Abstract
Essential hypertension (EH) results when the balance between vasoconstriction and vasodilation is shifted in favor of vasoconstriction. This balance is controlled by the interaction of genetic and epigenetic factors. When there is an unstable balance, vitamin D deficiency as an epigenetic factor triggers a shift to the side of vasoconstriction. In this article, we critically analyze clinical findings on the effect of vitamin D on blood pressure, combined with progress in molecular mechanisms. We find that vitamin D repletion exerts a clinically significant antihypertensive effect in vitamin D-deficient EH patients. Of note, a few trials reported no antihypertensive effect from vitamin D due to suboptimal study design. Short-term vitamin D supplementation has no effect on blood pressure in normotensive subjects. This could explain the mixed results and may provide a theoretical basis for future trials to identify beneficial effects of vitamin D in intervention for EH.
Keywords: Essential Hypertension, Vitamin D, Clinical Trial, Molecular Mechanism
Essential Hypertension (EH)
One billion people worldwide suffer from hypertension (HTN) [1]. Of these patients, 95% have hypertension of unknown etiology, called essential hypertension (EH), and cannot maintain normal blood pressure (BP) without daily treatment [2]. Many signaling pathways are involved in BP regulation in EH. These include the angiotensin II (Ang II)-sympathetic nerve-CD4+ T cell system [3], a pathway consisting of a series of genes participating in the control of renal salt handling [4], and pathways mediating constriction and dilation of vascular smooth muscle (VSM) cells [5, 6]. Dysfunction of any one of these pathways leads to increased VSM tone and remodeling in resistance arteries, resulting in high BP. However, the exact etiology and pathogenesis of EH is poorly understood, leading to nonspecific and less-effective treatment. In America, 50% of hypertensive patients (∼68 million) do not have their BP well-controlled and about 5 million patients are resistant to antihypertensive treatment with a combination of at least three antihypertensive medications [7]. The lack of well-controlled high BP leads to target organ damage. Thus, EH has become a major risk factor for heart attack, stroke, chronic heart failure, and renal failure [8].
From family and twin studies, the incidence of genetic HTN is estimated to be about 50% of EH. However, despite intense efforts, identified gene variants causing monogenic HTN represent less than 1% of EH. Because no common gene variants contribute a large effect size for this common disease, recent genetic discoveries have little utility in clinical practice [2]. With advances in genomic, epigenomic, transcriptomic, and proteomic research technologies, it is now possible to find novel rare genetic variants with significant effects on EH. These can be captured by larger genome-wide association studies combined with sequencing large targeted stretches of DNA, whole exome, or genome using next-generation sequencing techniques [9, 10]. In addition, research advances in epigenetic/epigenomic modification play a partial role in genetic hypertension; these include DNA methylation analysis at the genome level using microarray hybridization or next-generation sequencing, posttranslational modification of histone analyzing by the proteomic technique, and regulatory non-coding RNA analysis through the transcriptomic technique [11-13]. Environmental risk factors, such as stress, high-salt diet, obesity, and vitamin D deficiency have been considered key causes for the other 50% of EH. Advances in our knowledge of environmental factors/gene interactions might explain how environmental factors, including high-salt diet [6] and vitamin D deficiency [Chen et al, unpublished data], could influence BP by inducing target gene expression in specific tissues through specific signaling pathways. One of the advanced approaches in this field is determining the function of target genes, identified by microarray hybridization in global gene knockout and tissue-specific gene knockout animal models, in regulating BP.
In this article, we focus on the role of one potential environmental risk factor, vitamin D deficiency, in EH. We critically analyzed clinical trials on the effect of vitamin D supplementation on BP and reviewed the progress in our understanding of the molecular mechanisms underlying vitamin D deficiency-induced HTN. Combining these with our basic and clinical research, we propose a hypothesis that could explain the controversy in the literature regarding the results of previous clinical trials; this could provide a theoretical basis for future clinical trials.
Vitamin D Biology and Deficiency
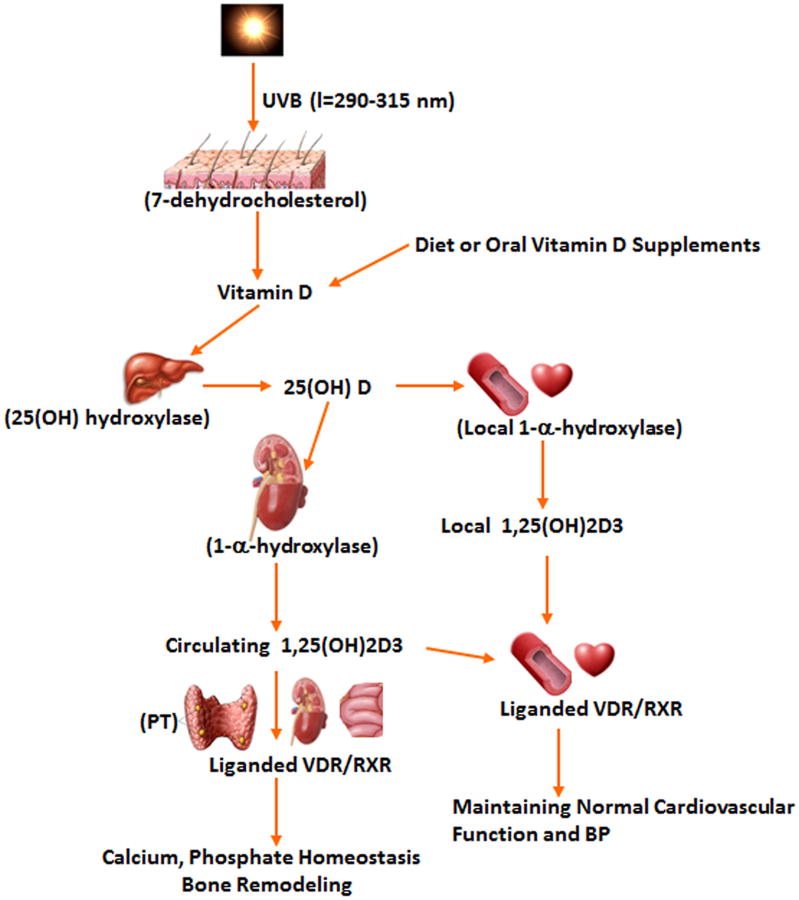
In human, the majority of naturally occurring vitamin D (∼80%) is synthesized in the skin from 7-dehydrocholesterol by ultraviolet (UV) B radiation. About 20% of vitamin D comes from dietary sources, such as oily fishes, egg yolks, fortified milk, cereal, juice, and yogurt (Fig. 1). Vitamin D from the skin, diet, or oral supplements including vitamin D2 and vitamin D3 are metabolized in the liver to 25-hydroxyvitamin D (25(OH)D). 25(OH)D, the main circulating vitamin D metabolite, is largely bound to vitamin D binding protein in serum, and has been widely accepted as the most reliable marker for determining vitamin D status [14] (vitamin D sufficient (25(OH)D ≥30 ng/ml [or ≥75 nmol/l]); vitamin D insufficient (25(OH)D 20-30 ng/ml [or 50-75 nmol/l]); or vitamin D deficient (25(OH)D <20 ng/ml [or <50 nmol/l])). These cut-off points are currently the most commonly used for vitamin D status [15]. The threshold of 20 ng/ml for vitamin D deficiency was recently endorsed by the Institute of Medicine (IOM) [16], even though there is some debate about this recommendation [17].
Figure 1.
Synthesis of natural product, vitamin D in the regulation of calcium, phosphorous, bone metabolism, BP and cardiovascular function. PT: Parathyroid glands. BP: blood pressure.
Circulating 25(OH)D is converted by renal or extrarenal 1α-hydroxylase into 1,25-dihydroxyvitamin D3 (VD3), which circulates at ∼1000-fold lower serum concentrations than 25(OH)D, but is the biologically active agonist for the vitamin D receptor (VDR). Serum levels of VD3 are mainly determined by renal VD3 production, which is closely associated with calcium homeostasis and parathyroid hormone (PTH) level. Lower calcium and high PTH levels will promote VD3 synthesis by increasing 1α-hydroxylase activity, whereas other factors, including fibroblast growth factor-23 and Klotho, suppress the renal conversion of 25(OH)D to VD3 through inhibition of 1α-hydroxylase expression [18]. Indeed, many other cell types, including those of the vascular wall and heart, express 1α-hydroxylase with subsequent conversion of 25(OH)D to the local production of VD3, which exerts its biological effects locally in an autocrine or paracrine manner (see below) [19-21]. It is unclear whether local 1α-hydroxylase expression is regulated by serum calcium and PTH levels.
In addition to the small intestine, parathyroid glands, and kidneys (the primary organs responsive to vitamin D), other tissues and organs (e.g. the cardiovascular system, skeletal muscle, and immune system) also respond to vitamin D. VDR is expressed in many tissues and organs, including those not typically associated with bone metabolism and calcium homeostasis. These include the heart, VSM, endothelium, and immune cells, including CD4+ T cells [19, 22-24]. VDR is a nuclear receptor that binds to VD3 with high affinity and specificity. When occupied by VD3, VDR is phosphorylated, which leads to a surface conformational change. Activated VDR then interacts with the retinoid X receptor (RXR) to form a heterodimer that binds to vitamin D-responsive elements in the region of the gene promoter [25, 26]. By recruiting complexes of either coactivators or corepressors [27, 28], activated VDR/RXR regulates the transcription of genes encoding proteins that exert the traditional and non-traditional functions of VD3 (e.g., maintaining musculoskeletal health, calcium homeostasis, and normal BP and cardiovascular function) [29, 30]. Therefore, the role of vitamin D in a cell, tissue, or organ depends on the local production or delivery of a sufficient amount of VD3, expression levels of VDR/RXR and co-receptor proteins, and cell-specific transcriptional responses to regulate genes that encode proteins mediating vitamin D signaling.
Although the role of locally synthesized VD3 remains unknown in systemic effects, the biological function of VD3 (local or systemic production) is clearly dependent on the availability of adequate substrate, the circulating 25(OH)D; therefore, circulating 25(OH)D is considered the best indicator of whole-body vitamin D status. Lower 25(OH)D (<20 ng/ml) leads to reduced VD3 production and subsequently impairs activated VDR-mediated biological function.
More than 1 billion people currently are at risk for vitamin D deficiency worldwide [15]. This pandemic of hypovitaminosis D can mainly be attributed to decreased exposure to sunlight (for example, reduced outdoor activities and air pollution), which is required for UVB-induced vitamin D production in the skin. Levels of UVB radiation diminish with increasing distance from the earth's equator during the winter months. Black people absorb more UVB in the melanin of their skin than white people; therefore, blacks require more sun exposure to produce the same amounts of vitamin D. In addition to musculoskeletal problems, vitamin D deficiency has been associated with an increased risk of cardiovascular events [31, 32], especially HTN [33, 34].
Vitamin D Deficiency and EH: Cross-sectional and Perspective Studies
The prevalence of hypovitaminosis D is directly attributable to higher latitudes because of less intense UVB radiation, colder climates due to less skin exposure, and darker skin as it impedes UVB penetration and reduces vitamin D production [35]. The fact that a higher incidence of EH occurs during the winter, in people living in higher latitudes, and in those with deep skin pigmentation living far from the equator [36] makes it reasonable to speculate that vitamin D deficiency may contribute to increased prevalence of EH. To test this hypothesis, Krause et al. [37] used UVB irradiation to treat patients with untreated mild EH and vitamin D deficiency. These investigators found that UVB radiation, but not UVA radiation, increased 25(OH)D levels and lowered BP in vitamin D-deficient patients with EH. Since 1998, this finding has raised considerable research interest in the relationship between vitamin D deficiency and EH.
Most of the major cross-sectional studies [38-45] (Table 1) showed that BP was inversely and significantly correlated with 25(OH)D levels. Burgaz et al. [46] conducted a meta-analysis, including four prospective studies and 14 cross-sectional studies, to evaluate the association between circulating 25(OH)D level and HTN. They found an inverse relationship between serum 25(OH)D concentration and HTN incidence, with an odds ratio of 0.73 for the highest versus the lowest category of blood 25(OH)D. Most but not all prospective findings, including eight main studies [47-54] listed in Table 1, supported an association between vitamin D deficiency and increased risk of EH. To provide a reliably quantified relationship, in 2013 Kunutsor et al. [55] performed a meta-analysis to investigate vitamin D and risk of future HTN. The studies included eight unique prospective cohorts with aggregate data on 283,537 non-overlapping participants and 55,816 incident HTN cases. The pooled relative risks of incident HTN per 10 ng/ml increments in baseline 25(OH)D levels was 0.88, suggesting that the risk of HTN is reduced by 12% with a 10 ng/ml increase in blood 25(OH)D levels.
Table 1. The relationship between 25(OH)D and blood pressure (BP) or prevalence of hypertension (HTN).
| Author (Ref) | Participants (N) | Follow-up (years) | Major findings related to BP or incident HTN |
|---|---|---|---|
| Scragg et al (44) | 12,644 | A reverse association between SBP and 25(OH)D | |
| Martins et al (42) | 15,088 | Lower 25(OH)D with Increased prevalence of HTN | |
| Bhandari et al (38) | 2,722 | Decreased 25(OH)D with Increased prevalence of HTN | |
| Forrest KYZ et al (40) | 4,495 | Reduced 25(OH)D levels association with HTN incidence | |
| Hintzpeter B et al (41) | 4,030 | Lower 25(OH)D levels association with HTN incidence | |
| Dorjgochoo et al (39) | 1,460 | Decreased 25(OH)D with Increased prevalence of HTN | |
| Sabanayagam et al (43) | 9,215 | A reverse association between SBP and 25(OH)D | |
| Skaaby et al et al (45) | 4,330 | No association between 25(OH)D and prevalence of HTN | |
| Forman et al (49) | 1,811 | 4 to 8 | Lower 25(OH)D related to high risk of incident HTN |
| Forman et al (48) | 1,484 | 8 | Reduced 25(OH)D related to high prevalence of HTN |
| Ke et al (52) | 2,271 | 4 | A reverse association between SBP and 25(OH)D |
| Griffin et al (50) | 559 | 14 | Lower 25(OH)D associated with increased risk of systolic HTN |
| Jorde et al (51) | 4,125 | 14 | No association between 25(OH)D and incident HTN |
| Wang et al (54) | 695 | 15.3 | An inverse association between plasma 25(OH)D and risk of HTN |
| Anderson et al (47) | 4,1504 | 1.3 | Lower 25(OH)D associated with higher incidence of HTN |
| Margolis et al (53) | 4,863 | 7 | No association between plasma 25(OH)D and risk of HTN |
To test whether 25(OH)D levels are causally associated with BP and HTN risk, recently Vimaleswaran et al. [56] conducted a mendelian randomization study and used variants of genes that affect 25(OH)D synthesis or substrate availability (vitamin D 25-hydroxylase and 7-dehydrocholesterol reductase) to meta-analyze 146,581 participants. They found that each 10% increase in genetically instrumented 25(OH)D concentration was associated with a decrease in diastolic BP (-0.29 mmHg, P=0.01) and systolic BP (-0.37 mmHg, P=0.052), and an 8.1% reduced odds of HTN (P=0.002). This study used a mendelian randomization approach that largely excludes confounding factors and reduces reverse causation [57], both of which are inherent weakness existing in the observational studies. The findings of this study further confirmed that increased 25(OH)D concentration may reduce the risk of HTN.
Effect of Vitamin D on EH: Randomized Clinical Trials
So far, there have been more than 40 randomized clinical trials to determine the effect of vitamin D supplementation on BP as primary or secondary end points. Since the mechanisms underlying vitamin D's effect on HTN have not yet been elucidated, most of the clinical trials have displayed suboptimal design and results have been mixed. Based on the lack of well-designed, large-scale trials, several meta-analyses have attempted to aggregate prior findings to evaluate the role of vitamin D supplementation in BP. The conclusions have been inconsistent. Wu et al. [58] performed a meta-analysis from four randomized controlled trials of oral vitamin D supplementation in BP. They found that vitamin D supplementation significantly reduced systolic BP by 2.44 mmHg, but not diastolic BP. Witham et al. [59] examined eight randomized controlled trials in participants with mean baseline BP >140/90 mmHg, showing a non-significant reduction in systolic BP and a small significant reduction in diastolic BP (3.1 mmHg). Pooled data from normotensive individuals showed no reduction in BP with any intervention. Most of the trials included in the meta-analysis were conducted more than 20 years ago and 25(OH)D levels were unknown in 50% of the trials. Pittas et al. [60] investigated the role of vitamin D or UVB radiation in BP regulation in 10 trials and found no significant effect of vitamin D on BP. However, when limiting their analyses to studies that used higher doses of vitamin D supplementation (at least 1,000 IU/day), they were able to detect a small but statistically significant effect. In contrast, Elamin et al. [61] found no significant BP-lowering effect of vitamin D supplements in a meta-analysis evaluating pooled cardiovascular outcomes across 14 randomized interventional trials, but made note that there was significant heterogeneity associated with this analysis. Recently, Kunutsor et al. [62] carried out a meta-analysis of 16 randomized clinical trials to evaluate the effect of vitamin D supplementation on BP. They showed no significant reduction of systolic and diastolic BP with vitamin D supplementation, providing evidence of heterogeneity and publication bias. Subgroup analysis displayed a significant reduction in diastolic BP in participants who had preexisting cardiometabolic disease.
Based on the pathophysiology of EH and critically reviewing the results from the published trials, combined with our preliminary findings in clinical trials and mechanistic research, we propose a hypothesis that is composed of two parts to explain the results in most of the published trials. This may also provide a theoretical basis for future trials.
In the first part, EH is an age-dependent complex common trait with interaction between environmental and genetic factors. The incidence of EH dramatically increases in men older than 45 years and women older than 55 years. As an environmental risk factor, vitamin D deficiency is highly likely to be an important trigger to shift the unstable balance between vasoconstriction and vasodilation signaling in favor of vasoconstriction, leading to HTN in vulnerable middle-aged people. Therefore, an appropriately high dose of vitamin D supplementation that normalizes blood 25(OH)D levels should reduce BP in hypertensive patients with vitamin D deficiency (Fig. 2B). This is supported by our ongoing trial (see below) and the first seven trials [37, 63-68] listed in Table 2, in which vitamin D supplementation raised 25(OH)D levels and significantly reduced BP in cohorts that included 50-100% hypertensive patients with vitamin D deficiency or insufficiency, almost all of whom were mean age 45 years or older. Most of these trials were recently conducted and well-designed. Of note, vitamin D repletion exerted a significant but limited amount of BP reduction in the seven trials in Table 2; these trials chose participants with BP <160/95 mmHg and most of them used antihypertensive medications. Actually, vitamin D has a better antihypertensive effect than was seen in the trials mentioned above. In 1988, Lind et al. [69] conducted a placebo-controlled trial evaluating the effect of 0.5 μg alpha-calcidol, a vitamin D analog, on BP in hypertensive patients with impaired glucose tolerance (mean age 62 years, n=26) for 12 weeks. They found a significant reduction of SBP/DBP from 171/95 to 150/88 mmHg after treatment. While current institutional ethics committees will not allow this study to be repeated by recruiting subjects with SBP>160 mmHg in placebo groups, the study's results provide evidence that vitamin D supplementation can lower SBP >20 mmHg in hypertensive patients.
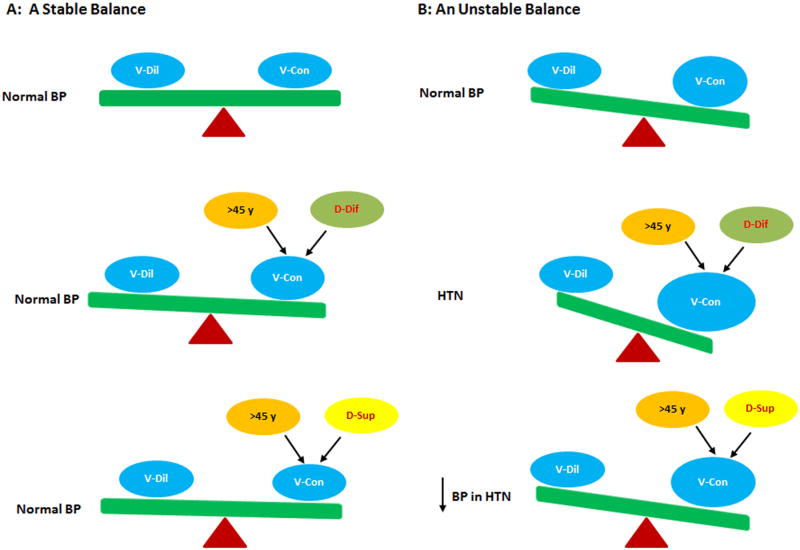
Figure 2.
The effect of vitamin D supplementation on BP. A: When people have a stable balance between vasodilatory (V-Dil) and vasoconstrictory (V-Con) factors to maintain normal BP, vitamin D deficiency (D-Dif) and vitamin D supplementation (D-Sup) in a relatively short period have a minimal effect on BP. B: When people have an unstable balance between vasodilatory and vasoconstrictory factors, vitamin D deficiency becomes a risk trigger to promote the development of HTN at age >45 years; vitamin D repletion is able to reduce BP in this setting.
Table 2. The effect of vitamin D supplementation on BP in hypertensive patients with vitamin D deficiency or insufficiency.
| Author (Ref) | Mean Age (Yrs) | % of HTN Subjects | Mean 25(OH)D (ng/ml) | Intervention | Findings (the changes in 25(OH)D and BP) | |
|---|---|---|---|---|---|---|
| 25(OH)D (ng/ml) | BP (mmHg) | |||||
| Krause et al (37) | 48 | 100 | 23 | Full body UVB (thrice-weekly, 6 wks, n=18) | 60 | ↓ 24-h SBP/DBP (-6/ -6) |
| Sudgen et al (67) | 64 | Not provided (82% taking anti-HTN drugs) | 15 | Vitamin D2 100,000IU once (8wks, n=34) | 21 | ↓ SBP (-14) |
| Witham et al (68) | 65 | 75 | 18 | Vitamin D2 1 or 200,000 IU once (8 wks, n=61) | 25 or 32 | ↓ SBP (- 8.2) or SBP(- 9.3) |
| Larsen et al (65) | 61 | 100 | 23 | Vitamin D3 3,000 IU daily (20 wks, n=112) | 44 | ↓ SBP (-6) and C-SBP(-7) 24-h SBP/DBP (-3/-1) In 92 vitamin D insufficient patients. ↓ 24-h SBP/DBP (-4/-3) |
| Chen et al (63) | 62 | 100 | 19 | Vitamin D3 2,000 IU daily (24 wks, n=126) | 34 | ↓24-h SBP/DBP (-6.2/-4.2) In 113 vitamin D insufficient patients ↓ 24-h SBP/DBP (-7.1/-5.7) |
| Mozaffari et al (66) | 43 | 100 | 18 | Vitamin D3 50,000 weekly (8 wks, n=42) | 52 | ↓ SBP/DBP (-6.4/-2.4) |
| Forman et al (64) | 51 | 50 | 16 | Vitamin D3 1,000, 2,000, 4,000 IU daily (12 wks, n=283) | 30,35 and 46 | SBP(-0.66, -3.4, -4.0) ↓ SBP(-1.4 per 1000 IU vitamin D3/d) |
| Arora et al (85) | 36 | 28 | 16 | Vitamin D3 4,000 IU daily (24 wks, n=383) | 33 | 24-h BP(-0.8/-1.2) |
| Scragg et al (91) | 48 | 15 | 29 | Vitamin D3 200,000 IU for 2 months, 100,100 IU/month for 16 months | 50 | SBP (- 0.6) |
represents a significantly reduction of BP compared with placebo group.
Several early trials showed an antihypertensive effect of vitamin D, even with the lack of reported vitamin D status [69-72]. However, in a few trials no effect of vitamin D on the reduction of BP in hypertensive cohorts (SBP ≥ 140 mmHg) was observed. The major reason for the negative results could be due to suboptimal study design. This could include high levels of background treatment with antihypertensive medications that overlap with vitamin D's antihypertensive role, cohorts with fewer than 40% of participants with vitamin D deficiency, and low or exceptionally high levels of vitamin D intake. Human study has shown that an overly high dose of vitamin D may have a different biological role [73]. Treatment of hypertensive patients, especially the elderly, with this dose of vitamin D may exert a different biological function or produce side effects to counteract its antihypertensive effect (see below). Recently, Beveridge et al. [74] performed a meta-analysis that included clinical trials that used vitamin D supplementation and reported BP. Similar to an earlier meta-analysis [59], vitamin D had no effect on normal BP in 30 trials. They also found no significant reduction of BP by vitamin D in the trials with participants whose mean baseline SBP was ≥140 mmHg. Their eTable 1 lists 15 studies with mean baseline SBP ≥140 mmHg. Seven showed a significant reduction in BP by vitamin D [67-72, 75], including one that reduced BP in some participants. One trial showed that both vitamin D and placebo significantly decreased BP in 65% of patients taking two or more antihypertensive drugs [76]. Seven trials failed to show a vitamin D effect, including four that administered 100,000 IU vitamin D to the elderly [77-79] or patients with resistant HTN [80], one that used a lower dose (600 IU) of vitamin D [81], and two that used vitamin D combined with several antihypertensive medications [82, 83]. Based on pooling the results of these improperly designed trials, any conclusion regarding vitamin D's antihypertensive role from the meta-analysis is questionable.
In the second part of the hypothesis, young people (<45 years old) with vitamin D deficiency are less likely to develop HTN because, unlike the VDR-null animal model, it is impossible for humans to develop compete vitamin D signaling deficiency. The residual vitamin D signaling and sufficient compensatory abilities from many different vascular protective factors (e.g., natriuretic peptides-cyclic guanosine monophosphate (cGMP) system) also can maintain a stable balance between vasoconstriction and vasodilation signaling to keep normal BP homeostasis. Therefore, vitamin D deficiency does not play an important role in normal BP regulation, and vitamin D repletion may exert a minimal effect on BP in normotensive individuals aged <45 years. This is supported by one study that showed that 17 hereditary vitamin D-resistant rickets patients with relatively severe vitamin D signaling deficiency at a mean age of 27 years demonstrated normal BP [84]; in addition no trials showed that vitamin D supplementation significantly lowered BP in normotensive cohorts with a limited number of hypertensive participants (<30%) aged <45 [85-88].
Although several studies had suboptimal designs and/or weaknesses, they showed that vitamin D supplementation for 18 months or less with a dose that significantly increases blood 25(OH)D levels to correct vitamin D deficiency or insufficiency had a minimal effect on BP in almost all normotensive cohorts aged >45 [89-94]. This suggests that, under normal BP status with a stable balance between vasoconstriction and vasodilation signaling, time-limited vitamin D supplementation plays a minimal role in BP regulation (Fig. 2A). However, it is possible that the well-designed, large, randomized, ongoing VITamin D and OmegA-3 TriaL (VITAL) will show a significant reduction of incident of HTN after five-year administration of vitamin D (2,000 IU daily) through eliminating vitamin D deficiency as a trigger in the development of EH in the cohort aged ≥50 years compared with the control groups [95]. Although some participants with normal baseline vitamin D levels, as well as control group members who take 800 IU vitamin D daily, may experience a decrease in the incidence of HTN, this is the largest trial to examine the potential preventive role of vitamin D supplementation in BP and the development of HTN. The first VITAL participants were randomized in Novermber 2011 and the randomization was completed in March 2014 [96].
Our two-part hypothesis is supported by our mechanistic research findings (see below). A combination of the two parts would most likely dilute and mask the antihypertensive effect of vitamin D supplementation on BP in patients with vitamin D deficiency-induced EH seen in the several meta-analyses above. The different results from prior trials were mainly dependent on how many hypertensive participants with vitamin D deficiency enrolled in the cohorts. As shown in Table 2, vitamin D usually has a minimal effect on the cohorts with mean BP in the normal range, including fewer than 30% hypertensive subjects [85, 91]. Of note, an appropriately high dose (at least >1,000 IU daily) of vitamin D supplementation to raise blood 25(OH)D levels is also essential to see an antihypertensive effect of vitamin D. To determine this, we analyzed recent major clinical trials below.
Larsen et al. [65] conducted a randomized, double-blind, placebo-controlled trial in Denmark during the winter to investigate the effect of 3,000 IU vitamin D daily on central, office, and 24-hour BP in 130 hypertensive patients with mean 25(OH)D of 23 ng/ml. After 20 weeks of treatment, mean 25(OH)D levels increased by 21 ng/ml in the treatment arm and decreased by 3ng/ml in the placebo arm. Central and office BP were reduced by 7/2 and 6/2 mmHg (p=0.007/0.15, 0.002/0.18 vs. placebo group), respectively. There was a small, non-significant reduction in 24-hour BP of 3/1 mmHg (p=0.26/0.18). However, in a subgroup analysis that included only the patients with 25(OH)D levels <32 ng/ml, the authors were able to detect that vitamin D supplementation significantly decreased 24-hour BP by 4/3 mmHg (p=0.05/0.01 vs. placebo group). This suggests that vitamin D supplementation predominantly benefits hypertensive patients with vitamin D deficiency. This study used an appropriately high dose of vitamin D with a high compliance rate. However, in addition to recruiting some patients with sufficient vitamin D levels, the trial chose only hypertensive patients with SBP <150 or DBP <95 mmHg, and ∼84% of participants took antihypertensive medication. All of these might have masked or diminished the effect of vitamin D on BP in hypertensive patients. These factors, along with a small sample size, may have significantly contributed to negative results in the change of 24-hour BP.
Recently, Chen et al. [63] performed a placebo-controlled randomized trial to investigate whether vitamin D supplementation (2,000 IU/day) for 6 months reduces blood pressure. They recruited 126 patients with stage 1-2 EH and vitamin D deficiency who received nifedipine (30 mg/d). There was a significant increase in mean 25(OH)D levels from baseline (19 ng/ml) to 6 months (34 ng/ml) and a reduction of 24h mean BP by 6.2/4.2 mmHg (p<0.001). In patients with vitamin D <30 ng/ml at baseline (n=113), 24h mean BP decreased by 7.1/5.7 mmHg (p<0.001), suggesting again that vitamin D supplementation is more effective in hypertensive patients with vitamin D deficiency or insufficiency. In this trial, the antihypertensive medicine nifedipine might have diminished the effect of vitamin D on BP in these Chinese hypertensive patients.
Consistent with the finding by Chen et al., Mozaffari-Khosravi et al. [66] conducted an eight-week, randomized, placebo-controlled trial. Results showed that vitamin D repletion significantly raised blood 25(OH)D levels and lowered BP in vitamin D-deficient hypertensive patients who were taking antihypertensive medication in an Iranian population (mean changes in SBP and DBP: -6.4 +/- 5.3 vs. 0.9 +/- 3.7 mmHg; -2.4 +/- 3.7 vs. 1.0+/- 2.7 mmHg, p<0.01, n=42). Again, the major weakness in the trial was that all patients were taking antihypertensive drugs, which might have reduced the role of vitamin D repletion in lowering BP in hypertensive patients.
The ideal trial to detect the maximum antihypertensive effect of vitamin D supplementation on vitamin D deficiency-induced HTN is the administration of vitamin D to patients with untreated stage 1 EH with vitamin D deficiency living in a high latitude area in winter. This would effectively determine the antihypertensive role of vitamin D, assuming that the trial includes untreated stage 2 hypertensive patients with vitamin D deficiency. However, ethics committees do not allow recruiting untreated stage II hypertensive patients to a placebo group. We conducted this kind of trial (n=40) in Liaoning, northern China, in winter, administering 3,000 IU vitamin D daily for three weeks to patients with a mean age of 53 years who had untreated stage 1 EH and vitamin D deficiency (their 25(OH)D levels were <20 ng/ml). The treatment significantly raised blood 25(OH)D levels and reduced systolic BP, but not diastolic BP. The negative results in diastolic BP may be due to a small sample size, short treatment period, and recruiting only stage 1 hypertensive patients compared with Chen et al.'s study [63]. There was no change in 25(OH)D levels and BP in the control group (unpublished data). Although we need a longer intervention to determine the maximum antihypertensive effect, the findings from this trial confirms the antihypertensive effect of vitamin D in a vulnerable vitamin D-deficient hypertensive population.
This is supported by a recent study from Forman et al. [64]. They performed a four-arm, double-blind, placebo-controlled, randomized trial, recruiting 283 black Americans (mean age of 51 years) to evaluate the effect of 1,000 IU, 2,000 IU, and 4,000 IU vitamin D daily or placebo on BP for a period of three months. Baseline 25(OH)D levels were about 16 ng/ml. Initial mean BP (122/78 mmHg) was relatively lower because only 50% of participants had HTN and 40% were taking antihypertensive medications. The difference between the systolic BP at baseline and after 3 months was +1.7 mmHg in the placebo group, -0.66 mmHg in the group with 1,000 IU vitamin D, -3.44 mmHg in the group with 2,000 IU vitamin D, and -4.0 mmHg in the group with 4,000 IU vitamin D (-1.4 mmHg for every 1,000 IU of additional vitamin D intake, p =0.04). For each 1 ng/ml increase in 25(OH)D levels, a significant reduction in systolic BP of 0.2 mmHg was detected. However, no significant reduction in diastolic BP was found, which might be due to recruiting only 50% of participants with HTN, as well as the 40% who were taking antihypertensive medication. Although the 50% of vitamin D-deficient normotensive participants masked the vitamin D antihypertensive effect when all data were pooled, systolic BP was still significantly reduced by 1.4 mmHg for each additional 1,000 IU/d of vitamin D. At the same time, this suboptimally designed trial showed that vitamin D supplementation significantly, but modestly, lowered BP in a vitamin D-deficient cohort, of whom only half were hypertensive. If a trial detecting the antihypertensive effect of vitamin D with the same dose and regime selects a cohort with fewer than 50% hypertensive patients, this would introduce a bias and lead to a negative result.
Arora et al. [85] recently published the results from the Daylight trial, which was conducted on this kind of cohort. The trial enrolled 534 participants aged 18 to 50 years (mean age of 36) with 25(OH)D <25 ng/ml and mean 24h BP 127/78 mmHg. Of these participants, 28% were hypertensive. They received 4,000 IU or 400 IU vitamin D daily for six months. Of these, 383 subjects (72%) completed the six-month study. At the end of the study, 25(OH)D levels were 33 ng/ml and 20 ng/ml in the high-dose versus low-dose treatment groups, respectively. Although none of the subjects took antihypertensive medication, six-month vitamin D supplementation failed to reduce BP in the study. The strengths of the design in this trial included an appropriately high dose of vitamin D daily, 24h BP monitoring, and selecting untreated hypertensive subjects. However, the major weakness was to include 72% of participants who were young (<40 years old) and not hypertensive. This may be the reason for the trial's negative results.
The Arora et al. study gave no clear explanation for investigating the antihypertensive role of six-month vitamin D supplementation in more than two-thirds of participants aged <40 years with mean systolic BP value <130 mmHg without type 2 diabetes mellitus and kidney disease. However, 72% of participants were clearly normotensive. It is not surprising that vitamin D supplementation in the cohort that included only 28% of hypertensive patients had a neutral effect on BP because combining the results from hypertensive and normotensive participants might have understated the antihypertensive effect of vitamin D. Of note, the key feature of this trial was to confirm the previous findings from three different groups [86-88] that vitamin D supplementation for six weeks to one year raised 25(OH)D levels and had a minimal effect on BP in normotensive cohorts that included fewer than 30% of hypertensive subjects aged <40 years with vitamin D deficiency or insufficiency.
In a recent trial by Scagg et al. [91], a similar enrollment was followed and similar results were obtained. They performed a randomized controlled trial to determine whether high doses of vitamin D taken for 18 months lowers BP in healthy adults. The 322 participants, included 48 hypertensive patients (14.9%), had a mean baseline BP of 123/76 mmHg with mean 25(OH)D level of 29 ng/ml, and mean age of 47 years. Participants received either placebo or vitamin D 200,000 IU for two months, followed by 100,000 IU monthly or placebo for 16 months. The 18-month treatment raised 25(OH)D levels to 40 ng/ml but had no effect on BP. However, there were several concerns with the conduct of this study. First, most of the recruited subjects were normotensive with mean 25(OH)D levels near normal; only 45 out of 322 participants had 25(OH)D <20 ng/ml. Second, the regime of vitamin D administration used was suboptimal and under debate. Both of these factors might have contributed to the negative findings, even though the inclusion of normotensive participants in vitamin D supplementation study would be a major confounding factor.
In 2013, Witham et al. [79] reported the findings of the VitDISH, raising another question about the regime of vitamin D supplementation. The trial enrolled 159 participants with a mean age of 77 years, mean BP 163/78 mmHg, and mean 25(OH)D levels of 18 ng/ml. Participants received 100,000 IU of oral vitamin D3 or placebo every three months for one year. The treatment increased 25(OH)D levels by 8 ng/ml, but failed to show a significant reduction in BP and arterial stiffness in the selected systolic hypertensive patients. Although these patients took one to two antihypertensive medications, their systolic BP was about 160 mmHg. Vitamin D supplementation should have reduced BP in these hypertensive patients with vitamin D deficiency. However, isolated systolic HTN is believed to result from vascular stiffness and calcification. Whether vitamin D reverses these pathologic changes remains unclear. In addition, it is not clear whether the polymorphism in VDR, 1-α-hydroxylase, and vitamin D binding protein affected the interventional results. The more important question is whether 100,000 IU of vitamin D four times a year is safe to treat 77-year-old people who have reduced metabolic activity. The tolerable upper level indicated in the IOM guidelines is 4,000 IU daily [16], though daily doses of vitamin D3 up to 10,000 IU are considered safe in healthy males. Higher doses of vitamin D metabolites and analogs have been shown to induce an osteoblastic phenotype in VSM cells [97], which has been demonstrated in animal models to induce calcification [98-100]. Though 100,000 IU of vitamin D may not produce acute vitamin D intoxication, characterized by hypercalcemia and hyperphosphatemia, it is difficult to rule out that a dose of vitamin D that is 25-fold higher than the daily tolerable dose produced a side effect on preexisting vascular calcification in 77-year-old people with isolated systolic HTN. This may have compromised the antihypertensive effect of vitamin D in this trial.
In support of this idea, Pfeifer et al. [75] treated 75-year-old people (mean BP 144/85 mmHg, 25(OH)D 10.1 ng/ml, n=74) with 800 IU vitamin D plus 1,200 mg calcium daily for eight weeks. They found a significant increase in 25(OH)D levels and a reduction in systolic BP by administration of vitamin D plus calcium compared with calcium alone. Obviously, well-designed placebo-controlled trials using 2,000 to 3,000 IU vitamin D alone daily to treat patients with isolated systolic HTN and vitamin D deficiency are warranted.
The most significant advantage of vitamin D supplementation over other antihypertensive medications in treating vitamin D deficiency-induced HTN is the fact that it is a natural product that treats this kind of HTN and is specifically directed at the underlying etiology. Sufficient vitamin D supplementation would clinically cure HTN. Other antihypertensive medications may also reduce vitamin D deficiency-induced HTN through the same final signaling pathway, but their effects are temporary and nonspecific, daily treatment is needed, and side effects are difficult to avoid. However, vitamin D supplementation combined with three or more antihypertensive medications that may overlay the role of vitamin D shows no effect on the reduction of BP in a hypertensive cohort. For example, Witham et al. [80] administered vitamin D to 34 patients with resistant HTN who had BP >140/90 mmHg and took three or more antihypertensive agents for six months. They found vitamin D supplementation did not reduce BP in these patients. In addition, the administration of intermittent and high-dose vitamin D in this trial might be a factor in the negative results.
Finally, patients with EH who do not have vitamin D deficiency usually caused by other risk factors may not benefit from vitamin D treatment. While several trials have shown that vitamin D reduced BP in vitamin D-deficient hypertensive patients [37, 63-65, 67, 68, 101], Pilz et al. [102] found no antihypertensive effect of vitamin D on a hypertensive cohort with 37% vitamin D-deficient patients (mean 25(OH)D level of 22 ng/ml). Similarly, Larsen et al. [65] found no significant BP reduction in a hypertensive cohort with mean 25(OH)D 23 ng/ml; however, in a post-hoc subgroup analysis of patients with 25(OH)D levels lower than 32 ng/ml, a significant BP reduction occurred during vitamin D supplementation. These results suggest that the administration of vitamin D to hypertensive cohorts with fewer than 40% participants with vitamin D deficiency will obscure the role of vitamin D in vitamin D-deficient EH patients. In Pilz's study [102], taking an average of two antihypertensive drugs also played a role in the negative results. In addition, while several recent trials showed that vitamin D reduced [103-105] or had no effect [106] on triglyceride levels, Pilz's study [102] found a modest increase in triglyceride levels within the normal physiological range. Whether the modest variation in triglyceride levels has any clinical significance needs further investigation.
Mechanistic Link between Vitamin D Deficiency and BP
Studies of EH caused by deficient vitamin D signaling are difficult in clinical settings, where establishment of vitamin D-deficient models is unrealistic and opportunities for invasive study are limited. Two genetically engineered mouse models and a diet-induced rat model have consistently shown that inhibition of vitamin D signaling leads to HTN. Several biological mechanisms relating vitamin D deficiency and HTN have been proposed. However, the exact mechanisms remain largely unknown.
Vitamin D Deficiency and Increased Renin Expression
Renin is synthesized and released by the juxtaglomerular (JG) cells of the kidney and plays an important role in BP regulation by stimulating the production of Ang II and aldosterone, both of which contribute to HTN. Therefore, modest (∼2-2.5 fold) elevation in renin levels in the mouse models [30, 107], which was not observed in the rat model with residual VDR signaling [108], has been proposed to account for high BP. However, this effect has not been rigorously confirmed experimentally. Our VDR-null mice developed HTN and also displayed a 2-fold elevation in renal renin levels [29]. However, while disruption of the peroxisome proliferator-activated receptor gamma locus in renin-producing JG cells led to a 2-fold increase in renin mRNA levels and plasma renin levels (a scenario similar to VDR-/- mice), JG cell-specific peroxisome proliferator-activated receptor gamma knockout mice display normal BP [109]. This indicates that 2-fold elevated renin expression in VDR-/- mice is less likely to mediate VDR deletion-induced HTN. JG cell-specific VDR transgenic mice reduced renin gene expression by 50% and displayed normal BP [110], further suggesting that a modest change in renin gene expression might not exert a significant influence on BP. Furthermore, treatment with VD3 or its analogues reduced BP in spontaneously hypertensive rats [111-114] with low or normal renin levels [115-118] and Ang II-induced mouse HTN, a renin-independent HTN model [119]. These results indicate that vitamin D reduces BP in hypertensive animals independent of renin levels. Taken together, the above evidence supports the notion that modestly elevated renin levels observed in mouse models might not play an important role in vitamin D signaling deficiency-induced HTN. Therefore, the molecular mechanisms underlying impaired vitamin D signaling-induced HTN remain to be elucidated.
Vitamin D Deficiency and High PTH and Low Calcium Levels
VDR-null and 1α-hydroxylase-null mice develop HTN accompanied by secondary hyperparathyroidism and low serum calcium levels, which are also commonly seen in patients with vitamin D deficiency. Low levels of ionized calcium can stimulate the calcium-sensing receptor of the parathyroid gland, leading to the release of PTH. Increased PTH levels have been shown to be associated with HTN [120-122]. Although the underlying mechanisms for the association between PTH and BP is unclear, the fact that intravenous infusion of PTH increased BP in healthy individuals [121] suggests that higher PTH levels may contribute to the development of HTN. However, both VDR knockout and 1α-hydroxylase-deficient mice still displayed hypertensive phenotypes when administered a special diet enriched with calcium and phosphorus [123] to maintain normal plasma calcium and phosphorous levels and near normal PTH levels. These data suggest that hypocalcemia and hyperparathyroidism play minor roles in vitamin D signaling deficiency-induced HTN.
Vitamin D Deficiency and Endothelial and VSM Cells in Vessel Walls
The fundamental characteristic of EH is increased peripheral resistance due to enhanced vascular tone. This is predominantly determined by direct or indirect dysfunction of endothelial and VSM cells. Vitamin D deficiency has been demonstrated to have a direct effect on the vasculature, leading to increased vascular resistance [124]. Although severe vitamin D signaling deficiency in several animal models has clearly been shown to lead to HTN, it remains largely unknown which vasomotor signaling dysfunction mediates vitamin D deficiency-induced HTN. Since our endothelial cell-specific VDR knockout mice display normal BP [125], the finding suggests that endothelial dysfunction plays a limited role in vitamin D deficiency-mediated HTN. We showed that VD3 negatively regulates endothelin (ET) and Ang II-induced VSM cell proliferation [126, 127], indicating that activated VDR deficiency might enhance ET and Ang II signaling-induced vasoconstriction. We also demonstrated that VD3 upregulated the expression of guanylyl cyclase (GC)-A in VSM cells [128], which would stimulate cGMP production, leading to vasodilation. These data imply that VDR signaling defects may downregulate GC-A expression and activity in VSM cells, subsequently diminishing GC-A/(cGMP) signaling, leading to impaired vasodilation. A recent study has shown that sodium nitroprusside-induced vessel dilation was significantly reduced in vitamin D-deleted female rats [129], suggesting that it may involve nitric oxide-sensitive GC in the nitric oxide -GC-cGMP pathway. Further studies will be needed to elucidate the specific signal responsible for dysfunction of vasoconstriction and vasodilation in VSM cells of vitamin D signaling-deficient animals.
Vitamin D Deficiency and Increased Sympathetic Nervous Activity
Increased central or renal sympathetic activation has been shown to be an important factor in the pathogenesis of human HTN. So far, no data have displayed that vitamin D deficiency directly affects sympathetic nervous activity. However, it is possible that vitamin D deficiency may enhance the downstream signaling response induced by increased sympathetic activities. For example, Ang II induces HTN through increased central nervous activity-mediated T cell activation and perivascular infiltration [130], and vitamin D has been shown to suppress effector T cell activity [131]. Thus, vitamin D deficiency may accelerate sympathetic outflow-stimulated T cell response.
Potential Candidate Genes Responsible for Vitamin D Deficiency-induced HTN
As mentioned above, vitamin D exerts its action through direct or indirect binding to its cognate nuclear VDR. Any gene with a genomic position occupied by activated VDR or indirectly regulated by VD3/VDR signaling leading to increased or decreased transcription and function involved in the regulation of BP should be a candidate molecule in the signaling pathway responsible for vitamin D deficiency-mediated HTN. For example, using chromatin immunoprecipitation with a next-generation sequencing approach, Ramagopalan et al. [132] defined activated VDR binding to and increased expression of potassium intermediate/small conductance calcium-activated channel, subfamily N, member 4, (KCNN4). KCNN4 encodes the KCa3.1 protein, which promotes VSM relaxation. Genetic deletion of KCNN4 led to elevation of BP [133, 134]. We also showed that activated VDR upregulated GC-A expression through directly binding to its promoter in VSM cells [128]. GC-A knockout mice developed HTN [135, 136]. Whether these two potential candidate genes contribute to vitamin D deficiency-induced HTN remains to be determined.
We deleted floxed VDR in the germline, generating global VDR knockout mice that are hypertensive [29]. We recently found a candidate gene by microarray analysis that increased its expression in VDR-null hypertensive mice. Double deletion of VDR and the candidate gene normalized VDR-null induced HTN (unpublished data), suggesting that its overexpression plays a critical role in the development of VDR-null-induced HTN. While the detailed molecular mechanism by which the candidate gene is regulated by vitamin D signaling is under investigation, we found that Ang II-induced HTN, and increased mesenteric resistance vessel tone and the candidate gene expression in the vascular walls, were significantly inhibited by the vitamin D analog paricalcitol. We also found that VD3 and paricalcitol suppressed Ang II-stimulated, but not basal promoter activities, mRNA, and protein levels of the candidate gene in VSM cells. Actually, the mice with deletion of the candidate gene display normal BP (unpublished data), suggesting that impaired basal expression of the gene can be well compensated physiologically in the regulation of normal BP. Taken together, these data suggest that VD3 exerts an antihypertensive role through reduction of the candidate gene overexpression and is unable to lower basal BP through the change in the basal gene expression. These support the clinical intervention results, as discussed above, that vitamin D supplementation reduced BP in hypertensive participants but not in normotensive individuals.
VSM cells and CD4+ T cells participate in BP regulation in EH. We have successfully developed smooth muscle-specific VDR knockout mice by crossing floxed VDR mice with mice expressing tamoxifen-inducible Cre under control of smooth muscle-specific myosin heavy chain (smMHC-CreERT2) (unpublished data). The generation of CD4+ T cell-specific VDR-null mice is ongoing. The progress in our basic and clinical research, combined with six large randomized, placebo-controlled trials ongoing in Europe (FIND, VIDAL, DOHealth, and HYPODD), the United States (VITAL), and New Zealand (ViDA) will promote a better understanding of vitamin D deficiency-induced HTN and determine the role of vitamin D supplementation in the prevention and treatment of EH in specific populations.
Conclusion
EH is caused by multiple factors acting through genetic and environmental determinants in an age-related manner. Vitamin D deficiency as an environmental risk factor favors increased vascular tone, which may not play an important role in the regulation of normal BP homeostasis but serves as a trigger to contribute to the development of EH in vulnerable middle-aged people (Fig. 2). Accumulating evidence from animal models and observational human studies strongly support the hypothesis that vitamin D deficiency contributes to EH. Pooled data from previous clinical trials have produced mixed results. The evidence provided in this article clearly shows that an appropriately high dose of vitamin D can normalize or nearly normalize blood 25(OH)D levels and significantly reduce BP in hypertensive cohorts with vitamin D deficiency. Of note, a few trials did not observe vitamin D's antihypertensive effect. However, these negative results could be due to suboptimal study design, including confounding effects of other antihypertensive medications that overlap with the antihypertensive effect of vitamin D, cohorts with <40% of participants with vitamin D deficiency, and either low or exceptionally high levels of vitamin D intake. Treatment of vitamin D-deficient or -insufficient normotensive individuals with vitamin D for a short period results in minimal effects on BP. However, it is possible that the ongoing five-year VITAL trial, which is using a high dose of vitamin D supplementation daily in a cohort at the age susceptible to EH, will prevent the development of HTN by eliminating vitamin D deficiency as a trigger for its development. The hypothesis, coupled with our clinical research progress and detailed mechanistic study, will provide a theoretical basis for a large clinical trial conducted in a well-defined population. If successful, vitamin D supplements for the treatment of EH in a specific population will not only be a significant breakthrough that dramatically improves clinical practice for EH intervention, but also substantially decreases the public heath burden of the management of EH.
Acknowledgments
Sources of support: NIH Grant HL096047 and State of Nebraska LB692 Clinical and Translational Research Grant (S Chen) and NIH R01 HL120659 (DK Agrawal).
Footnotes
Potential conflicts of interest/financial disclosure: None
All authors have read the journal's authorship agreement and publication policy and declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ open. 2013;3:e003423. doi: 10.1136/bmjopen-2013-003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S. Essential hypertension: perspectives and future directions. Journal of hypertension. 2012;30:42–5. doi: 10.1097/HJH.0b013e32834ee23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trott DW, Harrison DG. The immune system in hypertension. Advances in physiology education. 2014;38:20–4. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 5.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nature medicine. 2010;16:183–90. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- 6.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nature medicine. 2008;14:64–8. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 7.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–58. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nature medicine. 2011;17:1402–9. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 9.Ehret GB, Caulfield MJ. Genes for blood pressure: an opportunity to understand hypertension. European heart journal. 2013;34:951–61. doi: 10.1093/eurheartj/ehs455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natekar A, Olds RL, Lau MW, Min K, Imoto K, Slavin TP. Elevated blood pressure: Our family's fault? The genetics of essential hypertension. World journal of cardiology. 2014;6:327–37. doi: 10.4330/wjc.v6.i5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friso S, Carvajal CA, Fardella CE, Olivieri O. Epigenetics and arterial hypertension: the challenge of emerging evidence. Translational research : the journal of laboratory and clinical medicine. 2015;165:154–65. doi: 10.1016/j.trsl.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Liang M, Cowley AW, Jr, Mattson DL, Kotchen TA, Liu Y. Epigenomics of hypertension. Seminars in nephrology. 2013;33:392–9. doi: 10.1016/j.semnephrol.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques FZ, Booth SA, Charchar FJ. The emerging role of non-coding RNA in essential hypertension and blood pressure regulation. Journal of human hypertension. 2014 doi: 10.1038/jhh.2014.99. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Annals of epidemiology. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 16.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of clinical endocrinology and metabolism. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 18.Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, et al. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. The Journal of cell biology. 2008;182:459–65. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, et al. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–12. doi: 10.1161/HYPERTENSIONAHA.108.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–71. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 21.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. Journal of the American Society of Nephrology : JASN. 2002;13:621–9. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 22.Handel AE, Sandve GK, Disanto G, Berlanga-Taylor AJ, Gallone G, Hanwell H, et al. Vitamin D receptor ChIP-seq in primary CD4+ cells: relationship to serum 25-hydroxyvitamin D levels and autoimmune disease. BMC medicine. 2013;11:163. doi: 10.1186/1741-7015-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ, et al. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. The Journal of clinical investigation. 1989;83:1903–15. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsuhashi T, Morris RC, Jr, Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. The Journal of clinical investigation. 1991;87:1889–95. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Costa CH, Nakamura K, Ribeiro RC, Gardner DG. Vitamin D-dependent suppression of human atrial natriuretic peptide gene promoter activity requires heterodimer assembly. The Journal of biological chemistry. 1999;274:11260–6. doi: 10.1074/jbc.274.16.11260. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Wu J, Hsieh JC, Whitfield GK, Jurutka PW, Haussler MR, et al. Suppression of ANP gene transcription by liganded vitamin D receptor: involvement of specific receptor domains. Hypertension. 1998;31:1338–42. doi: 10.1161/01.hyp.31.6.1338. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Cui J, Nakamura K, Ribeiro RC, West BL, Gardner DG. Coactivator-vitamin D receptor interactions mediate inhibition of the atrial natriuretic peptide promoter. The Journal of biological chemistry. 2000;275:15039–48. doi: 10.1074/jbc.275.20.15039. [DOI] [PubMed] [Google Scholar]
- 28.Meyer MB, Pike JW. Corepressors (NCoR and SMRT) as well as coactivators are recruited to positively regulated 1alpha,25-dihydroxyvitamin D3-responsive genes. The Journal of steroid biochemistry and molecular biology. 2013;136:120–4. doi: 10.1016/j.jsbmb.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, et al. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–47. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. The Journal of clinical investigation. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGreevy C, Williams D. New insights about vitamin D and cardiovascular disease: a narrative review. Annals of internal medicine. 2011;155:820–6. doi: 10.7326/0003-4819-155-12-201112200-00004. [DOI] [PubMed] [Google Scholar]
- 32.Zittermann A. Vitamin D and cardiovascular disease. Anticancer research. 2014;34:4641–8. [PubMed] [Google Scholar]
- 33.Rostand SG. Vitamin D deficiency in the pathogenesis of hypertension: still an unsettled question. Current hypertension reports. 2014;16:464. doi: 10.1007/s11906-014-0464-6. [DOI] [PubMed] [Google Scholar]
- 34.Tamez H, Kalim S, Thadhani RI. Does vitamin D modulate blood pressure? Current opinion in nephrology and hypertension. 2013;22:204–9. doi: 10.1097/MNH.0b013e32835d919b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rostand SG. Vitamin D, blood pressure, and African Americans: toward a unifying hypothesis. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1697–703. doi: 10.2215/CJN.02960410. [DOI] [PubMed] [Google Scholar]
- 36.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 37.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–10. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 38.Bhandari SK, Pashayan S, Liu IL, Rasgon SA, Kujubu DA, Tom TY, et al. 25-hydroxyvitamin D levels and hypertension rates. Journal of clinical hypertension. 2011;13:170–7. doi: 10.1111/j.1751-7176.2010.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorjgochoo T, Ou Shu X, Xiang YB, Yang G, Cai Q, Li H, et al. Circulating 25-hydroxyvitamin D levels in relation to blood pressure parameters and hypertension in the Shanghai Women's and Men's Health Studies. The British journal of nutrition. 2012;108:449–58. doi: 10.1017/S0007114511005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutrition research. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. European journal of clinical nutrition. 2008;62:1079–89. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 42.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Archives of internal medicine. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 43.Sabanayagam C, Shankar A, Somasundaram S. Serum vitamin D level and prehypertension among subjects free of hypertension. Kidney & blood pressure research. 2012;35:106–13. doi: 10.1159/000330716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. American journal of hypertension. 2007;20:713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Skaaby T, Husemoen LL, Pisinger C, Jorgensen T, Thuesen BH, Fenger M, et al. Vitamin D status and changes in cardiovascular risk factors: a prospective study of a general population. Cardiology. 2012;123:62–70. doi: 10.1159/000341277. [DOI] [PubMed] [Google Scholar]
- 46.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. Journal of hypertension. 2011;29:636–45. doi: 10.1097/HJH.0b013e32834320f9. [DOI] [PubMed] [Google Scholar]
- 47.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. The American journal of cardiology. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–32. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 50.Griffin FC, Gadegbeku CA, Sowers MR. Vitamin D and subsequent systolic hypertension among women. American journal of hypertension. 2011;24:316–21. doi: 10.1038/ajh.2010.226. [DOI] [PubMed] [Google Scholar]
- 51.Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. 2010;55:792–8. doi: 10.1161/HYPERTENSIONAHA.109.143990. [DOI] [PubMed] [Google Scholar]
- 52.Ke L, Graubard BI, Albanes D, Fraser DR, Weinstein SJ, Virtamo J, et al. Hypertension, pulse, and other cardiovascular risk factors and vitamin D status in Finnish men. American journal of hypertension. 2013;26:951–6. doi: 10.1093/ajh/hpt051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolis KL, Martin LW, Ray RM, Kerby TJ, Allison MA, Curb JD, et al. A prospective study of serum 25-hydroxyvitamin D levels, blood pressure, and incident hypertension in postmenopausal women. American journal of epidemiology. 2012;175:22–32. doi: 10.1093/aje/kwr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Ma J, Manson JE, Buring JE, Gaziano JM, Sesso HD. A prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. European journal of nutrition. 2013;52:1771–9. doi: 10.1007/s00394-012-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. European journal of epidemiology. 2013;28:205–21. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 56.Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, et al. LifeLines Cohort Study i. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. The lancet Diabetes & endocrinology. 2014;2:719–29. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Afzal S, Nordestgaard BG. Low vitamin D and hypertension: a causal association? The lancet Diabetes & endocrinology. 2014;2:682–4. doi: 10.1016/S2213-8587(14)70119-6. [DOI] [PubMed] [Google Scholar]
- 58.Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. Southern medical journal. 2010;103:729–37. doi: 10.1097/SMJ.0b013e3181e6d389. [DOI] [PubMed] [Google Scholar]
- 59.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. Journal of hypertension. 2009;27:1948–54. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 60.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Annals of internal medicine. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2011;96:1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 62.Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon? European journal of epidemiology. 2014;29:1–14. doi: 10.1007/s10654-013-9874-z. [DOI] [PubMed] [Google Scholar]
- 63.Chen WR, Liu ZY, Shi Y, Yin da W, Wang H, Sha Y, et al. Vitamin D and nifedipine in the treatment of Chinese patients with grades I-II essential hypertension: a randomized placebo-controlled trial. Atherosclerosis. 2014;235:102–9. doi: 10.1016/j.atherosclerosis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–85. doi: 10.1161/HYPERTENSIONAHA.111.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo-controlled trial. American journal of hypertension. 2012;25:1215–22. doi: 10.1038/ajh.2012.111. [DOI] [PubMed] [Google Scholar]
- 66.Mozaffari-Khosravi H, Loloei S, Mirjalili MR, Barzegar K. The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin D deficiency: a randomized, double-blind, placebo-controlled trial. Blood pressure monitoring. 2014 doi: 10.1097/MBP.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 67.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabetic medicine : a journal of the British Diabetic Association. 2008;25:320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 68.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53:2112–9. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 69.Lind L, Lithell H, Skarfors E, Wide L, Ljunghall S. Reduction of blood pressure by treatment with alphacalcidol. A double-blind, placebo-controlled study in subjects with impaired glucose tolerance. Acta medica Scandinavica. 1988;223:211–7. [PubMed] [Google Scholar]
- 70.Lind L, Wengle B, Ljunghall S. Blood pressure is lowered by vitamin D (alphacalcidol) during long-term treatment of patients with intermittent hypercalcaemia. A double-blind, placebo-controlled study. Acta medica Scandinavica. 1987;222:423–7. doi: 10.1111/j.0954-6820.1987.tb10959.x. [DOI] [PubMed] [Google Scholar]
- 71.Lind L, Wengle B, Wide L, Ljunghall S. Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. American journal of hypertension. 1989;2:20–5. doi: 10.1093/ajh/2.1.20. [DOI] [PubMed] [Google Scholar]
- 72.Lind L, Wengle B, Wide L, Sorensen OH, Ljunghall S. Hypertension in primary hyperparathyroidism--reduction of blood pressure by long-term treatment with vitamin D (alphacalcidol). A double-blind, placebo-controlled study. American journal of hypertension. 1988;1:397–402. doi: 10.1093/ajh/1.4.397. [DOI] [PubMed] [Google Scholar]
- 73.Rossini M, Gatti D, Viapiana O, Fracassi E, Idolazzi L, Zanoni S, et al. Short-term effects on bone turnover markers of a single high dose of oral vitamin D(3) The Journal of clinical endocrinology and metabolism. 2012;97:E622–6. doi: 10.1210/jc.2011-2448. [DOI] [PubMed] [Google Scholar]
- 74.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA internal medicine. 2015 doi: 10.1001/jamainternmed.2015.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. The Journal of clinical endocrinology and metabolism. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 76.Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clinical nutrition. 2013;32:970–5. doi: 10.1016/j.clnu.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 77.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. European journal of clinical nutrition. 1995;49:640–6. [PubMed] [Google Scholar]
- 78.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circulation Heart failure. 2010;3:195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 79.Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA internal medicine. 2013;173:1672–9. doi: 10.1001/jamainternmed.2013.9043. [DOI] [PubMed] [Google Scholar]
- 80.Witham MD, Ireland S, Houston JG, Gandy SJ, Waugh S, Macdonald TM, et al. Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: randomized, controlled trial. Hypertension. 2014;63:706–12. doi: 10.1161/HYPERTENSIONAHA.113.02177. [DOI] [PubMed] [Google Scholar]
- 81.Muldowney S, Lucey AJ, Hill TR, Seamans KM, Taylor N, Wallace JM, et al. Incremental cholecalciferol supplementation up to 15 mug/d throughout winter at 51-55 degrees N has no effect on biomarkers of cardiovascular risk in healthy young and older adults. The Journal of nutrition. 2012;142:1519–25. doi: 10.3945/jn.111.154005. [DOI] [PubMed] [Google Scholar]
- 82.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376:1543–51. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 83.Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227:140–6. doi: 10.1016/j.atherosclerosis.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Tiosano D, Schwartz Y, Braver Y, Hadash A, Gepstein V, Weisman Y, et al. The renin-angiotensin system, blood pressure, and heart structure in patients with hereditary vitamin D-resistance rickets (HVDRR) Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:2252–60. doi: 10.1002/jbmr.431. [DOI] [PubMed] [Google Scholar]
- 85.Arora P, Song Y, Dusek J, Plotnikoff G, Sabatine M, Cheng S, et al. Vitamin D Therapy in Individuals with Pre-Hypertension or Hypertension: The DAYLIGHT Trial. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.011732. [DOI] [PubMed] [Google Scholar]
- 86.Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. Journal of internal medicine. 2010;267:462–72. doi: 10.1111/j.1365-2796.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 87.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabetic medicine : a journal of the British Diabetic Association. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 88.Salehpour A, Shidfar F, Hosseinpanah F, Vafa M, Razaghi M, Hoshiarrad A, et al. Vitamin D3 and the risk of CVD in overweight and obese women: a randomised controlled trial. The British journal of nutrition. 2012;108:1866–73. doi: 10.1017/S0007114512000098. [DOI] [PubMed] [Google Scholar]
- 89.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. European journal of nutrition. 2009;48:349–54. doi: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- 90.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. The American journal of clinical nutrition. 2006;83:754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 91.Scragg R, Slow S, Stewart AW, Jennings LC, Chambers ST, Priest PC, et al. Long-term high-dose vitamin D3 supplementation and blood pressure in healthy adults: a randomized controlled trial. Hypertension. 2014;64:725–30. doi: 10.1161/HYPERTENSIONAHA.114.03466. [DOI] [PubMed] [Google Scholar]
- 92.Scragg R, Wishart J, Stewart A, Ofanoa M, Kerse N, Dyall L, et al. No effect of ultraviolet radiation on blood pressure and other cardiovascular risk factors. Journal of hypertension. 2011;29:1749–56. doi: 10.1097/HJH.0b013e328349666d. [DOI] [PubMed] [Google Scholar]
- 93.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. The Journal of clinical endocrinology and metabolism. 2012;97:3557–68. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 94.Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. The American journal of clinical nutrition. 2009;89:1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 95.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemporary clinical trials. 2012;33:159–71. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pradhan AD, Manson JE. Update on the Vitamin D and OmegA-3 trial (VITAL) The Journal of steroid biochemistry and molecular biology. 2015 doi: 10.1016/j.jsbmb.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zebger-Gong H, Muller D, Diercke M, Haffner D, Hocher B, Verberckmoes S, et al. 1,25-Dihydroxyvitamin D3-induced aortic calcifications in experimental uremia: up-regulation of osteoblast markers, calcium-transporting proteins and osterix. Journal of hypertension. 2011;29:339–48. doi: 10.1097/HJH.0b013e328340aa30. [DOI] [PubMed] [Google Scholar]
- 98.Bas A, Lopez I, Perez J, Rodriguez M, Aguilera-Tejero E. Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:484–90. doi: 10.1359/JBMR.051211. [DOI] [PubMed] [Google Scholar]
- 99.Niederhoffer N, Bobryshev YV, Lartaud-Idjouadiene I, Giummelly P, Atkinson J. Aortic calcification produced by vitamin D3 plus nicotine. Journal of vascular research. 1997;34:386–98. doi: 10.1159/000159247. [DOI] [PubMed] [Google Scholar]
- 100.Rodriguez M, Martinez-Moreno JM, Rodriguez-Ortiz ME, Munoz-Castaneda JR, Almaden Y. Vitamin D and vascular calcification in chronic kidney disease. Kidney & blood pressure research. 2011;34:261–8. doi: 10.1159/000326903. [DOI] [PubMed] [Google Scholar]
- 101.Mozaffari-Khosravi H, Loloei S, Mirjalili MR, Barzegar K. The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin D deficiency: a randomized, double-blind, placebo-controlled trial. Blood pressure monitoring. 2015;20:83–91. doi: 10.1097/MBP.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 102.Pilz S, Gaksch M, Kienreich K, Grubler M, Verheyen N, Fahrleitner-Pammer A, et al. Effects of Vitamin D on Blood Pressure and Cardiovascular Risk Factors: A Randomized Controlled Trial. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.115.05319. [DOI] [PubMed] [Google Scholar]
- 103.Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clinical nutrition. 2014 doi: 10.1016/j.clnu.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 104.Munoz-Aguirre P, Flores M, Macias N, Quezada AD, Denova-Gutierrez E, Salmeron J. The effect of vitamin D supplementation on serum lipids in postmenopausal women with diabetes: A randomized controlled trial. Clinical nutrition. 2014 doi: 10.1016/j.clnu.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 105.Schnatz PF, Jiang X, Vila-Wright S, Aragaki AK, Nudy M, O'Sullivan DM, et al. Calcium/vitamin D supplementation, serum 25-hydroxyvitamin D concentrations, and cholesterol profiles in the Women's Health Initiative calcium/vitamin D randomized trial. Menopause. 2014;21:823–33. doi: 10.1097/GME.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids in health and disease. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney international. 2008;74:170–9. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 108.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. The American journal of physiology. 1990;258:E134–42. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 109.Desch M, Schreiber A, Schweda F, Madsen K, Friis UG, Weatherford ET, et al. Increased renin production in mice with deletion of peroxisome proliferator-activated receptor-gamma in juxtaglomerular cells. Hypertension. 2010;55:660–6. doi: 10.1161/HYPERTENSIONAHA.109.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney international. 2008;74:1577–81. doi: 10.1038/ki.2008.452. [DOI] [PubMed] [Google Scholar]
- 111.Borges AC, Feres T, Vianna LM, Paiva TB. Effect of cholecalciferol treatment on the relaxant responses of spontaneously hypertensive rat arteries to acetylcholine. Hypertension. 1999;34:897–901. doi: 10.1161/01.hyp.34.4.897. [DOI] [PubMed] [Google Scholar]
- 112.Borges AC, Feres T, Vianna LM, Paiva TB. Recovery of impaired K+ channels in mesenteric arteries from spontaneously hypertensive rats by prolonged treatment with cholecalciferol. British journal of pharmacology. 1999;127:772–8. doi: 10.1038/sj.bjp.0702581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feres T, Borges AC, Silva EG, Paiva AC, Paiva TB. Impaired function of alpha-2 adrenoceptors in smooth muscle of mesenteric arteries from spontaneously hypertensive rats. British journal of pharmacology. 1998;125:1144–9. doi: 10.1038/sj.bjp.0702177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kong J, Kim GH, Wei M, Sun T, Li G, Liu SQ, et al. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. The American journal of pathology. 2010;177:622–31. doi: 10.2353/ajpath.2010.091292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bolterman RJ, Manriquez MC, Ortiz Ruiz MC, Juncos LA, Romero JC. Effects of captopril on the renin angiotensin system, oxidative stress, and endothelin in normal and hypertensive rats. Hypertension. 2005;46:943–7. doi: 10.1161/01.HYP.0000174602.59935.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Campbell DJ, Duncan AM, Kladis A, Harrap SB. Angiotensin peptides in spontaneously hypertensive and normotensive Donryu rats. Hypertension. 1995;25:928–34. doi: 10.1161/01.hyp.25.5.928. [DOI] [PubMed] [Google Scholar]
- 117.Paliege A, Pasumarthy A, Mizel D, Yang T, Schnermann J, Bachmann S. Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. American journal of physiology Regulatory, integrative and comparative physiology. 2006;290:R694–700. doi: 10.1152/ajpregu.00219.2005. [DOI] [PubMed] [Google Scholar]
- 118.Yu H, Di Nicolantonio R. Altered age-dependent modulation of tissue renin messenger RNA levels in the spontaneously hypertensive rat. Journal of hypertension. 1996;14:871–80. doi: 10.1097/00004872-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 119.Chen S, Gardner DG. Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart. The Journal of steroid biochemistry and molecular biology. 2013;136:150–5. doi: 10.1016/j.jsbmb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 120.Fitzpatrick LA, Bilezikian JP, Silverberg SJ. Parathyroid hormone and the cardiovascular system. Current osteoporosis reports. 2008;6:77–83. doi: 10.1007/s11914-008-0014-8. [DOI] [PubMed] [Google Scholar]
- 121.Hulter HN, Melby JC, Peterson JC, Cooke CR. Chronic continuous PTH infusion results in hypertension in normal subjects. J Clin Hypertens. 1986;2:360–70. [PubMed] [Google Scholar]
- 122.Muhando CA, Kuguru BL, Wagner GM, Mbije NE, Ohman MC. Environmental effects on the distribution of corallimorpharians in Tanzania. Ambio. 2002;31:558–61. [PubMed] [Google Scholar]
- 123.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9831–5. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duprez D, De Buyzere M, De Backer T, Clement D. Relationship between vitamin D and the regional blood flow and vascular resistance in moderate arterial hypertension. Journal of hypertension Supplement : official journal of the International Society of Hypertension. 1993;11:S304–5. [PubMed] [Google Scholar]
- 125.Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension. 2014;64:1290–8. doi: 10.1161/HYPERTENSIONAHA.114.03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen S, Law CS, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. The Journal of steroid biochemistry and molecular biology. 2010;118:135–41. doi: 10.1016/j.jsbmb.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen S, Law CS, Grigsby CL, Olsen K, Gardner DG. A role for the cell cycle phosphatase Cdc25a in vitamin D-dependent inhibition of adult rat vascular smooth muscle cell proliferation. The Journal of steroid biochemistry and molecular biology. 2010;122:326–32. doi: 10.1016/j.jsbmb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen S, Ni XP, Humphreys MH, Gardner DG. 1,25 dihydroxyvitamin d amplifies type a natriuretic peptide receptor expression and activity in target cells. Journal of the American Society of Nephrology : JASN. 2005;16:329–39. doi: 10.1681/ASN.2004090797. [DOI] [PubMed] [Google Scholar]
- 129.Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. The Journal of physiology. 2011;589:4777–86. doi: 10.1113/jphysiol.2011.214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204:2449–60. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. Journal of immunology. 2009;182:4624–32. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome research. 2010;20:1352–60. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–32. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 134.Si H, Heyken WT, Wolfle SE, Tysiac M, Schubert R, Grgic I, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circulation research. 2006;99:537–44. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 135.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, et al. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–8. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 136.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14730–5. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]