Abstract
The abscopal effect is a phenomenon observed in the treatment of metastatic cancer where localized irradiation of a particular tumor site causes a response in a site distant to the irradiated volume. The mechanisms of the abscopal effect are speculated to be of several origins, including distant effects on p53, elaboration of inflammatory agents including cytokines, and, most recently, secondary to immune mechanisms. In this case report, we present a rare report of a patient with hepatocellular carcinoma with lung metastases who, after receiving radiation treatment to the liver, had a treatment response in the liver and a complete response in the lung. Recent advances in the understanding of the primary role of immune-modulated cytotoxicity, especially with the success of immune checkpoint inhibitors, have the potential to turn the abscopal effect from a rare phenomenon into a tool to guide antineoplastic therapy and provide a new line of research.
Keywords: abscopal effect, lung metastases, radiotherapy, liver cancer, spontaneous regression, immunomodulation, immune checkpoint inhibitors, sbrt, lung tumors
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide, and the third most common cause of death from cancer [1]. The abscopal effect is a phenomenon rarely observed in the treatment of metastatic cancer where localized irradiation of a particular tumor site causes a response in a site distant to the irradiated area. Here, we describe a case report of a gentleman with HCC and lung metastases who, after receiving only focal radiation treatment to the liver, had complete and sustained radiological regression of pulmonary metastases.
The London Health Sciences Centre Research Ethics Board approved this study (approval #16487E). Patient information was collected and released under an ethics approved prospective database.
Case presentation
In early December 2009, a 71-year-old man presented to the Emergency Department with dyspnea as well as right-sided chest wall and upper quadrant abdominal pain. He was diagnosed with pulmonary embolus and started on anticoagulation. His imaging investigations revealed multiple coalesced masses in the liver with the largest measuring 6 cm x 9 cm x 9 cm in the right lobe of the liver. Multiple lesions were also noted in the lung with bilateral pleural plaques consistent with asbestos exposure (Figure 1A). Alpha-fetoprotein (AFP) was significantly elevated at 11 460 µg/L (normal less than 5). In late December, a liver biopsy was performed and pathology was diagnostic for primary HCC. He was diagnosed with Stage IV T3N0M1 disease. His past medical history was only significant for hypertension and Type 2 diabetes. He was an ex-smoker and quit in 1968. He was a retired boilermaker with asbestos exposure.
Figure 1. The axial CT scans of the thorax before and after radiation of the liver.
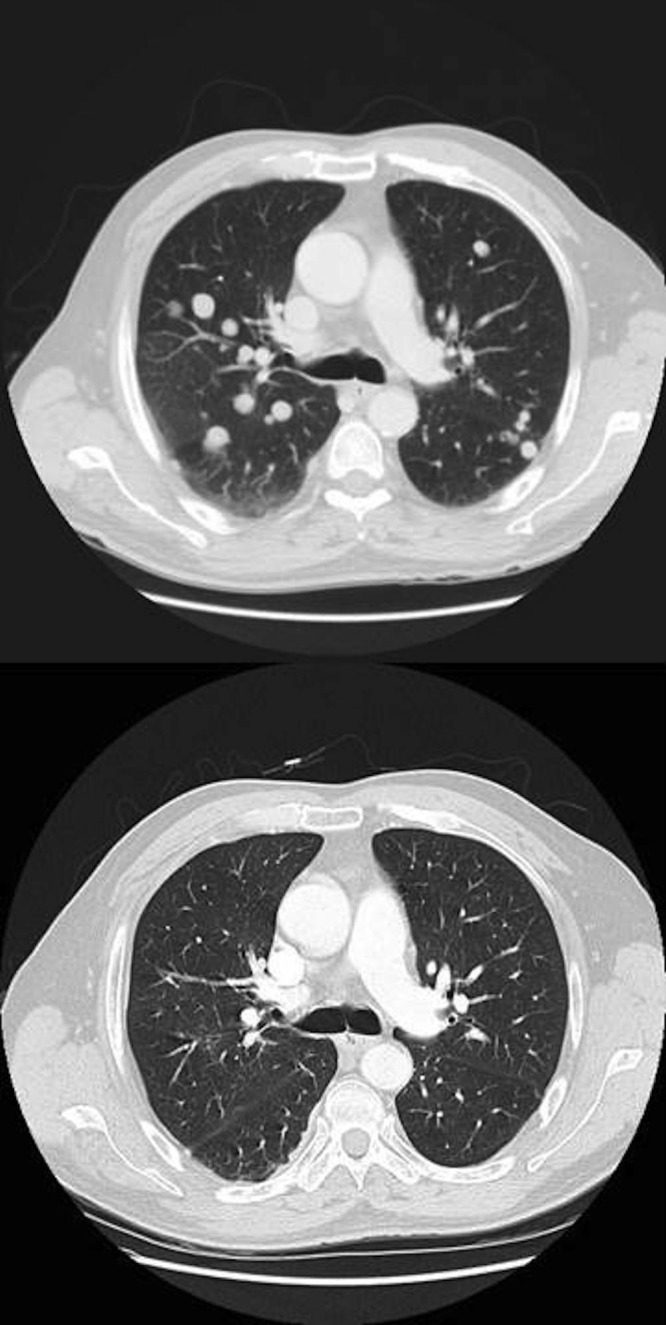
Figure 1A (top) The February 2010 axial CT thorax demonstrates multiple metastases before radiation .
Figure 1B (bottom) The August 2010 axial CT thorax demonstrates resolution of metastases five months after radiation .
In late January 2010, he met with a liver surgeon. A repeat CT scan of the thorax and abdomen in early February revealed the liver lesions had increased in size with the primary lesion now measuring greater than 14 cm. Furthermore, there was evidence of worsening metastatic disease in the lungs with innumerable lesions between 0.5 and 2 cm in size. These were non-calcified and non-cavitary lesions. With this rapid doubling time, there was some debate within the Multidisciplinary Tumor Board about the role of medical treatment with sorafenib. The patient was ECOG 1 and had only mild hepatomegaly. He had no jaundice or stigmata of liver disease but was experiencing significant fatigue. Liver enzymes were minimally elevated with ALT 31 IU/L (normal less than 40), AST 73 IU/L (normal less than 41), ALP 95 IU/L (normal less than 129), and total bilirubin 8.1 µmol/L (normal less than 17). His albumin was 35 g/L and INR was normal. His Child-Pugh Score was A6. However, the combination of his hypertension and anticoagulation raised the concern of increased bleeding risk and potential difficulty controlling hypertension while on sorafenib. Therefore, in conjunction with the patient, it was decided to go ahead with radiotherapy alone.
The patient underwent 70 Gy treatment in 15 fractions to the liver, which was completed in April 2010 (Figure 2). A follow-up CT abdomen in June 2010 showed the largest liver lesion had now shrunk from over 14 cm to 3 cm. The patient was clinically stable and asymptomatic. Biochemically, the AFP had decreased to 196.8 µg/L. In August 2010, the CT thorax and abdomen demonstrated stable disease in the liver. Furthermore, there was a dramatic complete resolution of the numerous pulmonary metastases with no new nodules or masses seen (Figure 1B). The patient received no other treatments up to this assessment point (August 2010), including no systemic treatments.
Figure 2. Radiation dosimetry plan for liver treatment.
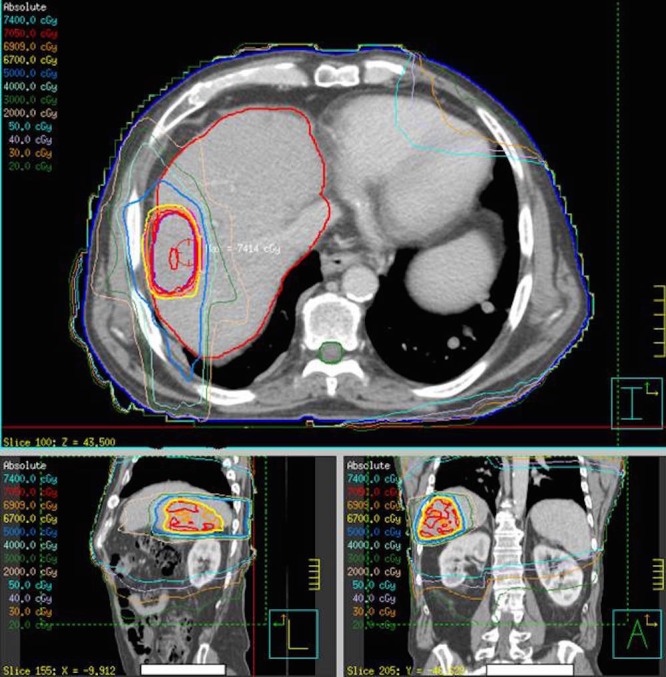
The patient underwent 70 Gy treatment in 15 fractions to the liver which was completed in April 2010.
From August 2010 till March 2012, the man enjoyed stable disease with normal liver laboratory results (including an AFP of 3.1) and no imaging evidence of disease recurrence. In March of 2012, a small recurrent nodule was found in the right liver and he underwent hepatic artery embolization with doxorubicin and cisplatin in May of 2012. This resulted in a complete response at that location.
Discussion
In 1953, Mole described the phenomena of abscopal effect as an “action at a distance from the irradiated volume, but within the same organism” [2]. It can result in a tumor in a non-irradiated area being spontaneously reduced. Since Mole’s original account, many cases of the abscopal effect have been described in multiple tumor sites, including lymphoma and melanoma [3-4]. Though there are some reports of abscopal effect in hepatocellular carcinoma, these primarily report regression of hepatocellular carcinoma itself due to radiation treatment delivered at a metastatic site or with systemic treatment [5-10]. To our knowledge, ours is the first case report of the abscopal effect with focal liver radiation causing regression of distant lung metastasis in a systemic treatment naïve patient with HCC.
One interesting report in the literature describes a man with HCC who had a mediastinal metastasis and a pulmonary mass in the right lower lobe. After receiving focused mediastinal radiation, the abscopal effect was observed with regression of the pulmonary mass, which was not in the irradiated field [10]. However, in this case, the patient was first treated with a transcatheter arterial embolization, and hence, it is unclear if this treatment may have contributed to the response. This is different from our case, in which the patient was naïve to any treatment and received radiation treatment to the liver only, with subsequent regression of distant lung metastases. A limitation of our case report is that there was no pathologic confirmation for the metastatic disease in the lungs. However, the CT images (Figure 1A) reported by the radiologists were convincing and consistent with the common “cannon ball” lesions seen in lung metastasis.
The mechanism of the abscopal effect is still not well understood, although major mechanistic categories have been proposed and opportunities for exploitation reviewed [11-37]. These include involvement of the immune system, cytokines, tumor growth inhibition, and tumoricidal effects [16, 25, 38-39]. One proposed mechanism is that the irradiation of one tumor site results in the release of circulating tumor antigens and inflammatory factors that then mediate an augmented immune response against non-irradiated malignant lesions that express the same tumor antigens [40-41]. A French study [42] investigated the radiation-induced inflammatory response in mice after total abdominal or total-body irradiation. A systemic inflammatory reaction was found after abdominal and total-body irradiation in these mice, concomitant with increased cytokine and chemokine production in the jejunum and also in the lungs [42].
More recently, the observation of unexpected abscopal response in patients receiving radiation during immunotherapy has attracted much interest. Many of these mechanisms have explicit support from animal studies [40, 43-50, 65]. There is considerable insight into the mechanisms of synergistic activity between radiation immunogenic cell death and other biological effects. There appears to be a promotion of T cell recruitment and function associated with irradiation [51-52]. Roles for TGF-β [53], involvement of p53 [54-57], and local tumor damage exposing previously hidden antigens have been implicated [40-41]. Upregulation of cytokines has also been demonstrated [38-39]. Immune checkpoint research has shown remarkable clinical results and may play a principle role when combined with radiation [15, 58-59]. CTLA-4, an immune checkpoint protein, has been shown to downregulate the immune system. In landmark work by Dewan, anti-CTLA-4 antibodies were assessed with and without radiation [59]. Dewan demonstrated that immune checkpoint inhibition alone or radiation alone did not result in an abscopal effect. However, a combination of fractionated radiation and anti-CTLA-4 results in an abscopal effect [59]. Inhibition of the secondary tumor was proportional to CD8+ T cells showing tumor specific IFN-gamma production. This synergy with immune checkpoint inhibitors can result in the net effect on antibodies targeting inhibitory receptors on T cells [4, 58-64]. In a 2015 review of 13 preclinical and 24 clinical papers, Reynders, et al. found that time to a documented abscopal response was within 24 months (median of 5 months). The response was maintained for 3-39 months (median of 13 months) [65].
Conclusions
We have reported a case of abscopal effect with focal radiation therapy to the liver causing regression of distant pulmonary metastasis in HCC. There is increasing evidence supporting the mechanisms of the abscopal effect as an immune-mediated process initiated by high-dose radiation. The recent elaboration and utility of immune checkpoint inhibitors in clinical practice provides significant optimism of a role of radiotherapy in combination with these therapies. This is a promising area of research and therapeutic development, which could lead us to take advantage of these phenomena in the treatment of cancer.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
London Health Sciences Centre Research Ethics Board issued approval 16487E
References
- 1.Cancer statistics, 2007. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Whole body irradiation radiobiology or medicine? Mole RH. Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 3.Unexpected consequence of splenectomy in composite lymphoma. The abscopal effect. Perego D, Faravelli A. Haematologica. 2000;85:211. [PubMed] [Google Scholar]
- 4.Immunologic correlates of the abscopal effect in a patient with melanoma. Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spontaneous regression of hepatocellular carcinoma with multiple lung metastases: a case report. Ikeda M OS, Ueno H, Okusaka T, Kuriyama H. Jpn J Clin Oncol. 2001;31:454–458. doi: 10.1093/jjco/hye092. [DOI] [PubMed] [Google Scholar]
- 6.Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I, Kohno S. Gut. 1998;43:575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Oquiñena S, Iñarrairaegui M, Vila JJ, Alegre F, Zozaya JM, Sangro B. Dig Dis Sci. 2009;54:1147–1153. doi: 10.1007/s10620-008-0447-z. [DOI] [PubMed] [Google Scholar]
- 8.A case of remission in metastatic lung tumor from hepatocellular carcinoma after combined CDDP and PSK therapy (Article in Japanese) Miyanaga O, Shirahama M, Ishibashi H. Gan No Rinsho. 1990;36:527–531. [PubMed] [Google Scholar]
- 9.Abscopal effect on hepatocellular carcinoma. Nakanishi M, Chuma M, Hige S, Asaka M. Am J Gastroenterol. 2008;103:1320–1321. doi: 10.1111/j.1572-0241.2007.01782_13.x. [DOI] [PubMed] [Google Scholar]
- 10.Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. J Med Case Rep. 2011;5:111. doi: 10.1186/1752-1947-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Localized radiation can induce systemic anti-cancer immune and non-immune responses and how we might utilize it. Ahmed M. Med Phys. 2015;42:3609. [Google Scholar]
- 12.Trial Watch: Radioimmunotherapy for oncological indications. Bloy N, Pol J, Manic G, Vitale I, Eggermont A, Galon J, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Oncoimmunology. 2014;3:0. doi: 10.4161/21624011.2014.954929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Blyth BJ, Sykes PJ. Radiat Res. 2011;176:139–157. doi: 10.1667/rr2548.1. [DOI] [PubMed] [Google Scholar]
- 14.The emerging roles of stereotactic ablative radiotherapy for metastatic renal cell carcinoma. Cheung P, Thibault I, Bjarnason GA. Curr Opin Support Palliat Care. 2014;8:258–264. doi: 10.1097/SPC.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 15.Synergy between radiotherapy and immunotherapy in the treatment of advanced malignancies: recent evidences of a new challenge in oncology (article in Italian) Corvò R, Belgioia L. Recent Prog Med . 2015;106:322–330. doi: 10.1701/1940.21088. [DOI] [PubMed] [Google Scholar]
- 16.Radiation as an immunological adjuvant: current evidence on dose and fractionation. Demaria S, Formenti SC. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Immunologically augmented cancer treatment using modern radiotherapy. Durante M, Reppingen N, Held KD. Trends Mol Med. 2013;19:565–582. doi: 10.1016/j.molmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Use of synchrotron medical microbeam irradiation to investigate radiation-induced bystander and abscopal effects in vivo. Fernandez-Palomo C, Bräuer-Krisch E, Laissue J, Vukmirovic D, Blattmann H, Seymour C, Schültke E, Mothersill C. Phys Med. 2015;31:584–595. doi: 10.1016/j.ejmp.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 20. Local immunomodulation for cancer therapy: Providing treatment where needed. Fransen MF, Ossendorp F, Arens R, Melief CJ. Oncoimmunology. 2013;2:0. doi: 10.4161/onci.26493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Frey B, Rubner Y, Kulzer L, Werthmöller N, Weiss EM, Fietkau R, Gaipl US. Cancer Immunol Immunother. 2014;63:29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Frey B, Rubner Y, Wunderlich R, Weiss EM, Pockley AG, Fietkau R, Gaipl US. Curr Med Chem. 2012;19:1751–1764. doi: 10.2174/092986712800099811. [DOI] [PubMed] [Google Scholar]
- 23.Immune modulation in advanced radiotherapies: Targeting out-of-field effects. Hanna GG, Coyle VM, Prise KM. Cancer Lett. 2015;368:246–251. doi: 10.1016/j.canlet.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Radiotherapy: Abscopal responses: pro-immunogenic effects of radiotherapy. Hutchinson L. Nat Rev Clin Oncol. 2015;12:504. doi: 10.1038/nrclinonc.2015.127. [DOI] [PubMed] [Google Scholar]
- 25.The controversial abscopal effect. Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. Cancer Treat Rev. 2005;31:159–172. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Optimizing cancer treatments to induce an acute immune response: radiation abscopal effects, PAMPs, and DAMPs. Ludgate CM. Clin Cancer Res. 2012;18:4522–4525. doi: 10.1158/1078-0432.CCR-12-1175. [DOI] [PubMed] [Google Scholar]
- 27.Dose and spatial effects in long-distance radiation signaling in vivo: implications for abscopal tumorigenesis. Mancuso M, Giardullo P, Leonardi S, Pasquali E, Casciati A, De Stefano I, Tanori M, Pazzaglia S, Saran A. Int J Radiat Oncol Biol Phys. 2013;85:813–819. doi: 10.1016/j.ijrobp.2012.07.2372. [DOI] [PubMed] [Google Scholar]
- 28. The radiation bystander effect and its potential implications for human health. Mancuso M, Pasquali E, Giardullo P, Leonardi S, Tanori M, Di Majo V, Pazzaglia S, Saran A. Curr Mol Med. 2012;12:613–624. doi: 10.2174/156652412800620011. [DOI] [PubMed] [Google Scholar]
- 29.Bystander effects and radiotherapy. Marín A, Martín M, Liñán O, Alvarenga F, López M, Fernández L, Büchser D, Cerezo L. Rep Pract Oncol Radiother. 2015;20:12–21. doi: 10.1016/j.rpor.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The effect of radiation on the immune response to cancers. Park B, Yee C, Lee KM. Int J Mol Sci. 2014;15:927–943. doi: 10.3390/ijms15010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stereotactic ablative radiotherapy (SABR) impact on the immune system and potential for future therapeutic modulation. Reese AS, Feigenberg SJ, Husain A, Webb TJ, Hausner PF, Edelman MJ, Feliciano J, Tkaczuk KH, Sharma NK. Mol Cell Pharmacol. 2013;5:19–25. [PMC free article] [PubMed] [Google Scholar]
- 32.Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Rödel F, Frey B, Multhoff G, Gaipl U. Cancer Lett. 2015;356:105–113. doi: 10.1016/j.canlet.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Author's view radiation and immunotherapy as systemic therapy for solid tumors. Seyedin SN, Tang C, Welsh JW. Oncoimmunology. 2015;4:0. doi: 10.4161/2162402X.2014.986402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Siva S, MacManus MP, Martin RF, Martin OA. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, Massarelli E, Hong D, Naing A, Diab A, Gomez D, Ye H, Heymach J, Komaki R, Allison JP, Sharma P, Welsh JW. Cancer Immunol Res. 2014;2:831–838. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Combinations of immunotherapy and radiation in cancer therapy. Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Immune modulation and stereotactic radiation: improving local and abscopal responses. Zeng J, Harris TJ, Lim M, Drake CG, Tran PT. Biomed Res Int. 2013;2013:658126. doi: 10.1155/2013/658126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J, Demaria S, Formenti SC. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 39.Bystander-type effects mediated by long-lived inflammatory signaling in irradiated bone marrow. Rastogi S, Coates PJ, Lorimore SA, Wright EG. Radiat Res. 2012;177:244–250. doi: 10.1667/rr2805.1. [DOI] [PubMed] [Google Scholar]
- 40.Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Shiraishi K, Ishiwata Y, Nakagawa K, Yokochi S, Taruki C, Akuta T, Ohtomo K, Matsushima K, Tamatani T, Kanegasaki S. Clin Cancer Res. 2008;14:1159–1166. doi: 10.1158/1078-0432.CCR-07-4485. [DOI] [PubMed] [Google Scholar]
- 41.Ionizing radiation alters Fas antigen ligand at the cell surface and on exfoliated plasma membrane-derived vesicles: implications for apoptosis and intercellular signaling. Albanese J, Dainiak N. Radiat Res. 2000;153:49–61. doi: 10.1667/0033-7587(2000)153[0049:irafal]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Abdominal radiation exposure elicits inflammatory responses and abscopal effects in the lungs of mice. Van der Meeren A MP, Vandamme M, Squiban C, Wysocki J, Griffiths N. Radiat Res. 2005;163:144–152. doi: 10.1667/rr3293. [DOI] [PubMed] [Google Scholar]
- 43.Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by GRP94/gp96 against squamous cell carcinoma in mice. Akutsu Y, Matsubara H, Urashima T, Komatsu A, Sakata H, Nishimori T, Yoneyama Y, Hoshino I, Murakami K, Usui A, Kano M, Ochiai T. Int J Oncol. 2007;31:509–515. [PubMed] [Google Scholar]
- 44.Abscopal effect of low-LET gamma-radiation mediated through Rel protein signal transduction in a mouse model of nontargeted radiation response. Aravindan S, Natarajan M, Ramraj SK, Pandian V, Khan FH, Herman TS, Aravindan N. Cancer Gene Ther. 2014;21:54–59. doi: 10.1038/cgt.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Hodge JW, Sharp HJ, Gameiro SR. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abscopal effects of X-irradiation on mouse bone marrow. Kurnick NB, Nokay N. Radiat Res. 1964;23:239–248. [PubMed] [Google Scholar]
- 47.Abscopal effects and bone-marrow repopulation in man and mouse. Kurnick NB, Nokay N. Ann N Y Acad Sci. 1964;114:528–537. doi: 10.1111/j.1749-6632.1964.tb53605.x. [DOI] [PubMed] [Google Scholar]
- 48.Oncogenic radiation abscopal effects in vivo: interrogating mouse skin. Mancuso M, Leonardi S, Giardullo P, Pasquali E, Tanori M, De Stefano I, Casciati A, Naus CC, Pazzaglia S, Saran A. Int J Radiat Oncol Biol Phys. 2013;86:993–999. doi: 10.1016/j.ijrobp.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 49.Abscopal effects of whole-body X-irradiation on compensatory hypertrophy of the rat kidney USNRDL-TR-783. Wachtel LW, Cole LJ. Res Dev Tech Rep. 1964;11:1–23. [PubMed] [Google Scholar]
- 50.Intratumoral injection of interleukin-2 augments the local and abscopal effects of radiotherapy in murine rectal cancer. Yasuda K, Nirei T, Tsuno NH, Nagawa H, Kitayama J. Cancer Sci. 2011;102:1257–1263. doi: 10.1111/j.1349-7006.2011.01940.x. [DOI] [PubMed] [Google Scholar]
- 51.Role of T lymphocytes in tumor response to radiotherapy. Demaria S, Formenti SC. Front Oncol. 2012;2:95. doi: 10.3389/fonc.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The TLR7 agonist imiquimod as an adjuvant for radiotherapy-elicited in situ vaccination against breast cancer. Demaria S, Vanpouille-Box C, Formenti SC, Adams S. Oncoimmunology. 2013;2:0. doi: 10.4161/onci.25997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.TGFbeta Is a master regulator of radiation therapy-induced a ntitumor immunity. Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, Demaria S. Cancer Res. 2015;75:2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radiation abscopal antitumor effect is mediated through p53. Camphausen K, Moses MA, Ménard C, Sproull M, Beecken WD, Folkman J, O'Reilly MS. Cancer Res. 2003;63:1990–1993. [PubMed] [Google Scholar]
- 55.The influence of p53 functions on radiation-induced inflammatory bystander-type signaling in murine bone marrow. Lorimore SA, Rastogi S, Mukherjee D, Coates PJ, Wright EG. Radiat Res. 2013;179:406–415. doi: 10.1667/RR3158.2. [DOI] [PubMed] [Google Scholar]
- 56.Abscopal effect of radiation therapy: Interplay between radiation dose and p53 status. Strigari L, Mancuso M, Ubertini V, Soriani A, Giardullo P, Benassi M, D'Alessio D, Leonardi S, Soddu S, Bossi G. Int J Radiat Biol. 2014;90:248–255. doi: 10.3109/09553002.2014.874608. [DOI] [PubMed] [Google Scholar]
- 57.Corrigendum: Abscopal effect of radiation therapy: interplay between radiation dose and p53 status. Strigari L, Mancuso M, Ubertini V, Soriani A, Giardullo P, Benassi M, D'Alessio D, Leonardi S, Soddu S, Bossi G. Int J Radiat Biol. 2015;91:294. doi: 10.3109/09553002.2014.997514. [DOI] [PubMed] [Google Scholar]
- 58.Exploiting the stress response to radiation to sensitize poorly immunogenic tumors to anti-CTLA-4 treatment. Demaria S, Pilones KA, Formenti SC, Dustin ML. Oncoimmunology. 2013;2:0. doi: 10.4161/onci.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Clin Cancer Res. 2009;2:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MO-FG-BRA-04: Leveraging the abscopal effect via new design radiotherapy biomaterials loaded with immune checkpoint inhibitors. Hao Y, Cifter G, Altundal Y, Sinha N, Moreau M, Sajo E, Makrigiorgos G, Ngwa W. Med Phys. 2015;42:3565. [Google Scholar]
- 61.PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, Mansfield AS, Furutani KM, Olivier KR, Kwon ED. Cancer Immunol Res. 2015;3:610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Combination of radiotherapy and immune checkpoint inhibitors. Pilones KA, Vanpouille-Box C, Demaria S. Semin Radiat Oncol. 2015;25:28–33. doi: 10.1016/j.semradonc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 63.In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. Vaccine. 2015;pub ahead of print:0–1. doi: 10.1016/j.vaccine.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Wu L, Wu MO, De la Maza L, Yun Z, Yu J, Zhao Y, Cho J, de Perrot M. Oncotarget. 2015;6:12468–12480. doi: 10.18632/oncotarget.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. Cancer Treat Review. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


