Abstract
Sensory loss leads to widespread adaptation of brain circuits to allow an organism to navigate its environment with its remaining senses, which is broadly referred to as cross-modal plasticity. Such adaptation can be observed even in the primary sensory cortices, and falls into two distinct categories: (1) recruitment of the deprived sensory cortex for processing the remaining senses, which we term “cross-modal recruitment”, and (2) experience-dependent refinement of the spared sensory cortices referred to as “compensatory plasticity.” Here we will review recent studies demonstrating that cortical adaptation to sensory loss involves LTP/LTD and homeostatic synaptic plasticity. Cross-modal synaptic plasticity is observed in adults, hence cross-modal sensory deprivation may be an effective way to promote plasticity in adult primary sensory cortices.
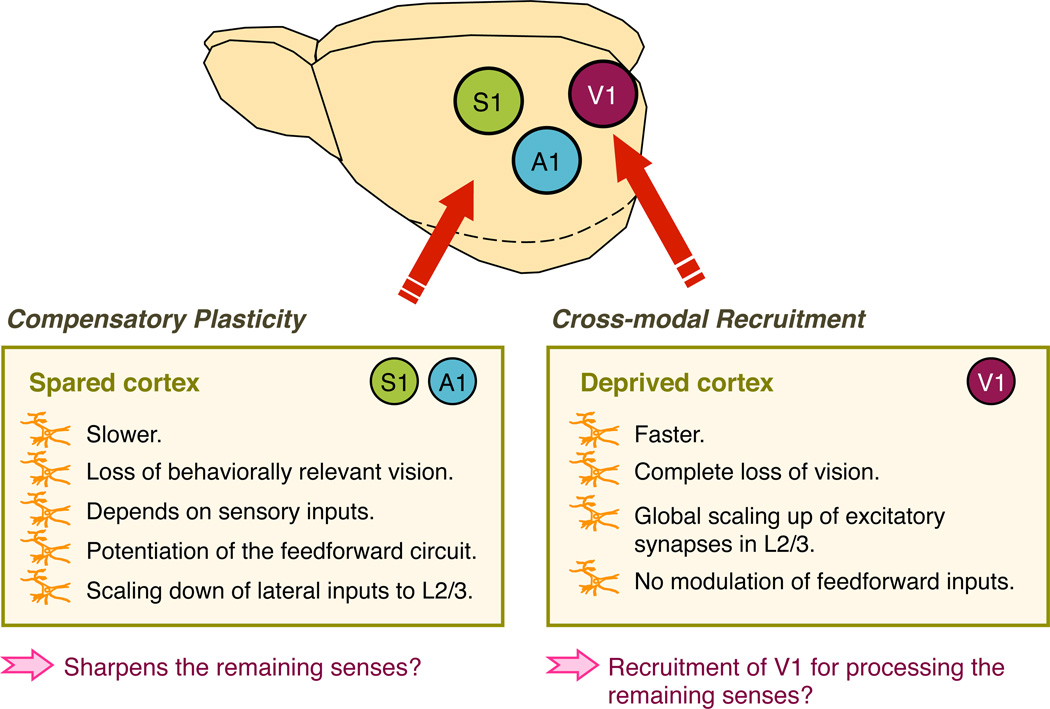
Recent studies highlight that primary sensory cortices do not work in isolation, but are influenced by other senses [1]. Specifically, the superficial layers of primary sensory cortices receive subthreshold inputs originating from other senses [2,3], which could form the basis for multisensory integration even at these early stages of sensory processing [1]. Such multisensory interactions between primary sensory cortices could serve as substrates for cross-modal plasticity in the event of losing a sensory modality. It is now well described that loss of a sensory modality leads to widespread compensatory plasticity across brain areas, which in some cases includes the primary sensory cortices [4]. These changes fall into two categories that together are broadly termed “cross-modal plasticity” [5] (Fig. 1): The first category is where the primary sensory cortex of the deprived sense is recruited by the remaining senses. Here we will refer to this as “cross-modal recruitment” to distinguish it from the second category of modifications, where the primary sensory cortices of the spared senses reorganize to allow for better processing. In this review, we will use the terminology “compensatory plasticity” to refer to this second category.
Figure 1. Two distinct aspects of cross-modal synaptic plasticity: cross-modal recruitment and compensatory plasticity.
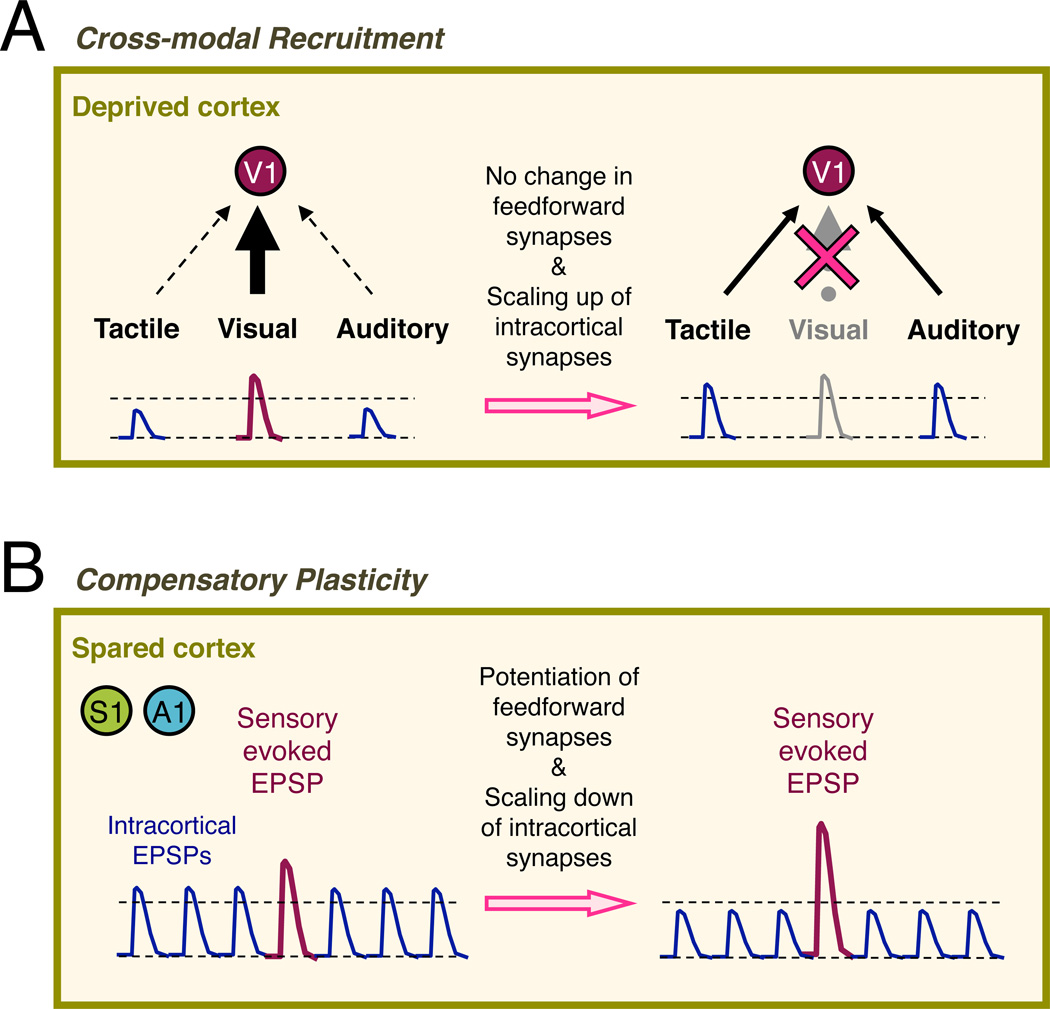
Loss of vision triggers two distinct modes of cortical plasticity. In the deprived cortex (i.e. V1), intracortical excitatory synapses onto L2/3 pyramidal neurons globally scale up without changes in the feedforward synapses from L4 neurons. In addition, there is no alteration in the strength of feedforward synapses from the lateral geniculate nucleus (LGN) to L4 neurons. Global plasticity of intracortical synapses follows the rules of multiplicative homeostatic synaptic scaling, hence enhances the gain across a large population of synapses. Such adaptation is predicted to enhance intracortical processing, which may include feedback inputs from high order visual areas that carry multisensory information. We propose that this may form the cellular basis for cross-modal recruitment of V1 for processing the remaining senses. Cross-modal plasticity in V1 requires complete loss of vision, which suggests that any residual vision may impede cross-modal recruitment.
In contrast, the spared sensory cortices (i.e. S1 and A1) undergo a different mode of synaptic plasticity, where feedforward excitatory synapses (both thalamocortical inputs to L4 and L4 inputs to L2/3) potentiate while intracortical excitatory synapses globally scale down. Such plasticity tips the balance to favor feedforward sensory processing at the expense of intracortical processing. Potentiation of the feedforward thalamocortical synapses requires within modality sensory inputs, which suggests that it is an experience-dependent synaptic plasticity akin to LTP. Cross-modal potentiation of thalamocortical synapses is observed in adults following acute loss of vision and is also observed in V1 following deafening. These suggest that this form of plasticity likely operate universally across different primary sensory cortices in adults. Unlike cross-modal synaptic plasticity of the deprived cortex, plasticity in the spared cortex can be triggered by a milder degradation of vision but requires a longer duration of vision loss. Compensatory plasticity in the spared cortices correlated with sharper tuning of S1BF and A1 neurons, which suggests that this form of synaptic plasticity may form the basis for sharpening the remaining senses.
Recruitment of the deprived cortex for processing the remaining senses has been reported following several types of sensory loss. For example, early onset blind individuals display activation of visual areas, including the primary visual cortex (V1), when reading braille [6,7] or comprehending ultra fast speech [8,9]. Also, in deaf individuals visual stimuli have been shown to activate auditory cortical areas, including the primary auditory cortex (A1) [10]. Such changes were initially thought to be long-term developmental adaptations to sensory loss [11], but similar changes have been reported for late onset blind individuals [7] and have also been observed after just a few days in blindfolded adults with braille training [4]. In any case, these studies highlight that deprived sensory cortices become re-engaged for processing remaining senses, and a narrow definition of “cross-modal plasticity” has been used in the literature to describe these changes [12]. However, here we will use the terminology “cross-modal recruitment” to distinguish this form of plasticity from the broader definition of cross-modal plasticity (Fig. 1). In addition to cross-modal recruitment of the deprived cortex, the enhanced processing of the spared senses may also depend on reorganization of the spared primary sensory cortices. For instance, there is an expansion of the representation in primary somatosensory cortex (S1) corresponding to the braille reading finger in blind subjects [13]. Such forms of plasticity that are triggered by cross-modal loss of another sense are often referred to as “compensatory plasticity” [12,14] (Fig. 1) to distinguish from the narrow definition of “cross-modal plasticity” or “cross-modal recruitment” mentioned above.
In this article, we will discuss recent studies examining the synaptic basis for both cross-modal recruitment and compensatory plasticity, focusing on the primary sensory cortices. One of the first demonstrations that synaptic changes are triggered by cross-modal sensory experience comes from a study of excitatory synaptic transmission across different primary sensory cortices following visual deprivation [15]. This study demonstrated that visual deprivation leads to opposite changes in the strength of excitatory synapses in the superficial layers of primary sensory cortices, such that they are increased in deprived cortex (i.e. V1) and decreased in spared cortices (both A1 and S1). Since then, several other studies have followed describing cortical circuit adaptations across different primary sensory cortices when one sensory modality is lost. We will review these findings, and provide our opinion that both LTP/LTD and homeostatic synaptic plasticity mechanisms interact to alter synaptic circuits in the deprived and spared cortices and allow cross-modal recruitment and compensatory plasticity following loss of sensation. In addition, we will summarize recent findings showing that cross-modal synaptic plasticity also operates in adults, and discuss whether a cross-modal sensory deprivation paradigm may be an effective tool to recover synaptic plasticity in the adult cortex.
Synaptic plasticity in the deprived cortex: A basis for cross-modal recruitment?
Several studies have shown that depriving all visually driven activity leads to homeostatic up-regulation of excitatory synapses of layer 2/3 neurons in V1. For instance, monocular inactivation via intraocular tetrodotoxin (TTX) injection causes excitatory synapses in L2/3 of the monocular zone of V1 to scale up [16,17]. Similarly, a few days of dark exposure or binocular enucleation, which deprives all visual activity, also scales up excitatory synapses of these neurons [15,18,19]. However, depriving vision via lid suture, which allows diffuse light through the closed eyelids and does not completely remove all visually driven activity [20], fails to scale up excitatory synapses in V1 L2/3 [17,19]. This does not mean that lid suture does not alter excitatory synapses in V1. As a matter of fact, it may cause a net LTD [17] or a combination of LTP and LTD at different synapses resulting in a net zero gain of average synaptic strength [19]. In any case, these examples suggest that V1 synapses adapt differentially to different modes of visual deprivation, such that a complete loss of visually driven activity causes homeostatic scaling up of excitatory synapses, while any residual visual activity, as with lid suture condition [20], fails to trigger global homeostatic scaling.
One of the main features of homeostatic synaptic scaling, which is assessed by measuring miniature excitatory postsynaptic currents (mEPSCs), is that it is a global phenomenon that occurs across a large number of synapses. This is based on the assumption that individual mEPSCs originate from independent synapses, because they are spontaneous fusion of presynaptic vesicles in the absence of action potentials, which is considered a very low probability event at individual synapses. Typically hundreds of mEPSCs are collected from a neuron to generate an average, hence the average mEPSC amplitude reflects the average strength of hundreds of synapses. We recently found that mEPSCs, which randomly sample many synapses, mainly reflect intracortical synapses [21], which are the majority of inputs to principal neurons in V1 [22]. Similar to the experience-dependent homeostatic plasticity of mEPSCs described above, visual deprivation increased the strength of lateral inputs to V1 L2/3 neurons without changes in the strength of feed-forward synapses from L4 [21]. This suggests that V1 adapts to loss of vision by specifically scaling up lateral intracortical inputs to L2/3 neurons. Interestingly, L2/3 neurons in V1 receive strong feedback intracortical inputs from higher order visual areas [23–25], and have been shown to produce subthreshold responses to and modulation of activity by auditory and tactile stimuli [2,26]. Therefore, it is possible that intracortical mEPSCs, which are regulated by visual deprivation, likely include inputs carrying other sensory inputs to V1. Indeed, multisensory cortical feedback has been postulated to play a role in cross-modal activation of V1 by braille reading [27].
Based on the above findings we propose that homeostatic scaling up of excitatory synapses would provide a cellular basis for cross-modal recruitment by increasing the gain of intracortical synapses, which carrying multisensory information, onto V1 L2/3 neurons. Our hypothesis is that following visual deprivation, previously subthreshold inputs carrying auditory and tactile information may become strong enough to summate and cross the threshold to activate V1 neurons (Fig. 2A). If this is the case, our prediction is that in the absence of vision, V1 neurons would respond with action potentials following auditory or tactile stimulation. Such modifications would provide a cellular substrate for V1 activation in blind individuals when reading braille [6,7]. While most of the human brain imaging studies have reported V1 activation with braille reading in early onset blind [7], a recent study suggests that such change can occur quite rapidly even in adults who are blindfolded for only a few days [4]. This is consistent with our idea that homeostatic synaptic plasticity mechanisms, which occur with only a few days of visual deprivation even in adults [18], may play a role in this process.
Figure 2. Potential functional implication of differential adaptation of intracortical and feedforward synapses in the superficial layers of deprived and spared cortices.
(A)In the deprived cortex, loss of vision triggers global homeostatic scaling up of intracortical excitatory synapses in L2/3 without changes in feedforward synapses from L4. This allows previously weak synapses carrying multisensory information from other senses to cross the threshold and activate L2/3 neurons. Such synaptic plasticity mechanisms may be a cellular basis for cross-modal recruitment of V1 by braille reading. Intracortical excitatory postsynaptic potentials (EPSPs) are depicted in blue and visually evoked EPSP is shown in magenta. Dotted lines represent resting potential and action potential threshold.
(B) In the spared cortices, such as in S1 and A1, vision loss leads to experience-dependent strengthening of feedforward synapses from L4 to L2/3. In addition, there is concomitant global homeostatic scaling down of intracortical excitatory synapses. Such synaptic modification would allow the spared cortex to preferentially process feedforward sensory inputs at the expense of intracortical processing. Intracortical EPSPs are depicted in blue and sensory evoked EPSP is shown in magenta. Dotted lines represent resting potential and action potential threshold.
Consistent with the notion of cross-modal recruitment, there is some evidence that V1 can be activated by other sensory inputs after loss of its primary inputs. For instance, monocular enucleation of adult rodents initially reduces the activity in the deprived monocular zone of V1, but activity gradually increases back to normal levels [28], which is dependent on whisker stimuli [29]. Interestingly, this reactivation initially occurs in the supragranular layers of V1 followed by a delayed recovery in the infragranular layers [28]. This provides an intriguing possibility that homeostatic synaptic plasticity in V1 L2/3 may allow strengthening of inputs carrying whisker information to reactivate V1. In essence, this kind of adaptation would allow V1, when deprived of its primary inputs, to process the remaining senses. Therefore, plasticity seen in the deprived sensory cortex could be the cellular basis for cross-modal recruitment seen in studies from blind and blind folded humans showing that activity in V1 is needed for braille reading [4,30]. The strict requirement that complete loss of visual inputs is required for homeostatic up-scaling of V1 intracortical synapses [19] could explain the results of a recent study showing an inverse correlation between the degree of residual vision and V1 activation by ultra fast speech comprehension [9].
Cross-modal adaptive synaptic plasticity in the spared cortices: A basis for sensory compensation?
It is not only the deprived sensory cortex that adapts to loss of its sensory inputs, but the spared sensory cortices also undergo synaptic changes (Table 1). While such changes at the level of primary sensory cortices are surprising, a recent study showed that there are subthreshold synaptic inputs between primary sensory cortices [2]. Therefore, plasticity of these inputs could potentially serve as a substrate for the observed cross-modal interaction. Initial reports of compensatory plasticity in the spared sensory cortices showed that the excitatory synapses in superficial layers change rather globally. For instance, the average amplitude of mEPSCs in L2/3 of A1 and S1 barrel fields (S1BF) decreases following a week of visual deprivation [15,19], which is also observed when visual deprivation is initiated in adulthood [31]. A more chronic visual deprivation from birth also similarly alters the amplitude of mEPSCs in L2/3 neurons [15]. However, when experiments are done before the initiation of the critical period, there is a global decrease in the frequency of mEPSCs across the primary sensory cortices rather than a change of mEPSC amplitude [32]. Collectively, these results suggest that L2/3 neurons respond globally across many synapses to adapt to cross-modal loss of sensory inputs.
Table 1.
Summary of cortical plasticity across ages and visual experience manipulations.
| Layer 2/3 Neurons in V1 |
Layer 4 Neurons in V1 |
Ocular Dominance Plasticity in V1 |
Cross-modal Synaptic Plasticity in S1 or A1 |
|
|---|---|---|---|---|
| Normal Juvenile (<p35) | Highly plastic for LTP/LTD [35] and scaling (≥ p21) [18] | Highly plastic for LTP/LTD [35] and scaling (p16–20) [16] | Dramatic ocular dominance plasticity (ODP) [56] | |
| Normal Adult (≥p35) | Plastic for LTP/LTD [35] and scaling [18] | Aplastic [16,35] | No ODP with a brief duration (3–4d) of MD [56] | |
| Dark Reared from Birth to Adulthood | Promotes LTP at the expense of LTD (≥p30) [57]. Scales up mEPSCs (≥p35) [15]. | No recovery of LTP (≥p20) or LTD (≥p30) [35] | ODP beyond traditional critical period [58] | Scales down mEPSCs in L2/3 of S1 (≥p35) [15]. |
| Dark Exposure in Adulthood | Promotes LTP at the expense of LTD (≥p30) [59]. Scales up mEPSCs and lateral inputs (≥p90) [18,21]. No change in synapses from L4 (≥p90) [21]. | No potentiation of thalamocortical (TC) synapses (≥p90) [31] | ODP in previously aplastic age (≥p90) [49] | Scales down mEPSCs and lateral inputs to A1 L2/3 (≥p90) [21]. Potentiates TC synapses in A1 L4 (≥p90) [31]. Potentiates L4 to L2/3 synapses in A1 (≥p90) [21]. |
| Monocular Enucleation in Adulthood | Gradual recovery of activity by whisker inputs (≥p120) [28]. | Gradual activation of S1 and A1 (≥p120) [29]. |
Note: Data summarized here are from rodents. Postnatal ages (p) for the observation are in parentheses. Synaptic plasticity referenced here are for excitatory synapses – feedforward synapses in the case of LTP/LTD – onto principal neurons of each layer.
Interestingly, cross-modal compensatory plasticity of excitatory synaptic strength in spared cortices can be dissociated from changes in V1 and requires within modality sensory inputs [19,31]. Even so, there is no corresponding gross increase in the level of sensory inputs arriving from the peripheral organs [19,31]. This suggests that central adaptation allows the same sensory inputs to trigger synaptic plasticity. In support of this, a few days of visual deprivation enhances the strength of thalamocortical synapses in A1 L4 [31], and also potentiates L4 to L2/3 synapses in A1 [21] as well as in S1BF [33]. Surprisingly, cross-modally induced potentiation of thalamocortical synapses was reported in adult mice [31], even though thalamocortical synaptic plasticity was previously thought to be restricted to early development around second postnatal week in rodents [34–36] (Table 1). Even so, cross-modal potentiation of thalamocortical synapses seems to be a general property of the adult cortex, because it is also observed in V1 L4 following a few days of deafening [31]. On the other hand, visual deprivation does not alter the strength of thalamocortical synapses in V1 L4 [31], suggesting that within modality change in sensory experience is not sufficient to alter thalamocortical synaptic strength in adults. However, a more drastic sensory loss such as denervation of primary sensory afferents can trigger thalamocortical synaptic plasticity in adult S1BF [37].
Cross-modal potentiation of feed-forward synapses allows the same bottom up sensory inputs to more strongly influence the activity of cortical neurons, and allows for better processing of the remaining senses. In line with this, visual deprivation decreases the neural threshold for sound intensity in A1 L4 neurons [31] and sharpens the neuronal receptive fields in A1 [31] and S1BF [33]. The fact that cross-modal potentiation depends on the spared senses [19,31] suggests that this may be a form of experience-dependent synaptic plasticity similar to LTP. In addition to changes in the strength of feed-forward synapses, recently we found that the strength of lateral intracortical excitatory synapses to A1 L2/3 decreases [21], which suggests that the cross-modal decrease in mEPSCs in A1 L2/3 [15,31] mainly reflects the more abundant lateral intracortical synapses to these neurons. These changes imply that there is differential cross-modal adaptation of the feed-forward and intracortical excitatory synaptic gain, where the former is potentiated at the expense of the latter (Fig. 2B). In addition to the alterations in the strength of intracortical synapses, cross-modal adaptation to vision loss includes spatial refinement of intracortical inputs to A1 L2/3 [38]. Computational modeling of such changes show that refinement of intracortical inputs combined with strengthening of feedforward synapses allow more reliable coding in A1 [38].
Cross-modal synaptic plasticity in adults: A basis for recovering adult cortical plasticity?
The limited early plasticity of thalamocortical inputs in L4 (Table 1) may form the basis for limited cortical plasticity later in life. For instance, ODP with brief duration of MD is quite limited to early development (Box 1), but a period of dark exposure later in life can recover juvenile-like ODP and restores visually evoked potentials (VEPs) from the deprived eye in L4 [39]. While there are reports of recovery of V1 neural responses to the deprived eye after reversal of MD in adults (Box 1), this is restricted to MD initiated after an initial period of visual experience [40–46]. Chronic MD initiated before or right after eye opening is much more resilient to recovery [39,41,47–50]. These findings support clinical observations that the recovery of visual acuity after removal of congenital cataracts in humans is limited to very early interventions [51]. At the cellular level, MD during early development leads to weakening of synaptic inputs serving the deprived eye in V1 [40,52,53], hence mechanisms to enhance synaptic potentiation will be relevant to allow the weaker deprived eye inputs to regain synaptic connectivity in V1. Since a few days of deafening leads to potentiation of thalamocortical synapses in V1 [31], it is plausible that cross-modal sensory deprivation may be an effective means to recover the strength of deprived eye inputs caused by chronic MD. Furthermore, because cross-modal sensory deprivation leads to potentiation of feed-forward synapses and enhances neuronal processing of sensory inputs to the spared cortices in adults [31], it may be a useful tool to enhance bottom-up processing of sensory information in the adult brain.
Highlights.
Sensory loss produces cross-modal plasticity across primary sensory cortices.
Deprived sensory cortices undergo synaptic plasticity for cross-modal recruitment.
Synaptic plasticity in spared sensory cortices conform to compensatory plasticity.
Cross-modal synaptic plasticity is readily observed in adults.
Conclusions
Here we summarized recent findings indicating that loss of a sensory modality produces two distinct modes of cross-modal synaptic plasticity (Fig. 1). In the deprived sensory cortex intracortical synapses undergo homeostatic potentiation, which we propose forms the basis for cross-modal recruitment. In the spared sensory cortices compensatory plasticity is triggered via experience-dependent potentiation of feedforward synapses and concomitant homeostatic scaling down of intracortical synapses, which we hypothesize enhances processing of the remaining senses. Both types of cross-modal synaptic plasticity are evident in adults indicating that cross-modal sensory deprivation may be an effective means to produce plasticity in the adult cortex.
Box 1. Ocular dominance plasticity as a model of critical period and adult cortical plasticity.
It is well documented that sensory loss dramatically alters synapses and circuits in deprived sensory cortices, especially during early development. Hubel and Wiesel first demonstrated that monocular deprivation (MD) of kittens causes V1 neurons to become exclusively driven by non-deprived eye [51], a phenomenon termed ocular dominance plasticity (ODP). Since its first description, ODP has been thoroughly studied and has served as a model of cortical plasticity. ODP can most reliably be elicited during periods of development during which the visual cortex is highly plastic, referred to as the “critical period”. As such, manipulations that alter the timing of critical period may be effective in altering the timeline for ODP, and molecular mechanisms underlying critical period timing may be key to reopening adult cortical plasticity.
Several factors have been identified as important for the closure of the critical period and proper sensory development (reviewed in [54]). In general, closure of the critical period is thought to be controlled by several anatomical, molecular, and neuromodulatory mechanisms. These include inhibition of axonal growth and dendritic spine turnover, regulation of inhibitory neuronal activity through perineuronal nets and signaling factors including endocannabinoids, alterations in genetic and epigenetic signaling pathways, and neuromodulators such as serotonin. Presumably, all of these factors work in concert to mature the cortical circuitry and restrict ODP to the critical period during development.
Though the adult cortex has long been thought to be resilient to change, recent evidence suggests that it retains the ability to modify with sensory experience [39–41,45]. In juveniles, cortical plasticity is critical for the developmental fine-tuning of the cortical circuits to the sensory environment, while in adults cortical plasticity acts as an adaptive mechanism, serving to recover function following injury or sensory loss. ODP, if present, generally requires longer periods of MD in adult animals than in the young [40]. But more often ODP in adults requires manipulations that remove the “brakes” on cortical plasticity. In brief, environmental enrichment [55], a period of dark exposure [39,49,50], or locomotion [45] has been shown to enable ODP in adults. In addition, genetic or pharmacological removal or suppression of identified molecular “brakes” can also enable ODP in adults. Some forms of adult plasticity may utilize different cellular substrates or cortical circuits compared to those used in juveniles. For instance, the ability of cortical synapses to undergo LTP/LTD and/or homeostatic synaptic plasticity with age is laminar specific (Table 1). Hence the circuits that support sensory experience-dependent synaptic plasticity may differ between juveniles and adults.
Acknowledgements
This work was supported by NIH grants (R01-EY014882 and R01-EY022720) to H-KL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References
- 1.Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2. Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F, Medini P. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 2012;73:814–828. doi: 10.1016/j.neuron.2011.12.026. ••Using in vivo whole-cell recordings this study demonstrated that auditory stimulation elicited inhibitory synaptic currents in the primary visual cortex. They further showed that such cross-modal interactions are common across primary sensory cortices.
- 3.Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merabet LB, Hamilton R, Schlaug G, Swisher JD, Kiriakopoulos ET, Pitskel NB, Kauffman T, Pascual-Leone A. Rapid and reversible recruitment of early visual cortex for touch. PLoS One. 2008;3:e3046. doi: 10.1371/journal.pone.0003046. •This study showed rapid recruitment of V1 with braille training in blindfolded adults. Furthermore, the authors showed using transcranial magnetic stimulation that V1 activity is needed for braille reading in blindfolded individuals.
- 5.Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 6.Sadato N, Pascual-Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- 7.Buchel C, Price C, Frackowiak RS, Friston K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain. 1998;121(Pt 3):409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich S, Hertrich I, Ackermann H. Ultra-fast speech comprehension in blind subjects engages primary visual cortex, fusiform gyrus, and pulvinar - a functional magnetic resonance imaging (fMRI) study. BMC Neurosci. 2013;14:74. doi: 10.1186/1471-2202-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich S, Hertrich I, Ackermann H. Training of ultra-fast speech comprehension induces functional reorganization of the central-visual system in late-blind humans. Front Hum Neurosci. 2013;7:701. doi: 10.3389/fnhum.2013.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott GD, Karns CM, Dow MW, Stevens C, Neville HJ. Enhanced peripheral visual processing in congenitally deaf humans is supported by multiple brain regions, including primary auditory cortex. Front Hum Neurosci. 2014;8:177. doi: 10.3389/fnhum.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadato N, Okada T, Honda M, Yonekura Y. Critical period for cross-modal plasticity in blind humans: a functional MRI study. Neuroimage. 2002;16:389–400. doi: 10.1006/nimg.2002.1111. [DOI] [PubMed] [Google Scholar]
- 12.Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neurosci Biobehav Rev. 2014;41:36–52. doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116(Pt 1):39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Rauschecker JP. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- 15. Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 2006;9:1001–1003. doi: 10.1038/nn1725. ••One of the first studies to pinpoint molecular mechanisms underlying cross-modal synaptic changes in multiple primary sensory cortical areas. This study showed that homeostatic synaptic scaling and bidirectional AMPA receptor regulation are involved in cross-modal synaptic plasticity.
- 16.Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- 17.Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He K, Petrus E, Gammon N, Lee HK. Distinct sensory requirements for unimodal and cross-modal homeostatic synaptic plasticity. J Neurosci. 2012;32:8469–8474. doi: 10.1523/JNEUROSCI.1424-12.2012. ••This study showed specific requirements for sensory experience to selectively modify spared and deprived cortex. In addition, this study showed that compensatory plasticity in the spared cortex is experience-dependent.
- 20.Blais BS, Frenkel MY, Kuindersma SR, Muhammad R, Shouval HZ, Cooper LN, Bear MF. Recovery from monocular deprivation using binocular deprivation. J Neurophysiol. 2008;100:2217–2224. doi: 10.1152/jn.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petrus E, Rodriguez G, Patterson R, Connor B, Kanold PO, Lee HK. Vision Loss Shifts the Balance of Feedforward and Intracortical Circuits in Opposite Directions in Mouse Primary Auditory and Visual Cortices. J Neurosci. 2015;35:8790–8801. doi: 10.1523/JNEUROSCI.4975-14.2015. ••This study demonstrated that cross-modal recruitment involves plasticity of lateral intracortical synapses, while compensatory plasticity acts on feedforward synapses.
- 22.Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W, Carrasquillo Y, Hooks BM, Nerbonne JM, Burkhalter A. Distinct balance of excitation and inhibition in an interareal feedforward and feedback circuit of mouse visual cortex. J Neurosci. 2013;33:17373–17384. doi: 10.1523/JNEUROSCI.2515-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coogan TA, Burkhalter A. Hierarchical organization of areas in rat visual cortex. J Neurosci. 1993;13:3749–3772. doi: 10.1523/JNEUROSCI.13-09-03749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder CE, Foxe J. Multisensory contributions to low-level, 'unisensory' processing. Curr Opin Neurobiol. 2005;15:454–458. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Pons T. Novel sensations in the congenitally blind. Nature. 1996;380:479–480. doi: 10.1038/380479a0. [DOI] [PubMed] [Google Scholar]
- 28.Van Brussel L, Gerits A, Arckens L. Evidence for cross-modal plasticity in adult mouse visual cortex following monocular enucleation. Cereb Cortex. 2011;21:2133–2146. doi: 10.1093/cercor/bhq286. [DOI] [PubMed] [Google Scholar]
- 29.Nys J, Aerts J, Ytebrouck E, Vreysen S, Laeremans A, Arckens L. The cross-modal aspect of mouse visual cortex plasticity induced by monocular enucleation is age dependent. J Comp Neurol. 2014;522:950–970. doi: 10.1002/cne.23455. [DOI] [PubMed] [Google Scholar]
- 30.Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 31. Petrus E, Isaiah A, Jones AP, Li D, Wang H, Lee HK, Kanold PO. Crossmodal induction of thalamocortical potentiation leads to enhanced information processing in the auditory cortex. Neuron. 2014;81:664–673. doi: 10.1016/j.neuron.2013.11.023. ••This study revealed that sensory deprivation potentiates thalamocortical synapses of spared cortices in adult animals, leading to a functional enhancement of spared sensory processing.
- 32.Zheng JJ, Li SJ, Zhang XD, Miao WY, Zhang D, Yao H, Yu X. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci. 2014;17:391–399. doi: 10.1038/nn.3634. [DOI] [PubMed] [Google Scholar]
- 33. Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, et al. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. ••This study linked sensory experience with neuromodulator function and molecular mechanisms of plasticity; specifically, the research showed that visual deprivation increases serotonin levels in multiple primary sensory cortices and leads to increased synaptic AMPA receptor content in spared cortices.
- 34.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 35.Jiang B, Trevino M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27:9648–9652. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu X, Chung S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP. Thalamocortical inputs show post-critical-period plasticity. Neuron. 2012;74:731–742. doi: 10.1016/j.neuron.2012.04.024. •Combining MRI and electrophysiological techniques, this study showed that denervation can induce thalamocortical plasticity in adult rodents.
- 38.Meng X, Kao JPY, Lee H-K, Kanold PO. Visual deprivation causes refinement of intracortical circuits in the auditory cortex. Cell Rep. doi: 10.1016/j.celrep.2015.07.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montey KL, Quinlan EM. Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nat Commun. 2011;2:317. doi: 10.1038/ncomms1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 41.Liao DS, Krahe TE, Prusky GT, Medina AE, Ramoa AS. Recovery of cortical binocularity and orientation selectivity after the critical period for ocular dominance plasticity. J Neurophysiol. 2004;92:2113–2121. doi: 10.1152/jn.00266.2004. [DOI] [PubMed] [Google Scholar]
- 42.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32:9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaneko M, Stryker MP. Sensory experience during locomotion promotes recovery of function in adult visual cortex. Elife. 2014;3:e02798. doi: 10.7554/eLife.02798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephany CE, Chan LL, Parivash SN, Dorton HM, Piechowicz M, Qiu S, McGee AW. Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. J Neurosci. 2014;34:11631–11640. doi: 10.1523/JNEUROSCI.0545-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 1965;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- 48.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- 50. Montey KL, Eaton NC, Quinlan EM. Repetitive visual stimulation enhances recovery from severe amblyopia. Learn Mem. 2013;20:311–317. doi: 10.1101/lm.030361.113. ••This study is one of the few that reports recovery from chronic MD initiated before eye opening through adulthood. Such form of chronic MD has previously been shown to be resistant to treatment, hence the non-invasive method of dark exposure used in this study is of clinical value.
- 51.Daw N. Visual development. New York: Plenum Press; 1995. [Google Scholar]
- 52.Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 53.Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 55.Bartoletti A, Medini P, Berardi N, Maffei L. Environmental enrichment prevents effects of dark-rearing in the rat visual cortex. Nat Neurosci. 2004;7:215–216. doi: 10.1038/nn1201. [DOI] [PubMed] [Google Scholar]
- 56.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 58.Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo Y, Huang S, de Pasquale R, McGehrin K, Lee HK, Zhao K, Kirkwood A. Dark exposure extends the integration window for spike-timing-dependent plasticity. J Neurosci. 2012;32:15027–15035. doi: 10.1523/JNEUROSCI.2545-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]