitrogen species in hantavirus cardiopulmonary syndrome. J Virol. 2002;76: 8347–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klingström J, Åkerström S, Hardestam J, Stoltz M, Simon M, Falk KI, et al. Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions. Eur J Immunol. 2006;36: 2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolb H, Kolb-Bachofen V. Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol Today. 1998;19: 556–561. [DOI] [PubMed] [Google Scholar]
- 19. Luo JQ, Wen JG, Zou HH, Chen XP, Zhang W. Endothelial nitric oxide synthase gene G894T polymorphism and myocardial infarction: A meta-analysis of 34 studies involving 21068 subjects. Plos One. 2014. January 30;9(1):e87196 10.1371/journal.pone.0087196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang K, Bai P, Shi S, Zhou B, Wang Y, Song Y, et al. The G894T polymorphism on endothelial nitric oxide synthase gene is associated with increased coronary heart disease among Asian population: evidence from a meta analysis. Thromb Res. 2012;130: 192–197. 10.1016/j.thromres.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 21. Yoshimur M, Yasue H, Nakayama M, Shimasaki Y, Sumida H, Sugiyama S, et al. A missense glu298asp variant in the endothelial nitric oxide synthase gene is associated with coronary spasm in the Japanese. Hum Genet. 1998;103: 65–69. [DOI] [PubMed] [Google Scholar]
- 22. Kobashi G, Yamada H, Ohta K, Kato E-H, Ebina Y, Fujimoto S. Endothelial nitric oxide synthase gene (NOS3) variant and hypertension in pregnancy. Am J Med Genet. 2001;103: 241–244. [PubMed] [Google Scholar]
- 23. Jachymova M, Horky K, Bultas J, Kozich V, Jindra A, Peleska J, et al. Association of the glu298-to-asp polymorphism in the endothelial nitric oxide synthase gene with essential hypertension resistant to conventional therapy. Biochem Biophys Res Commun. 2001;284: 426–430. [DOI] [PubMed] [Google Scholar]
- 24. Berger K, Stogbauer F, Stoll M, Wellmann J, Huge A, Cheng S, et al. The glu298asp polymorphism in the nitric oxide synthase 3 gene is associated with the risk of ischemic stroke in two large independent case-control studies. Hum Genet. 2007;121: 169–178. [DOI] [PubMed] [Google Scholar]
- 25. Yun Z, Yu-Ping Y, Zong-Wu T, Yang S, Fang Y, Fang S. Association of endothelial nitric oxide synthase gene polymorphisms with end-stage renal disease: a systematic review and analysis. Ren Fail. 2014;36(6): 987–993. 10.3109/0886022X.2014.900601 [DOI] [PubMed] [Google Scholar]
- 26. Xue C, Zhou CC, Sun LJ, He LL, Xu CG, Dai B, et al. Effects of nitric oxide synthase gene on end-stage renal disease progression in autosomal dominant polykystic disease. Nephrology. 2014;19(10):630–637. 10.1111/nep.12310 [DOI] [PubMed] [Google Scholar]
- 27. Qidwai T, Jamai F. Inducible nitric oxide synthase (iNOS) polymorphism and disease prevalence. Scand J Immunol. 2010;72: 375–387. [DOI] [PubMed] [Google Scholar]
- 28. De O S Mansur T, Goncalves FM, Martins-Oliveira A, Speciali JG, Dach F, Lacchini R, et al. Inducible nitric oxide haplotype associated with migraine and aura. Mol Cell Biochem. 2012;364: 303–308. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Feng K, Yue M, Lu X, Zheng Q, Zhang H, et al. A non-synonymous SNP in the NOS2 associated with septic shock in patients with sepsis in Chinese populations. Hum Genet. 2013;132: 337–346. 10.1007/s00439-012-1253-4 [DOI] [PubMed] [Google Scholar]
- 30. Wang SS, Davis S, Cerhan JR, Hartge P, Severson RK, Cozen W, et al. Polymorphisms in oxidative stress genes and risk for non-Hodgkin lymphoma. Carcinogenesis. 2006;27: 1828–1834. [DOI] [PubMed] [Google Scholar]
- 31. Kidney Disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2: 1–138. [Google Scholar]
- 32. Vapalahti O, Lundkvist A, Kallio-Kokko H, Paukku K, Julkunen I, Lankinen H et al. Antigenic properties and diagnostic potential of puumala virus nucleocapsid protein expressed in insect cells. J Clin Microbiol. 1996;34: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Libraty DH, Mäkelä S, Vik J, Hurme M, Vaheri A, Ennis FA, et al. The degree of leukocytosis and urine GATA-3 m-RNA levels are risk factors for severe acute kidney injury in Puumala virus nephropathia epidemica. PlosOne. 2012;7(4):e35402 10.1371/journal.pone.0035402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rasche FM, Uhel B, Kruger DH, Karges W, Czock D, Hampl W, et al. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg Infect Dis. 2004;10: 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Latus J, Kitterer D, Segerer S, Artunc F, Alscher MD, Braun N. Severe thrombocytopenia in hantavirus-induced nephropathia epidemica. Infection. 2015;43: 83–87. 10.1007/s15010-014-0699-9 [DOI] [PubMed] [Google Scholar]
- 36. Latus J, Schwab M, Tacconelli E, Pieper FM, Wegener D, Rettenmaier B, et al. Acute kidney injury and tools for risk-stratification in 456 patients with hantavirus-induced nephropathia epidemica. Nephrol Dial Transplant. 2015;30: 245–251. 10.1093/ndt/gfu319 [DOI] [PubMed] [Google Scholar]
- 37. Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 38. Marsden PA, Schappert KT, Chen HS, Flowers M, Sundell CL, Wilcox JN, et al. Molecular cloning and characterization of human endothelial nitric oxide synthase. FEBS Lett. 1992;307: 287–293. [DOI] [PubMed] [Google Scholar]
- 39. MacNaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993;196: 1330–1334. [DOI] [PubMed] [Google Scholar]
- 40. Förstermann U, Sessa WC. Nitrix oxide synthases: regulation and function. Eur Heart J. 2012;33(7): 829–837, 837a-837d. 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marsden PA, Heng HH Q, Scherer SW, Stewart RJ, Hall AV, Shi XM, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268: 17478–17488. [PubMed] [Google Scholar]
- 42. Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, de Leeuw PW, et al. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J Hypertens. 2002;20(10): 2023–2027. [DOI] [PubMed] [Google Scholar]
- 43. Hepojoki J, Vaheri A, Strandin T. The fundamental role of endothelial cells in hantavirus pathogenesis. Front Microbiol. 2014. December 22;5:727 10.3389/fmicb.2014.00727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Modlinger P, Wilcox C, Aslam S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Seminars in Nephrology. 2004;4: 354–365. [DOI] [PubMed] [Google Scholar]
- 45. Cherney DZ, Scholey JW, Zhou J, Zimpelmann J, Kennedy C, Burns KD, et al. Endothelial nitric oxide synthase gene polymorphisms and the renal hemodynamic response to L-arginine. Kidney Int. 2009;75(3): 327–332. 10.1038/ki.2008.574 [DOI] [PubMed] [Google Scholar]
- 46. Antonen J, Leppänen I, Tenhunen J, Arvola P, Mäkelä S, Vaheri A, et al. A severe case of Puumala hantavirus infection successfully treated with bradykinin antagonist, icatibant. Scand J Infect Dis. 2013;45(6): 494–496. 10.3109/00365548.2012.755268 [DOI] [PubMed] [Google Scholar]
- 47. Laine O, Leppänen I, Koskela S, Antonen J, Mäkelä S, Sinisalo M, et al. Severe Puumala hantavirus infection in a patient with lymphoproliferative disease treated with icatibant. Infect Dis (Lond). 2015;47(2): 107–111. 10.3109/00365548.2014.969304 [DOI] [PubMed] [Google Scholar]
- 48. Taylor SL, Wahl-Jensen V, Copeland AM, Jahrling PB, Schmaljohn CS. Endothelial cell permeability during hantavirus infection involves factor XII-dependent increased activation of the kallikrein-kinin system. PLoS Pathog. 2013;9(7): e1003470 10.1371/journal.ppat.1003470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lückhoff A, Pohl U, Mulsch A, Busse R. Differential role of extra- and intra-cellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988;95: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Connolly-Andersen AM, Hammargren E, Whitaker H, Eliasson M, Holmgren L, Klingström J, et al. Increased risk of acute myocardial infarction and stroke during hemorrhagic fever with renal syndrome: A self-controlled case series study. Circulation. 2014;129: 1295–1302. 10.1161/CIRCULATIONAHA.113.001870 [DOI] [PubMed] [Google Scholar]
- 51. Connolly-Andersen AM, Sundberg E, Ahlm C, Hultdin J, Baudin M, Larsson J, et al. Increased thrombopoiesis and platelet activation in hantavirus infected patients. J Infect Dis. 2015. March 11 10.1093/infdis/jiv161 [DOI] [PubMed] [Google Scholar]
- 52. Li J, Wu Z, Li X, Feng G, He L, Shi Y. The endothelial nitric oxide synthase gene is associated with coronary artery disease: a meta-analysis. Cardiology. 2010;116(4): 271–278. 10.1159/000316063 [DOI] [PubMed] [Google Scholar]
- 53. Hingorani AD, Liang CF, Fatibene J, Lyon A, Monteith S, Parsons A, et al. A common variant of the endothelial nitric oxide synthase (Glu298 —> Asp) is a major risk factor for a coronary artery disease in the UK.Circulation. 1999;100(14): 1515–1520. [DOI] [PubMed] [Google Scholar]
- 54. Angeline T, Isabel W, Tsongalis GJ. Endothelial nitric oxide gene polymorphisms, nitric oxide production and coronary artery disease risk in a South Indian population. Ex Mol Pathol. 2010;89 (3): 205–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.