Abstract
TB vaccine discovery has focused on IFN-γ both for the selection of antigens and vaccine delivery strategies. Recent breakthroughs in our understanding of requirement for immunological memory and the expression of immunity to TB in the lung now provides a framework for reconsidering that strategy. We will discuss the status of the TB vaccine field, recent insights into the role of central memory cells and the potential of tissue-resident memory cells in vaccine promoted protection against TB.
Introduction
Tuberculosis (TB) kills around 1.5 million people each year and although novel diagnostic methods and intense treatment schemes like DOTS (directly observed therapy short course) is widely implemented in high prevalence areas, worldwide mortality has not been reduced at the expected or desired rates [1].
Adding to the magnitude and complexity of the TB problem, mortality represents only the tip of the iceberg, as more than 2 billion people worldwide are clinically healthy but latently infected with Mycobacterium tuberculosis (Mtb). Latent TB infection (LTBI) provides an infinite source of potential reactivation disease and transmission. These numbers make TB the most widespread infectious agent and together with HIV the top cause of death from infectious diseases [1]. The HIV epidemic is the driving force of the TB epidemic in many countries and results in an increasing number of cases involving MDR-, XDR-and TDR (Multi-, eXtensively-, and Total-Drug Resistant) TB [1].
The Bacillus Calmette-Guérin (BCG) vaccine was developed a century ago and is used extensively in most parts of the world with the exception of Western Europe and North America. This vaccine has some protective effect in children but fails to protect against pulmonary tuberculosis in adults. Mtb and humans have co-evolved since the most early human origin [2], and this has allowed the pathogen to adapt and develop a refined set of countermeasures that represent a very difficult target for the immune system.
The result is a pathogen that is rarely cleared by the natural immune response but instead establishes a long-term chronic infection [3]. Attempts to identify adaptive immune responses that are missing or are insufficiently expressed in individuals that develop disease has so far not been successful [4, 5]. However, although clearly insufficient for the control of TB in individuals that develop disease the immune response raised during the natural infection still guides most attempts to develop vaccines.
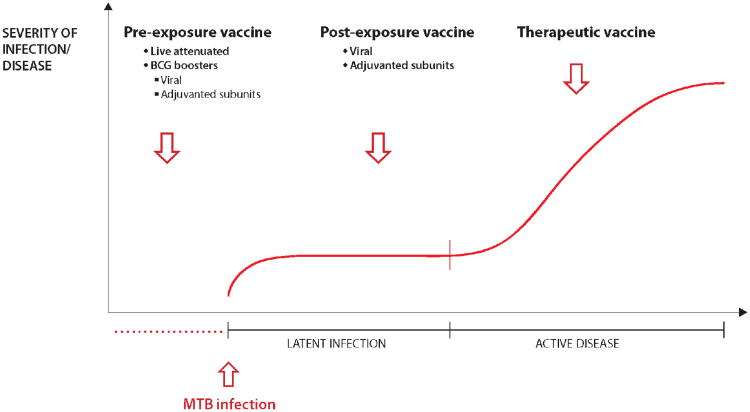
Vaccine strategies against TB
In the last 10 years, there has been substantial progress in the TB vaccine field, with more than a dozen novel vaccines in clinical trials. These vaccines can be divided into different categories depending on the time point of administration compared to the infection and/or prior BCG vaccination and the delivery system used (see Figure 1 for a schematic representation and description of the vaccines in clinical trials). Live mycobacterial vaccines such as recombinant BCG or attenuated Mtb are intended as replacements for neonatal BCG vaccination.
Figure 1. Different types of TB vaccines.
Vaccines are divided into different categories depending on the time point of administration and the delivery system used:
Pre-exposure vaccines are administered prior to infection with Mtb. Viable mycobacteria designed to replace BCG as prime vaccines are classical pre-exposure vaccines. There are two different vaccines in clinical trials; the recombinant BCG ΔureC hly+ (VPM1002) and attenuated mycobacteria with two gene deletions (MTBVAC) [6, 7].
BCG booster vaccines comprise Mtb protein antigens expressed in viral vectors or delivered in adjuvant. There are currently several different subunit vaccines in clinical trials based on viral delivery (MVA85A and Crucell Ad35) and protein in adjuvant (H4/IC31 and M72F/AS01E) [6, 7].
Post-exposure vaccines target adolescents and adults with LTBI. The ID93 and H56/IC31 subunit vaccines have been tailored for this strategy by integrating latency antigens of Mtb with the goal of enhancing immune pressure and control of infection to prevent reactivation of TB in latently infected individuals [6, 7].
Therapeutic vaccines could be used as an adjunct to conventional chemotherapy to shorten the treatment period and prevent recurrence of disease. The RUTI vaccine is a complex extract of mycobacterial antigens that is currently in clinical trials [6, 7].
Preventive booster vaccines aim to prolong and increase the efficacy of neonatal BCG vaccination and are subunit vaccines either based on recombinant antigens in adjuvants or expressed in live viral vectors such as MVA or adenovirus. Post-exposure vaccines are designed to be administered on top of already established LTBI and therefore include antigens that are upregulated by bacteria in this particular stage of the infection. The final strategy is the use of therapeutic vaccines in TB patients to complement or shorten conventional chemotherapy (Fig. 1). For a comprehensive overview of the current status of the different TB vaccines in clinical trials, see e.g. [6, 7].
Most of the novel vaccines are currently in clinical phase 1/2 trials to evaluate safety and immunogenicity. However, recently one of these new vaccines, a modified vaccinia virus expressing Ag85A (MVA85A) was the first new TB vaccine in for more than 60 years to undergo a clinical efficacy trial [8]. During its pre-clinical development program, this vaccine was demonstrated to boost powerful Th1 responses measured as IFN-γ EliSpots, which by most investigators in the field was seen not as a perfect correlate of protection but as the best measure of vaccine take.
The phase 2b clinical trial was conducted in South Africa and BCG-vaccinated infants were boosted with MVA85A and followed for three years. The outcome of the trial was very disappointing with no detectable improvement of protection against TB [8].
As recently pointed out [9], although the BCG-MVA booster strategy was also successful in promoting a very strong Th1 response in animals, it offered no significant increase in protection compared to BCG alone in various animal models. This lack of protection in the face of a strong TH1 response in both the preclinical models and in clinical trials has emphasized the need to reconsider the immunological requirements for TB vaccines. Our focus will be on the relevance of vaccine responses that are not characterized by high levels of systemic IFN-γ such as central memory and immunity expressed in the lung.
TB: Infection, Immunity and Antigens
Mtb is an airborne pathogen that can establish infection in susceptible individuals when aerosolized water droplets, even those containing very few infectious bacteria, are inhaled. The bacteria are thought to first be taken up by alveolar macrophages and are later acquired by other myeloid cells, including neutrophils and dendritic cells[10]. Importantly, virulent mycobacteria utilize cell surface lipids to restrict TLR-driven recruitment of host-protective immune cells to the initial site of infection [11]. Thus, for the first week after bacteria are inhaled, the host response to Mtb is probably mediated primarily by host cells resident in the lung prior to infection. Furthermore, Mtb manipulates cell death pathways to delay bacterial transport to the lung draining lymph node [12, 13], and upon arrival in the lymph node, Mtb induces an inflammatory milieu that promotes the expansion of highly suppressive pathogen-specific regulatory T cells [14]. These Mtb-driven processes slow the priming of Mtb-specific effector T cells in the lymph node, and delay their ultimate arrival at the site of infection in the lung [15].
The delayed onset of adaptive immunity in the lung is widely believed to facilitate the ability of Mtb to establish a niche for chronic infection [16, 17]. By the time T cells finally arrive at the site of infection they function to contain the bacteria, and play a role in granuloma formation, positioning themselves around infected macrophages (Figure 2). However, rarely are they able to eradicate infection. CD4 T are critically important for TB protection and the cytokines IFN-γ and TNFα are required for control of bacterial growth in both animal models and in man [3, 17]. These cytokines activate macrophages to control bacterial growth by a combination of reactive oxygen and nitrogen intermediates, lysosomal enzyme attack, antimicrobial peptides (such as cathelicidin) and autophagy [3, 17]. Th17 cells can accelerate the initial response and promote the recruitment of other cell types including neutrophils and CXCR5+ CD4 T cells to the site of infection through their secretion of IL17 [18, 19]. CD8 T cell responses increase later during infection often with a kinetic that is associated with increasing bacterial burden. Their role in protective immunity remain unresolved and recent data suggest that even very high numbers of vaccine promoted CD8 T cells specific for protective antigens fail to influence Mtb growth [20, 21].
Figure 2. Vaccine promoted immunity in the lung.
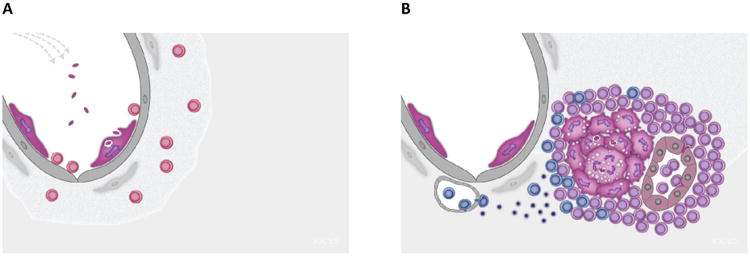
Vaccine protection against new infection with Mtb (Panel A) or long-term control of Mtb growth in the granuloma (Panel B).
Panel A: TRM's (in red) localized in the lung parenchyma and airways may be critical for preventing new infections or re-infections. TRM's are closely related to TEM's but do not circulate. They reside in peripheral tissues where they mediate a fast and efficient first line defence against invading pathogens. TRM's can be localized to the lung through aerosol delivery of vaccines.
Panel B: TCM's (in purple) mediate sustained control with bacterial growth in the granuloma. TCM's provide a self-renewing source of specific T cells that efficiently migrate into the parenchyma of the Mtb-infected lung. The granuloma acts as an ectopic lymphoid structure which provide the necessary components, such as HEV (to the right - inside the granuloma) to allow TCM's direct access to the granuloma. TEM's (in blue) rely on the conventional route into inflamed tissue through small capillaries along a chemokine gradient (to the left – outside the granuloma), but this port of entry seems to operate much less efficiently in the Mtb infected lung.
Mtb has evolved a set of strategies including arrest of phagosome maturation and lysosomal movements, modulation of cell death pathways, prevention of antigen presentation, and release of anti-inflammatory factors that allow it to evade elimination and establish a latent infection [22]. In this stage of the infection, part of the bacterial population transforms into a slow or non-replicative state (so-called dormant Mtb) [23]. LTBI represents a source of continuous antigen exposure that has profound influence on the long-term maintenance of immunity (see below). Mtb can resuscitate whereby the dormant bacteria transforms into a highly replicative and metabolically active stage. The molecular cues that triggers this transformation is still unknown. Alternatively, individuals with LTBI can develop active TB due to airborne infection with a new strain of Mtb, which may occur even more frequently than reactivation TB in regions of the world in which TB is endemic. It is unknown how ongoing LTBI alters the immune response to new Mtb infection.
The host recognizes multiple antigens during TB infection and the antigens expressed by Mtb changes in different stages of the infection [24, 25]. Therefore, in contrast to the findings for some viral diseases there is not one surface exposed antigen, which is critically important for protection. When comparing the many efforts over the last 25 years to discover novel antigens and in particular recent whole genome screening efforts, it is clear that certain gene families like the ESX gene family are immuno-dominant and very frequently and strongly recognized [26, 27]. Recent data suggest that some of these immuno-dominant antigens contain cryptic epitopes that are not targeted during the natural infection but can still induce a protective immune response [28, 29].
Protective immunity and memory
The goal of all TB vaccines currently in clinical trials is to induce memory T cells capable of mounting a protective response to subsequent Mtb challenge. Animal models suggest that this protective T cell response should have two principal components. First, it should be rapid and robust at the primary site of infection in the lung, and second, it should be durable [17]. Achieving both of these goals through vaccination requires careful consideration of the functional properties of distinct subsets of memory T cells, particularly their propensity to traffic to and to be retained within the lung, both prior to and during ongoing Mtb infection. Mounting a rapid frontline of defense in tissues, including the lung, has long been considered the domain of circulating effector T cells (TEM), but more recently the role of tissue-resident memory cells (TRM) has become increasingly appreciated [30]. Conversely, central memory T cells (TCM) are required to maintain a protective T cell response in the face of chronic antigenic stimulation [31]. Recent advances in our understanding of the advantages and limitations conferred by each of these memory subsets in the context of TB are discussed below.
The limitations of TEM
As the most differentiated of the TEM subsets, TEM of the T helper 1 (Th1) lineage are the most robust producers of IFN–γ [31]. Because of this property, the primary goal of most TB vaccine candidates has been to induce a large TEM population. TEM lack expression of the chemokine receptor CCR7, therefore they do not recirculate in the lymphoid system [31]. Rather they reside in the spleen, circulate in the blood, and enter and exit tissue sites, including the lung, as they patrol for foreign invaders. They represent a first line of defense; when tissues become infected, circulating TEM can be recruited quickly to these sites of inflammation. Despite these properties, the limited ability of TEM to prevent TB are becoming increasingly apparent. First, TEM circulating in the blood are poorly recruited to the lung during the early stages of infection [32]. This, at least in part, is likely to occur because of the dampening of TLR-mediated recruitment of immune cells to the lung by cell surface virulence lipids [11]. Later, during the chronic phases of TB, terminally differentiated effector T cells are excluded from the lung parenchyma and accumulate in the lung-associated vasculature [33]. This localization likely severely restricts their protective capacity, as optimal protection is provided only through cognate interactions between Mtb-specific T cells and infected cells, most of which reside in the lung parenchyma [34]. Finally, TEM are relatively short-lived, and fail to persist in the face of ongoing antigenic stimulation seen during the chronic phase of TB infection [35]. Therefore, vaccines, such as BCG, that prime a large proportion of TEM, confer insufficient levels of long-lived immune protection in animal models [36, 37].
The promise of TRM
In contrast to TEM, TRM comprise a newly defined subset of memory T cells that do not circulate, but take up residence in peripheral tissues, mucosal sites, and barrier surfaces, providing a frontline of defense at these locations [30]. Given the delayed recruitment of TEM from the circulation to the lung after airborne Mtb infection, vaccine-induced lung TRM may theoretically provide the best avenue for early Mtb control (Fig. 2A). Although the rules governing the induction, maintenance, and function of TRM are only beginning to be understood, respiratory viral infections are known to induce TRM that persist in lung tissue and the lung airways following infection clearance [38-40]. Both CD8 and CD4 TRM have been identified, but CD4 TRM seem to be more numerous [41]. Phenotypically, mouse CD4 TRM are distinguished from circulating memory T cell populations by their expression of CD69 and CD11a, whereas CD8 TRM also express CD103 [39, 40]. Importantly, it was recently demonstrated that the high expression of CD69 found on TRM functions to retain the cells in peripheral tissue by interfering with the sphingosine-1-phosphate receptor [42]. In addition to TRM of the Th1 lineage that may provide direct protection against Mtb infection, lung resident Th17 may also play an important role by inducing factors, including chemokines, that expedite the recruitment of Th1 TEM from the circulation into the lung [18, 19]. Given our current understanding, vaccines may need to deliver Mtb antigens directly to the lung mucosa to efficiently elicit lung TRM [41]. The role of TRM in TB protection still needs experimental confirmation but induction of this subset may have the potential to improve early control with infection in preventive vaccine approaches.
The need for TCM
In contrast to TEM and TRM, TCM express CD62L and CCR7 and circulate through lymphoid tissues, but generally have a limited ability to enter infected tissue sites [31]. As a result, protection provided by TCM is delayed and these cells are therefore generally seen as a potent second line of defense. Upon delivery of their cognate antigen to lymphoid tissue, TCM proliferate rapidly and differentiate into multiple effector cell types, including those capable of migrating to tissue sites of infection. Because of their proliferative capacity and their role as pluripotent progenitor cells, TCM are important mediators of long lasting immunity. Chronic TB infection diminish the TCM population by providing a continuous source of antigen that pushes Mtb specific T cell clones to terminal differentiation [36], whereas T cells specific for cryptic epitopes that are not expanded by the ongoing infection may have the ability to bypass this development [29].
The first indication that TCM may have a particularly important role in protective immunity to TB infection in the lung comes from adoptive transfer studies of immune CD4 T cells. The unexpected finding in this early study, was that whereas both CD62L high and low cells could transfer protection to the spleen of recipient mice, protection of the lung was mediated exclusively by T cells that expressed high levels of CD62L i.e. TCM's [43]. Recent studies examining the role of TCM in immunity against TB have revealed surprising insights into their trafficking patterns and protective capacity during Mtb infection that allow a rational explanation of this early observation. Despite ongoing stimulation by Mtb antigens, Mtb-specific CD4 T cells with many TCM-like properties are found in the lung during infection [44]. Their maintenance depends on intrinsic expression of ICOS, the transcription factor Bcl-6, and to a lesser extent, CXCR5, all components of signaling pathways operant within lymphoid follicles. The fact that these pathways operate within the lung parenchyma during TB highlights the role of the TB granuloma as a tertiary lymphoid structure within the lung (Fig. 2B) [45, 46].
Most, if not all, of the components that define lymph nodes, including CCL21, CXCL13, follicular dendritic cells, efferent and afferent lymphatics, and high endothelial venules are also present in granulomas [19]. The discovery that, upon adoptive transfer, cells with TCM-like properties [44] migrate directly into the parenchyma of the Mtb-infected lung whereas terminally differentiated effector T cells are excluded [33], was initially surprising and somewhat confusing. However, if movement of T cells into and out of the lung during TB is governed by the rules that dictate trafficking of T cells through granulomatous lymphoid structures; these results begin to make sense (Fig. 2A).
In further support of the idea that lymphoid follicles of the granulomas are critical for directing T cell trafficking and maintaining protective T cell populations, T cells lacking CXCR5, a chemokine receptor expressed by TCM that promotes migration into lymphoid follicles, are limited in their capacity to mediate protection [19]. Taken together, these results help to explain why vaccines that induce large numbers of TCM confer superior and more durable protection compared to other vaccines that induce much greater numbers of IFN-γ producing T cells [36, 47]. Not only do TCM have a high proliferative capacity and the ability for pluripotent differentiation, but also the biology of granulomas as lymphoid structures seems to promote migration of TCM into sites of infection within the lung.
Conclusions
Discovery of new and improved TB vaccines has been complicated and delayed by major gaps in our understanding of how immunity to TB manifests itself at the site of infection in the lung. Without this knowledge, too many efforts in TB vaccine discovery have been dominated by trial and error with the only conclusive read-out being the ability to protect against challenge in experimental animal models. Given that there is no direct correlation between protection and the level of systemic IFNγ promoted by experimental TB vaccines, we now need to develop the next generation of vaccines with specific focus on immunological memory expressed in the lung.
A successful vaccine strategy induce protective pulmonary cellular responses that can rapidly respond to Mtb and it seems logical that TRM localized in the lung parenchyma might be critical in this context. However, the evidence for improved efficacy by aerosol delivery of vaccines is still scarce and restricted to the use of airway virus like adenovirus for vaccine delivery [48]. In preclinical vaccine studies it is very important to keep in mind that choosing the aerosol route for vaccination may result in misleading interpretations as inflammation and innate immune activation at the port of entry may have both a positive and negative impact on resistance against subsequent challenge [49]. It is therefore critically important to define the role of TRM in TB prevention and in this context to discriminate between short-lived innate activation and long-lived adaptive immune responses. This work will depend on the development of effective approaches to pull the TRM's into the lung from the peripheral repertoire and to provide the necessary signals for their long-term maintenance at this site.
Given the efficiency with which TCM enter the TB infected lung parenchyma and the longevity of these responses, clearly this subset plays a decisive role in TB vaccines. Eliciting TCM to Mtb antigens that are expressed also during chronic infection may target bacteria localized in lung granulomas resulting in long-term control with infection of particular importance for post-exposure vaccines [50]. Although we still do not fully understand the vaccine parameters that govern the development of TCM's, several novel adjuvanted Tb subunit vaccines seems to strike a very desirable balance between TCM and TEM in both animal models [36, 51], and in clinical trials [52, 53]. BCG in contrast promotes a response that is much more skewed towards TEM [36, 37] and a recent study demonstrated that increased levels of TCM's were a prerequisite for the improved efficacy of a novel genetically engineered strain of BCG [54].
One important consequence of this recent improved understanding of immunity to TB in animal models is that to optimize vaccine promoted immune responses in clinical trials we need better tools for monitoring responses in the lung. Typically, TB vaccine clinical trials are designed to assess immune responses in blood samples collected at various time points after vaccination. Recent advances in our technical ability to monitor the expression of multiple cytokines and cell surface receptors have greatly improved our capacity to determine the TEM/TCM ratio in the blood. This, however, only provide limited insight into the local immune responses in the lung, especially those that fully develop only after the establishment of infection.
Even recent attempts to increase the relevance of in vitro assays through biologically relevant read-outs like mycobacterial grow-inhibition [55], may prove futile if the sample is obtained from the blood. It will therefore be critically important, both in preclinical animal models and in clinical trials, to move away from simple measurements of IFN-γ in the blood and towards quantifying TRM (prior to infection) and TCM (after infection) in the lung. Studies in animal models will be particularly important to understand the factors that govern the localization of vaccine-elicited immunity to the lung site of infection. Ultimately, hypotheses generated in animal models will need to be tested in human trials, and BAL has proven to be a useful technique for obtaining T cells resident in the lung airways. Due to its invasiveness, this approach may be more feasible in small clinical trials than for larger ones. It therefore remains a top priority to determine if blood assays can provide signatures of efficient vaccine-promoted immunity in the lung. Such assays would provide a big boost for future vaccine discovery, optimization and clinical evaluation.
Highlights.
Most TB vaccine candidates aim to amplify IFN-γ producing memory T cells in the blood
The frequency of IFN–γ producing T cells in blood does not correlate with protection
Animal models suggest vaccine-induced T cell responses should be rapid and sustained
Lung resident-memory T cells may prevent or curb early infection
Central memory T cells migrate to the site of infection and provide durable protection
Acknowledgments
We thank Karen Korsholm and Christina Gry Paulsen for help preparing the figures and Joshua Woodworth for critical reading of the manuscript.
Peter Andersen is supported by the European Union's Seventh Framework Programme (EU FP7) ADITEC (HEALTH-F4-2011-280873), the European Union's Research and Innovation Programme Horizon 2020 (EU H2020) TBVAC2020 (H2020-PHC-2014-2015/H2020-PHC-2014, Grant # 643381), the Innovative Medicines Initiative (IMI) Joint Undertaking “Biomarkers for Enhanced Vaccine Safety” BIOVACSAFE (IMI JU Grant No. 115308), the Danish National Advanced Technology Foundation (060-2009-3), and the Danish Research Council (10-094496).
Kevin Urdahl is supported by grants from the National Institutes of Health (U19 AI106761 and R01 AI076327) and from the Paul G. Allen Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• Special interest
•• Outstanding interest
- 1.Glaziou P, Sismanidis C, Floyd K, Raviglione M. Global Epidemiology of Tuberculosis. Cold Spring Harb Perspect Med. 2014 doi: 10.1101/cshperspect.a017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagneux S. Host-pathogen coevolution in human tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2012;367(1590):850–9. doi: 10.1098/rstb.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12(8):581–91. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 4.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182(8):1073–9. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner J, 3rd, Kaufmann SH. Recent advances towards tuberculosis control: vaccines and biomarkers. J Intern Med. 2014;275(5):467–80. doi: 10.1111/joim.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frick M. The TB Vaccines Pipeline: Where are we going, where have we been? 2013:263–283. [Google Scholar]
- 7.Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, Zumla AI, Maeurer M. Progress in tuberculosis vaccine development and host-directed therapies--a state of the art review. Lancet Respir Med. 2014;2(4):301–20. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 8•.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–8. doi: 10.1016/S0140-6736(13)60177-4. This large phase 2B trial demonstrated that despite significantly amplifying numbers of IFN-g producing T cells in the blood, the MVA85A vaccine did not improve protection against TB disease in BCG vaccinated infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beverley P. TB vaccine failure was predictable. Nature. 2013;503(7477):469. doi: 10.1038/503469e. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev. 2014;262(1):179–92. doi: 10.1111/imr.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505(7482):218–22. doi: 10.1038/nature12799. This report shows that an Mtb cell surface virulence lipid restricts TLR-mediated recognition of virulent mycobacteria and restricts recruitment of host-protective immune cells to the initial site of infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8(9):668–74. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramakrishnan L. Looking within the zebrafish to understand the tuberculous granuloma. Adv Exp Med Biol. 2013;783:251–66. doi: 10.1007/978-1-4614-6111-1_13. [DOI] [PubMed] [Google Scholar]
- 14.Shafiani S, Dinh C, Ertelt JM, Moguche AO, Siddiqui I, Smigiel KS, Sharma P, Campbell DJ, Way SS, Urdahl KB. Pathogen-specific Treg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity. 2013;38(6):1261–70. doi: 10.1016/j.immuni.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010;207(7):1409–20. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urdahl KB. Understanding and overcoming the barriers to T cell mediated immunity against tuberculosis. Semin Immunol. 2014;26(6):578–587. doi: 10.1016/j.smim.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 2013;6(5):972–84. doi: 10.1038/mi.2012.135. This report demonstrates that vaccine-induced, lung-resident Th17 cells confer protection against TB by promoting CXCL13-mediated recruitment of CXCR5+ CD4 T cells into lymphoid structures within the infected lung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013 doi: 10.1172/JCI65728. This report demonstrates that TB granulomas represent lymphoid structures that exhibit most, if not all, of the characteristics of lymph nodes. Furthermore, CXCR5+ CD4 T cells that home to the lymphoid follicles of these structures are critical for protective immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindenstrom T, Aagaard C, Christensen D, Agger EM, Andersen P. High-frequency vaccine-induced CD8(+) T cells specific for an epitope naturally processed during infection with Mycobacterium tuberculosis do not confer protection. Eur J Immunol. 2014;44(6):1699–709. doi: 10.1002/eji.201344358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Florido M, Pillay R, Gillis CM, Xia Y, Turner SJ, Triccas JA, Stambas J, Britton WJ. Epitope-specific CD4, but not CD8, T-cell responses induced by recombinant influenza A viruses protect against Mycobacterium tuberculosis infection. Eur J Immunol. 2014 doi: 10.1002/eji.201444954. [DOI] [PubMed] [Google Scholar]
- 22.Cambier CJ, Falkow S, Ramakrishnan L. Host Evasion and Exploitation Schemes of Mycobacterium tuberculosis. Cell. 2014;159(7):1497–1509. doi: 10.1016/j.cell.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–55. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogerson BJ, Jung YJ, LaCourse R, Ryan L, Enright N, North RJ. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology. 2006;118(2):195–201. doi: 10.1111/j.1365-2567.2006.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 2011;7(5):e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, Kim Y, Sidney J, James EA, Taplitz R, et al. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog. 2013;9(1):e1003130. doi: 10.1371/journal.ppat.1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181(11):7948–57. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Aagaard CS, Hoang TT, Vingsbo-Lundberg C, Dietrich J, Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J Immunol. 2009;183(4):2659–68. doi: 10.4049/jimmunol.0900947. These two studies shows that cryptic epitopes that are not promoted by the natural TB infection mediate an efficient protective immune response against Tb infection and that T cells to these epitopes have a better ability to maintain a TCM quality and a proliferative potential in the face of ongoing chronic infection. [DOI] [PubMed] [Google Scholar]
- 29•.Woodworth JS, Aagaard CS, Hansen PR, Cassidy JP, Agger EM, Andersen P. Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation. J Immunol. 2014;192(7):3247–58. doi: 10.4049/jimmunol.1300283. These two studies shows that cryptic epitopes that are not promoted by the natural TB infection mediate an efficient protective immune response against Tb infection and that T cells to these epitopes have a better ability to maintain a TCM quality and a proliferative potential in the face of ongoing chronic infection. [DOI] [PubMed] [Google Scholar]
- 30.Schenkel JM, Masopust D. Tissue-Resident Memory T Cells. Immunity. 2014;41(6):886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–63. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201(12):1915–24. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, Barber DL. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol. 2014;192(7):2965–9. doi: 10.4049/jimmunol.1400019. This is the first report to utilize invascular T cell labeling to study TB and demonstrates that Mtb-specific T cells capable of robust IFN-g production localize to the lung-associated vasculature rather than the lung parenchyma and have limited protective capacity due to their impaired ability to migrate into the infected lung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Srivastava S, Ernst JD. Cutting edge: Direct recognition of infected cells by CD4 T cells is required for control of intracellular Mycobacterium tuberculosis in vivo. J Immunol. 2013;191(3):1016–20. doi: 10.4049/jimmunol.1301236. This report shows that optimal control of Mtb requires cognate interactions between antigen-specific T cells and infected cells; protection provided by bystander cytokine production is limited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2010;107(45):19408–13. doi: 10.1073/pnas.1006298107. This report is the first to describe two distinct populations of Mtb-specific CD4 T cells during TB; 1) a self-renewing progenitor population, and 2) a terminally differentiated population that produces IFN-g robustly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Lindenstrom T, Knudsen NP, Agger EM, Andersen P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol. 2013;190(12):6311–9. doi: 10.4049/jimmunol.1300248. This study track the dynamic development of specific T cells in the organs during long term chronic TB infection and demonstrate a functional exhaustion of BCG promoted responses resulting in recrudescence of infection. An adjuvanted subunit vaccine boost expands a population of TCM cells that resist exhaustion and prolong immunity. [DOI] [PubMed] [Google Scholar]
- 37•.Lindenstrom T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182(12):8047–55. doi: 10.4049/jimmunol.0801592. This study demonstrate that an adjuvanted subunit vaccine compared to BCG, promotes a durable CD4 T cell response that protect against TB challenge for >1 year post vaccination and that this effect is mediated by CD4 TCM's that co-express IL2 and maintain a proliferative potential. [DOI] [PubMed] [Google Scholar]
- 38.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193(8):981–6. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–4. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7(3):501–10. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner DL, Farber DL. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol. 2014;5:331. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. Cutting Edge: CD69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J Immunol. 2015 doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 43.Andersen P, Smedegaard B. CD4(+) T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect Immun. 2000;68(2):621–9. doi: 10.1128/iai.68.2.621-629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Moguche AO, Shafiani S, Clemons C, Larson RP, Dinh C, Higdon LE, Cambier CJ, Sissons JR, Gallegos AM, Fink PJ, et al. ICOS and Bcl6-dependent pathways maintain a CD4 T cell population with memory-like properties during tuberculosis. J Exp Med. 2015;212(5):715–728. doi: 10.1084/jem.20141518. This report demonstrates that T cells located in the lung parenchyma during chronic phases of TB share properties with central memory cells, are maintained by signaling pathways operant within lymphoid follicles, and mediate superior protection compared to T cells that more robustly produce IFN-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204(2):217–28. doi: 10.1002/path.1628. [DOI] [PubMed] [Google Scholar]
- 46.Phuah JY, Mattila JT, Lin PL, Flynn JL. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. Am J Pathol. 2012;181(2):508–14. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Billeskov R, Christensen JP, Aagaard C, Andersen P, Dietrich J. Comparing adjuvanted H28 and modified vaccinia virus ankara expressingH28 in a mouse and a non-human primate tuberculosis model. PLoS One. 2013;8(8):e72185. doi: 10.1371/journal.pone.0072185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181(7):4955–64. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeyanathan M, Damjanovic D, Shaler CR, Lai R, Wortzman M, Yin C, Zganiacz A, Lichty BD, Xing Z. Differentially imprinted innate immunity by mucosal boost vaccination determines antituberculosis immune protective outcomes, independent of T-cell immunity. Mucosal Immunol. 2013;6(3):612–25. doi: 10.1038/mi.2012.103. [DOI] [PubMed] [Google Scholar]
- 50.Hoang T, Aagaard C, Dietrich J, Cassidy JP, Dolganov G, Schoolnik GK, Lundberg CV, Agger EM, Andersen P. ESAT-6 (EsxA) and TB10.4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS One. 2013;8(12):e80579. doi: 10.1371/journal.pone.0080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN. A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93. PLoS One. 2014;9(1):e83884. doi: 10.1371/journal.pone.0083884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA, O'Dee DM, Graves A, Thierry-Carstensen B, Andreasen LV, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32(52):7098–107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Idoko OT, Owolabi OA, Owiafe PK, Moris P, Odutola A, Bollaerts A, Ogundare E, Jongert E, Demoitie MA, Ofori-Anyinam O, et al. Safety and immunogenicity of the M72/AS01 candidate tuberculosis vaccine when given as a booster to BCG in Gambian infants: an open-label randomized controlled trial. Tuberculosis (Edinb) 2014;94(6):564–78. doi: 10.1016/j.tube.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, Kaufmann SH. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC∷hly vaccine's superior protection against tuberculosis. J Infect Dis. 2014;210(12):1928–37. doi: 10.1093/infdis/jiu347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fletcher HA, Tanner R, Wallis RS, Meyer J, Manjaly ZR, Harris S, Satti I, Silver RF, Hoft D, Kampmann B, et al. Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. Clin Vaccine Immunol. 2013;20(11):1683–9. doi: 10.1128/CVI.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]