Abstract
An immunochemical method for analyzing protein interactions with BrdUrd-substituted DNA was used to study binding of histones to nascent DNA in nuclei. The results indicate that in Ehrlich ascites tumor (EAT) cells, histone H1 deposits on newly replicated DNA simultaneously with or immediately after core histone deposition so that in chromatin replicated for 3 min, the stoichiometry of the histones is the same as in bulk chromatin. All histones, and especially histone H1, interact with nascent DNA more weakly than with bulk chromatin, although the efficiency of interaction via the globular domains seems to be the same for both types of chromatin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almouzni G., Clark D. J., Méchali M., Wolffe A. P. Chromatin assembly on replicating DNA in vitro. Nucleic Acids Res. 1990 Oct 11;18(19):5767–5774. doi: 10.1093/nar/18.19.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato A. T., Schindler R. K., Thomas C. A., Jr, Seale R. L. Dual nature of newly replicated chromatin. Evidence for nucleosomal and non-nucleosomal DNA at the site of native replication forks. J Biol Chem. 1981 Nov 25;256(22):11880–11886. [PubMed] [Google Scholar]
- Annunziato A. T., Seale R. L. Maturation of nucleosomal and nonnucleosomal components of nascent chromatin: differential requirements for concurrent protein synthesis. Biochemistry. 1982 Oct 26;21(22):5431–5438. doi: 10.1021/bi00265a008. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C., Wong M. L., Alberts B. M. In vitro replication through nucleosomes without histone displacement. Nature. 1990 Feb 22;343(6260):719–726. doi: 10.1038/343719a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Mobbs J. D., Marshall A. J. Chromatin structure: a property of the higher structures of chromatin and in the time course of its formation during chromatin replication. Nucleic Acids Res. 1976 Dec;3(12):3293–3304. doi: 10.1093/nar/3.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick M. E., Wassarman P. M., DePamphilis M. L. Application of nucleases to visualizing chromatin organization at replication forks. Methods Enzymol. 1989;170:290–316. doi: 10.1016/0076-6879(89)70053-7. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Dilworth S. M., Black S. J., Laskey R. A. Two complexes that contain histones are required for nucleosome assembly in vitro: role of nucleoplasmin and N1 in Xenopus egg extracts. Cell. 1987 Dec 24;51(6):1009–1018. doi: 10.1016/0092-8674(87)90587-3. [DOI] [PubMed] [Google Scholar]
- Fotedar R., Roberts J. M. Multistep pathway for replication-dependent nucleosome assembly. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6459–6463. doi: 10.1073/pnas.86.17.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili G., Levy A., Jakob K. M. Changes in chromatin structure at the replication fork. DNase I and trypsin-micrococcal nuclease effects on approximately 300- and 150-base pair nascent DNAs. J Biol Chem. 1983 Sep 25;258(18):11274–11279. [PubMed] [Google Scholar]
- Galili G., Levy A., Jakob K. M. Changes in chromatin structure at the replication fork. II The DNPs containing nascent DNA and a transient chromatin modification detected by DNAase I. Nucleic Acids Res. 1981 Aug 25;9(16):3991–4005. doi: 10.1093/nar/9.16.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss C., Sogo J. M. Chromatin replication. Bioessays. 1992 Jan;14(1):1–8. doi: 10.1002/bies.950140102. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. Histone segregation on replicating chromatin. Biochemistry. 1985 Nov 19;24(24):6930–6938. doi: 10.1021/bi00345a027. [DOI] [PubMed] [Google Scholar]
- Jackson V. In vivo studies on the dynamics of histone-DNA interaction: evidence for nucleosome dissolution during replication and transcription and a low level of dissolution independent of both. Biochemistry. 1990 Jan 23;29(3):719–731. doi: 10.1021/bi00455a019. [DOI] [PubMed] [Google Scholar]
- Jackson V., Marshall S., Chalkley R. The sites of deposition of newly synthesized histone. Nucleic Acids Res. 1981 Sep 25;9(18):4563–4581. doi: 10.1093/nar/9.18.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
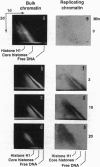
- Jackson V. Studies on histone organization in the nucleosome using formaldehyde as a reversible cross-linking agent. Cell. 1978 Nov;15(3):945–954. doi: 10.1016/0092-8674(78)90278-7. [DOI] [PubMed] [Google Scholar]
- Kapp L. N., Painter R. B. DNA replication fork movement rates in mammalian cells. Int Rev Cytol. 1982;80:1–25. doi: 10.1016/s0074-7696(08)60365-4. [DOI] [PubMed] [Google Scholar]
- Karpov V. L., Preobrazhenskaya O. V., Mirzabekov A. D. Chromatin structure of hsp 70 genes, activated by heat shock: selective removal of histones from the coding region and their absence from the 5' region. Cell. 1984 Feb;36(2):423–431. doi: 10.1016/0092-8674(84)90235-6. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Seiter A., Zentgraf H. Nucleosome assembly in vitro: separate histone transfer and synergistic interaction of native histone complexes purified from nuclei of Xenopus laevis oocytes. EMBO J. 1990 Apr;9(4):1309–1318. doi: 10.1002/j.1460-2075.1990.tb08240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
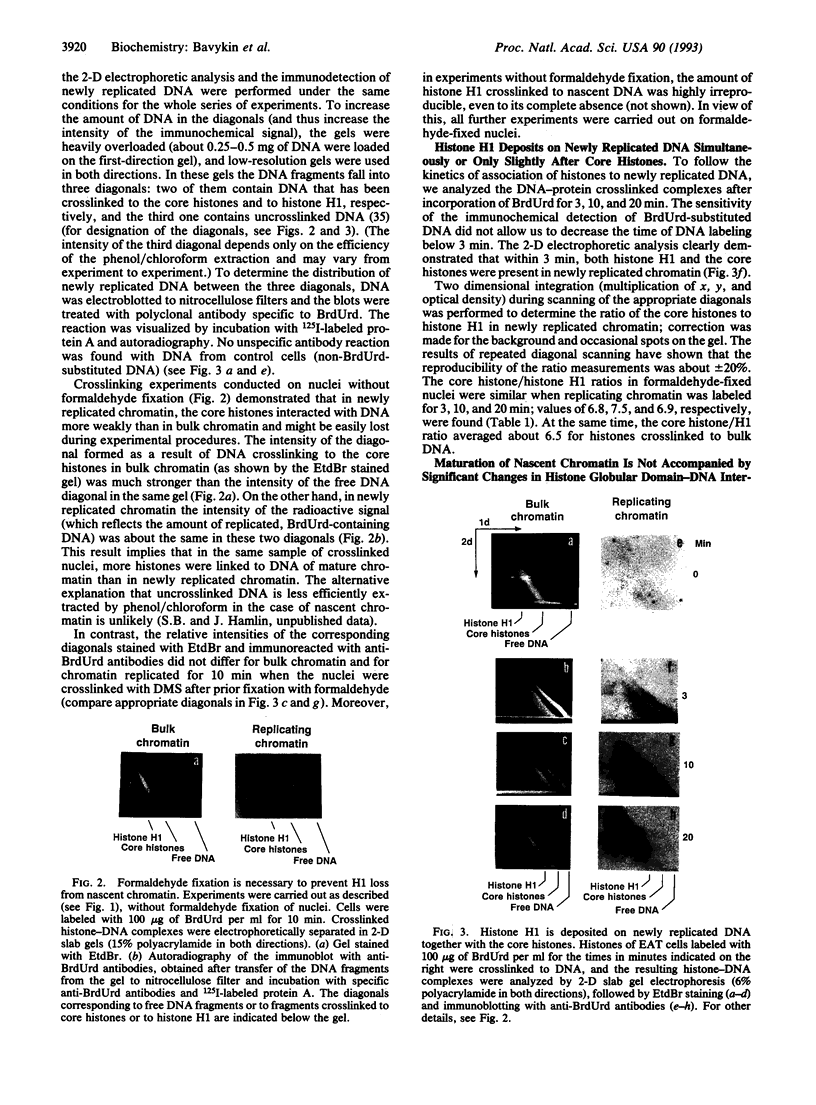
- Klempnauer K. H., Fanning E., Otto B., Knippers R. Maturation of newly replicated chromatin of simian virus 40 and its host cell. J Mol Biol. 1980 Feb 5;136(4):359–374. doi: 10.1016/0022-2836(80)90395-2. [DOI] [PubMed] [Google Scholar]
- Krude T., Knippers R. Transfer of nucleosomes from parental to replicated chromatin. Mol Cell Biol. 1991 Dec;11(12):6257–6267. doi: 10.1128/mcb.11.12.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffak I. M. Conservative segregation of nucleosome core histones. Nature. 1984 Jan 5;307(5946):82–85. doi: 10.1038/307082a0. [DOI] [PubMed] [Google Scholar]
- Levina E. S., Bavykin S. G., Shick V. V., Mirzabekov A. D. The method of crosslinking histones to DNA partly depurinated at neutral pH. Anal Biochem. 1981 Jan 1;110(1):93–101. doi: 10.1016/0003-2697(81)90117-2. [DOI] [PubMed] [Google Scholar]
- Levy A., Jakob K. M. Nascent DNA in nucleosome like structures from chromatin. Cell. 1978 Jun;14(2):259–267. doi: 10.1016/0092-8674(78)90112-5. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Bavykin S. G., Belyavsky A. V., Karpov V. L., Preobrazhenskaya O. V., Shick V. V., Ebralidse K. K. Mapping DNA-protein interactions by cross-linking. Methods Enzymol. 1989;170:386–408. doi: 10.1016/0076-6879(89)70058-6. [DOI] [PubMed] [Google Scholar]
- Nacheva G. A., Guschin D. Y., Preobrazhenskaya O. V., Karpov V. L., Ebralidse K. K., Mirzabekov A. D. Change in the pattern of histone binding to DNA upon transcriptional activation. Cell. 1989 Jul 14;58(1):27–36. doi: 10.1016/0092-8674(89)90399-1. [DOI] [PubMed] [Google Scholar]
- Perry C. A., Annunziato A. T. Histone acetylation reduces H1-mediated nucleosome interactions during chromatin assembly. Exp Cell Res. 1991 Oct;196(2):337–345. doi: 10.1016/0014-4827(91)90269-z. [DOI] [PubMed] [Google Scholar]
- Randall S. K., Kelly T. J. The fate of parental nucleosomes during SV40 DNA replication. J Biol Chem. 1992 Jul 15;267(20):14259–14265. [PubMed] [Google Scholar]
- Schlaeger E. J. Replicative conformation of parental nucleosomes: salt sensitivity of deoxyribonucleic acid-histone interaction and alteration of histone H1 binding. Biochemistry. 1982 Jun 22;21(13):3167–3174. doi: 10.1021/bi00256a021. [DOI] [PubMed] [Google Scholar]
- Seale R. L. In vivo assembly of newly synthesized histones. Biochemistry. 1981 Oct 27;20(22):6432–6437. doi: 10.1021/bi00525a023. [DOI] [PubMed] [Google Scholar]
- Seale R. L., Simpson R. T. Effects of cycloheximide on chromatin biosynthesis. J Mol Biol. 1975 May 25;94(3):479–501. doi: 10.1016/0022-2836(75)90216-8. [DOI] [PubMed] [Google Scholar]
- Setterfield G., Sheinin R., Dardick I., Kiss G., Dubsky M. Structure of interphase nuclei in relation to the cell cycle. Chromatin organization in mouse L cells temperature-sensitive for DNA replication. J Cell Biol. 1978 Apr;77(1):246–263. doi: 10.1083/jcb.77.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A., Tremethick D., Worcel A. Characterization of the repressed 5S DNA minichromosomes assembled in vitro with a high-speed supernatant of Xenopus laevis oocytes. Mol Cell Biol. 1988 Oct;8(10):4257–4269. doi: 10.1128/mcb.8.10.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. A., Jackson V., Chalkley R. Two-stage maturation process for newly replicated chromatin. Biochemistry. 1984 Mar 27;23(7):1576–1581. doi: 10.1021/bi00302a036. [DOI] [PubMed] [Google Scholar]
- Smith S., Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989 Jul 14;58(1):15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Smith S., Stillman B. Stepwise assembly of chromatin during DNA replication in vitro. EMBO J. 1991 Apr;10(4):971–980. doi: 10.1002/j.1460-2075.1991.tb08031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Stefanovsky V., Dimitrov S., Russanova V., Pashev I. Histones H1 and H4 are present near the replication fork. Mol Biol Rep. 1990 Nov;14(4):231–235. doi: 10.1007/BF00429890. [DOI] [PubMed] [Google Scholar]
- Svaren J., Chalkley R. The structure and assembly of active chromatin. Trends Genet. 1990 Feb;6(2):52–56. doi: 10.1016/0168-9525(90)90074-g. [DOI] [PubMed] [Google Scholar]
- Worcel A., Han S., Wong M. L. Assembly of newly replicated chromatin. Cell. 1978 Nov;15(3):969–977. doi: 10.1016/0092-8674(78)90280-5. [DOI] [PubMed] [Google Scholar]