Abstract
The error-related negativity (ERN) is an event-related potential that occurs approximately 50 ms after an erroneous response. The magnitude of the ERN is influenced by contextual factors, such as when errors are made during social evaluation. The ERN is also influenced by individual differences in anxiety, and it is elevated amongst anxious individuals. However, little research has examined how individual differences in anxiety interact with contextual factors to impact the ERN. Social anxiety involves fear and apprehension of social evaluation. The current study explored how individual differences in social anxiety interact with social contexts to modulate the ERN. The ERN was measured in 43 young adults characterized as either high or low in social anxiety while they completed a flanker task in two contexts: alone and during social evaluation. Results revealed a significant interaction between social anxiety and context, such that the ERN was enhanced in a social relative to a non-social context only among high socially anxious individuals. Furthermore, the degree of such enhancement significantly correlated with individual differences in social anxiety. These findings demonstrate that social anxiety is characterized by enhanced neural activity to errors in social evaluative contexts.
Social anxiety is defined by fear and anxiety of social scrutiny (Rapee & Heimberg, 1997; Rapee & Spence, 2004). Unlike other forms of anxiety (e.g., general distress; Clark & Watson, 1991), social anxiety is specific to socially threatening contexts (Geen, 1991; Rapee & Heimberg, 1997; Schlenker & Leary, 1982). In such contexts, socially anxious, relative to non-anxious individuals, exhibit greater anxious behavior and autonomic arousal, and report greater distress (Beidel, Turner, & Dancu, 1985; Furlan, DeMartinis, Schweizer, Rickels, & Lucki, 2001; Levin et al., 1993; Mauss, Wilhelm, & Gross, 2004). A number of physiological measures display elevated responses during social-evaluative contexts. The startle reflex, a measure of defensive reactivity to threat (Grillon, 2002; Landis & Hunt, 1939), is elevated during social-evaluative contexts and is related to individual differences in social anxiety (Cornwell, Johnson, Berardi, & Grillon, 2006), suggesting that social evaluation is particularly anxiogenic on a physiological basis among socially anxious individuals, which results in mobilization and defensive responding to social threats. Findings such as these inform neuroscientific theory of anxiety, since considerable research delineates the neural correlates of such physiological measures (Grillon, 2002). Social anxiety disorder (SAD), which involves heightened fear of social evaluation, is an extremely common and debilitating disorder affecting over 10% of the population (Kessler et al., 2005). Generalized anxiety disorder (GAD) and major depressive disorder (MDD) are commonly comorbid amongst individuals with SAD (Grant et al., 2005). Thus, it is critical to identify biomarkers specifically associated with social anxiety symptoms to better understand the etiology of SAD.
Socially anxious individuals demonstrate altered neural patterns including enhanced activation in the anterior cingulate cortex (ACC), particularly when processing socially salient information (Amir, Klumpp, et al., 2005; Lorberbaum et al., 2004). One potential biomarker associated with ACC activity is the error-related negativity (ERN; Dehaene, Posner, & Tucker, 1994; Holroyd, Dien, & Coles, 1998; Van Veen & Carter, 2002), which is a negative deflection in the event-related potential (ERP) waveform that occurs approximately 50 ms after an error (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). The ERN is thought to reflect the motivational response to error commission (Hajcak, 2012; Proudfit, Inzlicht, & Mennin, 2013), given that the ERN is enhanced when errors are perceived as more distressing and/or threatening (Hajcak & Foti, 2008; Hajcak, Moser, Yeung, & Simons, 2005; Riesel, Weinberg, Moran, & Hajcak, 2013). Contextual factors enhance the ERN, such as in contexts where accuracy is emphasized over speed (Gehring et al., 1993), when errors are punished (Riesel, Weinberg, Endrass, Kathmann, & Hajcak, 2012), or when errors incur monetary cost (Ganushchak & Schiller, 2008; Hajcak et al., 2005). The ERN is also enhanced by social motivational factors, such as when performance is critically evaluated (Hajcak et al., 2005), during interpersonal competition (Van Meel & Van Heijningen, 2010), or when errors are observed by a peer (Kim et al., 2005). Such contextual factors may be particularly salient among anxious individuals (Riesel et al., 2012). However, it remains unclear how individual differences in social anxiety interact with these social factors to influence the ERN.
A large body of literature links the ERN to individual differences in anxious behavior (see Moser, Moran, Schroder, Donnellan, & Yeung, 2013 for review). An enhanced ERN has been observed among individuals with an anxiety disorder (Carrasco, Hong, et al., 2013; Endrass, Riesel, Kathmann, & Buhlmann, 2014; Gehring, Himle, & Nisenson, 2000; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006; Weinberg, Olvet, & Hajcak, 2010), including SAD (Endrass et al., 2014). The ERN is related to subclinical symptoms of anxiety disorders, such as worry (Hajcak, McDonald, & Simons, 2003a; Moser, Moran, & Jendrusina, 2012), negative emotionality and affect (Hajcak, McDonald, & Simons, 2004; Luu, Collins, & Tucker, 2000) and behavioral inhibition (Amodio, Master, Yee, & Taylor, 2008; Lahat et al., 2014; McDermott et al., 2009), suggesting that the relation between the ERN and anxiety is driven by personality factors observed among all anxiety disorders (Moser et al., 2012; Proudfit et al., 2013). However, less is known about the relation between the ERN and individual differences in social anxiety.
In addition to the ERN, there are a number of ERP components associated with performance and error monitoring. The correct related negativity (CRN) is a negativity of small magnitude observed on correct motor responses which has a similar morphology and topography as the ERN (Ford, 1999; Gehring & Knight, 2000; Scheffers & Coles, 2000; Vidal, Burle, Bonnet, Grapperon, & Hasbroucq, 2003; Vidal, Hasbroucq, Grapperon, & Bonnet, 2000) but appears to be insensitive to motivational factors (Hajcak et al., 2005; Kim et al., 2005) and individual differences in anxiety (Gehring et al., 2000; Weinberg et al., 2010). A large positive ERP deflection, known as the positive error (Pe), is observed approximately 300 ms following an error response (Falkenstein et al., 1991) but relations between Pe and anxiety have been inconsistent, with most studies finding no relation between the Pe and anxiety (Endrass et al., 2008, 2014; Hajcak et al., 2003a; Weinberg et al., 2010).
The present study examines the interacting influences of social context and individual differences in social anxiety on the neural correlates of error monitoring (i.e., ERN, CRN, Pe). We recruited young adults who scored at the extremes on symptoms of social anxiety (Fresco et al., 2001; Liebowitz, 1987), and characterized participants as either high socially anxious (HSA) or low socially anxious (LSA). We measured the ERN and related components in participants across two different social motivational contexts. In one condition, participants played a computer game and committed errors while alone in a room (i.e., alone condition). In the other condition, participants played the same computer game and committed errors while being observed and evaluated by a peer (i.e., peer condition). We hypothesized that social anxiety would significantly interact with contexts in predicting the magnitude of the ERN, such that the ERN would be enhanced in the peer condition as compared to the alone condition only among the high socially anxious individuals. Among low socially anxious individuals, we predicted no differences in the ERN between conditions. Given the mixed results for the Pe and the CRN, we made no a priori hypotheses for these components.
Methods
Participants
The final sample included 43 participants (22 females). Undergraduate students in introductory psychology courses at University of Maryland (N= 792) completed the self-report version of the Liebowitz Social Anxiety Scale (LSAS-SR; Fresco, Coles, & Heimberg, 2001; Liebowitz, 1987). The LSAS-SR is a 24-item questionnaire that assesses the degree of anxiety and avoidance during social interaction and performance situations. Participants rate on a likert scale from 0 (none) to 3 (severe) how much anxiety is experienced in social situations and from 0 (never) to 3 (usually) how often they avoid these situations. Separate scales for anxiety (Total Anxiety) and avoidance (Total Avoidance) are created by summing relevant items. The LSAS-SR is widely used as a tool to measure the severity of social anxiety in both clinical and non-clinical samples and demonstrates excellent psychometric properties (Heimberg et al., 1999). Subjects were recruited for participation in the present study if they scored approximately ± 1 SD on the Total Anxiety scale of the LSAS-SR (M =24.63, SD = 12.8). Subjects were excluded for uncorrected visual impairments, inability to provide informed consent, insufficient number of errors, or extremely inaccurate task performance, but the study had no other exclusion criteria.
Forty-eight undergraduates (26 females) were selected for participation on the basis of their LSAS-SR scores. Twenty-five undergraduates (13 females) who scored high on LSAS-SR Total Anxiety comprised the high socially anxious group (HSA), and 23 undergraduates (12 females) who scored low on the LSAS-SR Total Anxiety comprised the low socially anxious (LSA) group. To limit the effect of performance feedback (e.g., feedback to improve accuracy) on influencing the ERN (e.g., Gehring et al., 1993), participants were excluded if accuracy was below 80% in any one condition (one participant). Participants were also excluded if fewer than 15 errors were committed (per Larson, Baldwin, Good, & Fair, 2010) in any one condition (two participants) . In addition, two participants were excluded due to technical errors. Therefore the final sample consisted of 22 (11 female) participants in the HSA group and 21 participants (11 female) in the LSA group (see Table 1). All participants received course credit for their participation, and the experimenter was blind to group assignment during experimental testing and data processing.
Table 1.
Descriptive statistics for the Low Socially Anxious (LSA) and High Socially Anxious (HSA) groups, and scores among each groups for symptoms of social anxiety, general anxiety, and depression.
| Low Socially Anxious |
High Socially Anxious |
|
|---|---|---|
| N | 21 (11 female) | 22 (11 female) |
| Age (years) | 19.51 (1.0) | 19.77 (1.2) |
| Liebowitz Social Anxiety Scale (Total Score) | 20.76 (19.0) | 71.41 (11.3) |
| Liebowitz Social Anxiety Scale (Total Anxiety) | 9.76 (3.3) | 41.68 (5.6) |
| Beck Depression Inventory | 4.48 (5.3) | 10.23 (7.4) |
| Penn State Worry Questionnaire | 42.33 (12.9) | 58.59 (10.5) |
Measures
Participants also completed the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990), a 24-item self-report questionnaire that assesses trait aspects of anxious apprehension (i.e., worry), which is closely aligned to measure general anxiety symptoms characteristic of GAD. The PSWQ demonstrates high internal reliability in normative and clinical populations, and has been known to accurately distinguish the construct of worry from other constructs of anxiety and depression (Brown, Antony, & Barlow, 1992; Davey, 1993; Gillis, Haaga, & Ford, 1995). Participants also completed the Beck Depression Inventory-II (BDI-II; Beck et al., 1996), a widely used measure of depression that assesses the presence and severity depressive symptomology across 21 items. The BDI-II has been utilized extensively in normative samples to asses individual differences in depressive symptoms (Bumberry, Oliver, & McClure, 1978).
Task and Materials
An adapted arrow version of the flanker task (Eriksen & Eriksen, 1974) was administered using e-prime software (Psychology Software Tools, Inc., Sharpsburg, PA). On each trial, participants viewed five horizontal arrowheads. On half of the trials, arrowheads were congruent (<<<<<, >>>>>) and on the other half of the trials the arrowheads were incongruent (<<><<, >><>>). The order of presentation of the arrowheads was presented randomly. The stimuli were approximately 13.8 cm in height and 2.6 cm in width. All stimuli were presented for 200 ms. An intertrial interval (ITI) was presented that varied randomly from 700–1100 ms following the response or after 800 ms from stimulus onset (whichever occurred first).
Procedure
Upon arrival to the laboratory, participants were greeted and consented by the experimenter and then seated next to a gender-matched undergraduate who was a confederate and who acted as a participant throughout the study. The experimenter informed both the participant and the confederate that they were participating in the same study, and both were given questionnaires to complete. After completing all questionnaires, participants were informed that they would be completing a computer game twice, each time in a different context. In one of the contexts, the participant and the confederate would complete the game alone in separate rooms (i.e., alone condition). In the other context, the participant and confederate would be studied in the same room, where one participant would be the “player” and the other participant would be the “observer” (i.e., peer condition). To determine who would be the player and the observer in the peer condition, participants drew numbers out of a hat (the actual participant always drew the number to be the player). After explaining both conditions, the confederate was led out of the room by a research assistant, and the experimenter explained the instructions of the computer task to the participant. The order of the conditions was counterbalanced across participants.
During the peer condition, the confederate was taken into the room after the participant was prepped for electroencephalogram (EEG) collection. The confederate was instructed to sit at a table with a computer that was at a 90° angle from the participant. When both the participant and the experimenter were seated in the room, the experimenter explained that the confederate would see the performance of the participant on their own computer screen (i.e., reaction times and accuracy). In addition, the confederate was given a clipboard and told to mark down every error that the participant made while completing the task. During the alone condition, the participant was told that the confederate was playing the computer game in an adjacent room.
Participants were seated approximately 30 inches from the monitor and were instructed to press the right button when the middle arrow was pointing to the right and press the left button when the middle arrow was pointing to the left on a hand-held button box. Participants performed a practice block of 30 trials. The experimental task consisted of 8 blocks of 52 trials (416 trials total). Two participants (1 participant from the HSA and 1 participant from the LSA group) completed 48 trials per block for a total of 386 trials. Prior to beginning the task, subjects were told to be as fast and accurate as possible. After each block, subjects received a short break and feedback about their performance (Weinberg et al., 2010). If performance was 75% or below, the message “Please be more accurate” was displayed. If performance was above 90%, participants received the message “Please respond faster”. No feedback was given for performance between 75% and 90%.
EEG Data Collection and Analysis
Continuous EEG was recorded using a 128-channel Geodesic Sensor Net and sampled at 250 Hz using EGI software (Electrical Geodesic, Inc, Eugene, OR). Before data collection, all electrode impedances were reduced to below 50 kΩ. All electrodes were referenced online to Cz and re-referenced to average reference off-line. Data were filtered off-line using a digital bandpass FIR filter from .3–30 Hz.
Reponses-locked trials were separately segmented for error and correct trials 400 ms before the response to 800 ms after the response (1200 ms total). Eye-blinks were removed from the segmented waveforms using independent component analysis (ICA) in ERP PCA Toolkit (Delorme & Makeig, 2004; Dien, 2010). Individual blinks were identified for each participant to create an average blink topography from all subjects. ICA components for each subject that correlated at .9 or above with the averaged blink topography and/or with the ERP PCA Toolkit supplied blink topography were removed. Following, a semi-automated procedure was utilized for artifact rejection and detection. Channels were marked bad if the fast average amplitude exceeded 100 µV or if the difference between a channel and neighboring channels was greater than 40 µV for an individual segment. Channels were marked globally bad if the correlation between neighboring channels was less than .30 or if the channel was bad on greater than 20% of trials. Trials were marked bad if more than 10% of channels were determined to be bad (alone condition: 1.7% trials; peer condition: 1.8% trials). Bad channels on remaining good trials were replaced using spherical spline interpolation (Perrin et al., 1989, 1990). Individual error trials were visually inspected for any remaining artifacts. All visual detection of artifact was done blind to group membership. There were no differences in the number of artifact-free error trials for the HSA group (M = 52.18, SD = 11.6) or for the LSA group (M =50.86 SD = 14.4) in the alone condition, t(41) = 0.33, p = .74, nor any differences between groups in the peer condition (HSA: M = 48.09, SD =14.0; LSA: M = 50.67 SD = 12.2), t(41) = 0.64, p = .52.
All correct and error trials were separately averaged for each participant and then baseline corrected to the average activity 400 ms before the response to 200 ms before the response. Channels were collapsed to create channel groups for each component by averaging activity over a group of channels in order to reduce multiple testing for independent channels and thus reducing type I error. The ERN and CRN were evaluated as the average activity at four fronto-central electrodes at the midline (6 [FCz], 7, 107, Cz), where the ERN was maximally negative. The Pe and correct Pe (i.e., Pe on correct trials) were evaluated as the average activity at seven centro-parietal electrodes (Cz, 31, 54, 55 [PCz], 62 [Pz], 79, 80), where the Pe was maximally positive. The ERN and CRN amplitudes were extracted as the mean activity 0–100 ms following the response for error and correct trials respectively. The Pe and correct Pe amplitudes were extracted as the average activity 200–400 ms following the response for error and correct trials respectively. To examine brain activity specific to errors, a difference wave was created by subtracting brain activity on correct trials from brain activity on error trials for the ERN (i.e., ERN - CRN; ΔERN). Similarly, a change score was calculated for the Pe by subtracting the correct Pe from the Pe (ΔPe). In addition, in order to examine the change in neural activity across conditions, neural activity from the alone condition was subtracted from neural activity in the peer condition for ERP measures of interest (e.g., Peer ERN – Alone ERN, Peer ΔERN – Alone ΔERN).
Trials with response times faster than 200 and slower than 800 were removed from analysis (alone condition: 1.1% trials; peer condition: 0.8% trials). The number of trials excluded due to extreme response times was not different between groups in the alone condition, t(41) = 1.68, p = .10, or in the peer condition, t(41) = 1.37, p = .18. Accuracy was calculated as the number of correct trials divided by the number total trials with a response. Response times were separately averaged for correct trials and error trials for each participant for each condition.
Data Analysis
To investigate differences in behavioral performance (i.e., reaction time, accuracy) between groups and conditions, mixed-model ANOVA’s were conducted with group (HSA, LSA) as the between-subjects factor, and condition (alone, peer) and response (correct, incorrect) as the within-subjects factor. Similarly, to examine differences in ERP measures, separate 2 (group) x 2 (condition) x 2 (response) mixed-model ANOVA’s were conducted for the ERN and for the Pe. For the ΔERN (i.e., ERN – CRN) and the ΔPe (i.e., Pe – correct Pe), separate 2 (group) x 2 (condition) mixed-model ANOVA’s were conducted. Degrees of freedom were adjusted using the Greenhouse-Geisser method for all within-subjects comparisons to reduce type I error. Significant group interaction effects were explored by conducting follow-up ANOVA’s and paired sample t-test separately for each group.
Next, the relation between the ERP measures and self-report measures were explored. First, a one-way MANOVA was conducted for the PSWQ, LSAS-SR, and the BDI to explore differences in social anxiety, general anxiety, and depressive symptoms between HSA and LSA groups. Second, the Pearson correlation coefficient was utilized to examine the relation between ERP measures and self-report questionnaires of anxiety and depression. Next, correlations were conducted to examine the relations between changes in ERP measures across the alone and peer conditions (e.g., Peer ERN – Alone ERN) and self-report measures. Last, to determine the unique contribution of the different types of anxiety in predicting the ERN, a multiple regression model was conducted with LSAS social anxiety and PSWQ general anxiety as predictors and change in ΔERN across conditions as the outcome variable. Significance was evaluated at the .05 level for all statistical analyses.
Results
Table 2 displays the reaction times and accuracy across the peer and alone conditions for HSA and LSA groups. Analysis of reaction time demonstrated a main effect of response, where participants across both groups and conditions exhibited a faster reaction time on error trials than correct trials, F(1,41) = 768.45, p < .001, η2 = .95. No other main or interaction effects reached significance. For analysis of accuracy, no main or interaction effects were significant (ps > .10).
Table 2.
Means for behavioral performance and event-related potential (ERP) measures for the high socially anxious group (HSA; n = 23) and the low socially anxious group (LSA; n = 21) for the peer and alone conditions.
| Low Socially Anxious | High Socially Anxious | |||
|---|---|---|---|---|
| Behavior Measures | Peer | Alone | Peer | Alone |
| Error Reaction Time (ms) | 295.01 (21.4) | 300.26 (21.6) | 296.10 (31.2) | 293.61 (29.2) |
| Correct Reaction time (ms) | 350.35 (31.0) | 356.92 (25.5) | 346.62 (34.7) | 344.21 (35.7) |
| Accuracy (%) | 87.45 (.03) | 87.32 (.03) | 87.87 (.03) | 86.76 (.03) |
| ERP’s (µV) | ||||
| ERN | −0.88 (3.3) | −0.90 (3.6) | −2.04 (3.5)* | −1.19 (3.2)* |
| CRN | 4.57 (3.3) | 4.80 (3.1) | 4.77 (2.8) | 4.83 (2.6) |
| ΔERN | −5.45 (3.8) | −5.69 (3.8) | −6.81 (3.6)* | −6.0 (3.0)* |
| Pe | 6.74 (3.6) | 7.05 (3.5) | 6.82 (4.0) | 6.13 (3.5) |
| Correct Pe | 0.25 (1.5) | 0.60 (1.4) | 0.61 (2.0) | 0.56 (1.7) |
| ΔPe | 6.49 (2.8) | 6.44 (2.7) | 6.21 (3.0) | 5.57 (2.7) |
p < .05 indicates a difference between the peer condition and the alone condition within each group.
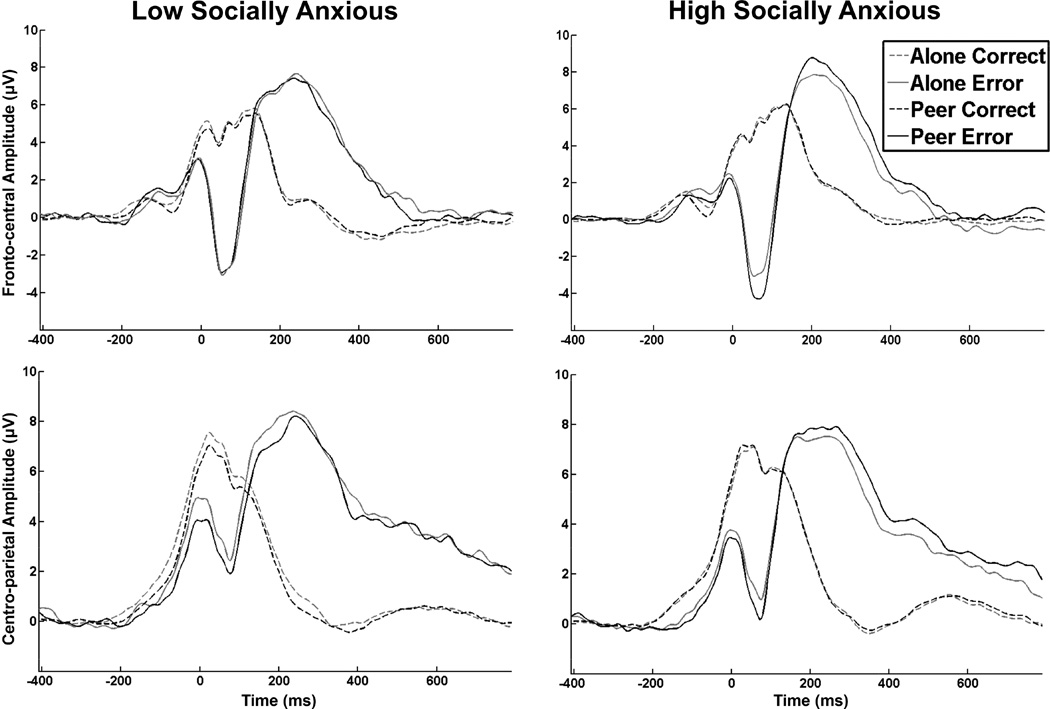
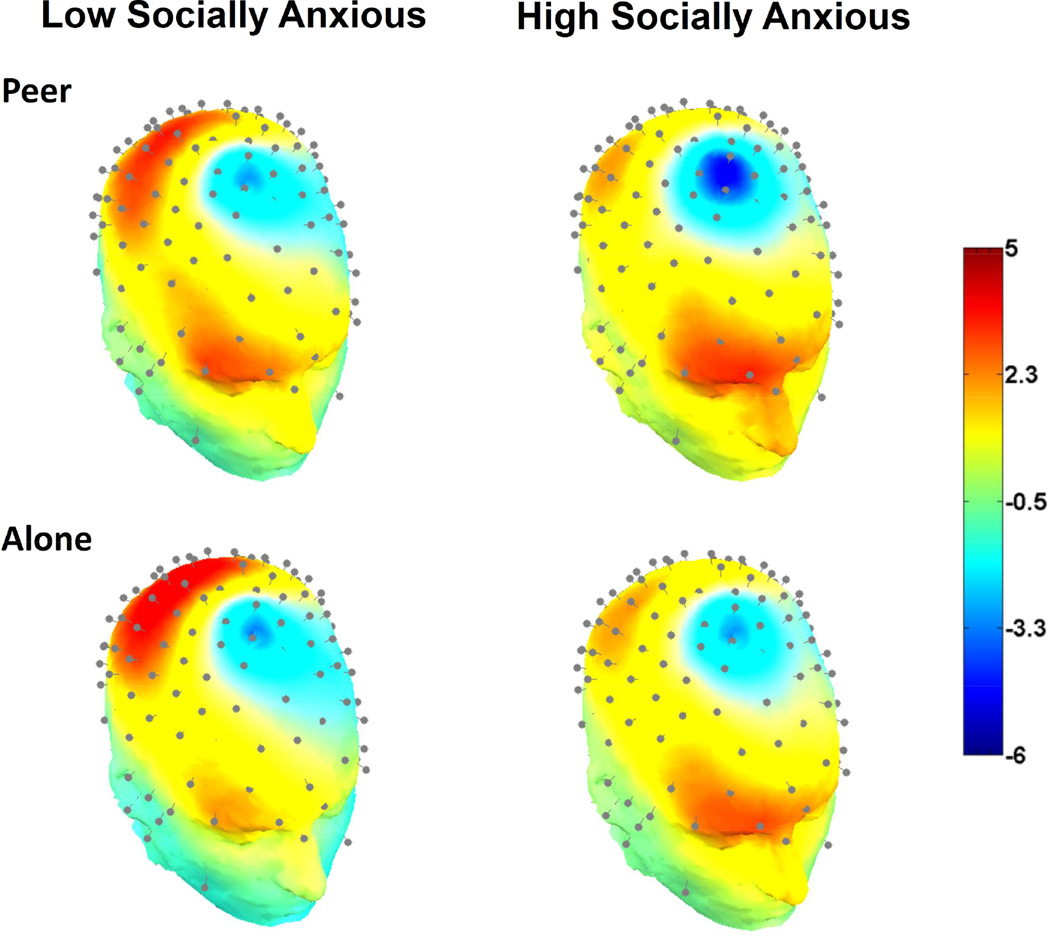
Figure 1 presents the ERP waveforms across the alone and peer conditions for HSA and LSA groups at the fronto-central electrode grouping (where the ERN was maximal) and at centro-parietal electrode grouping (where the Pe was maximal). Figure 2 presents the scalp topography of the ERN for the LSA and HSA groups across each condition. Means for all ERP measures for each condition for the HSA and LSA are presented in Table 2. Analysis of the neural activity during error responses (i.e., ERN) and correct responses (i.e., CRN) indicated a main effect of response, where the ERN was significantly larger (i.e., more negative) than the CRN across both conditions and groups, F(1, 41) = 126.91, p < .001, η2 =.76. However, this main effect was qualified by a significant 3-way (group x condition x response) interaction, F(1, 41) = 4.86, p = .033, η2 =.11, suggesting that the HSA and LSA groups demonstrated different patterns of the ERN and CRN across conditions.1 To explore this interaction, separate 2 (condition) x 2 (response) ANOVA’s were conducted for each anxiety group. For the LSA group, there were no differences in the ERN or the CRN between conditions, F(1, 20) = 0.10, p = .76, η2 =.01, nor any interaction between the ERN and CRN, F(1, 20) = 0.60, p = .54, η2 =.03. However, for the HSA group, there was a main effect of condition, suggesting enhanced neural activity in the peer condition than the alone condition, F(1, 21) = 8.01, p = .01, η2 =.28. This was qualified by a condition x response interaction, F(1, 21) = 5.09, p = .035, η2 =.20. Multiple paired-sample t-tests revealed that this interaction was primarily driven by enhancements of the ERN in the peer condition (M = −2.04, SD = 3.4) from the alone condition (M = −1.19, SD = 3.2) for the HSA group, t(21) = 3.57, p = .002, d =.85 (see Figure 2 for scalp topographies of the ERN for the HSA group). No differences in the CRN were observed between the peer and alone conditions for the HSA group, t(21) = 0.23, p = .82, d =.04.
Figure 1.
Response-locked event-related potential (ERP) waveforms on correct and error responses for the peer and alone conditions for low socially anxious (left) and high socially anxious (right) groups. The top row is the fronto-central electrode grouping, where the error-related negativity (ERN) was maximal. The bottom row is the centro-parietal electrode grouping, where the positive error (Pe) was maximal.
Figure 2.
Scalp topographies of the error-related negativity (ERN) for the low socially anxious group (left) and the high socially anxious group (right) during the peer condition (top) and the alone condition (bottom) at 68 ms post-response.
For analysis of the ΔERN, there was a significant condition x group interaction, F(1, 41) = 4.86, p =.033, η2 =.11. Separate follow-up paired-sample t-tests for each group revealed that the HSA group demonstrated a larger ΔERN in the peer condition (M = −6.81, SD = 3.6) than the alone condition (M = −6.01, SD = 3.0), t(21) = 2.26, p = .035, d =.56, whereas there were no differences for the ΔERN between conditions for the LSA group, t(20) = 0.78, p = .45, d = .17.
For the Pe, the repeated measures ANOVA for the centro-parietal electrode grouping (where the Pe was maximal) indicated a main effect of response where the Pe on error trials was significantly larger than the Pe on correct trials across both conditions and groups, F(1, 41) = 222.28, p < .001, η2 =.84. This effect was qualified by a significant condition x group interaction, F(1, 41) = 4.13, p = .049, η2 =.09. However, follow-up ANOVA’s indicated that there were no main effects of condition for the HSA group, F(1, 21) = 2.96, p = .10, η2 =.12, or for the LSA group, F(1, 20) = 1.49, p = .24, η2 =.07.
Table 1 displays the means and standard deviations of the self-report questionnaires. The one-way MANOVA revealed a significant multivariate main effect for group, suggesting that the HSA group reported more social anxiety (LSAS-SR), general anxiety (PSWQ), and depressive symptoms (BDI) than the LSA group, Wilks’ λ = .114, F(3, 39) = 101.30, p < .001, η2 = .88. As expected, there were significant correlations among all questionnaire measures. Social anxiety symptoms were positively related to both symptoms of general anxiety, r(41) = .60, p <.001, and depressive symptoms, r(41) = .40 p =.008. General anxiety symptoms were also positively related to depressive symptoms, r(41) = .45 p =.002.
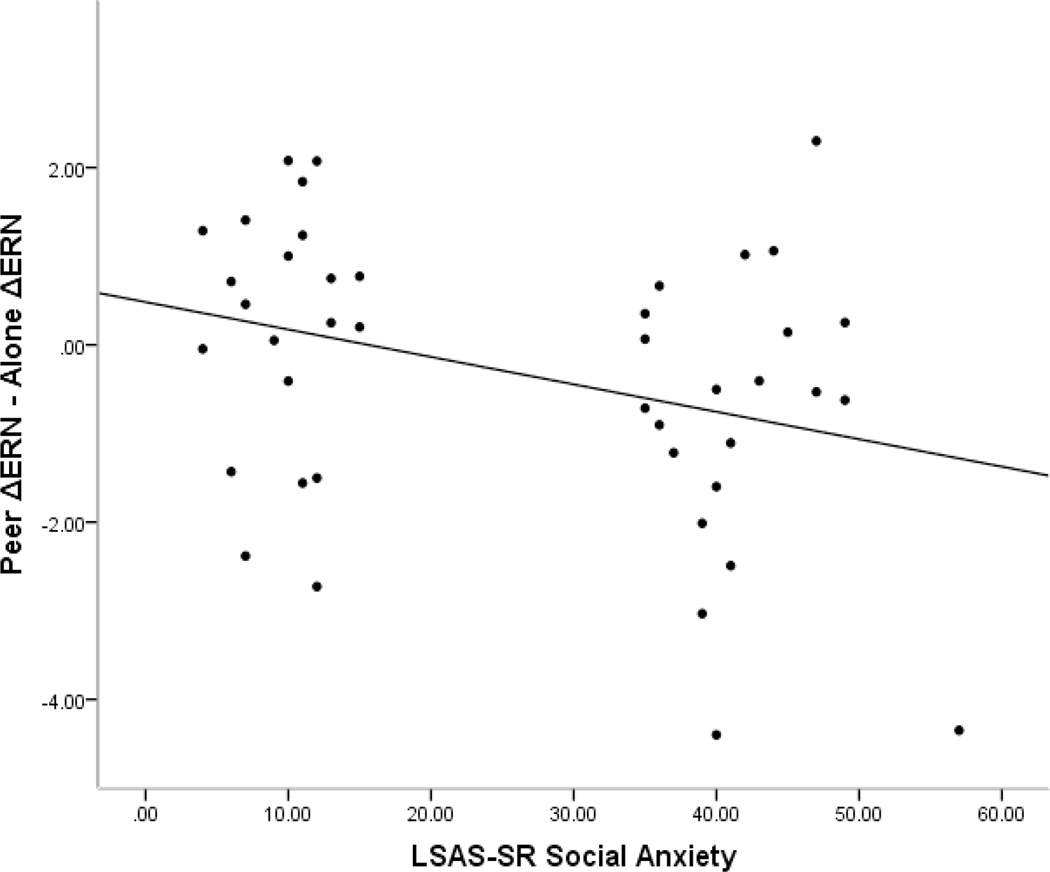
Table 3 displays the Pearson correlation coefficients between ERP measures and self-report questionnaires. Across the whole sample, ERP measures from both conditions were unrelated to measures of social anxiety, general anxiety, and depression (ps > .18). However, changes in the ΔERN across conditions (Peer ΔERN – Alone ΔERN) was negatively correlated with social anxiety symptoms, such that a larger ΔERN in the peer condition relative to alone condition was related to more social anxiety symptoms, r(41) = −.33, p = .030 (see Figure 3).2 The change in the ΔERN across conditions was also marginally associated with general anxiety symptoms, r(41) = −.30, p = .051. The regression model revealed that neither the LSAS social anxiety nor the PSWQ general anxiety uniquely predicted the ΔERN across conditions when both scales were included in the same model (ps > .20). For the ΔPe, changes across conditions (Peer ΔPe – Alone ΔPe) were positively correlated with general anxiety symptoms, r(41) = −.31, p = .047.
Table 3.
Pearson correlation coefficient for event-related potential (ERP) and self-report measures. The top rows represent ERP’s during the alone condition. The bottom rows represent the differences in ERP measures between the alone and peer conditions (i.e., Peer – Alone).
| LSAS-SR | PSWQ | BDI-II | |
|---|---|---|---|
| Alone | |||
| ERN | .01 | −.14 | −.07 |
| CRN | .03 | −.03 | .05 |
| ΔERN | −.03 | −.12 | −.12 |
| Pe | −.13 | −.07 | −.18 |
| Correct Pe | −.03 | −.07 | −.09 |
| ΔPe | −.15 | −.05 | −.23 |
| Social Effect (Alone - Peer) |
|||
| ERN | −.30† | −.17 | −.05 |
| ΔERN | −.33* | −.30† | −.05 |
| Pe | .25 | .21 | −07 |
| ΔPe | .17 | .31* | .01 |
p < .05
p <
Figure 3.
Scatter-plot depicting the relation between the Total Anxiety scale of the LSAS-SR and the change in ΔERN across the peer and alone conditions (Peer ΔERN – Alone ΔERN). A negative value indicates a larger (i.e., more negative) ΔERN in the peer condition as compared to the alone condition.
Discussion
The goal of the present study was to examine error-related brain activity in socially anxious individuals across social and nonsocial contexts. We explored whether highly socially anxious individuals exhibit greater enhancements of the error-related negativity (ERN) in a social than nonsocial context as compared to low socially anxious individuals. As hypothesized, we found a significant interaction between social anxiety and context, such that the ERN and the ΔERN were enhanced in the social context as compared to the nonsocial context only among the high socially anxious individuals. Furthermore, the degree to which the ERN was enhanced in social contexts significantly correlated with social anxiety symptoms. These findings suggest that social context uniquely modulates the ERN in socially anxious individuals.
Current theory suggests that the ERN represents a defensive response to error commission (Hajcak & Foti, 2008; Proudfit et al., 2013). Errors are an aversive event that cause a range of physiological changes associated with enhanced vigilance to threat, such as heart rate deceleration (Hajcak, McDonald, & Simons, 2003b), elevated skin conductance (Hajcak et al., 2003b), pupil dilation (Critchley, Tang, Glaser, Butterworth, & Dolan, 2005), and potentiation of the startle reflex (Hajcak & Foti, 2008; Riesel et al., 2013). Errors committed in a social context are particularly distressing, especially for socially anxious individuals (Hewitt et al., 2003; Schlenker & Leary, 1982), reflecting enhanced vigilance to perceived social threats. A number of studies have found that socially anxious individuals exhibit enhanced attention and vigilance to social information, such as biases toward orienting to threatening facial expressions (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Mogg & Bradley, 2002; Mogg, Philippot, & Bradley, 2004; Pishyar, Harris, & Menzies, 2004), and biases in interpreting social contexts as threatening (Amir, Beard, & Bower, 2005; Constans, Penn, Ihen, & Hope, 1999). Furthermore, social anxiety symptoms are reduced following training to avoid orienting toward social threats (Amir et al., 2009).
The present study adds to the growing literature that the ERN is sensitive to contextual factors (Hajcak et al., 2005; Kim et al., 2005; Van Meel & Van Heijningen, 2010). In the present study, we found that the ERN was influenced by social contextual factors only among high socially anxious individuals. One likely reason we did not observe an enhanced ERN in social contexts in both high and low socially anxious individuals is that we recruited participants who scored at the extremes in social anxiety, which maximized the role of individual differences in influencing the ERN. Other work also suggests that individual differences moderate motivational influences of the ERN (Pailing & Segalowitz, 2004). For example, Dikman and Allen (2000) compared the ERN in subjects characterized as high or low in socialization across conditions when errors were punished or when correct responses were rewarded. The authors found that low socialized individuals exhibited a reduced ERN in the punishment condition than the reward condition whereas high socialized individuals demonstrated no differences between conditions. Similarly, Riesel and colleagues (2012) found that the degree to which the ERN was enhanced when errors were punished was related to anxiety symptoms. Thus, the present findings extend such prior work specifically to individual-level and contextual social factors.
A large body of research has demonstrated that an enhanced ERN is characteristic of individuals with anxiety (Endrass et al., 2008; Gehring et al., 2000; Weinberg, Klein, & Hajcak, 2012; Weinberg et al., 2010). However, in the present study, no differences in the ERN were observed between high and low socially anxious individuals in nonsocial contexts. In the one published study examining social anxiety and the ERN in adults, Endrass, and colleagues (2014) found that both adults with social anxiety disorder (SAD) and adults with obsessive compulsive disorder (OCD) demonstrated an enhanced ERN as compared to healthy comparisons. However, social anxiety symptoms were unrelated to the magnitude of the ERN, suggesting that other symptoms common to both disorders may explain the observed enhanced ERN. It has been suggested that the enhanced ERN observed among many anxiety disorders is driven by symptoms of general distress/anxious apprehension (Moser et al., 2012, 2013; Simons, 2010; Weinberg et al., 2010), which is a core symptom of most anxiety disorders (Clark & Watson, 1991; Watson, 2005). Thus, future research should further explore whether social anxiety symptoms within clinical anxiety disorders are also related to the magnitude of the ERN.
The degree to which the ERN was enhanced in social contexts from nonsocial contexts (i.e., Peer ΔERN - Alone ΔERN) was most strongly associated with social anxiety symptoms, but also related to general anxiety symptoms. This finding is not surprising given that social anxiety and general anxiety are highly correlated and comorbid (Grant et al., 2005; Mennin, Heimberg, & Jack, 2000; Watson, 2005). We also observed a strong correlation between LSAS social anxiety and PSWQ general anxiety. Thus, it is difficult to determine if an enhanced ERN observed amongst socially anxious individuals in social contexts was primarily driven by social anxiety symptoms or more general anxiety symptoms (i.e., worry). However, it is important to note that social concerns are one of the main worries reported by adults (Ladouceur et al., 2002), and social worries are critical in the etiology of SAD (Clark & Wells, 1995; Rapee & Heimberg, 1997). Thus, many of the worries reported by highly socially anxious individuals may represent worries related to social factors. The PSWQ, our primary measure of anxious apprehension (i.e., general worry), does not differentiate social from nonsocial worries. Thus it is difficult to resolve this issue in the present study. This issue of specificity of the ERN and anxiety relation was recently examined by Zambrano-Vazquez and Allen (2014), who recruited high obsessive compulsive subjects and high worry subjects and found that only the high worry subjects exhibited an enhanced ERN. Future research on specificity on the relation between social anxiety and the ERN is needed.
The ERN is a candidate biological endophenotype for anxiety disorders, mediating early genetic risk and the later development of anxiety (Gottesman & Gould, 2003; Olvet & Hajcak, 2008). The magnitude of the ERN is relatively stable within an individual though childhood (Meyer, Bress, & Proudfit, 2014) and adulthood (Olvet & Hajcak, 2009; Weinberg & Hajcak, 2011), and it is heritable (Anokhin, Golosheykin, & Heath, 2008). Furthermore, an enhanced ERN is characteristic of individuals with personal or family history of anxiety disorders (Carrasco, Harbin, et al., 2013; Carrasco, Hong, et al., 2013; Gehring et al., 2000; Riesel, Endrass, Kaufmann, & Kathmann, 2011). The relation between anxiety and the ERN emerges in childhood (Carrasco, Harbin, et al., 2013; Ladouceur et al., 2006; Meyer et al., 2013; Meyer, Weinberg, Klein, & Hajcak, 2012), suggesting that the ERN may reflect a biological marker of early dispositional responses to threat (Proudfit et al., 2013). Indeed, children characterized as behaviorally inhibited, a temperament identified in early childhood and characterized by fear of novel social stimuli (Fox et al., 2001; Kagan, Reznick, Snidman, Gibbons, & Johnson, 1988), demonstrate an enhanced ERN at 7 years of age (Lahat et al., 2014). However, little research has explored whether individual differences in temperament, such as behavioral inhibition, may interact with contextual factors in predicting the magnitude of the ERN. Brooker & Buss (2014) examined the ERN in temperamentally fearful children and found that harsh parenting moderated the relation between fearfulness at age two and the ERN at age four such that there was a positive association between the ERN and fearfulness only among subjects exposed to greater harsh parenting. Future studies should utilize similar contextual modulation procedures to determine if an elevated ERN in social contexts may predict the emergence of later social anxiety symptoms among behaviorally inhibited children.
There are a number of limitations in the present study that should be addressed. First, no information on psychiatric diagnoses were collected. Thus it is unknown whether participants in the current study met diagnosis for SAD or any other psychiatric disorders or whether the high socially anxious group are representative of patients with SAD. Using the LSAS total score, a clinical cut-off of 60 or above has been suggested to represent high probability of an SAD diagnosis (Mennin et al., 2002). In the present study, 21 out of the 22 participants in the high socially anxious group scored above this cut-off, whereas no participants reached this cut-off in the low socially anxious group. In addition, we did not collect any information on medication status or brain injury, which are factors known to influence the ERN (de Bruijn, Sabbe, Hulstijn, Ruigt, & Verkes, 2006; Swick & Turken, 2002), thus making it unknown whether such variables influenced the present findings. Future should utilize a similar social manipulation procedure in patients with SAD and healthy comparisons as well as collect more extensive information on medication use and neurological impairments.
It is also important to note that although we found that the ERN was robustly modulated by social motivational factors among high socially anxious individuals, it is unknown whether socially anxious individuals perceived errors that occurred during social observation and evaluation as more socially threatening or anxiety inducing than the low socially anxious individuals. Thus, it is difficult to know whether an enhanced ERN in social contexts among high socially anxious individuals was due to enhanced social motivation, anxious arousal, worry, or some other factor. Future research should document state changes in motivation and anxiety across different contexts to better delineate psychological mechanisms that enhances the ERN. Lastly, by matching the confederate by the gender of the participant, we were unable to explore the effect of gender in influencing the ERN. Although social anxiety symptoms reported during opposite-gender and same-gender interactions are highly correlated (Robins et al., 1988), there is evidence that opposite-gender situations may cause greater physiological arousal among both socially anxious adults and healthy controls (Turner, Beidel, & Larkin, 1986). Thus, an enhanced ERN among social contexts may be greater during opposite-gender social observation and evaluation. Future research should explore this possibility.
In sum, the present study investigated whether highly socially anxious individuals exhibit differences in error monitoring, as measured by the ERN and related ERP components, across social and nonsocial contexts as compared to less socially anxious individuals. Findings revealed socially anxious individuals exhibit an enhanced ERN when errors were committed during social evaluative contexts as compared to errors committed alone. No differences were observed between social and nonsocial contexts for less socially anxious individuals. Furthermore, individual differences in social anxiety were related to the degree to which the ERN was elevated in social contexts from nonsocial contexts. These findings suggest that social anxiety is characterized by an enhanced ERN during the observation and evaluation of errors, which is suggestive of enhanced defensive reactivity and vigilance to social situations.
Acknowledgments
This research was partially supported by the National Institutes of Health (Grant Nos. HDR3717899 to Nathan A. Fox and 5T32HD007542 to Melanie Killen). Daniel S. Pine is supported by the NIMH-Intramural Research Program.
Footnotes
To examine the influence of extreme values on these ANOVA results, the relatively conservative Robust ANOVA procedure (Keselman, Wilcox, & Lix, 2003) was utilized in the ERP Toolkit as part of a secondary analysis (Dien, 2010). The omnibus Robust ANOVA revealed a main effect of response, TWJt/c(1.0,32.6)=123.71, p < 0.001. This was qualified by a condition x response x anxiety group interaction approaching significance TWJt/c(1.0,36.7)=3.69, p = 0.059. Follow-up analyses utilizing the Robust ANOVA procedure also yielded similar results.
Although several data points in the bivariate correlation were influential observations, analysis of residuals revealed no outliers.
The authors declare no competing financial interests.
References
- Amir N, Beard C, Bower E. Interpretation bias and social anxiety. Cognitive Therapy and Research. 2005;29:433–443. doi: 10.1007/s10608-009-9258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, Chen X. Attention training in individuals with generalized social phobia: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77:961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry. 2005;57:975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: Implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beidel DC, Turner SM, Dancu CV. Physiological, cognitive and behavioral aspects of social anxiety. Behaviour Research and Therapy. 1985;23:109–117. doi: 10.1016/0005-7967(85)90019-1. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Toddler fearfulness is linked to individual differences in error-related negativity during preschool. Developmental Neuropsychology. 2014;39:1–8. doi: 10.1080/87565641.2013.826661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Antony MM, Barlow DH. Psychometric properties of the Penn State Worry Questionnaire in a clinical anxiety disorders sample. Behaviour Research and Therapy. 1992;30:33–37. doi: 10.1016/0005-7967(92)90093-v. [DOI] [PubMed] [Google Scholar]
- Bumberry W, Oliver MJ, McClure JN. Validation of the Beck Depression Inventory in a university population using psychiatric estimate as the criterion. Journal of Consulting and Clinical Psychology. 1978;46:150–155. [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depression and Anxiety. 2013;30:39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Hong C, Nienhuis JK, Harbin SM, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and other anxiety disorders. Neuroscience Letters. 2013;541:214–218. doi: 10.1016/j.neulet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social phobia: Diagnosis, assessment, and treatment. New York: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Constans JI, Penn DL, Ihen GH, Hope DA. Interpretive biases for ambiguous stimuli in social anxiety. Behaviour Research and Therapy. 1999;37:643–651. doi: 10.1016/s0005-7967(98)00180-6. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson L, Berardi L, Grillon C. Anticipation of public speaking in virtual reality reveals a relationship between trait social anxiety and startle reactivity. Biological Psychiatry. 2006;59:664–666. doi: 10.1016/j.biopsych.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Davey GCL. A comparison of three worry questionnaires. Behaviour Research and Therapy. 1993;31:51–56. doi: 10.1016/0005-7967(93)90042-s. [DOI] [PubMed] [Google Scholar]
- De Bruijn ERA, Sabbe BGC, Hulstijn W, Ruigt GSF, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Research. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dikman ZV, Allen JJB. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46:1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Endrass T, Riesel A, Kathmann N, Buhlmann U. Performance monitoring in obsessive-compulsive disorder and social anxiety disorder. Journal of Abnormal Psychology. 2014;123:705–714. doi: 10.1037/abn0000012. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: The broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Fox AN, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, Goetz D. The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Furlan PM, DeMartinis N, Schweizer E, Rickels K, Lucki I. Abnormal salivary cortisol levels in social phobic patients in response to acute psychological but not physical stress. Biological Psychiatry. 2001;50:254–259. doi: 10.1016/s0006-3223(00)01126-4. [DOI] [PubMed] [Google Scholar]
- Ganushchak LY, Schiller NO. Motivation and semantic context affect brain error-monitoring activity: An event-related brain potentials study. NeuroImage. 2008;39:395–405. doi: 10.1016/j.neuroimage.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Geen RG. Social motivation. Annual Review of Psychology. 1991;42:377–399. doi: 10.1146/annurev.ps.42.020191.002113. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gillis MM, Haaga DAF, Ford GT. Normative values for the Beck Anxiety Inventory, Fear Questionnaire, Penn State Worry Questionnaire, and Social Phobia and Anxiety Inventory. Psychological Assessment. 1995;7:450–455. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Blanco C, Stinson FS, Patricia S, Goldstein RB, Huang B. The epidemiology of social anxiety disorder in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Journal of Clinical Psychiatry. 2005;66:1351–1361. doi: 10.4088/jcp.v66n1102. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Hajcak G. What we’ve learned from mistakes insights from error-related brain activity. Current Directions in Psychological Science. 2012;21:101–106. [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: Defensive motivation and the error-related negativity. Psychological Science. 2008;19:103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003a;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003b;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, Liebowitz MR. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychological Medicine. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- Hewitt PL, Flett GL, Sherry SB, Habke M, Parkin M, Lam RW, Stein MB. The interpersonal expression of perfection: Perfectionistic self-presentation and psychological distress. Journal of Personality and Social Psychology. 2003;84:1303–1325. doi: 10.1037/0022-3514.84.6.1303. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MGH. Error-related scalp potentials elicited by hand and foot movements: Evidence for an output-independent error-processing system in humans. Neuroscience Letters. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Development. 1988;59:1580. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Wilcox RR, Lix LM. A generally robust approach to hypothesis testing in independent and correlated groups designs. Psychophysiology. 2003;40:586–596. doi: 10.1111/1469-8986.00060. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T. Error-related negativity in children: Effect of an observer. Developmental Neuropsychology. 2005;28:871–883. doi: 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur R, Freeston MH, Fournier S, Dugas MJ, Doucet C. The social basis of worry in three samples: high-school students, university students, and older adults. Behavioural and Cognitive Psychotherapy. 2002;30:427–438. [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C, Hunt W. The startle pattern. x. Oxford, England: Farrar & Rinehart; 1939. [Google Scholar]
- Larson MJ, Baldwin SA, Good DA, Fair JE. Brief reports: Temporal stability of the error-related negativity (ERN) and post-error positivity (Pe): The role of number of trials. Psychophysiology. 2010;47:1167–1171. doi: 10.1111/j.1469-8986.2010.01022.x. [DOI] [PubMed] [Google Scholar]
- Levin AP, Saoud JB, Strauman T, Gorman JM, Fyer AJ, Crawford R, Liebowitz MR. Responses of “generalized” and “discrete” social phobics during public speaking. Journal of Anxiety Disorders. 1993;7:207–221. [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, George MS. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Mauss I, Wilhelm F, Gross J. Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition and Emotion. 2004;18:631–642. [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM, Heimberg RG, Schneier FR, Davies SO, Liebowitz MR. Screening for social anxiety disorder in the clinical setting: Using the Liebowitz Social Anxiety Scale. Journal of Anxiety Disorders. 2002;16:661–673. doi: 10.1016/s0887-6185(02)00134-2. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Jack MS. Comorbid generalized anxiety disorder in primary social phobia: Symptom severity, functional impairment, and treatment response. Journal of Anxiety Disorders. 2000;14:325–343. doi: 10.1016/s0887-6185(00)00026-8. [DOI] [PubMed] [Google Scholar]
- Meyer A, Bress JN, Proudfit GH. Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology. 2014;51:602–610. doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, Klein DN. Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology. 2013;41:1257–1266. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Jendrusina AA. Parsing relationships between dimensions of anxiety and action monitoring brain potentials in female undergraduates. Psychophysiology. 2012;49:3–10. doi: 10.1111/j.1469-8986.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in Human Neuroscience. 2013:7. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Research. 2009;1284:89–99. doi: 10.1016/j.brainres.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Corrigenda: EEG 02274. Electroencephalography and Clinical Neurophysiology. 1990;76:565. [Google Scholar]
- Pishyar R, Harris LM, Menzies RG. Attentional bias for words and faces in social anxiety. Anxiety, Stress & Coping. 2004;17:23–36. [Google Scholar]
- Proudfit GH, Inzlicht M, Mennin DS. Anxiety and error monitoring: The importance of motivation and emotion. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Spence SH. The etiology of social phobia: Empirical evidence and an initial model. Clinical Psychology Review. 2004;24:737–767. doi: 10.1016/j.cpr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: Evidence from unaffected first-degree relatives. The American Journal of Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49:239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Moran T, Hajcak G. Time course of error-potentiated startle and its relationship to error-related brain activity. Journal of Psychophysiology. 2013;27:51–59. [Google Scholar]
- Robins CJ. Sex role perceptions and social anxiety in opposite-sex and same-sex situations. Sex Roles. 1986;14:383–395. [Google Scholar]
- Scheffers MK, Coles MGH. Performance monitoring in a confusing world: Error-related brain activity, judgments of response accuracy, and types of errors. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Schlenker BR, Leary MR. Social anxiety and self-presentation: A conceptualization model. Psychological Bulletin. 1982;92:641–669. doi: 10.1037/0033-2909.92.3.641. [DOI] [PubMed] [Google Scholar]
- Simons RF. The way of our errors: Theme and variations. Psychophysiology. 2010;47:1–14. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SM, Beidel DC, Larkin KT. Situational determinants of social anxiety in clinic and non clinic samples:Physiological and cognitive correlates. Journal of Consulting and Clinical Psychology. 1986;54:523–527. doi: 10.1037//0022-006x.54.4.523. [DOI] [PubMed] [Google Scholar]
- Van Meel CS, Van Heijningen CAA. The effect of interpersonal competition on monitoring internal and external error feedback. Psychophysiology. 2010;47:213–222. doi: 10.1111/j.1469-8986.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vidal F, Burle B, Bonnet M, Grapperon J, Hasbroucq T. Error negativity on correct trials: A reexamination of available data. Biological Psychology. 2003;64:265–282. doi: 10.1016/s0301-0511(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the “error negativity” specific to errors? Biological Psychology. 2000;51:109–128. doi: 10.1016/s0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Longer term test-retest reliability of error-related brain activity. Psychophysiology. 2011;48:1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology. 2012;121:885–896. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Zambrano-Vazquez L, Allen JJB. Differential contributions of worry, anxiety, and obsessive compulsive symptoms to ERN amplitudes in response monitoring and reinforcement learning tasks. Neuropsychologia. 2014;61:197–209. doi: 10.1016/j.neuropsychologia.2014.06.023. [DOI] [PubMed] [Google Scholar]