Abstract
Objective
Stressful family environments early in life have negative effects on physical health. However, less is known about the health effects of positive aspects of families. We examined the associations between maternal responsiveness and immune markers among youth with asthma and identified youth expressions of positive affect as a potential mechanism of these associations.
Methods
Forty-three youths with asthma (26 males; aged 10-17) wore the Electronically Activated Recorder (EAR) for four days to assess maternal responsiveness and youth expressions of affect from audio-recordings of daily life. Trained coders rated EAR sound files for expressions of maternal responsiveness and affect displayed by the youth. Peripheral blood mononuclear cells were isolated, cultured, and assayed to determine stimulated levels of interleukin(IL)-5, IL-13, and interferon(IFN)- γ.
Results
Greater maternal responsiveness was associated with decreased stimulated production of IL-5 (r = −.38, p = .012) and IL-13 (r = −.33, p = .031). Greater total positive affect in youth was linked with decreased stimulated production of IL-5 (r = −.46, p = .002) and IL-13 (r = −.37, p = .014). Total negative affect among youth was unrelated to immune responses. There was a significant indirect effect of maternal responsiveness via positive affect in youth on lower levels of IL-5 (95% CI = −3.41, −.03) and IL-13 (95% CI = −2.34, −.01) when adjusting for caregiver-youth conflict and negative affect among youth.
Conclusions
These results indicate the importance of positive family interactions for youth and provide preliminary evidence for a mechanism through which parenting can influence immune responses in youths with asthma.
Keywords: Electronically Activated Recorder (EAR), Inflammation, Positive affect, Maternal responsiveness, Asthma, Health
INTRODUCTION
Research has clearly demonstrated links between negative family interactions and poor physical health (1, 2). A growing literature suggests that positive interactions may have a beneficial impact on child development and health by influencing immune functioning as well as by decreasing the onset and progression of inflammatory disorders such as asthma (3, 4). However, much less is known about the health benefits of positive family interactions for developing youth or the mechanisms through which these relationships might occur, despite recent calls for research clarifying the links between child and adolescent development and chronic illness (2). The present study seeks to clarify the relationship between positive parenting behaviors and youth health—specifically immune responses among youth with asthma—and identify a potential psychological mechanism of this relationship.
Research on child development has shown that parental behaviors, specifically maternal responsiveness, are critical during infant years and are predictive of beneficial cognitive and emotional outcomes later in childhood (5, 6). Parental responsiveness is a key aspect of the parent-child relationship that includes daily exchanges of action and reaction by youth and caregivers, in which caregivers respond in an appropriate, prompt, and contingent manner to youth behavior (7). Unresponsive parenting comprises behaviors that are inconsistent, delayed, or unreliable and is linked with a number of psychological vulnerabilities throughout development (8, 9). Harsh and unresponsive parenting behaviors have also been associated with poor health outcomes in youth and into adulthood (10, 11). Research has focused on the direct impact of negative parenting strategies and family dynamics on immune function, such that perceived lack of support from parents is linked to increased stimulated concentrations of interleukin (IL)-5, interferon (IFN)-γ, and eosinophil production in youth with asthma (12).
Fewer studies have examined the impact of positive parenting behaviors on youth outcomes. Nurturing and attentive maternal behaviors are linked with positive emotional outcomes and fewer externalizing problems (13, 14). Additionally, positive parenting behaviors have been shown to mediate the relationship between cumulative risk during childhood and subsequent problem behavior (15). Despite the favorable impact of positive parenting behaviors on emotional and behavioral outcomes, the research determining the direct impact of parental positive behaviors on youth health is scarce.
Emerging research suggests that positive affect may be a mechanism through which daily parent-child interactions—specifically maternal responsiveness—may impact immune functioning and, thus, diseases of the immune system such as asthma and markers associated with inflammation (3, 16). Positive affect is commonly defined as a level of pleasurable engagement with one’s surrounding environment including brief time-limited durations or displays of a trait-like disposition (17, 18). Broadly, research with adults has demonstrated a relationship between reported feelings of positive affect and superior health outcomes (19-21). Positive affect is associated with a variety of cellular immune responses in adults, from increases in stimulated production of IL-2 and IL-3 to decreases in tumor necrosis factor-alpha (18, 22, 23). Although a handful of studies have examined the relationship between of positive affect and immunity, none to our knowledge has examined the relationship between expressions of positive affect (e.g., observed positive affect expressed in social interactions) and immunity.
Asthma is a chronic disease characterized by inflammation and airway hyper-responsiveness that currently affects over 10 million youth in the United States and is the third most leading cause of hospitalization (24, 25). Research has focused on the relationship between stress and asthma, linking TH1, TH2, and/or pro-inflammatory cytokines which accentuate airway inflammation found during asthma exacerbations (26). In studies using peripheral blood analyses, young adults with asthma following stressful situations tend to have heightened IL-5 production, a TH2 cytokine linked to inflammation in asthma (27). In vitro analyses with stimulated cytokines demonstrated that adolescents with asthma from lower socioeconomic neighborhoods had greater levels of stimulated IL-5, IL-13 (a TH2 cytokine), and IFN- γ (a TH1 cytokine associated with initiating cellular immune responses and protection against infections) and, further, this relationship was partially explained by stress levels (28-30). Rather than focus on negative psychosocial factors, the work described here sought to examine positive factors in family life, including responsive parenting, and their associations with immune responses.
The Current Study
The goals of the present study were to determine if naturalistically-observed maternal responsive behaviors, assessed using the Electronically Activated Recorder (EAR; described in detail in below), are associated with immune responses in youth with asthma and to determine if youth expressions of positive affect in daily life provide a mechanistic explanation of associations between maternal responsiveness and immune responses. We expected that greater displays of maternal responsive behavior and displays of youth positive affect would be inversely associated with stimulated levels of cytokine production, including IL-5, IL-13, and IFN-γ. We also expected an indirect effect of maternal responsiveness on the immune responses through displays of youth positive affect. Although not a central focus of the current study as it is less novel and better understood, we also examined the relationship between youth expressed negative affect in daily life and the study variables.
Method
Participants
Forty-three children and adolescents aged 10 to 17 with asthma and their primary caregivers took part in the study as part of the pilot study for a larger longitudinal study investigating the effect of family environments on child health, the Asthma in the Lives of Families Today (ALOFT) Study (See Table 1)1. Participants were recruited from November 2010 until August 2012. Primary caregivers included 41 mothers, one father, and one aunt. Participants were recruited through Metro-Detroit hospitals, clinics, and area schools. Families were informed that the purpose of the study was to better understand the relationship between aspects of daily life and asthma. Families were eligible for the study if the youth was between the ages of 10 and 17 with a diagnosis of mild to severe asthma. Families were excluded if the participating youth was currently using oral steroid medication(s), diagnosed with a chronic condition other than asthma (e.g., endocrine disorders, immunodeficiency, and cardiovascular disease), or diagnosed with a medical condition that may interfere with immune system function (e.g., pregnancy, chemotherapy, or radiotherapy in the past year). Written assent and consent were obtained from the participating youth and their parent(s), respectively.
Table 1.
Descriptive Statistics of Youth Participants and their Households (N = 43)
| Mean (SD) or % | |
|---|---|
| Age | 12.6 (1.6) |
| Sex (Male) | 60.5% |
| Race | |
| Black | 55.8% |
| White | 41.9% |
| Other | 2.3% |
| Parental Income Per Year | |
| $0-$7,825 | 27.9% |
| $7,826-$31,850 | 46.5% |
| $31,851-$64,250 | 18.6% |
| $64,251-$97,925 | 4.7% |
| $97,926-$174,850 | 2.3% |
| Single Parent Home | 44% |
| Degree of Asthma Control | |
| Well Controlled | 72.1% |
| Moderately Controlled | 18.6% |
| Poorly Controlled | 9.3% |
| % beta-agonist prescribed | 76.7% |
| % inhaled corticosteroid prescribed | 67.4% |
| Stimulated Cytokine Concentrations | |
| IL-5 (pg/ml) | 866 (588) |
| IL-13 (pg/ml) | 1,047 (620) |
| IFN- γ (pg/ml) | 114,687 (149,137) |
Procedures
The participating youth and caregiver visited the laboratory, where they completed a number of questionnaires on a laboratory computer, individual interviews, and pulmonary function tests. Youth participants then wore the EAR for four days. Following the four-day monitoring period, a peripheral blood draw was conducted on each youth participant. Youth and caregivers were compensated for their time. The project was approved by the Wayne State University Institutional Review Board.
Electronically Activated Recorder (EAR)
In order to assess maternal responsiveness and youth expressions of affect in daily life, each child/adolescent wore the EAR (33) as they went about their daily lives. Previous research with the EAR suggests good psychometric properties, including sampling patterns that are generalizable to an individual’s daily behavior, satisfactory test-retest reliability levels, and high inter-rater reliability when using well defined coding schemes (34). Moreover, the EAR has been utilized in studies of daily behavior in family research (11, 35).
Two versions of the EAR were utilized in this study2. Participants were told to place the device in their front pocket or to wear the device on a belt clip provided. Following the laboratory session, the youth participant wore the EAR for four days, two weekdays and two weekend days. Youth were instructed to wear the EAR continuously from the time they woke up until bedtime. Recordings captured 50 seconds of sound every nine minutes. All participants were provided the option of receiving a CD containing their recordings and were given the opportunity to delete files prior to coding. Three participants requested their CDs and none of these participants chose to delete audio files.
EAR data was coded using the Everyday Child Home Observation (ECHO; 36) coding system that specified the youth’s current location, activity, mood, and behaviors related to specific types of parent-child interactions. Inter-coder reliability was determined by a set of training recordings (512 50-second recordings) independently coded by the eleven research assistants. Intraclass correlations (ICCs) based on a two-way random effects model were calculated for each coded behavior. Prior EAR studies have reported ICCs utilizing the 2,k method with values ranging from .12 for behaviors like reading to 1.0 for talking, reflecting two way random average ICCs across all raters, to which our values are comparable or better (e.g., 37, 38, 39). These reliabilities are typically high due to estimating the reliability for an average measure consisting of k codings—analogous to a questionnaire with x items. Using the 2,1 method (two-way random single measure) of computing ICCs is a more conservative approach and represents the reliability for single raters. Both ICC estimations are displayed in Table 2 to allow for comparison with the typical magnitude of EAR inter-rater reliabilities in previous studies (2, k, to which our estimates compare very favorably) and to provide estimates of the “true” reliability of these EAR codes using the 2,1 method. As expected, the reliability estimates using the 2,1 method are lower than when using the 2, k method. Participants had an average of 201.81 (SD = 74.80) waking audio files and an average of 88.91 (SD = 40.75) talking files. Scores for each EAR-observed behavior reflect a mean of the total recordings in which the behavior was observed during waking hours. The mean values of the summed composites, standard deviations, ranges, internal consistency values, and intra-class correlation coefficients are displayed in Table 2. EAR coders listen to all participant files prior to coding. During this time, they identify the youth participant and caregiver based upon the frequency in which speaker is found in sound files and a small snippet of spoken language recorded during the laboratory visit. Family members, neighbors, and other individuals in the youth participant environment are identified by contextual cues in the recording files (e.g., location, topic of conversation).
Table 2.
Electronically Activated Recorder (EAR) Composites (N = 43)
| EAR Behaviors | Mean (SD) | Range | Internal Consistency (α) |
Inter-rater Reliability (ICC [2,k]) |
Inter-rater Reliability (ICC [2,1]) |
|---|---|---|---|---|---|
| Maternal Responsiveness | 3.20 (0.33) | 3.00-4.68 | 0.78 | 0.93 | 0.55 |
| Caregiver-Youth Conflict | .03 (.03) | .00-.11 | 0.33 | 0.83-0.97 | 0.30-0.73 |
| Total Youth Positive Affect | 6.44 (1.35) | 4.51–9.73 | 0.85 | 0.90-0.93 | 0.44-0.55 |
| Youth Positive Affect Without Caregiver Present |
6.12 (1.67) | 4.09-10.36 | 0.81 | 0.90-0.93 | 0.44-0.55 |
| Total Youth Negative Affect | 5.47 (0.21) | 5.14-5.93 | 0.43 | 0.92-0.95 | 0.43-0.58 |
| Youth Negative Affect Without Caregiver Present |
5.39 (0.27) | 5.03-6.00 | 0.54 | 0.92-0.95 | 0.43-0.58 |
Note: Scores for each EAR-observed behavior reflect a mean of the total recordings in which the behavior was observed during waking hours. Individual EAR-observed behaviors were then summed to build the EAR composites.
Maternal responsiveness was assessed via a composite measure that included the sum of individual EAR-observed behaviors, including warmth, emotional support, and expressions of pride provided to the youth from their female caregiver. Although one caregiver-youth dyad enrolled in the current study included a father, this youth lived in a two-parent home and his mother participated in his caregiving and was included in this composite. All observed behaviors were coded on a 1-5 scale, allowing for half point scores. Warmth was defined as a state characterized by affection and/or kindness (1= not at all warm; 5 = extremely warm, both tone of voice and content). Examples of warmth toward the youth participant include “Don’t worry baby, I’m going to get you a sweater before you get sick because it is cold down here” and “Bless you my child.” Emotional support given towards participating youth was defined as providing support when the participating child/adolescent was in a state of distress or emotional need, including empathy, concern, caring, love, and trust (1 = absence; 5 = extremely supportive and empathic, both in tone of voice and content). Emotional support examples include “Don’t worry, Mommy is going to help you” and “You’re almost done.” Pride expressed for youth was defined as a feeling of deep pleasure or satisfaction as a result of the participating child/adolescent’s own achievements, qualities, or possessions (1 = not proud at all; 5 = extremely proud, both tone of voice and content). Examples of pride expressed for youth include “Awesome, I’m so proud of you” and “You’re doing real good sweetie.” The maternal responsiveness composite mean was 3.20 (SD = .33, range: 3.00 – 4.68, α = .78).
Total youth-expressed positive affect was assessed via a composite measure of summed individual EAR-observed expressed positive affect codes in daily interactions including happy, interested, excited, and proud. All observed affect was coded on a 1-5 scale, also allowing for half point ratings. “Happy” was defined as expressions of pleasure. Examples of happy affect include “I love this TV show” and “My basketball game was so much fun.” “Interested” was defined as showing curiosity, attention about something or someone, and/or increased involvement with a person or situation. Interested examples include “I can’t wait to hear how it went” and “Cool, tell me more.” “Excited” was defined as expressing enthusiasm and eagerness. Excited examples include “Oh my gosh, I am so excited” and “Go, go, go, tell me when you want to switch.” “Proud” was defined as expressing deep pleasure or satisfaction as a result of one’s own achievements, qualities, or possessions. Proud examples include “Dude, I got a 26 and 13, I’m a beast” and “I just had to see something and I was pretty right.” Total positive affect expressed by the youth composite mean was 6.44 (SD = 1.35, range: 4.51 – 9.73, α = .85).
A second composite was computed regarding youth positive affect that comprises the sum of individual EAR-observed expressed positive affect codes in daily interactions without the female caregiver present. The codes included in the composite are happy, interested, excited, and proud and these were also coded on a 1-5 scale allowing for half points. All files including the primary caregiver were eliminated from the current measure. Positive affect expressed by the youth without the caregiver present composite mean was 6.12 (SD = 1.67, range: 4.09-10.36, α = .81). Notably, the range of positive affect expressed not in the presence of the mother was much greater.
Youth-expressed negative affect was assessed via a composite measure of summed individual EAR-observed negative affect codes in daily interactions including sad, angry, upset, worried, and distressed. All observed affect was coded on a 1-5 scale, also allowing for half points. “Sad” was defined as a low volume of voice, slowness of speech, sometimes including crying, expressing feelings of disadvantage, loss, despair, helplessness, or sorrow. “Angry” was defined by use of abrupt, biting words, emotion characterized by antagonism toward someone or something. “Upset” was defined by a feeling characterized by unsettled, disappointment, frustration, or hurt feelings expressed. “Worried” was defined by showing signs of anxiety, fear, or uneasiness. This also included expressing concern for the self and others. “Distressed” was characterized by a state of suffering in the body or mind, exhibiting signs of stress including but not limited to crying, yelling, and speaking in a higher pitch with increasing volume. Total negative affect expressed by the youth composite mean was 5.44 (SD = .22, range: 5.05 – 5.94, α = .43).
An additional composite was computed regarding youth negative affect that comprises the sum of individual EAR-observed expressed negative affect codes in daily interactions without the female caregiver present. The codes included in the composite are sad, angry, upset, worried, and distressed and these were also coded on a 1-5 scale allowing for half points. All EAR files including the primary caregiver were eliminated from the current measure. Negative affect expressed by the youth without the caregiver present composite mean was 5.39 (SD = .27, range: 5.03-6.00, α = .54).
The affect composites and person-level aggregates of EAR-observed emotion codes exhibited a small range overall. However, among individual EAR files from participants, there was a full range from 1-5 on all codes with the exception of “worried” which ranged from 1-4.
Caregiver-youth conflict was also assessed via the EAR and used as a measure of negative parenting behaviors in the current study. The caregiver-youth conflict composite included coded conflict specifically between mother/female caregiver and participating youth, yelling by the mother/female caregiver, and yelling by the youth with each code rated as present (1) or absent (0). This composite is limited to the experience of conflict between the youth and their caregiver (M = .03, SD = .03, range: .00-.11, α = .39). Although the inter-rater reliabilities of the specific EAR conflict codes all within acceptable limits (e.g., above .70 for 2,k), the alpha levels for the caregiver-youth conflict composite was low due to small correlations between youth yelling (r = .13, p = .40) and mother yelling (r = .15, p = .35) with mother-youth conflict. However, in previous work, we identified that the yelling codes were significantly correlated with youth daily reports of negative caregiver-youth interactions (31). Thus, on both theoretical grounds and for parsimony of conducting analyses, we retained the yelling codes in the conflict composite.
Biological assays
The morning following the four-day monitoring period, an 8 mL peripheral blood sample was collected from each youth participant into Vacutainer Cell Preparation Tubes containing sodium heparin (Becton Dickinson and Co., East Rutherford, NJ). Following a modified version of the protocol used by Miller et al. (12), peripheral blood mononuclear cells (PBMCs) were isolated and resuspended in RPMI-1640 medium with HEPES supplemented with 10% Fetal Bovine Serum (FBS; Sigma-Aldrich, St. Louis, MO) at a concentration of 3 × 106 cells/mL. PBMCs were treated with 1μl of 100% ethanol and final concentrations of 25 ng/mL phorbol 12-myristate 13-acetate and 1 μg/mL ionomycin calcium salt dissolved in DMSO (all from Sigma-Aldrich), then incubated at 37°C and 5% CO2. After 48 hours of culture, cell suspensions were centrifuged, and supernatants were collected and frozen at −80°C. Concentrations of IL-5, IL-13, and IFN- γ were quantified by means of ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. Inter-assay variations were 4.6% for each analyte, and intra-assay variations for IL-5, IL-13, and IFN- γ were 6.3%, 3.9%, and 3.6%, respectively. Due to missing data, analyses with IFN- γ only included 42 youth participants. Supernatants were also initially assayed for IL-4, but yielded inconsistent and unreliable assay concentration results. Therefore, IL-4 assessment was dropped from the study.
Asthma Control
Degree of asthma control was determined from the National Asthma Education and Prevention Program, Expert Panel Report 2 guidelines (NAEPP/EPR2, 40). The NAEPP guidelines divides FEV1% predicted into three different levels – above 80% predicted typical of mild intermittent and persistent asthma and was classified in the current study as well-controlled, 60%-80% predicted typical of moderate asthma and was classified as moderately controlled, and below 60% predicted typical of severe asthma and classified as poorly controlled. The youth in the sample completed a pulmonary function test during the lab session. Current prescribed inhaled corticosteroid and β-adrenergic agonist medications were abstracted from medical records to account for the impact of medications. Dummy codes were created for both medication classes with 0 “no medication prescribed” and 1 “medication prescribed”.
Potential Confounds
Based on theoretical grounds and prior work, caregiver-youth conflict and youth expressions of negative affect were identified as key covariates in the current analyses3. Caregiver-youth conflict was used as a way to control for negative parenting behaviors that may be occurring in daily life and determine if maternal responsive behaviors are uniquely contributing to asthma-related immune responses above and beyond caregiver-youth conflict. Furthermore, expressions of negative affect in daily life was selected as a covariate due to the associations between negative affect and health outcomes but also the literature supporting positive affect and negative affect as independent constructs (Pressman & Cohen, 2005). Thus, by adjusting for expression of negative affect, we can consider the independent contributions of positive affect to health.
Statistical analyses
All analyses were performed using SPSS for Mac (version 20.0). IL-5, IL-13, and IFN- γ concentrations in stimulated supernatants and the maternal responsiveness composite score were base-10 log transformed prior to analyses to better approximate normal distributions; following transformation, skewness values were all less than 1. Pearson correlations were conducted to examine the individual relationships among demographic variables, maternal responsiveness, youth expressed affect, and immune markers. Multiple regression analyses were conducted to determine if maternal responsiveness predicted asthma-related immune responses when adjusting for parent-child conflict and expressions of youth negative affect.
We then examined whether there was an indirect effect on the relationship between maternal responsive behavior with each stimulated cytokine concentration via of youth expressed positive affect. To test for indirect effects, we conducted regression analyses using the procedure proposed by Preacher and Hayes. To calculate the indirect effects, we used Hayes’ (41) methods with the PROCESS macro using a bootstrapping procedure (20,000 samples).
Results
Associations between maternal responsiveness, youth affect, and immune responses
Maternal responsiveness was strongly associated with total youth positive affect (r = .70, p < .001) and youth positive affect without their caregiver present (r = .61, p = .001), but was unrelated to youth age, sex, or negative affect (Table 3). Greater youth displays of positive affect without the caregiver present was significantly associated with younger age (r = −.37, p = .015) and male sex (r = −.47, p = .001).
Table 3.
Pearson Correlation Coefficients between Demographic and Study Variables (N = 43)
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Youth Age | .02 | .05 | .24 | .03 | .07 | −.25 | −.05 | .07 | −.25 | −.37* | −.19 | −.23 | .11 | .00 | .32* |
| 2. Youth Sex | .05 | .04 | −.03 | .04 | −.02 | −.17 | .29 | −.27† | −.47** | −.06 | −.36* | .20 | .21 | .11 | |
| 3. Number of Parents | .02 | −.21 | .09 | −.14 | −.27† | −.03 | −.15 | −.29† | −.03 | −.09 | .23 | −.08 | .11 | ||
| 4. Parental Yearly Income | −.15 | .17 | −.04 | .03 | −.02 | .05 | −.04 | −.06 | −.06 | .01 | .05 | .21 | |||
| 5. Beta-Agonist Prescribed | .15 | −.02 | .13 | −.14 | .00 | .01 | .00 | .02 | .09 | .07 | .02 | ||||
| 6. Inhaled Glucocorticosteroid Prescribed | .03 | −.06 | .02 | −.01 | −.11 | .31 | .26 | .09 | .04 | .13 | |||||
| 7. Asthma Control | −.06 | −.27† | .08 | .16 | .03 | .08 | −.28 | −.05 | −.12 | ||||||
| 8. Maternal Responsiveness | −.20 | .70** | .61** | .36* | .45** | −.38* | −.33* | −.14 | |||||||
| 9. Caregiver-Youth Conflict | −.27† | −.31* | .24 | −.03 | .15 | .06 | .13 | ||||||||
| 10. Total Youth Positive Affect | .85** | .39* | .41** | −.46** | −.37* | −.21 | |||||||||
| 11. Youth Positive Affect Without Caregiver Present | .31* | .51** | −.47** | −.30* | −.30 | ||||||||||
| 12. Total Youth Negative Affect | .78** | −.11 | .06 | −.07 | |||||||||||
| 13. Youth Negative Affect Without Caregiver Present | −.15 | .06 | .01 | ||||||||||||
| 14.IL-5 | .75** | .53** | |||||||||||||
| 15. IL-13 | .49** | ||||||||||||||
| 16. IFN-G |
Note: †p < .10.
p < .05.
p < .01. For sex, males coded = 1, females coded =2. Due to missing data, correlations with IFN- γ only included 42 youth participants.
There were no differences between male and female levels of stimulated IL-5, IL-13, and IFN-γ production. Higher levels of maternal responsiveness were significantly associated with lower levels of stimulated IL-5 (r = −.38, p = .012) and IL-13 (r = −.33, p = .031) concentrations, which would be viewed as beneficial in individuals with asthma (Table 3). Total expressions of youth positive affect (r = −.46, p = .002) and without the caregiver present (r = −.47, p = .001) were significantly associated with lower levels of IL-5. The total youth expressions of positive affect (r = −.37, p = .014) and without the caregiver present (r = −.30, p = .049) were also significantly correlated with lower levels of IL-13. The total youth expressions of negative affect composite and without the caregiver present were unrelated to asthma-related immune responses. The cytokines were all positively correlated with one another (p < .001), with the strongest correlation between IL-5 and IL-13. This is as would be expected given IL-5 and IL-13 are both TH2 cytokines linked to inflammation among individuals with asthma. Greater stimulated IFN-γ concentrations were significantly related to greater stimulated levels of IL-5 (p < .001) and IL-13 (p = .002) but were unrelated to maternal responsiveness or positive affect (though in the expected direction). The stimulated levels of the individual cytokines were not correlated with youth sex, negative affect, degree of asthma control, parental income, prescribed beta-agonist, or prescribed inhaled corticosteroid.
We then conducted multiple regression analyses to examine the independent effects of maternal responsiveness on asthma-related immune responses. As displayed in Table 4, maternal responsiveness was entered as a predictor along with mother-youth conflict and youth negative affect. Increased maternal responsiveness uniquely predicted decreased levels of IL-5 (β = −.37, t = −2.19, p = .034) when adjusting for mother-youth conflict and youth expressions of negative affect. Additionally, increased maternal responsiveness also uniquely predicted decreased levels of IL-13 (β = −.44, t = −2.61, p = .013) when adjusting for mother-youth conflict and youth expressions of negative affect. Maternal responsiveness was unrelated IFN- γ, though in the expected direction (β = −.08, t = −.42, p = .67).
Table 4.
Multiple Regression Analyses with Maternal Responsiveness and Covariates Predicting Asthma-Related Immune Responses (N = 43)
| Predictor Variables | IL-5 β |
IL-13 β |
IFN-γ β |
|---|---|---|---|
| Step 1 | R2 = .14, F(1, 41) = 6.92* | R2 = .11, F(1, 41) = 5.01* | R2 = .02, F(1, 41) = .75 |
| Maternal Responsiveness | −.38* | −.33* | −.14 |
| Step 2 | ΔR2 = .01, F(3, 39) = 2.28† | ΔR2 = .05, F(3, 39) = 2.36† | ΔR2 = .02, F(3, 39) = .45 |
| Maternal Responsiveness | −.37* | −.44* | −.08 |
| Caregiver-Youth Conflict | .07 | −.09 | .14 |
| Total Youth Negative Affect | .01 | .24 | −.09 |
Note: †p < .10.
p < .05.
p < .01. Due to missing data, analyses with IFN- γ only included 42 youth participants.
Indirect Effects of Youth Positive Affect
We then tested for the presence of indirect effects between maternal responsiveness and lower immune responses to antigen stimulation via youth expressed positive affect. Each biomarker was tested individually.
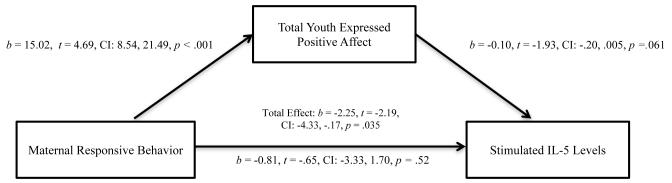
We used the PROCESS macro in SPSS to simultaneously estimate the indirect effect of maternal responsiveness through the total youth positive affect composite on simulated levels of IL-5 when adjusting for mother-youth conflict and youth expressions of negative affect (41, 42). As displayed in Figure 1, analyses indicated that there was a significant indirect effect of maternal responsiveness through the total youth expressions of positive affect on levels of stimulated IL-5 (95% CI = −3.41, −.03). The indirect effect of maternal responsiveness through the total youth expressions of positive affect on levels of stimulated IL-13 was significant (95% CI = −2.34, −.01) when adjusting for mother-youth conflict and youth expressions of negative affect. The indirect effect of maternal responsiveness through the total youth expressions of positive affect on levels of stimulated IFN- γ was not significant when adjusting for mother-youth conflict and youth expressions of negative affect (95% CI = −5.37, 1.69).
Figure 1.
Indirect effect of maternal responsiveness on levels of stimulated IL-5 through total displays of youth positive affect controlling for caregiver-youth conflict and expressions of negative affect (N = 43).
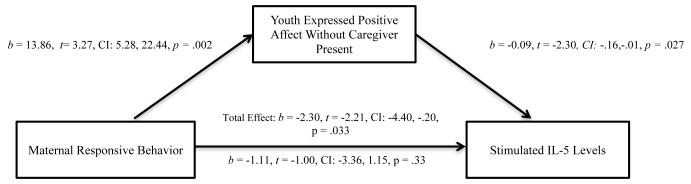
Next, we tested whether the relationship between maternal responsiveness, youth positive affect, and the asthma-related immune responses held when using youth positive affect without the caregiver present instead of the total positive affect composite to determine if this effect was unique to interactions with a caregiver. As shown in Figure 2, there was a significant indirect effect of maternal responsiveness through youth expressions of positive affect without the caregiver present on levels of stimulated IL-5 (95% CI = −3.11, −.10) when adjusting for caregiver-youth conflict and expressions of negative affect not in the presence of the caregiver. However, there was not a significant indirect effect of maternal responsiveness through youth expressions of positive affect without the caregiver present on levels of stimulated IL-13 (95% CI = −1.84, .10) when adjusting for caregiver-youth conflict and expressions of negative affect not in the presence of the caregiver.
Figure 2.
Indirect effect of maternal responsiveness on levels of stimulated IL-5 through displays of youth positive affect without the caregiver present controlling for caregiver-youth conflict and expressions of negative affect without the caregiver present (N = 43).
Discussion
The findings from this study showed that increased maternal responsiveness and displays of youth positive affect in daily life are linked to lower production of IL-5 and IL-13, potentially important biomarkers in the management of asthma. Furthermore, youth expressions of positive affect provided a mechanistic explanation for the relationship between maternal responsiveness and stimulated levels of IL-5 and IL-13. That these effects held whether examining total youth expressed positive affect or expressions of positive affect when youth were not in the presence of their female caregiver for IL-5 provides preliminary results that the links between positive affect and IL-5 may be robust across different social contexts. In other words, the association between youth positive affect and IL-5 was not driven simply by positive affect expressed in the presence of the female caregiver. Further, the fact that these results held when adjusting for negative aspects of daily family life, including caregiver-youth conflict and youth expressions of negative affect, provides more evidence for the independent health effects of positive family interactions—and positive emotions more broadly—in daily life.
For individuals with asthma where inflammation and allergic reactions can cause airway hyper-responsiveness and difficulty breathing (43), a reduced immune response leading to lower levels of IL-5 and IL-13 is desired. Although production of IL-5 and IL-13 were very strongly correlated, the indirect effect model predicting IL-13 via expressions of youth positive affect not in the presence of the caregiver was not significant; however, the indirect effect of maternal responsiveness on IL-13 via total expressions of youth positive was significant. It is possible that with a greater sample size, the indirect effect model with expressions of positive affect without the caregiver would be significant. In contrast to how negative interactions such as stress and strained family relationships can increase levels of potentially-damaging immune and/or pro-inflammatory biomarkers, these findings suggest that positive family interactions are associated with reduced, and therefore potentially less-damaging immune responses. These associations support the hypothesis that increased maternal responsiveness and positive affect expressed by youth may improve asthma symptoms over time by reducing the responsiveness of the immune system to exposures likely to trigger the well-characterized TH2 responses involving IL-5 and IL-13, cytokines related to increased inflammation and airway obstruction with key relevance for asthma (44).
The current analyses do not help to clarify the contribution of IFN- γ to asthma pathophysiology. The current role of IFN- γ is not well-defined in the literature in terms of the contribution to asthma symptoms and morbidity (12, 30). We found that greater caregiver responsivesness and greater expressed positive affect were not significantly related to stimulated IFN- γ concentrations but the associations were in the same direction as the associations between those psychological variables and IL-5 and IL-13. Similar to our results, children with asthma in low socioeconomic status family with greater stress exhibit a similar significant pattern of stimulated TH2 cytokines and no relationship with TH1 cytokines (28). Also consistent with the pattern of results found in our study, individuals with asthma who are subjected to academic examination stress display activated TH2 cytokines but unchanged TH1 cytokine expression (27). However, recent studies have shown links between stress and increases in IFN- γ in individuals with asthma (29, 45). It is possible that positive family interactions do not influence TH1 responses via IFN- γ, in contrast to the research that has demonstrated a link between stress, family relationships, and activated immune responses via the TH1 and TH2 pathway (12, 29, 46).
Additionally, these data contribute to the growing literature on the relationship between positive affect and superior health outcomes, showing links between positive affect and lower levels of stimulated IL-5 and IL-13. Notably, this is the only study to our knowledge to examine how expressions of youth positive affect are associated with health-related biological processes. Theories of positive affect assert that positive affect may promote resilience and endurance, bolster physical and psychological resources (47), and encourage positive health behaviors (48), which, in turn, may help to reduce stress and promote recovery from stressors. Consistent with the adult literature that has demonstrated a relationship between self-reported positive affect and improved immune function (49), these findings show that expressions of positive affect are also important and associated with a “healthier” immune response.
Overall, these observations highlight the potential health benefits of responsive and supportive family relationships. Parental responsiveness has been viewed as a crucial family component in the management of youth asthma. These data, coupled with accumulating evidence demonstrating the direct negative impact of adverse family experiences on asthma pathogenesis, make a strong case for further exploration of family-health links among youth with asthma (12). Additionally, these data also converge with research focusing on resilience in youth raised in risky family environments. Building on Chen and Miller’s (50) “Shift and Persist” model, which offers an explanation as to why some children can accept, adapt, and endure high degrees of life stress early in life, this work provides novel evidence for family characteristics that may mitigate the deleterious health effects of recurrent and severe adversity early in life.
The current study has limitations that are worth noting. First, this study has a fairly small sample size with limited statistical power and does not include a control group comprised of healthy youth; previous research has highlighted the different impact of family interactions on immune processes in healthy children (12, 28). Also, while utilizing an acoustic naturalistic observation strategy provides a number of benefits, the method only allows coding of verbal and audible support in the environment; this method cannot assess aspects of family relationships that are not heard (e.g., body language, physical touch, or written words). The EAR data also only captured family interactions through a 4-day period, which may limit our ability to observe family dynamics as they change and evolve over longer periods of time. These analyses focused only on maternal responsiveness and positive affect, but have established a foundation from which to explore other positive aspects in family life that may impact physical health. Additionally, the current study did not have a record of the actual medications used during the EAR sampling period. Prescribed medication levels used in the current study may not be an accurate reflection of medications taken provided the challenges associated with adhering to a maintenance medication for youth with asthma (51). Given the prescribed amount of medication may not be an adequate measure, it is possible that caregivers that were more responsive to the youth directed more attention to anti-inflammatory medication adherence and could potentially explain the lower immune response found in the current study. Further, the current data are cross-sectional and, although we found evidence for indirect effects, it is possible that these relationships are bidirectional and/or change over time. Youth in the current study are moving through a critical age in development, it is possible that these relationships change over time or that caregivers are more responsive to youth who express more positive affect. Finally, the current study did not assess youth personality as a potential contributor to the effects observed. It is possible that youth with certain trait-level characteristics, such as optimism, may express positive affect more in daily life and thus trait-level characteristics may ultimately account for improvement in asthma-related immune responses. Future work should aim to clarify the influence of various trait-level characteristics on expressions of positive affect and the relationship to health. This type of work could also clarify whether stable personality traits are stronger predictors of health than daily micro-level family interactions, such as those assessed in the current study.
The current work utilizes the TH1/TH2 paradigm as an avenue to understand the complex mechanisms underlying immune processes found in youth with asthma (30). Notably, this paradigm was developed out of animal models focusing on the contribution of IL-4 and IL-5 to allergic processes and limits the investigation of immune processes at play. Although there is strong research support for aspects of the TH1/TH2 paradigm, research utilizing human samples has not successfully replicated all the findings from animal studies supporting this paradigm (29, 52). Moreover, the TH1/TH2 paradigm does not investigate the influence of other T helper cell subsets (TH9, TH17, and TH22) found to influence asthma (30, 53, 54).
Future work should focus on clarifying the biological pathways that are involved with positive family interactions. One interesting potential direction for research is investigating whether maternal responsiveness and other positive parental behaviors enhance cortisol’s ability to regulate the immune response and simultaneously defend against glucocorticoid insensitivity in immune cells (12). Preliminary evidence from our group points to influence of positive caregiver behavior and interpersonal conflict on HPA axis functioning via diurnal cortisol patterns, such that positive maternal parenting behavior was associated with steeper (more “healthy”) diurnal cortisol slope in youth with asthma whereas interpersonal conflict was associated with flatter (less “healthy”) diurnal cortisol slope (55). By further investigating the potential downregulation of glucocorticoid receptor expression and functioning, research could continue to clarify the links between family interactions, cortisol secretion, and asthma. We theorize that youth in families characterized by high levels of positive behaviors should show increased glucocorticoid receptor gene expression and protein synthesis, and, in turn, greater down regulation of inflammation and immune response by the HPA axis. Furthermore, future work can clarify the contribution of other T helper cell subsets or T regulatory cells that were not examined in the current analyses. As interest in T regulatory cells’ contribution to TH2 cytokine production and asthma exacerbations increases, additional research could work to clarify how these cellular processes contribute to the relationship between family interactions and asthma. Moreover, extending this work to medically healthy children may provide more insights into the influence of positive family interactions on health.
A second critical direction of this work is examining how positive family behaviors impact immune function over time, and, in turn, impact asthma morbidity. As a corollary to extensive prior work showing that increased adversity in youth is associated with a number of negative biological and health consequences, the findings from this study suggest that responsive parenting may presage improvements in children’s health-related biology during development. Taking the longer view, future research is needed to determine whether responsive parenting in late childhood and adolescence can lead to physical and psychological health benefits in adulthood. Parental behaviors that exhibit appropriate and contingent support may help to buffer the effects of stress—both daily stress and stress due to illness—and positively impact a child’s ability to cope with stress into adulthood.
If replicated with larger samples and longitudinal designs, there are potential clinical implications of this work. Whether fostering parental responsiveness could lead to clinically significant improvements in either acute or chronic inflammation could be tested by integrating biological assessments into existing intervention protocols, many of which are geared toward helping parents to be more responsive to their children. Early intervention work in the parent-child relationship, focusing on improvement in maternal responsiveness, attentiveness, and control, has demonstrated resulting changes in ability to self-soothe, sociability, and cognitive exploration in infancy (56). Other programs have had success with enhancing support and sensitivity in at-risk samples to improve emotional and academic outcomes (57, 58); therefore, future work could assess the impact of these types of interventions on cellular processes and physical health outcomes.
Taken together, the findings from this study support the idea that positive and supportive family interactions have the potential to promote physical health in children and adolescents. Our data point to a plausible psychological mechanism regarding the way in which parental behaviors can influence youth health, such that maternal responsive behaviors, through youth positive affect expressed in daily life, may lead to positive health outcomes. This work helps to provide a more comprehensive understanding of how positive behaviors in the family, in addition to previously identified negative family characteristics, influence immune responses.
Acknowledgments
Source of Funding: This work has been supported by funding from the National Institutes of Health Grant (RO1HL114097 [Tobin, Saleh, Kane, and Slatcher]) and a Wayne State University Junior Faculty Grant in the Social and Behavioral Sciences. We thank all the research assistants in the Close Relationships Lab for their help with data collection.
Glossary
- IL
interleukin
- IFN
interferon
- EAR
Electronically Activated Recorder
Footnotes
Other papers from the same project have examined naturalistically observed conflict and asthma symptoms (31) and socioeconomic status and youth behaviors (32). The present analyses do not overlap with analyses from those other papers.
The first version used in this study (HP iPAQ 110) is 4.59 × 2.71 × .54 inches and weights 4.08 ounces. The second version used in this study (Apple iPod touch, 8GB) is 4.4 × 2.3 × 0.28 inches and weights 3.56 ounces.
We thank an anonymous reviewer for suggesting that caregiver-youth conflict and expressions of negative affect be included as covariates.
Conflicts of Interest: The authors have no conflict of interest.
Contributor Information
Erin T. Tobin, Department of Psychology, Wayne State University
Heidi S. Kane, School of Behavioral and Brain Sciences, University of Texas at Dallas
Daniel J. Saleh, Department of Psychology, Wayne State University
Derek E. Wildman, Department of Molecular and Integrative Physiology, University of Illinois
Elizabeth Crabb Breen, Cousins Center for Psychoneuroimmunology, Semel Institute for Neuroscience and Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles
Elizabeth Secord, Allergy/Immunology Department, Children’s Hospital of Michigan
Richard B. Slatcher, Department of Psychology, Wayne State University
References
- 1.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–66. [PubMed] [Google Scholar]
- 2.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–97. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Reviews Immunology. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 4.Dockray S, Steptoe A. Positive affect and psychobiological processes. Neuroscience & Biobehavioral Reviews. 2010;35:69–75. doi: 10.1016/j.neubiorev.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ainsworth M, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- 6.Bornstein MH. Maternal responsiveness: Characteristics and consequences. Jossey-Bass; 1989. [Google Scholar]
- 7.Bornstein MH, Manian N. Maternal responsiveness and sensitivity reconsidered: Some is more. Development and psychopathology. 2013;25:957–71. doi: 10.1017/S0954579413000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isabella RA, Belsky J. Interactional synchrony and the origins of infant-mother attachment: A replication study. Child development. 1991;62:373–84. [PubMed] [Google Scholar]
- 9.Shaw DS, Keenan K, Vondra JI. Developmental precursors of externalizing behavior: Ages 1 to 3. Developmental Psychology. 1994;30:355. [Google Scholar]
- 10.Gottman JM, Katz LF. Effects of marital discord on young children’s peer interaction and health. Developmental Psychology. 1989;25:373–81. [Google Scholar]
- 11.Slatcher RB, Robles TF. Preschoolers’ everyday conflict at home and diurnal cortisol patterns. Health Psychology. 2012;31:834–8. doi: 10.1037/a0026774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller GE, Gaudin A, Zysk E, Chen E. Parental support and cytokine activity in childhood asthma: The role of glucocorticoid sensitivity. Journal of Allergy and Clinical Immunology. 2009;123:824–30. doi: 10.1016/j.jaci.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Bridges LJ, Grolnick WS. The development of emotional self-regulation in infancy and early childhood. In: Eisenberg N, editor. Review of perosnality and social psychology. Sage Publications; Thousand Oaks, CA: 1995. pp. 185–211. [Google Scholar]
- 14.Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations Among Positive Parenting, Children’s Effortful Control, and Externalizing Problems: A Three-Wave Longitudinal Study. Child development. 2005;76:1055–71. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trentacosta CJ, Hyde LW, Shaw DS, Dishion TJ, Gardner F, Wilson M. The relations among cumulative risk, parenting, and behavior problems during early childhood. Journal of Child Psychology and Psychiatry. 2008;49:1211–9. doi: 10.1111/j.1469-7610.2008.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Emotional style and susceptibility to the common cold. Psychosomatic Medicine. 2003;65:652–7. doi: 10.1097/01.psy.0000077508.57784.da. [DOI] [PubMed] [Google Scholar]
- 17.Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13:205–34. [Google Scholar]
- 18.Pressman SD, Cohen S. Does Positive Affect Influence Health? Psychological Bulletin. 2005;131:925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 19.Avia MD, Kanfer FH. Coping with aversive stimulation: The effects of training in a self-management context. Cognitive Therapy and Research. 1980;4:73–81. [Google Scholar]
- 20.Codispoti M, Gerra G, Montebarocci O, Zaimovic A, Augusta Raggi M, Baldaro B. Emotional perception and neuroendocrine changes. Psychophysiology. 2003;40:863–8. doi: 10.1111/1469-8986.00104. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Pressman SD. Positive affect and health. Current Directions in Psychological Science. 2006;15:122–5. [Google Scholar]
- 22.Mittwoch-Jaffe T, Shalit F, Srendi B, Yehuda S. Modification of cytokine secretion following mild emotional stimuli. Neuroreport. 1995;6:789–92. doi: 10.1097/00001756-199503270-00021. [DOI] [PubMed] [Google Scholar]
- 23.Berk LS, Felten DL, Tan SA, Bittman BB, Westengard J. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Alternative therapies in health and medicine. 2001;7:62–76. [PubMed] [Google Scholar]
- 24.Center for Disease Control and Prevention . Vital and Health Statistics. Centers for Disease Control and Prevention; Washington DC: 2011. [Google Scholar]
- 25.Akinbami L. The state of childhood asthma, United States, 1980-2005. Advanced Data. 2006:1–24. [PubMed] [Google Scholar]
- 26.Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain, behavior, and immunity. 2007;21:993–9. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to anitgen challenge. American journal of respiratory and critical care medicine. 2002;165:1062–7. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 28.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117:1014–20. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic Status, Stress, and Immune Markers in Adolescents With Asthma. Psychosomatic Medicine. 2003;65:984–92. doi: 10.1097/01.psy.0000097340.54195.3c. [DOI] [PubMed] [Google Scholar]
- 30.Trueba AF, Ritz T. Stress, asthma, and respiratory infections: pathways involving airway immunology and microbial endocrinology. Brain, behavior, and immunity. 2013;29:11–27. doi: 10.1016/j.bbi.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Tobin ET, Kane HS, Saleh DJ, Naar-King S, Poowuttikul P, Secord E, Pierantoni W, Simon V, Slatcher RB. Naturalistically Observed Conflict and Youth Asthma Symptoms. 2015;34:622–31. doi: 10.1037/hea0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imami L, Tobin ET, Kane HS, Saleh DJ, Lupro TH, Slatcher RB. Effects of Socioeconomic Status on Maternal and Child Positive Behaviors in Daily Life Among Youth with Asthma. Journal of Pediatric Psychology. 2014;40:55–65. doi: 10.1093/jpepsy/jsu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehl MR, Pennebaker JW, Crow MD, Dabbs J, Price JH. The Electronically Activated Recorder (EAR): A device for sampling naturalistic daily activities and conversations. Behavior Research Methods, Instruments, and Computers. 2001;33:517–23. doi: 10.3758/bf03195410. [DOI] [PubMed] [Google Scholar]
- 34.Mehl MR, Robbins ML, Deters FG. Naturalistic observation of health-relevant social processes: the electronically activated recorder methodology in psychosomatics. Psychosomatic Medicine. 2012;74:410–7. doi: 10.1097/PSY.0b013e3182545470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slatcher RB, Trentacosta CJ. Influences of parent and child negative emotionality on young children’s everyday behaviors. Emotion. 2012;12:932–42. doi: 10.1037/a0027148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slatcher RB, Tobin ET. Everyday Child Home Observation Coding System. 2012.
- 37.Tenney ER, Vazire S, Mehl MR. This Examined Life: The Upside of Self-Knowledge for Interpersonal Relationships. PloS one. 2013;8:e69605. doi: 10.1371/journal.pone.0069605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehl MR, Vazire S, Holleran SE, Clark CS. Eavesdropping on happiness: Well-being is related to having less small talk and more substantive conversations. Psychological Science. 2010;21:539–41. doi: 10.1177/0956797610362675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehl MR, Gosling SD, Pennebaker JW. Personality in its natural habitat: Manifestations and implicit folk theories of personality in daily life. Journal of Personality and Social Psychology. 2006;90:862–77. doi: 10.1037/0022-3514.90.5.862. [DOI] [PubMed] [Google Scholar]
- 40.Expert Panel 2: National Heart Lung and Blood Institute NIoH . Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: 1997. NIH Publication No. 97-4053. [Google Scholar]
- 41.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 42.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press; New York, NY: 2013. [Google Scholar]
- 43.Kaugars AS, Klinnert MD, Bender BG. Family Influences on Pediatric Asthma. Journal of Pediatric Psychology. 2004;29:475–91. doi: 10.1093/jpepsy/jsh051. [DOI] [PubMed] [Google Scholar]
- 44.Hamelmann E, Gelfand EW. IL-5-induced airway eosinophilia–the key to asthma? Immunological reviews. 2001;179:182–91. doi: 10.1034/j.1600-065x.2001.790118.x. [DOI] [PubMed] [Google Scholar]
- 45.Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosomatic Medicine. 2009;71:378–84. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosomatic Medicine. 1999;61:175–80. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Fredrickson BL. What good are positive emotions? Review of general psychology. 1998;2:300. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith AW, Baum A. The influence of psychological factors on restorative function in health and illness. Social psychological foundations of health and illness. 2003:431–57. [Google Scholar]
- 49.Laidlaw TM, Booth RJ, Large RG. The variability of Type I hypersensitivity reactions: The importance of mood. Journal of psychosomatic research. 1994;38:51–61. doi: 10.1016/0022-3999(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 50.Chen E, Miller GE. Shift-and-Persist Strategies: Why Low Socioeconomic Status Isn’t Always Bad for Health. Perspectives on Psychological Science. 2012;7:135–58. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bender B, Wamboldt F, O’Connor SL, Rand C, Szefler S, Milgrom H, Wamboldt MZ. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Annals of Allergy, Asthma & Immunology. 2000;85:416–21. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 52.Kang DH, Fox C. Th1 and Th2 cytokine responses to academic stress. Research in nursing & health. 2001;24:245–57. doi: 10.1002/nur.1027. [DOI] [PubMed] [Google Scholar]
- 53.Jutel M, Akdis CA. T-cell subset regulation in atopy. Current allergy and asthma reports. 2011;11:139–45. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Research and Therapy. 2009;11:257. doi: 10.1186/ar2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kane HS, Tobin ET, Saleh DJ, Goetz S, Carre J, Wildman DE, Naar-King S, Secord E, Slatcher RB. Naturalistically Observed Conflict, Positive Parenting Behaviors, and Diurnal Cortisol Patterns in Youth with Asthma. under review for publication.
- 56.van den Boom DC. The influence of temperament and mothering on attachment and exploration: An experimental manipulation of sensitive responsiveness among lower-class mothers with irritable infants. Child Development. 1994;65:1457–77. doi: 10.1111/j.1467-8624.1994.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 57.Niccols A. ‘Right from the Start’: randomized trial comparing an attachment group intervention to supportive home visiting. Journal of Child Psychology and Psychiatry. 2008;49:754–64. doi: 10.1111/j.1469-7610.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 58.Ford RM, McDougall SJ, Evans D. Parent-delivered compensatory education for children at risk of educational failure: Improving the academic and self-regulatory skills of a Sure Start preschool sample. British journal of psychology. 2009;100:773–97. doi: 10.1348/000712609X406762. [DOI] [PubMed] [Google Scholar]