Abstract
Background
Lateral distal femur (LDF) scans by dual energy x-ray absorptiometry (DXA) are often feasible in children for whom other sites are not measurable. Pediatric reference data for LDF are not available for more recent DXA technology.
Aims
To assess older pediatric LDF reference data, construct new reference curves for LDF bone mineral density (BMD), and demonstrate the comparability of LDF BMD to other measures of BMD and strength assessed by DXA and by peripheral quantitative computed tomography (pQCT).
Methods
LDF, spine and whole body scans of 821 healthy children, 5 to 18 years of age, recruited at a single center were obtained using a Hologic Delphi/Discovery system. Tibia trabecular and total BMD (3% site), cortical geometry (38% site) (cortical thickness, section modulus, strain strength index) were assessed by pQCT. Sex and race-specific reference curves were generated using LMS-ChartMaker and Z-scores calculated and compared by correlation analysis.
Results
Z-scores for LDF BMD based on published findings demonstrated overestimation or underestimation of the prevalence of low BMD-for-age depending on the region of interest considered. Revised LDF reference curves were generated. The new LDF Z-scores were strongly and significantly associated with weight, BMI, spine and whole body BMD Z-scores, and all pQCT Z-scores.
Conclusion
These findings demonstrate the comparability of LDF measurements to other clinical and research bone density assessment modes, and enable assessment of BMD in children with disabilities, who are particularly prone to low trauma fractures of long bones, and for whom traditional DXA measurement sites are not feasible.
Keywords: BMD, distal femur, DXA, children, bone densitometry, reference data
Introduction
Children with physical disabilities such as cerebral palsy, spina bifida, muscular dystrophy, and spinal cord injuries that limit ambulation are typically osteopenic 1-3. This in turn results in fractures with minimal, or in some cases even unrecognized trauma. Femoral shaft and distal metaphyseal fractures are particularly common4,5. Assessment of bone density in these conditions is made difficult by several factors. Contractures of the lower limbs are prevalent and prevent laying in a fully supine position for optimal whole body and proximal femur (hip) measurements by dual energy x-ray absorptiometry (DXA). In addition, the anatomy of the proximal femur is frequently distorted in these conditions due to dysplasia, subluxation, or hip dislocation. Clinical care of hip disorders in these conditions sometimes requires osteotomy procedures and internal fixation with metallic implants, further interfering with DXA bone density assessment in this region.
Bone density measurement in the lumbar spine is also problematic in children with many common physical disabilities. The anatomy is often distorted due to scoliosis, which if surgically treated will have metallic fixation that interferes with DXA imaging. An additional point regarding bone density measurements in the lumbar spine is the lack of relevance to fracture risk in this population A prospective, longitudinal study in children with quadriplegic cerebral palsy found that DXA measures of lumbar spine areal bone mineral density (aBMD) were not predictive of subsequent fracture risk 6. This somewhat surprising observation likely relates to the finding that aBMD measures in the femur and spine correlate poorly in a child with low BMD 7. Fractures in children with physical disabilities typically occur in the long bones, most commonly the femur and tibia 8,9. In marked distinction to elderly adults, osteoporotic compression fractures of the spine are uncommon in nonambulatory children.
In order to address these difficulties in obtaining clinically meaningful assessments of bone health in children with disabilities an alternative technique was developed utilizing DXA measurements of the distal femur projected in the lateral plane10,11. Advantages of this technique are that the femur is the most common site of fracture, children with severe contractures can be comfortably positioned, and metallic fixation is rarely utilized in this region. Further, subregional analyses allow separate assessment of regions rich in cortical versus cancellous bone.
Existing reference data for bone density of the distal femur is based upon a relatively small sample of healthy children who were measured with the older pencil beam DXA technology 11. The purpose of this report is to provide more robust pediatric DXA lateral distal femur (LDF) aBMD reference data utilizing contemporary fan-beam technology. These LDF reference data were compared to DXA measures of areal BMC and aBMD of the spine and whole body, the sites recommended for clinical assessment of bone density in children 12. In addition, LDF bone density was compared to tibia measures of trabecular and cortical volumetric BMD (vBMD) and geometry measured by peripheral quantitative computed tomography (pQCT). Unlike DXA aBMD measures, which are based on a two-dimensional bone image, pQCT provides a three-dimensional vBMD measure, distinct estimates of trabecular and cortical vBMD, and measures of bone geometry known to relate to bone strength13. These comparisons were performed as a relative validation of the LDF measurement with respect to other commonly used clinical and research methods for bone density assessment.
Methods
Sample
Study participants consisted of healthy children, 5 to 18 years of age, enrolled in the Reference Project on Skeletal Development at The Children's Hospital of Philadelphia. Subjects were recruited through the pediatric practices of The Children's Hospital of Philadelphia, newspaper advertisements and community fliers. Children were excluded for chronic health conditions (e.g., renal, endocrine, gastrointestinal disorders) and medication use (e.g., glucocorticoids) that might affect growth or development (premature birth), dietary intake (medications affecting appetite), or bone density (restricted ambulation). Children were not excluded on the basis of fracture history, since fractures occur in 25 to 50% of otherwise healthy children14,15. Parents or guardians of subjects less than 18 years of age provided written informed consent, and the subjects provided assent. Subjects 18 years of age provided informed consent. The protocol was approved by the Internal Review Board of The Children's Hospital of Philadelphia.
Assessment of Growth and Pubertal Development
Height was measured to the nearest 0.1cm with a stadiometer (Holtain, Crymych, UK) and weight to the nearest 0.1kg a digital scale (Scaletronix). All measurements were obtained in triplicate by a trained anthropometrist using standardized techniques 16 with the subject wearing light clothing and with shoes and hair adornments removed. The mean of the three measurements was used in the analysis. The stage of pubertal development was determined using a validated self-assessment questionnaire 17,18 and classified according to Tanner 19.
DXA Measures of Bone Density
Areal bone mineral density (aBMD) measurements were obtained by dual energy x-ray absorptiometry (DXA) with a Delphi/Discovery (Hologic, Bedford, MA) densitometer. All measurements were obtained with the same device and analyzed using software version 12.3. The DXA exam included scans of the lumbar spine and whole body following standardized positioning, acquisition and analysis techniques. The coefficient of variation (%CV) for DXA measurements of the spine and whole body in children range from 0.64 to 1.20 20.
The lateral distal femur scan was obtained as described in Henderson et al. 21; positioning of the subject is illustrated in Figure 1a, and placement of the regions of interest (ROIs) is shown in Figures 1b and 1c. Briefly, the patient is placed in a side lying position on the scanning table, on the side being measured, with the femur following the length of the table. The other thigh is flexed out of the field of view. The lateral distal femur is analyzed for three regions of interest: region 1 is placed at the anterior half of distal metaphysis, region two is metadiaphyseal, and region 3 is diaphyseal. Region size is based on the diaphyseal width; all three regions are the same height. To assure consistency in lateral distal femur scan acquisition and analysis techniques, a subset of 40 randomly selected scans were reviewed by an independent investigator (HK). In addition, all scans were inspected by one investigator (BZ) for movement, interfering factors, and analysis consistency (placement of regions of interest) to assure technical quality of all scans.
Figure 1.
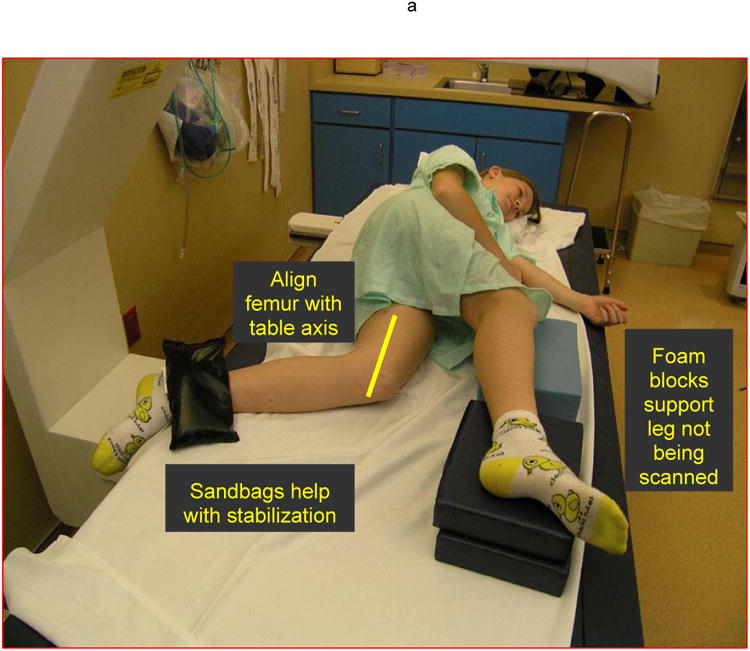
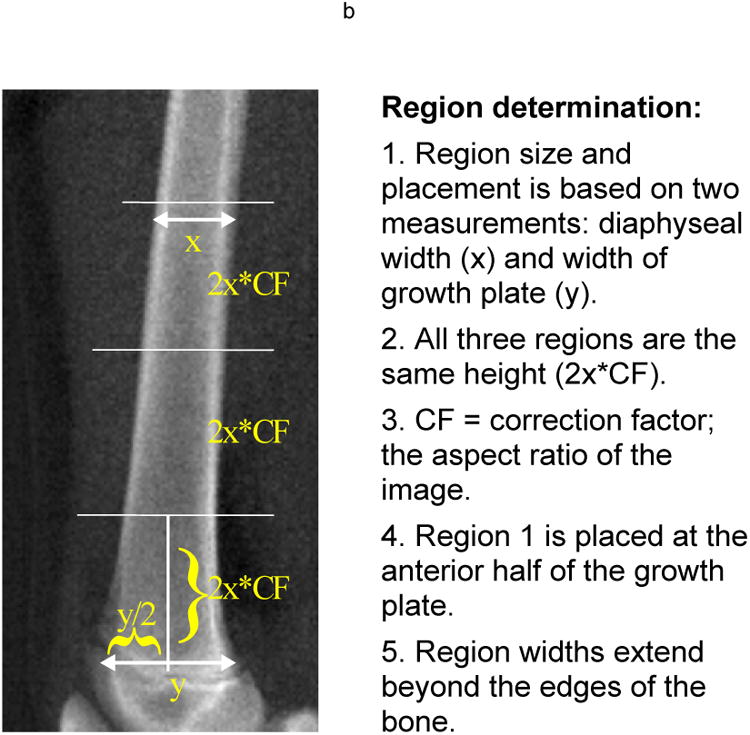
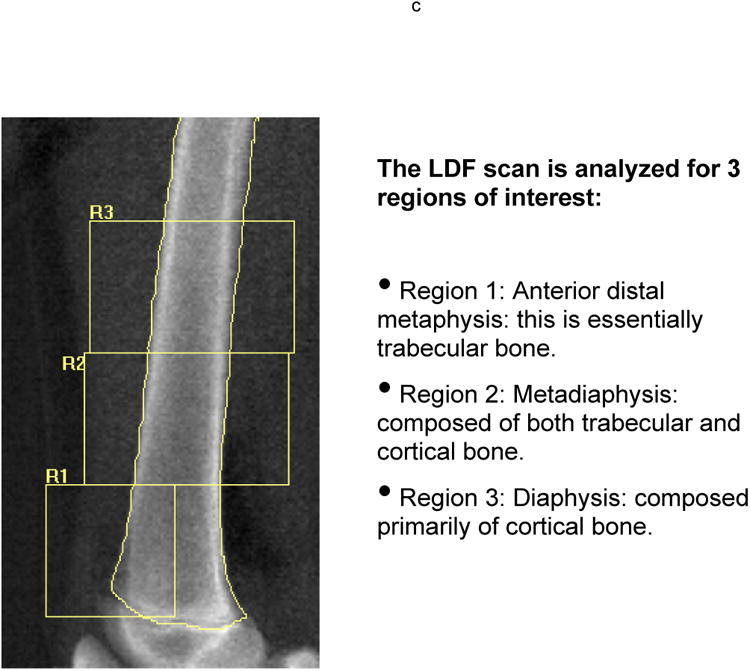
(A) Patient positioning for the left lateral distal femur scan showing the child in a side-lying position with positioning devices (foam blocks and sand bags) to assist in attaining a comfortable and stable position. The femur is centered on the table and parallel to the edge. The forearm scan mode is used to obtain the scan. (B) Analysis of the scan requires insertion of region of interest boxes. The width and height of each region of interest box is illustrated in the figure. (C) The three regions of interest are illustrated in the figure.
pQCT Measures of Volumetric BMD and Bone Strength
Volumetric BMD (vBMD) and bone strength of the distal tibia were assessed by peripheral quantitative computed tomography (pQCT) in a subset of the healthy reference sample using a Stratec XCT2000 device (Orthometrix, White Plains, NY) with a voxel size of 0.4mm and scan speed of 25mm/sec. An anthropometric measure of tibia length (mm) (from the distal tip of the medial malleolus to the superior edge of the medial tibial plateau) was obtained using sliding calipers (Rosscraft, Vancouver, BC). A scout view was obtained to place the reference line at the proximal border of the distal tibia growth plate, and measurements were obtained at regions located at the distal 3% and 38% of tibia length. Scans were analyzed using the Stratec 5.50 software. At the 3% site, scans were analyzed for total and trabecular vBMD1. At the 38% site, scans were analyzed for cortical thickness, section modulus and the strain strength index (SSI)2. The coefficient of variation (%CV) for selected pQCT measures were as follows: trabecular density 1.4%, cortical thickness 1.4%, SSI 2.8% 22.
Statistical Analysis
Descriptive statistics and graphical displays were generated to characterize the data and assess the distributions. Height, weight and body mass index (BMI) were converted to Z-scores (standard deviation scores) based on the Centers for Disease Control and Prevention Growth Charts 23.
Z-scores for lateral distal femur aBMD were calculated based on previously published reference data for lateral distal femur aBMD11. For males and females separately, the student's T-test was used to determine whether the distribution of Z-scores was significantly different than expected for a healthy reference samples, i.e., a mean of zero and standard deviation of one.
To assess the need for sex and race-specific reference curves, multiple linear regression analyses were used to determine whether significant group differences existed. Subjects were categorized as black vs. non-black according to self-reported race. Based on these analyses (results not presented), it was determined that separate sex- and race-specific reference curves were needed.
Reference curves for lateral distal femur aBMD measurements were generated using the “LMS” method 24 that accounts for the non-linearity, heteroscedasticity and skewness of aBMD data in growing children. Sex and race specific curves were constructed for aBMD of regions 1 through 3 using the LMS Chartmaker Program version 2.325. The LMS method fits three parameters (LMS) as cubic splines by nonlinear regression. The three parameters represent the median (M), standard deviation (S), and power in the Box-Cox transformation (L) that vary as a function of age. These parameters are used to construct centile curves using the following formula:
| Equation 1 |
where the L, M, and S are age-specific, and the Z is the Z-score that corresponds to a given percentile (e.g., Z=0 is the 50th percentile). For an individual with a measurement X, a Z-score is calculated using the age-specific L, M and S parameters and the following formula:
| Equation 2 |
Fit of the curves was evaluated by graphical inspection of the centile curves relative to the raw data and by Q-Q plots.
The LMS method was also used to generate sex and race-specific reference curves for all other densitometric measures on the same sample of subjects. Z-scores for DXA measures of spine aBMC and aBMD, total body aBMC and aBMD, and pQCT measures of trabecular and total vBMD at the 3% site of the tibia relative to age, and for cortical thickness, section modulus and strain-strength index relative to tibia length were calculated. Additional statistical analyses included correlations among the Z-scores for growth, lumbar spine, whole body and lateral distal femur aBMD, and pQCT measures of vBMD and bone strength.
Results
Lateral distal femur results were available for 821 of the 854 subjects recruited. The subjects for who lateral distal femur BMD scans were excluded for technical limitations (less than 4%) did not differ in age, sex, race or spine aBMD Z-score from the remainder of the sample. Characteristics of the sample are shown in Table 1. Similar to other reports of U.S. children26,27, Z-scores for height, weight and BMI were above zero signaling that their growth differed somewhat from the CDC Growth Charts. This sample was similar in height Z-score, but had lower weight and BMI Z-scores than the sample used in the development of the original LDF reference data (0.6±1.0 for both Z-scores) 11. Descriptive statistics (mean and standard deviation) for all bone measures are given in Table 2. The study subjects had DXA bone density values that were similar to U.S. national reference data26 (available only for ages 7 to 17: spine aBMD Z-score: -0.07±1.0, n=612; whole body aBMD Z-score: 0.09±1.0, n=668). Due to the limitations of the scan field length relative to femur dimensions, fewer subjects had results for lateral distal femur Region 3 (n=625) compared to Region 1 (n=821).
Table 1. Sample Characteristics (n=821).
| Gender | 52% Female |
| Race | 45% Black / African American |
| Sexual Maturity | |
| Tanner 1 | 34% |
| Tanner 2 | 14% |
| Tanner 3 | 15% |
| Tanner 4 | 23% |
| Tanner 5 | 15% |
| Age, y* | 11.4 (3.5) |
| Height Z-score* | 0.3 (0.9) |
| Weight Z-score* | 0.4 (1.0) |
| BMI Z-score* | 0.3 (1.0) |
mean (sd)
Table 2. Bone Density Measures by DXA and pQCT.
| Measure | n | Mean | s.d. |
|---|---|---|---|
| Lateral Distal Femur | |||
|
| |||
| Region1 aBMD, gm/cm2 | 821 | 0.88 | 0.19 |
| Region2 aBMD, gm/cm2 | 818 | 0.96 | 0.21 |
| Region3 aBMD, gm/cm2 | 625 | 0.93 | 0.23 |
|
| |||
| Lumbar Spine and Whole Body DXA | |||
|
| |||
| Lumbar Spine aBMC, gm | 810 | 35.1 | 17.0 |
| Lumbar Spine aBMD, gm/cm2 | 810 | 0.73 | 0.19 |
| Whole Body aBMC, gm | 814 | 1393 | 554 |
|
| |||
| Tibia pQCT | |||
|
| |||
| Tibia Total vBMD (3% site), gm/cm3 | 546 | 307 | 39 |
| Tibia Trabecular vBMD (3% site), gm/cm3 | 546 | 248 | 31 |
| Tibia Cortical Thickness, mm | 587 | 4.47 | 0.90 |
| Tibia Section Modulus, mm3 | 587 | 1210 | 537 |
| Tibia Strain Strength Index, mm4 | 587 | 1128 | 508 |
aBMD: areal bone mineral density
vBMD: volumetric bone mineral density
Z-scores for aBMD of the lateral distal femur based on previously published reference data11 acquired with a pencil-beam device are shown in Table 3. All Z-scores were significantly different from zero, and varied by sex, race and site. Thus, use of this reference data may lead to overestimation of the prevalence of low BMD-for-age (defined by the International Society for Clinical Densitometry12 as Z-score < -2.0) in some groups, and overestimation in other groups, depending on the region of interest considered.
Table 3. Lateral Distal Femur BMD Z-Scores (Regions 1 and 2) For The Current Sample Based On Previously Published Reference Data11.
| Race | Gender | Region 1 BMD Z-score | Region 2 BMD Z-score | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean | Sd | p | Mean | sd | P | ||
| All | All | -0.13 | 1.11 | <0.001 | 0.52 | 1.07 | <0.001 |
| Non-Black | Females | -0.46 | 1.09 | <0.001 | 0.21 | 1.07 | 0.003 |
| Males | -0.39 | 0.82 | <0.001 | 0.25 | 0.82 | <0.001 | |
| Black | Females | 0.25 | 1.31 | 0.01 | 0.94 | 1.16 | <0.001 |
| Males | 0.23 | 1.02 | 0.003 | 0.84 | 1.01 | <0.001 | |
p-value based on a t-test to determine if the mean is significantly different from zero.
The new reference curves for lateral distal femur aBMD resulted in Z-scores with a mean of zero and standard deviation of one, as expected. The L, M, and S parameters and percentile distributions are given in Table 4a to 4c, and the distributions of lateral distal femur aBMD are shown in Figures 2 to 4. Because of the small number of subjects in the 5 year old age group (n=13, divided by race and sex), the values in Table 4a to 4c were restricted to ages 6 to 18. The L, M (50th percentile) and S values in Table 4 can be used to calculate a Z-score for an individual child using equations 1 and 2 given above.
Table 4.
| a. L, M, S parameters and percentile distributions for Region 1 BMD
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NON BLACK | ||||||||||||||
|
|
||||||||||||||
| Females | Males | |||||||||||||
|
|
||||||||||||||
| Age | L | S | 3rd | 10th | M 50th | 90th | 97th | L | S | 3rd | 10th | M 50th | 90th | 97th |
|
|
||||||||||||||
| 6.0 | -0.78 | 0.12 | 0.52 | 0.56 | 0.65 | 0.78 | 0.85 | -1.83 | 0.12 | 0.53 | 0.56 | 0.64 | 0.77 | 0.87 |
| 7.0 | -0.77 | 0.12 | 0.52 | 0.56 | 0.66 | 0.78 | 0.86 | -1.51 | 0.12 | 0.55 | 0.58 | 0.67 | 0.80 | 0.90 |
| 8.0 | -0.74 | 0.12 | 0.53 | 0.57 | 0.67 | 0.80 | 0.88 | -1.20 | 0.12 | 0.57 | 0.60 | 0.70 | 0.84 | 0.94 |
| 9.0 | -0.69 | 0.13 | 0.55 | 0.59 | 0.69 | 0.82 | 0.91 | -0.90 | 0.13 | 0.59 | 0.63 | 0.73 | 0.88 | 0.98 |
| 10.0 | -0.61 | 0.13 | 0.57 | 0.61 | 0.72 | 0.86 | 0.95 | -0.64 | 0.13 | 0.61 | 0.66 | 0.78 | 0.93 | 1.03 |
| 11.0 | -0.51 | 0.13 | 0.61 | 0.66 | 0.78 | 0.94 | 1.04 | -0.39 | 0.13 | 0.64 | 0.69 | 0.82 | 0.98 | 1.08 |
| 12.0 | -0.38 | 0.14 | 0.66 | 0.71 | 0.85 | 1.03 | 1.14 | -0.13 | 0.13 | 0.66 | 0.72 | 0.86 | 1.03 | 1.12 |
| 13.0 | -0.26 | 0.14 | 0.70 | 0.76 | 0.92 | 1.12 | 1.24 | 0.12 | 0.13 | 0.68 | 0.75 | 0.89 | 1.07 | 1.17 |
| 14.0 | -0.16 | 0.15 | 0.72 | 0.80 | 0.96 | 1.18 | 1.30 | 0.38 | 0.13 | 0.70 | 0.78 | 0.94 | 1.11 | 1.21 |
| 15.0 | -0.07 | 0.15 | 0.74 | 0.82 | 1.00 | 1.23 | 1.36 | 0.65 | 0.13 | 0.73 | 0.81 | 0.98 | 1.16 | 1.25 |
| 16.0 | 0.00 | 0.15 | 0.76 | 0.85 | 1.04 | 1.28 | 1.41 | 0.91 | 0.13 | 0.76 | 0.85 | 1.02 | 1.20 | 1.29 |
| 17.0 | 0.08 | 0.16 | 0.78 | 0.87 | 1.07 | 1.32 | 1.46 | 1.17 | 0.12 | 0.79 | 0.88 | 1.06 | 1.23 | 1.31 |
| 18.0 | 0.14 | 0.16 | 0.80 | 0.89 | 1.10 | 1.36 | 1.51 | 1.43 | 0.12 | 0.83 | 0.92 | 1.10 | 1.26 | 1.34 |
|
|
||||||||||||||
| BLACK | ||||||||||||||
|
|
||||||||||||||
| Females | Males | |||||||||||||
|
|
||||||||||||||
| age | L | S | 3rd | 10th | M 50th | 90th | 97th | L | S | 3rd | 10th | M 50th | 90th | 97th |
|
|
||||||||||||||
| 6.0 | -1.29 | 0.13 | 0.53 | 0.57 | 0.66 | 0.81 | 0.92 | 0.26 | 0.08 | 0.63 | 0.70 | 0.78 | 0.82 | 0.87 |
| 7.0 | -1.29 | 0.13 | 0.57 | 0.61 | 0.71 | 0.87 | 0.99 | 0.26 | 0.10 | 0.62 | 0.72 | 0.82 | 0.88 | 0.94 |
| 8.0 | -1.29 | 0.13 | 0.61 | 0.65 | 0.76 | 0.93 | 1.06 | 0.26 | 0.12 | 0.62 | 0.73 | 0.86 | 0.93 | 1.01 |
| 9.0 | -1.29 | 0.13 | 0.64 | 0.69 | 0.81 | 1.00 | 1.13 | 0.26 | 0.14 | 0.62 | 0.75 | 0.90 | 0.98 | 1.07 |
| 10.0 | -1.29 | 0.13 | 0.68 | 0.73 | 0.86 | 1.05 | 1.20 | 0.26 | 0.15 | 0.62 | 0.76 | 0.94 | 1.04 | 1.14 |
| 11.0 | -1.29 | 0.14 | 0.71 | 0.77 | 0.90 | 1.11 | 1.26 | 0.26 | 0.16 | 0.63 | 0.79 | 0.98 | 1.09 | 1.21 |
| 12.0 | -1.29 | 0.14 | 0.74 | 0.80 | 0.94 | 1.16 | 1.32 | 0.26 | 0.17 | 0.64 | 0.82 | 1.03 | 1.15 | 1.28 |
| 13.0 | -1.29 | 0.14 | 0.77 | 0.82 | 0.97 | 1.20 | 1.36 | 0.26 | 0.17 | 0.67 | 0.86 | 1.08 | 1.21 | 1.35 |
| 14.0 | -1.29 | 0.14 | 0.78 | 0.84 | 0.99 | 1.23 | 1.40 | 0.26 | 0.17 | 0.70 | 0.90 | 1.13 | 1.26 | 1.40 |
| 15.0 | -1.29 | 0.14 | 0.80 | 0.86 | 1.01 | 1.25 | 1.42 | 0.26 | 0.17 | 0.74 | 0.94 | 1.17 | 1.31 | 1.45 |
| 16.0 | -1.29 | 0.14 | 0.81 | 0.87 | 1.02 | 1.27 | 1.45 | 0.26 | 0.16 | 0.79 | 0.99 | 1.22 | 1.35 | 1.49 |
| 17.0 | -1.29 | 0.14 | 0.82 | 0.88 | 1.04 | 1.29 | 1.47 | 0.26 | 0.15 | 0.84 | 1.04 | 1.26 | 1.39 | 1.52 |
| 18.0 | -1.29 | 0.14 | 0.83 | 0.89 | 1.05 | 1.31 | 1.50 | 0.26 | 0.14 | 0.90 | 1.09 | 1.30 | 1.42 | 1.55 |
| b. L, M, S parameters and percentile distributions for Region 2 BMD
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NON BLACK | ||||||||||||||
|
|
||||||||||||||
| Females | Males | |||||||||||||
|
|
||||||||||||||
| Age | L | S | 3rd | 10th | M 50th | 90th | 97th | L | S | 3rd | 10th | M 50th | 90th | 97th |
|
|
||||||||||||||
| 6.0 | 0.88 | 0.09 | 0.57 | 0.61 | 0.70 | 0.79 | 0.83 | -0.68 | 0.08 | 0.60 | 0.64 | 0.71 | 0.80 | 0.85 |
| 7.0 | 0.84 | 0.09 | 0.58 | 0.63 | 0.72 | 0.81 | 0.85 | -0.68 | 0.09 | 0.63 | 0.66 | 0.74 | 0.83 | 0.89 |
| 8.0 | 0.78 | 0.10 | 0.60 | 0.64 | 0.74 | 0.83 | 0.88 | -0.68 | 0.09 | 0.65 | 0.69 | 0.77 | 0.87 | 0.93 |
| 9.0 | 0.72 | 0.10 | 0.61 | 0.66 | 0.76 | 0.86 | 0.91 | -0.68 | 0.09 | 0.67 | 0.71 | 0.80 | 0.92 | 0.98 |
| 10.0 | 0.62 | 0.10 | 0.64 | 0.69 | 0.79 | 0.90 | 0.96 | -0.68 | 0.10 | 0.70 | 0.74 | 0.84 | 0.97 | 1.04 |
| 11.0 | 0.47 | 0.11 | 0.67 | 0.73 | 0.85 | 0.97 | 1.04 | -0.68 | 0.10 | 0.73 | 0.78 | 0.88 | 1.02 | 1.10 |
| 12.0 | 0.27 | 0.11 | 0.73 | 0.79 | 0.92 | 1.07 | 1.15 | -0.68 | 0.11 | 0.76 | 0.81 | 0.93 | 1.07 | 1.16 |
| 13.0 | 0.06 | 0.12 | 0.79 | 0.86 | 1.01 | 1.19 | 1.29 | -0.68 | 0.11 | 0.79 | 0.85 | 0.97 | 1.14 | 1.24 |
| 14.0 | -0.12 | 0.13 | 0.83 | 0.90 | 1.07 | 1.27 | 1.39 | -0.68 | 0.12 | 0.83 | 0.89 | 1.03 | 1.21 | 1.32 |
| 15.0 | -0.26 | 0.13 | 0.86 | 0.94 | 1.12 | 1.34 | 1.47 | -0.68 | 0.12 | 0.87 | 0.93 | 1.08 | 1.29 | 1.41 |
| 16.0 | -0.38 | 0.14 | 0.89 | 0.97 | 1.16 | 1.40 | 1.54 | -0.68 | 0.13 | 0.91 | 0.98 | 1.15 | 1.37 | 1.51 |
| 17.0 | -0.47 | 0.14 | 0.91 | 0.99 | 1.19 | 1.44 | 1.60 | -0.68 | 0.13 | 0.95 | 1.02 | 1.21 | 1.45 | 1.61 |
| 18.0 | -0.54 | 0.14 | 0.93 | 1.01 | 1.21 | 1.48 | 1.65 | -0.68 | 0.14 | 0.99 | 1.07 | 1.27 | 1.54 | 1.72 |
|
|
||||||||||||||
| BLACK | ||||||||||||||
|
|
||||||||||||||
| Females | Males | |||||||||||||
|
|
||||||||||||||
| age | L | S | 3rd | 10th | M 50th | 90th | 97th | L | S | 3rd | 10th | M 50th | 90th | 97th |
|
|
||||||||||||||
| 6.0 | -1.03 | 0.10 | 0.59 | 0.62 | 0.71 | 0.82 | 0.88 | -1.53 | 0.07 | 0.68 | 0.71 | 0.77 | 0.85 | 0.89 |
| 7.0 | -1.03 | 0.10 | 0.62 | 0.66 | 0.75 | 0.87 | 0.95 | -1.29 | 0.08 | 0.70 | 0.73 | 0.81 | 0.91 | 0.97 |
| 8.0 | -1.03 | 0.10 | 0.66 | 0.70 | 0.80 | 0.93 | 1.01 | -1.08 | 0.09 | 0.71 | 0.75 | 0.84 | 0.97 | 1.04 |
| 9.0 | -1.03 | 0.11 | 0.70 | 0.75 | 0.85 | 0.99 | 1.08 | -0.89 | 0.10 | 0.72 | 0.76 | 0.87 | 1.01 | 1.10 |
| 10.0 | -1.03 | 0.11 | 0.75 | 0.80 | 0.91 | 1.06 | 1.16 | -0.69 | 0.11 | 0.73 | 0.78 | 0.90 | 1.06 | 1.16 |
| 11.0 | -1.03 | 0.11 | 0.80 | 0.85 | 0.98 | 1.14 | 1.25 | -0.44 | 0.13 | 0.74 | 0.80 | 0.94 | 1.12 | 1.23 |
| 12.0 | -1.03 | 0.11 | 0.85 | 0.90 | 1.04 | 1.22 | 1.34 | -0.15 | 0.14 | 0.76 | 0.83 | 1.00 | 1.20 | 1.31 |
| 13.0 | -1.03 | 0.11 | 0.88 | 0.94 | 1.09 | 1.28 | 1.41 | 0.17 | 0.14 | 0.79 | 0.87 | 1.05 | 1.27 | 1.39 |
| 14.0 | -1.03 | 0.12 | 0.91 | 0.97 | 1.12 | 1.33 | 1.46 | 0.49 | 0.15 | 0.81 | 0.91 | 1.11 | 1.34 | 1.46 |
| 15.0 | -1.03 | 0.12 | 0.93 | 0.99 | 1.15 | 1.36 | 1.50 | 0.81 | 0.14 | 0.83 | 0.94 | 1.16 | 1.39 | 1.51 |
| 16.0 | -1.03 | 0.12 | 0.95 | 1.01 | 1.17 | 1.39 | 1.53 | 1.13 | 0.14 | 0.86 | 0.98 | 1.21 | 1.44 | 1.55 |
| 17.0 | -1.03 | 0.12 | 0.96 | 1.03 | 1.19 | 1.41 | 1.56 | 1.46 | 0.14 | 0.90 | 1.03 | 1.27 | 1.49 | 1.60 |
| 18.0 | -1.03 | 0.12 | 0.98 | 1.04 | 1.21 | 1.44 | 1.59 | 1.79 | 0.13 | 0.94 | 1.08 | 1.33 | 1.54 | 1.64 |
| c. L, M, S parameters and percentile distributions for Region 3 BMD
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NON BLACK | ||||||||||||||
|
|
||||||||||||||
| Females | Males | |||||||||||||
|
|
||||||||||||||
| Age | L | S | 3rd | 10th | M 50th | 90th | 97th | L | S | 3rd | 10th | M 50th | 90th | 97th |
|
|
||||||||||||||
| 6.0 | -0.25 | 0.07 | 0.66 | 0.68 | 0.75 | 0.82 | 0.85 | 0.32 | 0.05 | 0.68 | 0.71 | 0.76 | 0.81 | 0.84 |
| 7.0 | -0.25 | 0.07 | 0.67 | 0.70 | 0.77 | 0.85 | 0.89 | 0.32 | 0.06 | 0.70 | 0.73 | 0.79 | 0.86 | 0.90 |
| 8.0 | -0.25 | 0.08 | 0.69 | 0.72 | 0.80 | 0.88 | 0.93 | 0.32 | 0.07 | 0.71 | 0.75 | 0.83 | 0.91 | 0.96 |
| 9.0 | -0.25 | 0.08 | 0.71 | 0.74 | 0.83 | 0.92 | 0.97 | 0.32 | 0.08 | 0.74 | 0.78 | 0.87 | 0.97 | 1.02 |
| 10.0 | -0.25 | 0.08 | 0.73 | 0.77 | 0.86 | 0.97 | 1.03 | 0.32 | 0.09 | 0.77 | 0.82 | 0.92 | 1.04 | 1.10 |
| 11.0 | -0.25 | 0.09 | 0.77 | 0.82 | 0.92 | 1.04 | 1.11 | 0.32 | 0.10 | 0.80 | 0.85 | 0.97 | 1.10 | 1.17 |
| 12.0 | -0.25 | 0.10 | 0.81 | 0.87 | 0.99 | 1.14 | 1.22 | 0.32 | 0.10 | 0.82 | 0.88 | 1.01 | 1.15 | 1.23 |
| 13.0 | -0.25 | 0.12 | 0.85 | 0.92 | 1.07 | 1.25 | 1.35 | 0.32 | 0.10 | 0.85 | 0.92 | 1.05 | 1.21 | 1.29 |
| 14.0 | -0.25 | 0.12 | 0.89 | 0.96 | 1.13 | 1.33 | 1.45 | 0.32 | 0.10 | 0.88 | 0.95 | 1.10 | 1.26 | 1.34 |
| 15.0 | -0.25 | 0.13 | 0.92 | 1.00 | 1.18 | 1.40 | 1.53 | 0.32 | 0.11 | 0.92 | 0.99 | 1.14 | 1.31 | 1.41 |
| 16.0 | -0.25 | 0.13 | 0.95 | 1.03 | 1.22 | 1.45 | 1.59 | 0.32 | 0.11 | 0.96 | 1.03 | 1.19 | 1.37 | 1.47 |
| 17.0 | -0.25 | 0.13 | 0.98 | 1.06 | 1.25 | 1.49 | 1.63 | 0.32 | 0.11 | 1.00 | 1.08 | 1.25 | 1.43 | 1.53 |
| 18.0 | -0.25 | 0.13 | 1.00 | 1.09 | 1.28 | 1.53 | 1.67 | 0.32 | 0.11 | 1.04 | 1.12 | 1.30 | 1.49 | 1.59 |
|
|
||||||||||||||
| BLACK | ||||||||||||||
|
|
||||||||||||||
| Female | Males | |||||||||||||
|
|
||||||||||||||
| age | L | S | 3rd | 10th | M 50th | 90th | 97th | L | S | 3rd | 10th | M 50th | 90th | 97th |
|
|
||||||||||||||
| 6.0 | -0.82 | 0.09 | 0.65 | 0.69 | 0.77 | 0.88 | 0.94 | -0.59 | 0.06 | 0.73 | 0.75 | 0.82 | 0.89 | 0.92 |
| 7.0 | -0.82 | 0.09 | 0.69 | 0.72 | 0.81 | 0.93 | 0.99 | -0.59 | 0.07 | 0.75 | 0.78 | 0.86 | 0.94 | 0.99 |
| 8.0 | -0.82 | 0.09 | 0.73 | 0.77 | 0.86 | 0.98 | 1.05 | -0.59 | 0.08 | 0.77 | 0.81 | 0.89 | 0.99 | 1.05 |
| 9.0 | -0.82 | 0.09 | 0.77 | 0.81 | 0.92 | 1.04 | 1.12 | -0.59 | 0.08 | 0.79 | 0.83 | 0.93 | 1.04 | 1.11 |
| 10.0 | -0.82 | 0.09 | 0.82 | 0.87 | 0.98 | 1.11 | 1.19 | -0.59 | 0.09 | 0.81 | 0.86 | 0.96 | 1.10 | 1.17 |
| 11.0 | -0.82 | 0.09 | 0.88 | 0.93 | 1.05 | 1.19 | 1.28 | -0.59 | 0.10 | 0.83 | 0.88 | 1.00 | 1.15 | 1.24 |
| 12.0 | -0.82 | 0.09 | 0.93 | 0.98 | 1.11 | 1.26 | 1.35 | -0.59 | 0.11 | 0.84 | 0.90 | 1.03 | 1.20 | 1.30 |
| 13.0 | -0.82 | 0.09 | 0.97 | 1.03 | 1.15 | 1.31 | 1.41 | -0.59 | 0.12 | 0.86 | 0.92 | 1.07 | 1.26 | 1.37 |
| 14.0 | -0.82 | 0.09 | 1.00 | 1.06 | 1.19 | 1.35 | 1.45 | -0.59 | 0.12 | 0.87 | 0.94 | 1.10 | 1.32 | 1.45 |
| 15.0 | -0.82 | 0.09 | 1.02 | 1.08 | 1.21 | 1.38 | 1.48 | -0.59 | 0.13 | 0.89 | 0.96 | 1.14 | 1.37 | 1.52 |
| 16.0 | -0.82 | 0.09 | 1.04 | 1.09 | 1.23 | 1.40 | 1.50 | -0.59 | 0.14 | 0.91 | 0.98 | 1.18 | 1.43 | 1.60 |
| 17.0* | -0.82 | 0.09 | 1.05 | 1.11 | 1.24 | 1.42 | 1.52 | |||||||
| 18.0* | -0.82 | 0.09 | 1.06 | 1.12 | 1.26 | 1.43 | 1.53 | |||||||
too few male subjects in this age range
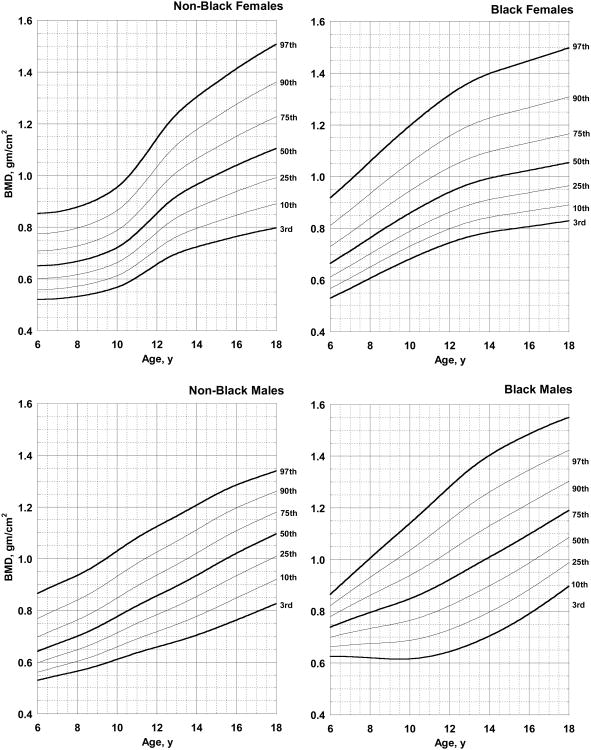
Figure 2.
Reference curves for areal bone mineral density for Region 1. The reference curves are based on 244 non-black females, 183 black females, 212 non-black males and 182 black males.
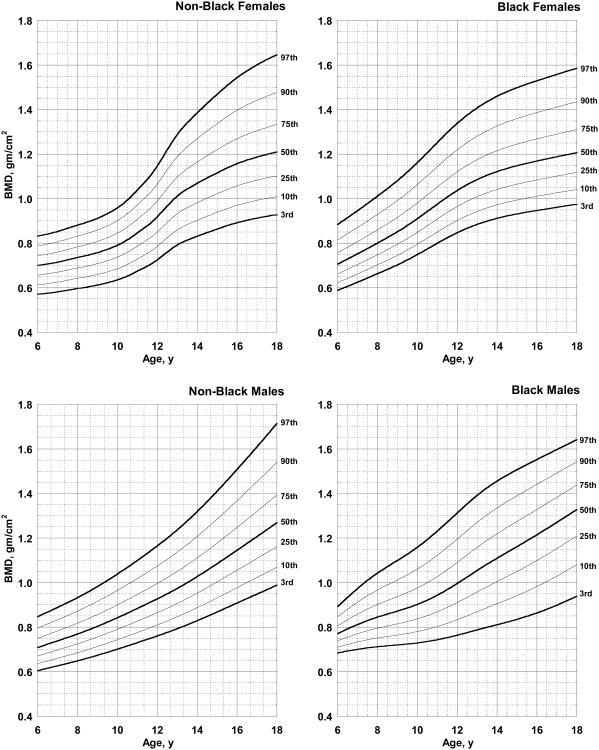
Figure 4.
Reference curves for areal bone mineral density for Region 3. The reference curves are based on 184 non-black females, 146 black females, 157 non-black males and 138 black males. The curve for black males is restricted to ages 6 to 16 because there was insufficient representation of subjects in this age range.
DXA spine and whole body scans were available for 838 subjects, and pQCT scans were available for 566 subjects at the 3% site and 610 subjects at the 38% site. Since values for these measures increased with age and body size in a non-linear fashion, Z-scores were computed for all measures to account for these expected changes. The mean ± sd was 0±1 for each of these Z-scores, e.g., aBMD of the spine and whole body and pQCT measures of vBMD and bone strength, as expected. Correlations among Z-scores for growth/body size (height, weight, BMI), aBMD and vBMD and strength measures are shown in Table 5. These correlation coefficients illustrate that lateral distal femur aBMD Z-scores were significantly associated with other DXA measures of aBMD recommended for clinical assessment in children12. The lateral distal femur Z-scores, like other aBMD Z-scores, were positively and significantly associated with growth (height, weight and BMI) Z-scores indicating that children who were large for their age had greater aBMC and aBMD for their age. Also of note, lateral distal femur Z-scores, like the spine and whole body Z-scores, correlated well with pQCT measures of vBMD and bone strength, especially the measures of trabecular and total vBMD.
Table 5. Correlations Among Z-Scores*.
| Region 1 aBMD | Region 2 aBMD | Spine aBMD | WB aBMC | |
|---|---|---|---|---|
| Region 2 aBMD | 0.81 | 1.00 | 0.63 | 0.68 |
| Region 3 aBMD | 0.72 | 0.91 | 0.58 | 0.65 |
|
| ||||
| Height | 0.26 | 0.26 | 0.32 | 0.60 |
| Weight | 0.54 | 0.48 | 0.44 | 0.64 |
| BMI | 0.50 | 0.44 | 0.36 | 0.45 |
|
| ||||
| Spine aBMC | 0.55 | 0.61 | 0.84 | 0.85 |
| Spine aBMD | 0.61 | 0.63 | 1.00 | 0.78 |
| WB aBMC | 0.66 | 0.68 | 0.78 | 1.00 |
| WB aBMD | 0.68 | 0.72 | 0.77 | 0.87 |
|
| ||||
| Trabecular vBMD | 0.61 | 0.64 | 0.45 | 0.46 |
| Total vBMD | 0.57 | 0.63 | 0.46 | 0.42 |
| Cortical Thickness | 0.44 | 0.47 | 0.40 | 0.31 |
| Section Modulus | 0.39 | 0.36 | 0.35 | 0.37 |
| Strain Strength Index | 0.36 | 0.34 | 0.35 | 0.37 |
Note: all correlations are statistically significant p<0.0001
Discussion
For many children with disabilities and at-risk for the co-morbidity of low BMD, traditional assessment techniques such as spine or whole body imaging by DXA are often not an option due to deformity, indwelling hardware or difficulties with positioning. In addition, lower limb fractures are particularly common among children with conditions affecting mobility, such as cerebral palsy and Duchenne muscular dystrophy 4,5, so the distal femur is of significant clinical interest. Distal femur scans offer an excellent alternative for most of these children. Previously published reference data for the lateral distal femur were based on a smaller sample of 256 healthy children, primarily Caucasian 11. These scans were obtained on older technology, the Hologic (QDR1000 and 2000 models) scanners in pencil-beam mode. The current generation of fan-beam technology DXA instruments offer more rapid scan times; a factor that is critical in the success of obtaining interpretable scans in children, especially those with significant physical or cognitive disabilities. In addition, changes in DXA instruments and software are known to have a significant impact on aBMD results in children because of their smaller bone size and soft tissue distribution 28,29. The results of this study demonstrated that the distribution of lateral distal femur aBMD in this large sample of healthy children measured in the latest generation of Hologic software and hardware in fan-beam mode differed from the previously published data. Thus, when selecting reference data for the evaluation of measurements in children, it is important to consider the hardware and software versions used12.
Optimally, pediatric reference data should be based on a sample of healthy children that is sufficiently large to characterize the age and sex related variability in the outcome measure, and on statistical techniques that accurately characterize the variability in the measure. Racial differences in aBMD during childhood add further complexity to the development of reference data. For most BMD measures, the age-related changes are non-linear and the variability increases with age. The reference ranges presented here were based on a total of 821 children, with at least 180 children in each sex and race group. The statistical technique used to generate the reference ranges was the same as that used for the creation of the CDC growth charts23 and the U.S. reference data for aBMD26. This approach enables calculation of exact centiles and Z-scores using equations 1 and 2 (above) and the L, M, and S values from Table 4. A possible limitation of the reference data presented is that all subjects were evaluated at a single geographical location drawing on subjects from urban and suburban communities. Potential regional and device-specific variation in lateral distal femur aBMD could not be assessed. In addition, because of limitations in the length of the scan field relative to the size of the femur, not all regions of interest could be measured in all subjects.
Separate reference curves were presented for Black vs. Non-Black children. Racial differences in BMD are widely recognized in adults and children 30-34. However, the clinical application of race-specific reference curves should be carefully considered. The International Society for Clinical Densitometry recommends the use of reference curves based on Caucasian samples for adults for all race and ethnic groups 35. For children, the use of race-specific curves is recommended12 since they may be useful in identifying children who are not attaining their genetic potential for bone mineral accrual. However, the use of race-specific reference curves for determination of fracture risk in children is unknown and requires further investigation.
Z-scores for lateral distal femur aBMD were compared to Z-scores for growth, traditional DXA measures of aBMD, and pQCT measures of vBMD and bone strength. While none of these comparisons attest to the accuracy of lateral distal femur aBMD measurements since they are measuring different aspects of skeletal and somatic growth and development, they do provide important insights. First, the lateral distal femur aBMD Z-scores were significantly associated with body size measures, as were other aBMD Z-scores. In other words, children who were large for their age also had higher aBMD for their age. Of note, the association between height Z-score and lateral distal femur aBMD Z-score was somewhat lower than the corresponding associations between height Z-score and spine and whole body aBMD Z-score. This is likely due to the technique used in the analysis of the lateral distal femur image, in which bone size is taken into account in defining the region of interest. This is not done in spine or whole body DXA measurements. Since many children at-risk for low BMD also have growth failure, it is an important consideration that this measure of aBMD status is less influenced by height status. It is also noteworthy that the correlations between lateral distal femur aBMD and weight and BMI Z-score were higher than for other aBMD measures. One possible explanation is that the distribution of weight-bearing forces are more concentrated on the lateral distal femur than on the spine or whole body, so the strength of this association may reflect the impact of weight-bearing physical activity on BMD of the femur.
Lateral distal femur aBMD Z-scores were also strongly associated with Z-scores of distal tibia total and trabecular vBMD, and mid-shaft cortical thickness, section modulus and SSI obtained by pQCT. These pQCT measures offer more detail than DXA various aspects of bone strength (trabecular bone at the ultradistal 3% site and cortical bone in the mid-shaft at the 38% site) and therefore provide further evidence of the utility and validity of the lateral distal femur scan to characterize bone strength. The use of Z-scores in the analysis has the advantage of removing the age or size effects associate with the unadjusted bone measurements. Z-scores are an indicator of status relative to peers of the same age and sex. The correlations among Z-scores demonstrated, for example, the degree to which a healthy child who has a high lateral distal femur aBMD for age will also have a high trabecular vBMD relative to same age peers. The correlation results also demonstrated that the lateral distal femur aBMD Z-scores performed as well or better than spine and whole body Z-scores in relation to pQCT measures of trabecular and cortical bone in healthy children.
Unlike DXA measures of aBMD, pQCT vBMD Z-scores were not influenced by height status 36. Although these pQCT measures were obtained on the tibia rather than the femur (due to the configuration of the pQCT device), the significant associations between the lateral distal femur aBMD Z-scores and pQCT outcome measures indicates the degree to which lateral distal femur DXA aBMD measurements are generalizable to other measures obtained with a method that is strongly predictive of fracture risk in experimental situations37,38.
In summary, reference curves for lateral distal femur aBMD measurements to be used for clinical care are presented based on a large multi-ethnic sample of healthy children. This technique provides an excellent opportunity for bone health assessment in children for whom spine or whole body measurements are not feasible. A critical issue for any measure of bone health is the relationship to fracture. Futures studies are needed to evaluate the ability of lateral distal femur aBMD measurements to predict fracture, especially among children for whom traditional DXA measurement sites are not feasible.
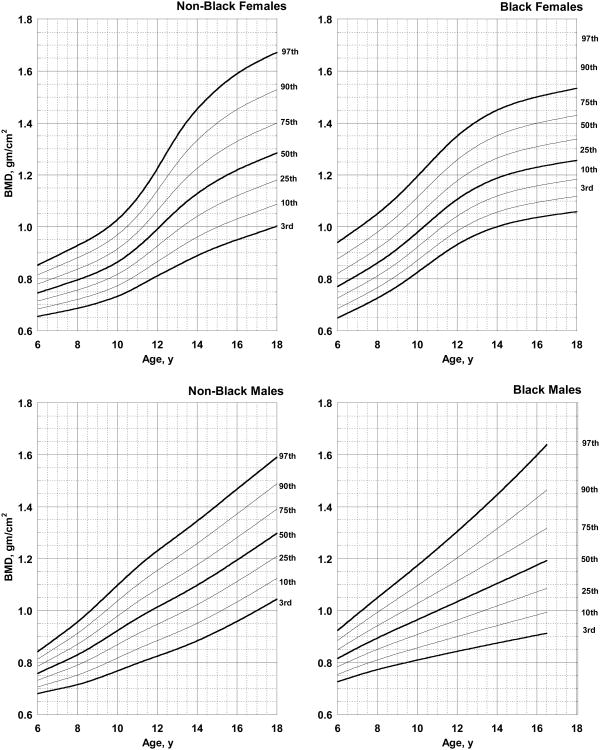
Figure 3.
Reference curves for areal bone mineral density for Region 2. The reference curves are based on 244 non-black females, 183 black females, 211 non-black males and 180 black males.
Acknowledgments
This paper would not have been possible without the generous contribution of the children and their families who participated in the Reference Project on Skeletal Development in Childhood, the support of the Joseph Stokes, Jr. Research Institute of The Children's Hospital of Philadelphia, and the Clinical and Translational Research Center of The Children's Hospital of Philadelphia/University of Pennsylvania (UL1 -RR024134). The authors also thank the contribution of the following research staff who contributed to this project: Susan Kaup, Adriana Natolli, Chantal Dilzer, Margarita Gomelsky, Samantha Ferrante, Gail Jackson and Heather Mitchell.
Footnotes
pQCT analysis parameters used: contour mode 1, peel mode 1, inner threshold=200, outer threshold=600.
pQCT analysis parameters used: contour mode 1, peel mode 2, cortmode 2, threshold = 711.For the SSI measure only, the threshold was set to 300.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. Journal of Pediatric Orthopedics. 2000;20:71–4. [PubMed] [Google Scholar]
- 2.Henderson RC, Kairalla J, Abbas A, Stevenson RD. Predicting low bone density in children and young adults with quadriplegic cerebral palsy. Developmental Medicine and Child Neurology. 2004;46:416–9. doi: 10.1017/s0012162204000672. [DOI] [PubMed] [Google Scholar]
- 3.Quan A, Adams R, Ekmark E, Baum M. Bone mineral density in children with myelomeningocele. Pediatrics. 1998;102:E34. doi: 10.1542/peds.102.3.e34. [DOI] [PubMed] [Google Scholar]
- 4.McDonald DG, Kinali M, Gallagher AC, Mercuri E, Muntoni F, Roper H, Jardine P, Jones DH, Pike MG. Fracture prevalence in Duchenne muscular dystrophy. Dev Med Child Neurol. 2002;44:695–8. doi: 10.1017/s0012162201002778. [DOI] [PubMed] [Google Scholar]
- 5.Presedo A, Dabney KW, Miller F. Fractures in patients with cerebral palsy. J Pediatr Orthop. 2007;27:147–53. doi: 10.1097/BPO.0b013e3180317403. [DOI] [PubMed] [Google Scholar]
- 6.Henderson RC. Bone density and other possible predictors of fracture risk in children and adolescents with spastic quadriplegia. Dev Med Child Neurol. 1997;39:224–7. doi: 10.1111/j.1469-8749.1997.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 7.Henderson RC. The correlation between dual-energy X-ray absorptiometry measures of bone density in the proximal femur and lumbar spine of children. Skeletal Radiol. 1997;26:544–7. doi: 10.1007/s002560050283. [DOI] [PubMed] [Google Scholar]
- 8.McIvor WC, Samilson RL. Fractures in patients with cerebral palsy. Journal of Bone and Joint Surgery. 1966;48:858–66. [PubMed] [Google Scholar]
- 9.Leet AI, Mesfin A, Pichard C, Launay F, Brintzenhofeszoc K, Levey EB, DS P. Fractures in children with cerebral palsy. Journal of Pediatric Orthopedics. 2006;26:624–7. doi: 10.1097/01.bpo.0000235228.45539.c7. [DOI] [PubMed] [Google Scholar]
- 10.Harcke HT, Taylor A, Bachrach S, Miller F, Henderson RC. Lateral femoral scan: an alternative method for assessing bone mineral density in children with cerebral palsy. Pediatric Radiology. 1998;28:241–6. doi: 10.1007/s002470050341. [DOI] [PubMed] [Google Scholar]
- 11.Henderson RC, Lark RK, Newman JE, Kecskemthy H, Fung EB, Renner JB, Harcke HT. Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur. AJR American Journal of Roentgenology. 2002;178:439–43. doi: 10.2214/ajr.178.2.1780439. [DOI] [PubMed] [Google Scholar]
- 12.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, Moyer-Mileur L, Shepherd J, Specker B, Ward K, et al. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Jones IE, Williams SM, Dow N, Goulding A. How many children remain fracture-free during growth? a longitudinal study of children and adolescents participating in the Dunedin Multidisciplinary Health and Development Study. Osteoporos Int. 2002;13:990–5. doi: 10.1007/s001980200137. [DOI] [PubMed] [Google Scholar]
- 15.Rovner AJ, Zemel BS, Leonard MB, Schall JI, Stallings VA. Mild to moderate cystic fibrosis is not associated with increased fracture risk in children and adolescents. J Pediatr. 2005;147:327–31. doi: 10.1016/j.jpeds.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Lohman T, Roche AF, Martorell R. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 17.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth and Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 18.Schall JI, Semeao EJ, Stallings VA, Zemel BS. Self-assessment of sexual maturity status in children with Crohn's disease. Journal of Pediatrics. 2002;141:223–9. doi: 10.1067/mpd.2002.125907. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM. Growth at Adolescence. Blackwell Scientific Publication; Oxford: 1962. [Google Scholar]
- 20.Shepherd J, Fan B, Sherman M, Gilsanz V, Horlick M, Kalkwarf H, Lappe J, Mahboubi S, Zemel B, Fredrick M, et al. Pediatric DXA precision varies with age. Abstract presented at the American Society of Bone Mineral Research Meetings; Seattle, WA. 2004. [Google Scholar]
- 21.Henderson RC, Lark RK, Renner JB, Fung EB, Stallings VA, Conaway M, Stevenson RD. Dual X-ray absorptiometry assessment of body composition in children with altered body posture. J Clin Densitom. 2001;4:325–35. doi: 10.1385/jcd:4:4:325. [DOI] [PubMed] [Google Scholar]
- 22.Zemel BS, Paulhamus D, Dilzer C, Stallings VA, Shabbout M, Leonard MB. Precision of peripheral quantitative computed tomography measures of the tibia in children (Abstract) Presented at the American Society for Bone Mineral Research. 2004 S232. [Google Scholar]
- 23.Kuczmarski R, Ogden CL, Grummer-Strawn LM. Advance Data from Vital and Health Statistics. National Center for Health Statistics; Jun 8, 2000. CDC Growth Charts - United States. [Google Scholar]
- 24.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Statistics in Medicine. 1992;11:1305–19. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 25.Cole TJ, Pan HLMS Chartmaker Pro. A program for calculating age-related reference centiles using the LMS method. 2.3. Child Growth Foundation; 2006. [Google Scholar]
- 26.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, et al. The Bone Mineral Density in Childhood Study (BMDCS): Bone Mineral Content and Density According to Age, Sex and Race. Journal of Clinical Endocrinology and Metabolism. 2007 doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 28.Zemel B, Petit M. In: Evaluation in Bone Densitometry in Growing Patients. Sawyer A, Barchrach L, Fung E, editors. Humana Press; Totowa, NJ: 2007. pp. 115–126. [Google Scholar]
- 29.Shypailo RJ, Ellis KJ. Bone assessment in children: comparison of fan-beam DXA analysis. J Clin Densitom. 2005;8:445–53. doi: 10.1385/jcd:8:4:445. [DOI] [PubMed] [Google Scholar]
- 30.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. Journal of Clinical Endocrinology and Metabolism. 1999;84:4702–12. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 31.Gilsanz V, Roe TF, Mora S, Costin G, Goodman WG. Changes in vertebral bone density in black girls and white girls during childhood and puberty. New England Journal of Medicine. 1991;325:1597–600. doi: 10.1056/NEJM199112053252302. [DOI] [PubMed] [Google Scholar]
- 32.Gilsanz V, Skaggs DL, Kovanlikaya A, Sayre J, Loro ML, Kaufman F, Korenman SG. Differential effect of race on the axial and appendicular skeletons of children. Journal of Clinical Endocrinology and Metabolism. 1998;83:1420–7. doi: 10.1210/jcem.83.5.4765. [DOI] [PubMed] [Google Scholar]
- 33.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporosis International. 1998;8:468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 34.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay RL. Proximal femur bone mineral levels of US adults. Osteoporosis International. 1995;5:389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 35.Leib ES, Lewiecki EM, Binkley N, Hamdy RC. Official positions of the International Society for Clinical Densitometry. J Clin Densitom. 2004;7:1–6. doi: 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 36.Leonard MB, Zemel BS. Current concepts in pediatric bone disease. Pediatric Clinics of North America. 2002;49:143–73. doi: 10.1016/s0031-3955(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 37.Ferretti JL, Capozza RF, Zanchetta JR. Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone. 1996;18:97–102. doi: 10.1016/8756-3282(95)00438-6. [DOI] [PubMed] [Google Scholar]
- 38.Muller ME, Webber CE, Bouxsein ML. Predicting the failure load of the distal radius. Osteoporos Int. 2003;14:345–52. doi: 10.1007/s00198-003-1380-9. [DOI] [PubMed] [Google Scholar]