Abstract
Background
Statins substantially reduce cardiovascular mortality and appear to have beneficial effects independent of their lipid lowering properties. We evaluated the hypothesis that statin use may modulate the secretion of aldosterone, a well-known contributor to cardiovascular disease.
Methods and Results
We measured adrenal hormones in two intervention studies. In study 1 in hypertensive subjects, aldosterone was analyzed at baseline and after angiotensin-II stimulation (AngII) on both high (HS) and low sodium (LS) diets (1122 observations, 15% on statins > 3 months). Statin users had 33% lower aldosterone levels in adjusted models (p < 0.001). Cortisol was not modified by statins. In secondary analyses, the lowest aldosterone levels were seen with lipophilic statins and with higher doses. Statin users had lower blood pressure (BP) and reduced salt sensitivity of BP (p=0.001). In study 2, aldosterone was measured in diabetic patients on a HS diet, before and after AngII stimulation (143 observations, 79% statin users). Again, statin users had 26% lower aldosterone levels (p =0.006), particularly those using lipophilic statins. Ex vivo studies in rat adrenal glomerulosa cells confirmed that lipophilic statins acutely inhibited aldosterone, but not corticosterone, in response to different secretagogues.
Conclusions
Statin use among hypertensive and diabetic subjects was associated with lower aldosterone secretion in response to AngII and LS diet in two human intervention studies. This effect appeared to be most pronounced with lipophilic statins and higher doses. Future studies to evaluate whether aldosterone inhibition may partially explain the robust cardioprotective effects of statins are warranted.
Keywords: statin, aldosterone, cortisol, salt sensitive hypertension, adrenal hormones, sodium
INTRODUCTION
Statins are an established first line treatment for hypercholesterolemia and the most widely prescribed class of drug in the world, with more than 20 million users among the US population1. Randomized trials provide strong evidence that statins reduces the incidence of major coronary events, ischemic stroke and mortality, even in subjects without established cardiovascular disease2,3. Recent studies provide evidence that some of the clinical benefits of statins may be related to mechanisms independent of their lipid lowering effects that could be specific for certain statins 4. Supporting the concept of pleiotropic actions are the described positive cardiovascular outcomes observed with acute, short-term statin treatment before coronary procedures or early treatment in acute coronary syndromes 5,6. Also, in a recent randomized trial, atorvastatin (a lipophilic statin) outperformed rosuvastatin (hydrophilic statin) in renoprotective effects in diabetic subjects despite lower lipid concentrations in the latter arm 7. The so called pleiotropic effects of statins involve plaque stabilization, myocardial remodeling and immunomodulation8. The clinical translation of these effects is well documented by high-quality studies showing that statins improve endothelial and cardiac function9, prevent atrial fibrillation10, deep vein thrombosis11 and microalbuminuria7 , decrease blood pressure (BP)12 and reduce inflammation13. Interestingly, these statin effects are not consistently observed with low cholesterol diets14, nor with other lipid lowering medications such as niacin, ezetimibe, CETP inhibitors or fibrates11,15,16. To date the multifactorial mechanisms involved in statin’s antithrombotic, antiinflammatory and cardioprotective effects have not been fully elucidated and the hypothetical pleiotropic effect of statins are still a matter of debate.
In addition to dyslipidemia, there is strong evidence supporting a role for renin-angiotensin-aldosterone system (RAAS) dysregulation and mineralocorticoid receptor (MR) activation in the pathogenesis of atherosclerosis, coronary disease, atrial fibrillation and heart failure. These effects are in addition to the well-known role of aldosterone signaling on salt sensitivity of BP and hypertension17, 18. For instance, in patients with coronary disease, aldosterone levels predict ischemic events and long-term mortality19. Consistently, lower aldosterone levels predict better cardiovascular health in a non-high risk community study such as the Framingham cohort 20. Further, several trials have shown that the inhibition of RAAS - particularly MR blockade- has remarkable similarities with the described pleiotropic effects of statins, specifically related to improvements in atherosclerotic plaque, cardiovascular remodeling, BP and endothelial dysfunction21. Despite these clinical similarities, there is insufficient information related to a potential aldosterone inhibitory effect of statins.
In the present study, our primary aim was to evaluate the hypothesis that chronic statin use is associated with lower aldosterone levels. Further, using ex vivo experiments in adrenal zona glomerulosa (ZG) cells we provide complementary information demonstrating a novel role of statins in modulating aldosterone secretion.
MATERIALS AND METHODS
A. Human studies
1) Study 1: Discovery Cohort
Participants were studied within the HyperPATH Protocol, consisting of individuals with mild to moderate hypertension (HTN) evaluated in response to sodium intake and adrenal secretagogues. The protocol includes rigorous control of several factors that influence RAAS, incorporating antihypertensive medication washout, body positioning and diurnal variation under strictly controlled diets.
We excluded in this study participants with known or suspected secondary HTN such as primary hyperaldosteronism, Cushing syndrome or renovascular HTN. Participants with coronary disease, stroke, psychiatric illness, drug abuse and severe HTN were also excluded as previously described22. Users of other non-statin medication for dyslipidemia were excluded. Also, to avoid confounding by indication, we excluded in study 1 all diabetic subjects. Each institutional review board approved the protocol and informed consent was obtained before enrollment.
Chronic statin use was considered if participants were on a statin for at least three months prior to the study interventions. Since, lipophilic statins are taken up by many tissues, including adrenal cells 23, we determined whether lipophilicity influenced adrenal secretion by classifying subjects in three groups: no statin use, low-moderate lipophilic statin (atorvastatin, fluvastatin, lovastatin) or high lipophilic statin (simvastatin) 23, 24. Hydrophilic statins were excluded in this categorization because of the small sample size in study 1. To explore a dose-dependent effect, statins users were classified according to their LDL reduction capacity 25 (See Supplemental Material for expanded methods section ).
Human Protocol
Details of this protocol have been published previously22,26,27. For the run in phase, all recruited subjects completed a screening visit. To control for the influence that medications may play in aldosterone secretion, all angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB) or mineralocorticoid receptor antagonists (MRA) were discontinued 1 month and all other anti-hypertensive medications were discontinued at least 2 weeks prior the start of the study. Only if necessary, subjects were placed on amlodipine for BP control due to the neutral effect on aldosterone.
During the intervention phase, each subject was provided with a caffeine-alcohol-free diet containing 100-mEq/day potassium, 1000 mg/day calcium, and 200-mEq/day sodium. On the sixth day of this high salt (HS) diet, participants were admitted to an inpatient research unit. Blood samples were obtained at 0800 h to measure aldosterone, cortisol, plasma renin activity (PRA), electrolytes, lipid profile, glucose and insulin using standardized and validated methods as previously described (HS baseline, intervention 1)22,26,27. To examine the adrenal response of aldosterone to the physiologic secretagogue, Angiotensin II infusion (AngII, Bachem AG, Switzerland) was then administered (3 ng/kg per min for 60 min, HS stimulation, intervention 2). After completion of 5–7 days on HS, the same subject was placed on the same diet with reduced sodium to 10 mEq/ day (low sodium (LS) diet 10 mEq/ day) in a crossover intervention (LS baseline, intervention 3), that also increases aldosterone secretion. After 1 week of LS diet we measured adrenal steroids after AngII infusion (LS stimulation, intervention 4). Sodium balance was confirmed by urinary sodium (HS > 150 mEq/24h and LS < 30 mEq/24h). Also, 24h urinary aldosterone and cortisol were collected as an estimation of adrenal daily production. The protocols were standardized across all study sites (Boston, Salt Lake City, Paris).
Analysis of blood pressure and other hemodynamic measures
BP was examined using systolic arterial pressure in repeated measure analysis. Also, we analyzed the effect of statins on salt sensitivity of BP by comparing the difference between baseline HS and LS measurements. We calculated pulse pressure (PP, systolic BP minus diastolic BP) as a surrogate marker of peripheral vascular compliance on HS and LS diets.
2) Study 2: Replication cohort
Enrolled subjects were part of a randomized trial evaluating the role of MRA in diabetic cardiovascular disease (ClinicalTrials.gov NCT00865124). We included these participants, prior spironolactone randomization, as they were evaluated using the same protocol regarding sodium and AngII interventions.
Description of this protocol has been published previously 28. The main differences compared to protocol in Study 1 is that all subjects had well-controlled type 2 diabetes and were on an ACE inhibitor. At the start of the 3-month run-in phase, subjects started enalapril 20 mg/day, other anti-hypertensive medications were tapered off, and amlodipine 5–10 mg/day was added if needed. Simvastatin was started if the subject was not on a statin and LDL was >100 mg/dl at the screening visit. In the context of the run-in-phase of a randomized trial, all subjects were evaluated with the same criteria for starting statin therapy, avoiding a selection bias. Diabetic medications were adjusted to target HbA1c level ≤ 7%.
Study participants received the same controlled HS diet described in Study 1 five days before admission to an inpatient unit. Baseline adrenal steroids measurements (HS baseline, intervention 1), and after AngII infusion were performed (HS stimulation, intervention 2) as described in Study 1. Subjects were categorized as no statin use, hydrophilic (pravastatin and rosuvastatin) or lipophilic statin use (atorvastatin, fluvastatin, lovastatin and simvastatin)24. Statins were again classified according to their LDL reduction capacity, including now rosuvastatin users 25.
Laboratory analysis
Brigham and Women’s Hospital served as the central laboratory for all lab processing and all assays used have been extensively reported previously 22,26,27. All subjects had PRA, aldosterone and cortisol measured in triplicate in each of the intervention points.
B. Animal protocol and in vitro studies
Ex vivo adrenal studies were designed to determine whether statins would acutely modulate aldosterone production. Adrenals were isolated from male Wistar rats maintained on HS (Na=1.6%) for 1 week. ZG cells were isolated from adrenal tissues as previously described by carefully dissecting the capsular (glomerulosa) portion 29. In brief, ZG cells were incubated for digestion at 37°C for 50 minutes in modified Krebs-Ringer bicarbonate solution, centrifuged for 10 minutes at 4°C, washed and re-suspended for another 30 minutes at 37°C. Final cell suspension with pellet dilution obtained 1 to 2×105-isolated cells/0.5ml with a potassium content of 3.7 mM. Cells were incubated in duplicate, measuring basal and stimulated aldosterone in the presence of AngII (10−7 M, N = 20 experiments) and Potassium (4.7 mM, N = 6 experiments). Samples were pre-incubated for 15 min with one of three different statins, and: pravastatin (10−5 M=10 uM), atorvastatin (10−5 M) obtained from Sigma and hydroxy acid simvastatin (active metabolite of simvastatin, 10−5 M, Santa Cruz Biotechnology). To the best of our knowledge this is the first study exploring the effect of statins on aldosterone secretion in adrenal cells ex-vivo. Thus, the statin concentrations used in our studies were based on those concentrations used in previously published in vitro studies in other tissues 30,31 and on our initial observation that different statin doses had similar effects on aldosterone production, thus we chose and intermediate dose. Of note, this ex-vivo protocol was an exploratory study to help understand the potential mechanism of our human results and was not intended to mimic usual plasma levels of AngII or statins levels achieved in humans.
Aldosterone was measured at baseline and 1 hour after stimulation using RIA kit (SIEMENS, Los Angeles, CA) as described by our group 29. Data were normalized to the number of cells in each incubate and reported as percentage of change. Zona fasciculata (ZF) cells were isolated following the same protocol measuring basal and stimulated corticosterone in the presence of ACTH (10−12 M) 32. The Animal Care Committee at Harvard approved all of the experimental procedures.
Statistical analyses
Baseline analyses included Student’s t test and Chi-square; continuous variables are presented as mean +/− standard deviation (SD) and categorical variables as percentage of the total sample. Due to the unique characteristic of our protocol where several measurements are available in the same subject in four different intervention settings, we performed a mixed model linear regression analysis, thus allowing adjustment for confounders and addressing the effect of statin within and between subjects. Two models were tested and these covariates (fixed effects) were chosen for their clinical importance and significance in univariate analysis whereas identity was the random effect (See Supplemental Material). In study 2, similar repeated measure analyses were performed (See Supplemental Material). P values ≤ 0.05 were considered statistically significant. All human analyses were performed using STATA 13. Animal data were analyzed using one-way ANOVA, followed by Tukey’s post hoc analysis, using the GraphPad Prism 6 software.
RESULTS
1) Study 1: Adrenal secretion in hypertensive subjects by statin use
Characteristics of hypertensive participants
After applying the inclusion-exclusion criteria, a total of 1122 aldosterone measurements were available from the HyperPATH cohort. These measurements represent available repeated analyses from 317 subjects at 4 different intervention points (26% on HS baseline, 18% HS AngII stimulation, 28% LS baseline and 28% LS AngII stimulation). 175 out of the 1122-aldosterone measurements (15.6 %) were obtained in individuals with confirmed chronic statin use. The proportions of patients on the different statins were simvastatin 43%, atorvastatin 23%, lovastatin 19%, pravastatin 9% and fluvastatin 6%. When categorized by type of treatment, 84.8% of the observations were in individuals without statin treatment, and 8.3% in those on a low-moderate lipophilic statin (atorvastatin, fluvastatin or lovastatin) and a 6.9% on a high lipophilic statin treatment (simvastatin). When categorized by dose, an 84.% of aldosterone measurements where categorized as no statin use, 7.8% a low statin dose and a 7.7% as moderate/high statin dose.
Subjects on statins were similar to those without statin treatment regarding gender, race, sodium intake, potassium and PRA on HS and LS diet (Table 1). Subjects using statins were slightly older and heavier thus age and BMI were included as covariates in the adjusted models. When compared to participants with no treatment, statin users had significantly lower values of unadjusted baseline aldosterone, AngII-stimulated aldosterone and 24h urinary aldosterone on a HS diet (all p <0.001). While on LS diet the effect of statins resembled the effect seen on a HS diet (Table 2, Supplemental Figure). Aldosterone to renin ratio was not different when analyzed by statin use on both HS and LS diet. Of note, cortisol levels had no significant changes when comparing by statin use groups in all measurements (baseline, stimulated, and 24h urinary collection on both diets (Table 2).
Table 1.
Baseline characteristics of hypertensive subjects categorized by statin use on high and low sodium diets.
| n=317 | No Statin Group (85%) | Statin Group (15%) | p value |
|---|---|---|---|
| Age (years) | 49.1 ± 7.1 | 53.9 ± 6.6 | 0.001 |
| Female (%) | 43.5 | 46.3 | 0.76 |
| Body Mass Index (kg/m2) | 28.6 ± 4.4 | 30.8 ± 4.2 | 0.001 |
| Systolic Blood Pressure on HS | 146.6 ± 18.6 | 141.0 ± 18.4 | 0.07 |
| Systolic Blood Pressure on LS | 131.4 ± 16.1 | 127.9 ± 14.4 | 0.16 |
| Urinary sodium on HS* diet (24h mmol) | 232.9 ± 68.4 | 228.6 ± 70.2 | 0.70 |
| Urinary sodium on LS* diet (24h mmol) | 13.9 ± 7.6 | 14.7 ± 7.8 | 0.54 |
| HS plasma potassium (mmol/L) | 4.15 ± 0.3 | 4.14 ± 0.3 | 0.87 |
| LS plasma potassium (mmol/L) | 4.17 ± 0.3 | 4.12 ± 0.3 | 0.32 |
| HS Plasma renin activity (ng/mL*h) | 0.51 ± 0.4 | 0.35 ± 0.3 | 0.18 |
| LS Plasma renin activity (ng/mL*h) | 2.49 ± 2.8 | 1.91 ± 1.7 | 0.34 |
| HS LDL Cholesterol mmol/L | 3.21 ± 0.9 | 3.16 ± 1.1 | 0.80 |
Data reported as mean ± SD, except as noted. HS indicates high sodium; LS, low sodium
Table 2.
Unadjusted effects of statin use on aldosterone and cortisol levels on sodium diet and angiotensin II interventions.
| Hormonal measurements 317 subjects | Control group on HS* (85%) | Statin Group on HS* (15%) | p value | Control group on LS* (85%) | Statin Group on LS* (15%) | p value |
|---|---|---|---|---|---|---|
| Baseline Aldosterone nmol//L (ng/mL) | 0.14 ± 0.09 (5.0 ± 3.3) | 0.09 ± 0.04 (3.6 ± 1.5) | 0.008 | 0.47 ± 0.27 (17.3 ± 9.9) | 0.29 ± 0.16 (10.5 ± 5.9) | <0.001 |
| Baseline Cortisol nmol/L (ug/dL ) | 278.7 ± 113.1 (10.1 ± 4.1) | 289.7 ± 93.8 (10.5 ± 3.4) | 0.69 | 314.5 ± 93.4 (11.4 ± 3.8) | 10.1 ± 4.1 11.1 ± 3.1 | 0.70 |
| Stimulated Aldo nmol//L (ng/mL) | 0.39 ± 0.21 (13.9 ± 7.7) | 0.30 ± 0.13 (11.0 ± 4.8) | 0.003 | 1.08 ± 0.54 (39.0 ± 19.5) | 0.70 ± 0.33 (25.3 ± 11.9) | <0.001 |
| Stimulated Cortisol nmol/L (ug/dL ) | 259.3 ± 124.2 (9.4 ± 4.5) | 251.1 ± 91.0 (9.1 ± 3.3) | 0.74 | 262,1 ± 113.1 (9.5 ± 3.2) | 223.8 ± 66.2 (9.10 ± 2.4) | 0.26 |
| Urinary Aldosterone ug/24h | 11.7 ± 7.8 | 8.3 ± 4.6 | <0.001 | 38.4 ± 23.3 | 27.0 ± 13.7 | <0.001 |
| Urinary Cortisol ug/24h | 68.5 ± 32.2 | 66.2 ± 48.3 | 0.69 | 49.4 ± 24.8 | 50.0 ± 34.5 | 0.90 |
Data reported as mean ± SD, except as noted. HS indicates high sodium; LS, low sodium
Repeated measures analyses in hypertensive patients by statin use
As a formal test of our hypothesis we examined the effect of statin use on aldosterone levels in repeated measures analysis using all four measurements of aldosterone while adjusting for potential confounders. Statins users had lower aldosterone levels in repeated measures analysis, with a predicted decrease by 33% in model 1 and by 32% in model 2 (both p <0.001) (Table 3). We also confirmed that the aldosterone levels were significantly decreased by statins when categorizing by HS (baseline and AngII stimulated, a decrease of 18% in both models) or LS (baseline and AngII stimulated, a decrease of 35%) with a higher effect on LS (Table 3). Consistently, we found a significant interaction for the effect of statin use by dietary sodium type, with greater aldosterone differences while on LS, both at baseline (p=0.02 for interaction) and after AngII stimulation (p< 0.001 for the interaction). In contrast, cortisol levels were not affected by statin use. Further, we observed that available LDL levels or systolic BP did not modify the effect of statins on aldosterone when included in the model as a sensitivity analysis. No interaction between statin use on aldosterone with age, BMI, gender or race was observed.
Table 3.
Effect of statins on aldosterone and cortisol levels in repeated measures analysis adjusted by confounders in hypertensive subjects.
| Adrenal hormones 1122 observations |
Model 1* | Model 2† | ||
|---|---|---|---|---|
| Statin effect:β (Predicted change) | p value | Statin effect:β (Predicted change) | p value | |
| Aldosterone ng/dl (HSb, HSstim, LSb, LSstim)‡ | −4.61 (↓33%) | p <0.001 | −4.29 (↓32%) | p <0.001 |
| Cortisol ug/dL (HSb, HSstim, LSb, LSstim)‡ | −0.23 | p =0.667 | −1.64 | p =0.763 |
| Aldosterone ng/dl on HS (b,stim)§ | −1.72 (↓18%) | p <0.001 | −1.53 (↓18%) | p <0.001 |
| Aldosterone ng/dl on LS b,stim) || | −6.67 (↓35%) | p <0.001 | −6.17 (↓34%) | p <0.001 |
Mixed Model 1: adjusted by age, BMI, gender and urinary sodium.
Mixed Model 2: covariates in model 1 plus race, site, and sodium diet order
High sodium (HS) baseline (b), HS Angiotensin II stimulation (stim), Low sodium (LS) b, LS stim (1122 observations)
High sodium baseline (b), HS Angiotensin II stimulation (stim) (494 observations)
Low sodium baseline (b), LS Angiotensin II stimulation (stim) (628 observations)
Non-invasive hemodynamic measurements by statin use
Use of statins was significantly associated with a decrease in systolic BP in repeated measure analysis (BP at baseline and after AngII on both HS and LS, combined effect of 5.57 mmHg, p=0.001) even when adjusted by confounders, and when analyzed on each diet. Further, statin users had reduced salt sensitivity of BP (systolic HS – systolic LS, β −5.85 mmHg, p<0.001, Table 4). Also, pulse pressure was significantly decreased in statin users when combining the four intervention measurements (β −2.95 mmHg, p=0.004, Table 4).
Table 4.
Effect of statin use on systolic blood pressure, salt sensitivity and pulse pressure.
| Blood Pressure (BP) analysis 1070 observations | Model 2† | |
|---|---|---|
| Statin effect (Adjusted β) | p value | |
| Systolic BP in all 4 measurements (HSbase,HSstim,LSbase,LSstim)* mmHg | −5.57 | p =0.001 |
| Systolic BP on HS (HSbase,HSstim) mmHg | −6.03 | p =0.001 |
| Systolic BP on LS (LSbase,LSstim) mmHg | −5.46 | p = 0.001 |
| Salt sensitivity of BP (Systolic HSbase – Systolic LSbase) mmHg | −5.85 | p <0.001 |
| Pulse pressure in all 4 measurements (Systolic-Diastolic) mmHg | −2.95 | p =0.004 |
High sodium (HS) baseline (base), HS Angiotensin II stimulation (stim), Low sodium (LS)
Mixed Model adjusted by age, BMI, gender, urinary sodium (LS, HS), race, site and randomization order.
Effect of type and dose of statin
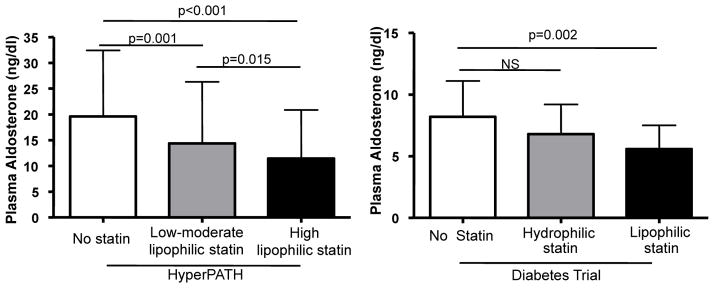
We observed a significant decrease in aldosterone levels related to higher lipophilicity characteristics (1050 observations, β −3.11 ng/mL, p-trend ≤ 0.001). In addition, we observed a significant decrease in aldosterone upon adjusted repeated measures analysis, comparing high lipophilic statin users to non statin users (β= −6.26 ng/mL, p ≤ 0.001) and low-moderate lipophilic statin to non statin users (β= −3.04 ng/mL, p =0.001) as observed in Figure 1. Also, high lipophilic statin users had lower aldosterone levels than low-moderate lipophilic statin users (β= −3.23 ng/mL, p =0.015, Figure 1). Regarding a dose effect, we observed a consistent effect on aldosterone levels (1045 observations, β= −2.87 ng/mL, p-trend ≤ 0.001) and when comparing moderate-high dose to no statin use (β= −5.81 ng/mL, p ≤ 0.001) and low dose to no statin use (β= −2.71 ng/mL, p =0.004). Interestingly, when both lipophilicity and dose were included as explanatory variables in the adjusted model, only lipophilicity remained significant. Neither type nor dose of statin was associated with changes in cortisol levels.
Figure 1.
Adjusted mean ± SD changes in aldosterone levels categorized by type of statins in hypertensive and diabetic subjects. HyperPATH: 1050 observations; Diabetes Trial: 143 observations.
2) Study 2: Adrenal secretion in diabetic subjects on liberal sodium diet by statin use
Characteristics of Type 2 diabetic participants
After the inclusion-exclusion criteria were applied, a total of 143 observations in 79 diabetic subjects were available for analysis. As expected in this population, chronic statin use was highly prevalent (69%). The proportion of subjects on the different statins were similar to those in study 1 with simvastatin being the most commonly used (65%), followed by atorvastatin (14%), rosuvastatin (8%), pravastatin (5%) and lovastatin (3%). Subjects on statins were comparable to those without statin treatment regarding age, gender, race, BMI, sodium intake and years of diabetes (Supplementary Table 1).
Adrenal hormones secretion on liberal sodium intake in diabetic patients by statin use
Consistent with the findings in study 1, statin use was associated with lower unadjusted aldosterone levels at baseline and after AngII stimulation on HS diet (Supplementary Table 1). As all subjects in study 2 were on an ACEI, these results suggest that statins may act downstream from the angiotensin II receptor (AT1R). Again, cortisol levels were similar (baseline and stimulated) when comparing diabetic subjects with or without statin use (Supplementary Table 1). Plasma renin levels and potassium levels were not different by statin use.
Repeated measures analysis in diabetic patients by statin use
As in study 1, we tested the effect of statin use on aldosterone levels using repeated measurements of aldosterone (baseline aldosterone and AngII-stimulated aldosterone) for each subject on HS diet. Statin use associated with decreased aldosterone levels even when adjusted by confounders in both models 1 and 2 (Table 5). This effect was maintained when including LDL levels and systolic BP in the model as a sensitivity analysis. Consistently, cortisol levels were not modified by statin use (Table 5). Similar to the results in study 1, lower aldosterone levels were related in a stepwise fashion to higher lipophilicity (adjusted β −1.11 ng/mL, p-trend =0.003). Further, aldosterone levels in diabetic subjects were lower in those receiving a lipophilic statin as compared with those not on a statin (adjusted β −2.19 ng/mL, p =0.002, Figure 1) but not when comparing hydrophilic statin to non user (adjusted β −1.63 ng/mL, p =0.15, Figure 1). We also observed a dose response effect when subjects were classified into the following 3 groups: no statin, low dose statin or moderate-high dose statin, (β −1.15 ng/mL, p for the trend = 0.002). When comparing only the moderate-high dose statin to non-users we observed 30% lower adjusted aldosterone levels (p= 0.002), but again no effect on cortisol.
Table 5.
Effect of statins on aldosterone and cortisol levels in repeated measures analysis adjusted by confounders (Diabetes replication study).
| 143 observations | Model 1* | Model 2† | ||
|---|---|---|---|---|
| Statin effect:β (Predicted change) | p value | Statin effect: β (Predicted change) | p value | |
| Aldosterone ng/dl (baseline, AngII stim) | −2.13 (↓26%) | p =0.009 | −2.20 (↓26%) | p=0.006 |
| Cortisol ug/dL (baseline, AngII stim) | −0.24 | p =0.78 | 0.14 | p =0.87 |
Mixed Model 1: adjusted by age, BMI, gender and urinary sodium.
Mixed Model 2: covariates in model 1 plus race, diabetes duration and amlodipine use for hypertension control.
Ex vivo Studies
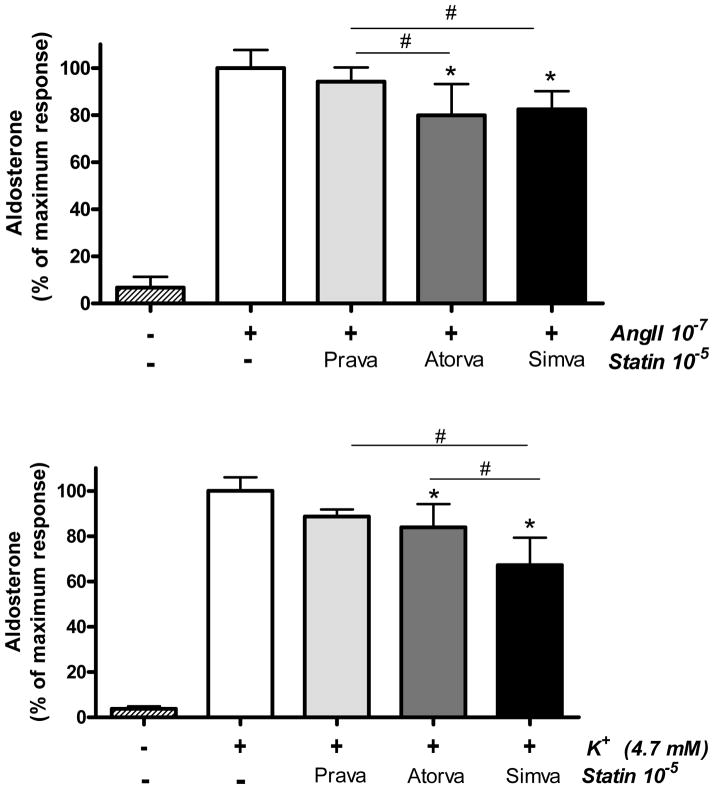
To confirm whether the lower aldosterone levels observed in statin users could be explained by a direct effect of statins on adrenal secretion (as opposed to an increased aldosterone metabolism in response to statins), we performed ex vivo studies in rodent adrenal cells. In this system, zona glomerulosa (ZG) aldosterone production is increased in response to various secretagogues, including AngII (which uses the AT1R pathway) and potassium (which acts via an AT1R-independent mechanism). Notably we observed that the acute statin effect on aldosterone secretion was dependent on type of statin. Of note, the lipophilic statins, simvastatin and atorvastatin, reduced both potassium- and AngII-stimulated aldosterone production, whereas the hydrophilic statin pravastatin did not have a significant effect on aldosterone acute secretion (Figure 2). In the potassium stimulated aldosterone studies, simvastatin had significantly greater blocking effect than pravastatin and atorvastatin.
Figure 2.
Effects of acute statin treatment on aldosterone secretion in ex vivo mouse zona glomerulosa cells (ZG). Aldosterone levels were assessed in response to AngII 10-7 M or potassium (K+) 4.7 mM in the absence or presence of 3 different statins (all 10-5 M). Data represents means ± SD. *p<0.001 compared to AngII or K+; # P<0.001 compared between type of statin.
In addition, we verified that statins did not acutely affect corticosterone production in zona fasciculata cells. Further, statins did not decrease the precursor of aldosterone (corticosterone) in ZG cells, thus suggesting that statins could be modulating the late pathway of aldosterone steroidogenesis.
DISCUSSION
In these short-term RAAS intervention studies, we demonstrate that chronic statin use is associated with reduced aldosterone levels in both hypertensive and diabetic subjects. This association is evident on both high and low sodium diets, in response to an infusion of the aldosterone secretagogue AngII and when measured in 24h urine samples. The effect on aldosterone was specific, as cortisol levels were not modified in any intervention setting. Interestingly, both human cohort data suggest that lipophilic statins could be more efficient in blocking aldosterone secretion, results that were confirmed ex vivo.
In the past decades, accumulating evidence has shown that aldosterone dysregulation participates in hypertension, coronary disease, vascular injury and heart failure9,21. Since statins are one of the most studied drugs in cardiovascular prevention and treatment, there is growing literature discussing the potential interaction between statins, the RAAS and related medications, beyond the well described anti-inflammatory and cardioprotective effects of statins 21. For instance, a synergistic and beneficial effect of statins with both AngII blockers and ACE inhibitors has been described in patients with hypertension, diabetes and heart failure33, 34,35. Further, atorvastatin and simvastatin in humans, both lipophilic statins, reduced angiotensin II sensitivity and downregulate AT1R 36. Thus, it has been assumed that this synergistic effect is secondary to an effect mediated directly by angiotensin II. However, previous studies have not considered the possibility that this potential synergism maybe secondary to an effect on aldosterone production. To the best of our knowledge our study is the first to analyze adrenal secretion of aldosterone and cortisol by statin use in the setting of prolonged washout of medications that affect the RAAS, strictly controlled sodium diet and AngII infusion. However, our results showing a specific effect on aldosterone secretion but not cortisol, as shown by our human and ex vivo data, are supported by several previous studies. An early report, evaluating whether statins impaired adrenal steroidogenesis, showed that nine months of simvastatin significantly decreased aldosterone but not cortisol levels37. Moreover, in an animal intervention study, long-term simvastatin treatment on a high sodium diet significantly reduced plasma aldosterone levels38. More recently, Palmer et al observed that starting a statin post-myocardial infarction was associated with a significant decrease in aldosterone levels, though values were not adjusted by sodium intake or medications affecting the RAAS39. Of note, torcetrapib, a CETP inhibitor for dyslipidemia, was halted from clinical use because of higher mortality. Treatment with the CETP inhibitor led to increased aldosterone levels and subsequent studies showed that the CETP inhibitor induces aldosterone synthase in adrenal cells. 40. On the other hand, fenofibrate did not changed aldosterone levels in a similar protocol of high sodium diet intervention41. In relation to cortisol, there are numerous well-designed studies, including cortisol levels after ACTH stimulation, that demonstrate that typical statin use does not affect cortisol levels42. Intriguingly renin levels did not change despite a consistent decrease in aldosterone levels. Even though a moderate decrease in aldosterone could be insufficient to upregulate renin secretion, the specific mechanism(s) for the apparent impaired negative feedback warrant further assessment.
There are several novel and exciting results in our work that support a potential role for statins in modulating aldosterone secretion. First, the results are consistent in different intervention settings within the same subjects as well as in different clinical settings, such as hypertension and diabetes. Also, we found dose-response effect for statin use with lower aldosterone levels at higher doses, providing greater evidence for causality. In relation to the type of statins, our data suggest that lipophilic statins, particularly simvastatin, rather than hydrophilic statins, are associated with lower aldosterone levels. The biological plausibility of this finding is supported by the following: 1) different affinity for adrenal tissue described by type of statin showing that radiolabeled simvastatin has a high adrenal uptake (even higher than liver) compared to pravastatin23; 2) previous preliminary reports showing aldosterone levels were modified by simvastatin37 but not pravastatin 43; 3) our ability to replicate our initial findings in a second cohort; and 4) our demonstration that only certain type of statins acutely reduce aldosterone secretion ex vivo.
In relation to our exploratory ex vivo experiments, we observed in adrenal cells that the type of statin affected acute aldosterone production. Lipophilic statins had a greater effect in blocking aldosterone production than pravastatin, a hydrophilic statin. Further, our finding that potassium-stimulated aldosterone could be blocked acutely by statins (up to a 33% reduction), with simvastatin as the most potent and pravastatin the least potent, suggests that statins act downstream from the AT1R to decrease aldosterone production. Consistent with this concept, we found that statin use was associated with lower aldosterone levels in our replication cohort where all individuals were being treated with the same type and dose of ACE inhibition drug. Although we did not find previous studies examining the effect of statins in adrenal cells, our results are consistent with an in vitro study in human mesangial cells showing that atorvastatin suppressed acute aldosterone production induced by angiotensin II44. The specific mechanism for aldosterone inhibition, which is beyond the scope of this initial study, remains to be elucidated, but in rats long-term pitavastatin treatment suppressed the expression of aldosterone synthase in the kidney 45. Reduced cholesterol availability and/or isoprenylation of signaling proteins have been postulated as potential mechanisms for statins modulating steroidogenesis46. Consistent with the concept that statins affect steroidogenesis, a recent meta-analysis showed that statins could lower testosterone in men and in women with polycystic ovary syndrome 47. The mechanisms by which statins affect aldosterone production remain to be elucidated and these studies will need to examine and contrast the acute effects of statins on adrenal steroidogenesis as well as chronic in vivo effects.
We additionally showed that statins were associated with lower BP, as previously reported, but also with less salt sensitivity of BP and pulse pressure. As both sodium reabsorption and arterial stiffness have been related to aldosterone dysregulation, these observations could be, at least in part, secondary to aldosterone secretion modulation by statins 48. A recent study showed that simvastatin outperformed pravastatin in reducing AngII induced hypertension, which is consistent with our human and ex vivo results 49.
From a clinical perspective we believe that even a moderate decrease of aldosterone may be relevant. For example, the LURIC study showed that a quartile increase in normal aldosterone levels significantly increased cardiovascular mortality after 7 years of follow up 50. It could be that a modest decrease in aldosterone secretion may be a new and non-traditional effect of statins that may partially explain their robust cardioprotective actions not consistently observed with other lipid lowering interventions.
Despite novel findings our study has some limitations. First, statin intervention was not randomized and unmeasured confounding variables associated with statin users cannot be ruled.
Second, the acute dietary interventions and AngII infusions have no long term follow up, despite the fact our protocols are the ideal setting to measure aldosterone since they were designed to control all factors that could modulate adrenal secretion. Future studies should make sure that there is not a compensatory increase in ACTH to maintain cortisol levels. In addition, while initial data suggest that statins may modulate the late pathway of aldosterone synthesis, the specific mechanism for the aldosterone decreasing effect of chronic statin use needs to be fully elucidated. Finally, these results should be validated in prospective randomized studies with controlled RAAS modulators and in different populations where statins are routinely used.
Conclusions
In the present study we demonstrate that chronic statin users have lower aldosterone levels in two studies that evaluate RAAS modulation by sodium diet and AngII. The effect of statins on adrenal secretion appears to be specific for aldosterone and related to the lipophilicity and dose of statin. Future confirmation studies and the assessment of potential mechanisms for this novel finding are warranted.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the staff of the human research centers in which these intervention studies were performed. We also want to thank the Research Ventures & Licensing Office at Brigham and Women’s Hospital and the Partners Innovation fund for their support and assistance.
Funding Sources: This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and NCATS, NIH Award UL1 TR001102) as well as the following grants from the National Institutes of Health HL104032 (LP), K23HL111771 (AV), K24 HL103845 (GKA), R01 HL087060-01 (GKA), HL-69208 (GHW), T32HL007609 (GHW), R01HL11476 (GHW), funds from INSERM and the French Ministry of Health (XJ), from the Doris Duke Charitable Foundation (AV) and from the Chilean National Science and Technology Research Fund (FONDECYT) 1130427 (RB), 1150437 (RB), 1150327(RB) and CORFO 13CTI-21526 -P1 (RB).
Footnotes
Disclosures: None.
References
- 1.Mann DM, Woodward M, Ye F, Krousel-Wood M, Muntner P. Trends in medication use among us adults with diabetes mellitus: Glycemic control at the expense of controlling cardiovascular risk factors. Arch Intern Med. 2009;169:1718–1720. doi: 10.1001/archinternmed.2009.296. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margaritis M, Channon KM, Antoniades C. Statins as regulators of redox state in the vascular endothelium: Beyond lipid lowering. Antioxid Redox Signal. 2014;20:1198–1215. doi: 10.1089/ars.2013.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PY, Lee PT, Chang WT, Tai YL, Chao TH, Lee CH, Li YH, Chen JH, Tsai LM, Liao JK. Evidence of pleiotropy by statins: Leukocyte rho kinase (rock) activity and pretreated statin before percutaneous coronary interventions are clinical vascular outcome predictors. Int J Cardiol. 2014;176:250–253. doi: 10.1016/j.ijcard.2014.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinlay S, Schwartz GG, Olsson AG, Rifai N, Leslie SJ, Sasiela WJ, Szarek M, Libby P, Ganz P Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study I. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the miracl study. Circulation. 2003;108:1560–1566. doi: 10.1161/01.CIR.0000091404.09558.AF. [DOI] [PubMed] [Google Scholar]
- 7.de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, Monyak JT, Parving HH, Remuzzi G, Sowers JR, Vidt DG. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (planet i): A randomised clinical trial. The lancet. Diabetes & endocrinology. 2015;3:181–190. doi: 10.1016/S2213-8587(14)70246-3. [DOI] [PubMed] [Google Scholar]
- 8.Ma S, Ma CC. Recent development in pleiotropic effects of statins on cardiovascular disease through regulation of transforming growth factor-beta superfamily. Cytokine Growth Factor Rev. 2011;22:167–175. doi: 10.1016/j.cytogfr.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhang S, Jiang H, Sun A, Zou Y, Ge J. Effects of statin treatment on cardiac function in patients with chronic heart failure: A meta-analysis of randomized controlled trials. Clin Cardiol. 2011;34:117–123. doi: 10.1002/clc.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–835. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 11.Ramcharan AS, Van Stralen KJ, Snoep JD, Mantel-Teeuwisse AK, Rosendaal FR, Doggen CJ. Hmg-coa reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J Thromb Haemost. 2009;7:514–520. doi: 10.1111/j.1538-7836.2008.03235.x. [DOI] [PubMed] [Google Scholar]
- 12.Strazzullo P, Kerry SM, Barbato A, Versiero M, D'Elia L, Cappuccio FP. Do statins reduce blood pressure?: A meta-analysis of randomized, controlled trials. Hypertension. 2007;49:792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Liao JK. Pleiotropic effects of statins. - basic research and clinical perspectives. Circ J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalsen A, Lehmann N, Pithan C, Knoblauch NT, Moebus S, Kannenberg F, Binder L, Budde T, Dobos GJ. Mediterranean diet has no effect on markers of inflammation and metabolic risk factors in patients with coronary artery disease. Eur J Clin Nutr. 2006;60:478–485. doi: 10.1038/sj.ejcn.1602340. [DOI] [PubMed] [Google Scholar]
- 15.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 16.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 17.Lieb W, Pencina MJ, Jacques PF, Wang TJ, Larson MG, Levy D, Kannel WB, Vasan RS. Higher aldosterone and lower n-terminal proatrial natriuretic peptide as biomarkers of salt sensitivity in the community. Eur J Cardiovasc Prev Rehabil. 2011;18:664–673. doi: 10.1177/1741826710389406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durante A, Peretto G, Laricchia A, Ancona F, Spartera M, Mangieri A, Cianflone D. Role of the renin-angiotensin-aldosterone system in the pathogenesis of atherosclerosis. Curr Pharm Des. 2012;18:981–1004. doi: 10.2174/138161212799436467. [DOI] [PubMed] [Google Scholar]
- 19.Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, Collet JP, Beygui F, Hennache B, Ennezat PV, Juthier F, Richard F, Dallongeville J, Hillaert MA, Doevendans PA, Jude B, Bertrand M, Montalescot G, Van Belle E. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J. 2012;33:191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 20.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: Associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the framingham offspring study. Circulation. 2014;130:1676–1683. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 21.Drapala A, Sikora M, Ufnal M. Statins, the renin-angiotensin-aldosterone system and hypertension - a tale of another beneficial effect of statins. J Renin Angiotensin Aldosterone Syst. 2014;15:250–258. doi: 10.1177/1470320314531058. [DOI] [PubMed] [Google Scholar]
- 22.Chamarthi B, Williams JS, Williams GH. A mechanism for salt-sensitive hypertension: Abnormal dietary sodium-mediated vascular response to angiotensin-ii. J Hypertens. 2010;28:1020–1026. doi: 10.1097/HJH.0b013e3283375974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nezasa K, Higaki K, Matsumura T, Inazawa K, Hasegawa H, Nakano M, Koike M. Liver-specific distribution of rosuvastatin in rats: Comparison with pravastatin and simvastatin. Drug Metab Dispos. 2002;30:1158–1163. doi: 10.1124/dmd.30.11.1158. [DOI] [PubMed] [Google Scholar]
- 24.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam Clin Pharmacol. 2005;19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35:139–151. doi: 10.1111/j.1365-2710.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 26.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metabol. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, Adler GK. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61:886–893. doi: 10.1161/HYPERTENSIONAHA.111.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg R, Rao A, Baimas-George M, Hurwitz S, Foster C, Shah R, Jerosch-Herold M, Kwong R, Di Carli M, Adler GK. Mineralocorticoid Receptor Blockade Improves Coronary Microvascular Function in Individuals with Type 2 Diabetes Mellitus. Diabetes. 2015;64:236–42. doi: 10.2337/db14-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pojoga L, Kolatkar NS, Williams JS, Perlstein TS, Jeunemaitre X, Brown NJ, Hopkins PN, Raby BA, Williams GH. Beta-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension. 2006;48:892–900. doi: 10.1161/01.HYP.0000244688.45472.95. [DOI] [PubMed] [Google Scholar]
- 30.Lin YC, Chiang CH, Chang LT, Sun CK, Leu S, Shao PL, Hsieh MC, Tsai TH, Chua S, Chung SY, Kao YH, Yip HK. Simvastatin attenuates the additive effects of TNF-α and IL-18 on the connexin 43 up-regulation and over-proliferation of cultured aortic smooth muscle cells. Cytokine. 2013;62:341–51. doi: 10.1016/j.cyto.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XS, Ren JH, Lu JP, Fan Y. Atorvastatin protects against angiotensin ii-induced injury and dysfunction in human umbilical vein endothelial cells through bradykinin 2 receptors. J Cardiovasc Pharmacol. 2010;56:171–176. doi: 10.1097/FJC.0b013e3181e5f2e2. [DOI] [PubMed] [Google Scholar]
- 32.Braley LM, Menachery AI, Brown EM, Williams GH. Comparative effect of angiotensin ii, potassium, adrenocorticotropin, and cyclic adenosine 3',5'-monophosphate on cytosolic calcium in rat adrenal cells. Endocrinology. 1986;119:1010–1019. doi: 10.1210/endo-119-3-1010. [DOI] [PubMed] [Google Scholar]
- 33.Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, Kang MH, Ahn TH, Choi IS, Shin EK. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687–3692. doi: 10.1161/01.CIR.0000143085.86697.13. [DOI] [PubMed] [Google Scholar]
- 34.Koh KK, Quon MJ, Han SH, Ahn JY, Jin DK, Kim HS, Kim DS, Shin EK. Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension. 2005;45:1088–1093. doi: 10.1161/01.HYP.0000166722.91714.ba. [DOI] [PubMed] [Google Scholar]
- 35.Maejima Y, Nobori K, Ono Y, Adachi S, Suzuki J, Hirao K, Isobe M, Ito H Heart Failure by Coadministration of S, Angiotensin IIRBTI. Synergistic effect of combined hmg-coa reductase inhibitor and angiotensin-ii receptor blocker therapy in patients with chronic heart failure: The hf-costar trial. Circ J. 2011;75:589–595. doi: 10.1253/circj.cj-10-0804. [DOI] [PubMed] [Google Scholar]
- 36.Nickenig G, Baumer AT, Temur Y, Kebben D, Jockenhovel F, Bohm M. Statin-sensitive dysregulated at1 receptor function and density in hypercholesterolemic men. Circulation. 1999;100:2131–2134. doi: 10.1161/01.cir.100.21.2131. [DOI] [PubMed] [Google Scholar]
- 37.Ide H, Fujiya S, Aanuma Y, Agishi Y. Effects of simvastatin, an hmg-coa reductase inhibitor, on plasma lipids and steroid hormones. Clin Ther. 1990;12:410–420. [PubMed] [Google Scholar]
- 38.Bayorh MA, Ganafa AA, Eatman D, Walton M, Feuerstein GZ. Simvastatin and losartan enhance nitric oxide and reduce oxidative stress in salt-induced hypertension. Am J Hypertens. 2005;18:1496–1502. doi: 10.1016/j.amjhyper.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Palmer BR, Pilbrow AP, Frampton CM, Yandle TG, Skelton L, Nicholls MG, Richards AM. Plasma aldosterone levels during hospitalization are predictive of survival post-myocardial infarction. Eur Heart J. 2008;29:2489–2496. doi: 10.1093/eurheartj/ehn383. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, Dietz JD, Xia C, Knight DR, Loging WT, Smith AH, Yuan H, Perry DA, Keiser J. Torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. Endocrinology. 2009;150:2211–2219. doi: 10.1210/en.2008-1512. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert K, Nian H, Yu C, Luther JM, Brown NJ. Fenofibrate lowers blood pressure in salt-sensitive but not salt-resistant hypertension. J Hypertens. 2013;31:820–829. doi: 10.1097/HJH.0b013e32835e8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobs AS, Schrott H, Davidson MH, Bays H, Stein EA, Kush D, Wu M, Mitchel Y, Illingworth RD. Effects of high-dose simvastatin on adrenal and gonadal steroidogenesis in men with hypercholesterolemia. Metabolism. 2000;49:1234–1238. doi: 10.1053/meta.2000.7716a. [DOI] [PubMed] [Google Scholar]
- 43.Glorioso N, Troffa C, Filigheddu F, Dettori F, Soro A, Parpaglia PP, Collatina S, Pahor M. Effect of the hmg-coa reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999;34:1281–1286. doi: 10.1161/01.hyp.34.6.1281. [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa T, Matsuzawa Y, Suematsu S, Saito J, Omura M, Kino T. Effect of atorvastatin on aldosterone production induced by glucose, ldl or angiotensin ii in human renal mesangial cells. Arzneimittelforschung. 2010;60:445–451. doi: 10.1055/s-0031-1296310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toba H, Mitani T, Takahashi T, Imai N, Serizawa R, Wang J, Kobara M, Nakata T. Inhibition of the renal renin-angiotensin system and renoprotection by pitavastatin in type1 diabetes. Clin Exp Pharmacol Physiol. 2010;37:1064–1070. doi: 10.1111/j.1440-1681.2010.05436.x. [DOI] [PubMed] [Google Scholar]
- 46.Ortega I, Cress AB, Wong DH, Villanueva JA, Sokalska A, Moeller BC, Stanley SD, Duleba AJ. Simvastatin reduces steroidogenesis by inhibiting cyp17a1 gene expression in rat ovarian theca-interstitial cells. Biol Reprod. 2012;86:1–9. doi: 10.1095/biolreprod.111.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schooling CM, Au Yeung SL, Freeman G, Cowling BJ. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013;11:57. doi: 10.1186/1741-7015-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juncos LI, Juncos LA, Garcia NH. The antihypertensive actions of statins: Modulation by salt intake. Am J Hypertens. 2012;25:1140–1148. doi: 10.1038/ajh.2012.105. [DOI] [PubMed] [Google Scholar]
- 49.Drapala A, Aleksandrowicz M, Zera T, Sikora M, Skrzypecki J, Kozniewska E, Ufnal M. The effect of simvastatin and pravastatin on arterial blood pressure, baroreflex, vasoconstrictor, and hypertensive effects of angiotensin ii in sprague-dawley rats. J Am Soc Hypertens. 2014;8:863–871. doi: 10.1016/j.jash.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: The ludwigshafen risk and cardiovascular health (luric) study. Eur Heart J. 2010;31:1237–1247. doi: 10.1093/eurheartj/ehq019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.