Abstract
Background
Adequate maternal thyroid function during pregnancy is necessary for normal fetal brain development, making pregnancy a critical window of vulnerability to thyroid disrupting insults. Sodium/iodide symporter (NIS) inhibitors, namely perchlorate, nitrate, and thiocyanate, have been shown individually to competitively inhibit uptake of iodine by the thyroid. Several epidemiologic studies examined the association between these individual exposures and thyroid function. Few studies have examined the effect of this chemical mixture on thyroid function during pregnancy.
Objectives
We examined the cross sectional association between urinary perchlorate, thiocyanate and nitrate concentrations and thyroid function among healthy pregnant women living in New York City using weighted quantile sum (WQS) regression.
Methods
We measured thyroid stimulating hormone (TSH) and free thyroxine (FreeT4) in blood samples; perchlorate, thiocyanate, nitrate and iodide in urine samples collected from 284 pregnant women at 12 (± 2.8) weeks gestation. We examined associations between urinary analyte concentrations and TSH or FreeT4 using linear regression or WQS adjusting for gestational age, urinary iodide and creatinine.
Results
Individual analyte concentrations in urine were significantly correlated (Spearman’s r 0.4–0.5, p < 0.001). Linear regression analyses did not suggest associations between individual concentrations and thyroid function. The WQS revealed a significant positive association between the weighted sum of urinary concentrations of the three analytes and increased TSH. Perchlorate had the largest weight in the index, indicating the largest contribution to the WQS.
Conclusions
Co-exposure to perchlorate, nitrate and thiocyanate may alter maternal thyroid function, specifically TSH, during pregnancy.
Keywords: Perchlorate, Chemical mixtures, Thyroid Function, Pregnancy
INTRODUCTION
Pregnancy represents a period of unique vulnerability to the adverse effects of thyroid disrupting chemicals. During pregnancy, maternal thyroid function increases to meet the needs of both mother and developing fetus. Physiologic changes associated with pregnancy require the maternal thyroid gland to increase production of thyroid hormones by 40% to 100% (Stagnaro-Green, 2011). Until the fetal thyroid matures towards the end of gestation, the developing fetus relies on maternal thyroid hormones to guide normal brain development (Burrow et al., 1994). Maternal thyroid insufficiency during gestation has been associated with adverse neurodevelopmental health outcomes in offspring. Frank hypothyroidism (serum thyrotropin concentrations ≥99.7th percentile) during early pregnancy is associated with adverse outcomes ranging from delayed cognitive function and low intelligence scores to cretinism (i.e. severely stunted physical and mental growth) in offspring (Haddow et al., 1999). More subtle changes in maternal thyroid function during critical windows of development may also predict poor psychomotor and cognitive outcomes in children (Pop et al., 1999). Because thyroid hormone is important for the synchrony of brain development, and fetal thyroid hormone production is not sufficient until late in pregnancy, pregnant women may be more vulnerable to the thyroid disrupting effects of some environmental chemicals (Howdeshell, 2002). Indeed, among the small number of mothers who were hypothyroid or hypothyroxinemic during pregnancy (n = 44), high perchlorate exposure was associated with increased odds of offspring IQ being in the lowest 10% at 3 years of age (Taylor et al., 2014). In the current paper, we investigate exposure to thyroid disrupting compounds and estimate the contribution of perchlorate, nitrate and thiocyanate to adverse changes in maternal thyroid function among healthy pregnant women.
Perchlorate, nitrate and thiocyanate are environmental chemicals known to inhibit iodine uptake at the sodium iodide symporter (NIS) located in the basolateral membrane of thyroid follicular cells (Cao et al., 2010; NRC, 2005; Steinmaus et al., 2007; Tran et al., 2008; Ward et al., 2010; Wolff, 1998). Iodine uptake at the NIS is essential for thyroid hormone synthesis and inadequate iodine is the major cause for disturbance in the hypothalamus-pituitary-thyroid (HPT) axis leading to hypothyroidism (IOM, 2001; Yen, 2001; Zimmermann, 2009). Human exposure to these chemicals occurs mainly through diet and drinking water (Blount and Valentin-Blasini, 2006; Dasgupta et al., 2006; Huber et al., 2011; Lau et al., 2013; Murray et al., 2008). Cigarette smoke is likely the major source of thiocyanate exposure for the non-occupationally exposed population ((ATSDR), 2006). Diet may be an important source of exposure to thiocyanate among non-smokers as it is also found naturally in Brassica genus vegetables, such as cauliflower, broccoli, kale and Brussel sprouts (Han and Kwon, 2009). Perchlorate, both a naturally occurring and man-made chemical used to produce rocket fuel, fireworks, and explosives, is widespread in the U. S. (Blount et al., 2007; Dasgupta et al., 2006; Steinmaus et al., 2013) and worldwide (Dyke et al., 2007; Ozpinar et al., 2014; Taylor et al., 2014; Zhang et al., 2010). Large-scale studies of the U.S. population suggest environmental perchlorate exposure is associated with altered thyroid function, namely decreased free thyroxine (free T4) and increased thyroid stimulating hormone (TSH) (Blount et al., 2006; Steinmaus et al., 2010; Suh et al., 2013). In one recent study of pregnant woman, exposure to perchlorate during pregnancy has been associated with changes in maternal thyroid function (Charatcharoenwitthay et al 2014), though other population studies including pregnant women do not show demonstrate similar associations (Pearce et al., 2011; Taylor et al., 2014). Nitrate and thiocyanate were detected in nearly all spot urine samples collected from NHANES 2001–2002 participants (Suh et al., 2013). Levels of exposure to nitrate and thiocyanate are much higher than perchlorate, though experimental studies suggest nitrate and thiocyanate are less potent inhibitors of NIS activity than perchlorate (Tonacchera et al., 2004). Exposure to nitrate or thiocyanate has been associated with decreased thyroid function in human populations (Blount et al., 2006; Brauer et al., 2006; Tajtakova et al., 2006).
As perchlorate, nitrate and thiocyanate are ubiquitous in the environment and potentially act through the same mechanism of action, it is useful to account for the effects of exposure to the mixture of these chemicals on thyroid function (De Groef et al., 2006; Tarone et al., 2010). An in vitro study considering the effects of exposure to a mixture of these three compounds suggests they interact in a simple additive fashion (Tonacchera et al., 2004). Such model predictions from in vitro studies can place perspective on health risks associated with environmental exposure to trace amounts of these compounds in humans, but similar dosing studies are not ethical in humans, thus we rely on data from epidemiologic studies. In the epidemiologic literature, analytical methods which typically evaluate associations with individual chemicals may not be appropriate to examine the effect of exposure to a chemical mixture on maternal thyroid function due to (1) correlated exposures, (2) low level exposures (particularly multiple compounds below an individual observable effect level, but which in combination produce an observable effect), and (3) differences in potency (i.e. chemicals with the highest body burdens are not necessarily the most potent/toxic) (Gennings et al., 2013). Weighted quantile sum (WQS) regression is an approach recently proposed to examine mixture effects in epidemiologic studies. This method can be used to estimate the total exposure burden due to a mixture of correlated contaminants and identify influential compounds in the mixture.
The objective of this cross sectional study was to assess the relationships between perchlorate, thiocyanate and nitrate concentrations and maternal thyroid function among healthy pregnant women enrolled in the New York City area between 2009–2010. We compare traditional regression approaches to weighted quantile sum (WQS) regression to examine the associations between exposure to a mixture of these compounds and maternal thyroid function.
METHODS
Study design
The study sample comprises healthy pregnant women aged 16–35 years participating in the Endocrine Disruption in Pregnant Women: Thyroid Disruption and Infant Development Study. Enrollment took place in two prenatal clinics in New York City between September 2009 and December 2010. Eligible subjects consisted of pregnant women with singleton pregnancies seeking prenatal care before approximately 12 weeks gestational age (based on reported date of last menstrual period and the earliest ultrasound), who were free of clinical thyroid disorders, were not taking any thyroid medication, and reported no history of thyroid cancer. Subjects were also excluded if they had medical complications (including chronic hypertension, diabetes, or epilepsy), or reported use of street drugs and/or alcohol during pregnancy. Baseline data collected on mothers enrolled into the study included demographic characteristics, measures of social circumstances (marital status, home ownership, income, education, medical insurance coverage), number of previous pregnancies, infections during the current pregnancy, and pre-pregnancy weight and height. The Institutional Review Board (IRB) of Columbia University approved this study protocol. Written informed consent was obtained from all subjects. The Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) involvement was limited to analyzing coded specimens and interpreting results.
Biological sampling data
During a routine prenatal visit conducted during the first half of pregnancy, blood and spot urine samples were collected from all participants. A single 7.5 mL serum separator tube was drawn for testing of thyroid hormones (TSH and Free T4). Following venipuncture, blood was clotted at room temperature for 30–45 minutes, centrifuged for 10 minutes, then stored cold (2–8°C) until shipment to the Collaborative Studies Clinical Laboratory, Minneapolis, Minnesota. TSH and Free T4 were measured using a chemiluminescent immunoassay (Vitros Immunoassay System, OrthoClinical Diagnostics, Rochester, New York).
Aliquots of urine were shipped on dry ice to the Division of Laboratory Sciences at the Centers for Disease Control and Prevention for measurement of perchlorate, thiocyanate, nitrate, and iodide using ion chromatography tandem mass spectrometry (Blount and Valentin-Blasini, 2006; Blount and Valentin-Blasini, 2007). Urinary creatinine was measured using an automated colorimetric method (COBAS Creatinine plus assay, Roche Diagnostics Corporation, Indianapolis, Indiana). Reported results for all CDC assays met the division’s quality control and quality assurance performance criteria for accuracy and precision (Caudill et al., 2008).
Of 316 women enrolled into the study, baseline demographic information, thyroid function measures and maternal urine samples were available for 293 subjects. Nine subjects were further excluded due to thyroid markers indicating hyperthyroidism (TSH < 0.08 mU/L and free T4 > 1.90 ng/dL). The final sample size for the current analysis is thus 284. Sensitivity analyses including the 9 hyperthyroid subjects did not change the direction of the results.
Statistical analysis
Descriptive Statistics
We used Spearman’s correlation coefficient to examine associations between creatinine-adjusted urinary exposure variables (perchlorate, thiocyanate, nitrate and iodide) and to examine relationships between creatinine adjusted urinary exposure variables and thyroid function outcome variables (serum TSH and Free T4). The concentration of perchlorate measured in one subject was under the limit of detection (LOD). The concentration of thiocyanate measured in one subject was under the LOD. For these two subjects, the LOD for each analyte under the LOD was used. We also examined the associations between additional covariates known or likely to be associated with thyroid function or urinary NIS inhibitor exposure.
Associations between exposure and thyroid outcomes
Thyroid stimulating hormone (TSH) was natural log transformed to minimize the influence of extreme values. In regression models, creatinine was always included as a covariate in the model to control for urinary dilution. Additionally, we included variables associated with the exposure or the outcomes of interest at a significance level of 20% (p < 0.20). Covariates included maternal education, annual family income, ethnicity (Hispanic or not Hispanic), cigarette smoking during pregnancy (self-reported yes/no), maternal reported pre-pregnancy body mass index (BMI), gestational age at the time of urine collection (and the quadratic term) and urinary iodide concentration (natural log scale).
Multiple linear regression analyses examined the association between each natural log transformed urinary exposure measure (perchlorate, thiocyanate, or nitrate) individually with Free T4 or log TSH using the core model described above. Exposure variables were treated; a) continuously (Model 1), b) as categorical variables with 4 quartiles (Model 2), using the lowest quartile of exposure as the reference, and c) as dichotomous variables comparing the highest quartile of exposure to all other categories of exposure. Based on evidence suggesting the potential effect of perchlorate exposure on thyroid function is dependent on the presence of other environmental NIS inhibitors (including nitrate and thiocyanate) and by iodine intake (De Groef et al., 2006; Tarone et al., 2010), we examined 2 by 2 interactions between exposures as follows; perchlorate by nitrate, perchlorate by thiocyanate, perchlorate by iodide, nitrate by thiocyanate, nitrate by iodide, and thiocyanate by iodide.
Multiple logistic regression was used to examine the association between each natural log transformed urinary exposure measure individually with the odds of each thyroid hormone being in the 10th percentile of function. TSH was dichotomized at the highest 10% compared to the remaining 90%. Free T4 was dichotomized as the lowest 10% compared to the remaining 90% (Koopman-Esseboom et al., 1994).
Weighted Quantile Sum (WQS) regression was used to estimate the association between exposure to the mixture of perchlorate, thiocyanate and nitrate and thyroid function. The WQS regression was described previously (Carrico et al., 2014). WQS estimates the empirical weights for each exposure based on the associations between the individual exposures and the outcome of interest and constructs a weighted index, of exposure. This index is used as a single exposure variable in linear regression models estimating the relationship between exposure to the three chemicals and free T4 or TSH. The index is interpretable as an estimation of the mixture effect (i.e., the slope associated with the index) where the weights identify the bad actors and ‘zero out’ components with no (or negligible) association (Billionnet et al., 2012). We a priori hypothesized that the WQS index would have a positive association with log TSH and an inverse association with Free T4.
RESULTS
Demographics
Sociodemographic characteristics of the mothers participating in this study are presented in Table 1. Most subjects were enrolled during the first half of pregnancy (mean weeks of gestation at sample collection = 12.2 (range 5 to 23 weeks). This cohort is predominately Hispanic (69%). The mean maternal age at enrollment was 29 (range 16–43 years). At the time of enrollment, the majority of women (84%) had completed high school. Of these, 44% were seeking or had obtained a college degree and 16% were seeking or had obtained a graduate degree. Despite high educational attainment, 63% reported an annual family income < $25,000. Most women (70%) were multiparous; the median number of previous pregnancies was 1. Few women (2.1%) reported smoking during pregnancy.
Table 1.
Sociodemographic characteristics of 284 mothers enrolled during the first half of pregnancy from New York City prenatal clinics between 2009–2010, New York City
| Variable | N (%) |
|---|---|
| Ethnicity | |
| Hispanic | 196 (69%) |
| Non Hispanic | 88 (31%) |
| Maternal age (mean years ± SD) | 29 ± 6.3 |
| Maternal education | |
| Less than high school diploma | 47 (17%) |
| High school or equivalent | 67 (24%) |
| College Degree (or some college) | 123 (44%) |
| Graduate Degree | 45 (16%) |
| Household income | |
| <$25,000 | 179 (63%) |
| $25,000–50,000 | 30 (11%) |
| >$50,000 | 75 (26%) |
| Prepregnancy BMI | |
| Underweight (< 18.5) | 18 (6%) |
| Normal (18.5 to 24.9) | 158 (56%) |
| Overweight (25–29.9) | 70 (25%) |
| Obese (> 30) | 38 (13%) |
| Parity (≥1) | 198 (69.7%) |
| Gestational age at urine/blood collection (mean weeks ± SD) | 12.2 ± 2.8 |
| Cigarette smoking | 6 (2.1%) |
| Thyroid Function Category*,** | |
| Euthyroid | 237 (83%) |
| Subclinical hypothyroid | 24 (9%) |
| Clinical/overt hypothyroid | 3 (1%) |
| Hypothyroxinemia | 20 (7%) |
Thyroid function categories provided in Supplementary Table 1
All subclinical or clinical hyperthyroid subjects were excluded from analyses (N = 9)
Thyroid function
Thyroid stimulating hormone (TSH) and Free T4 were measured in maternal blood samples collected in the first half of pregnancy, mean ± standard error TSH = 1.53 ± 0.07 mU/L and free T4 = 1.01 ± 0.01 ng/dL (Table 2). Consistent with our recruitment strategy, most subjects (83%) had thyroid measurements in the normal range for pregnancy (TSH 0.08 to 3.00 mU/L; Free T4 0.86 to 1.90 ng/dL).
Table 2.
Mean and Standard Error (± SE) levels of thyroid stimulating hormone (TSH) and free T4 in maternal serum and perchlorate, nitrate, thiocyanate, and iodide in maternal urine collected during the first half of pregnancy (N= 284).
| % > LOD | Mean ± SE | 25th | 50th | 75th | 95th | |
|---|---|---|---|---|---|---|
| Maternal Serum | ||||||
| TSH (mU/L) | 100 | 1.53 ± 0.07 | 0.80 | 1.23 | 1.89 | 4.13 |
| Free T4 (ng/dL) | 100 | 1.01 ± 0.01 | 0.95 | 1.01 | 1.08 | 1.20 |
| Maternal Urine (μg/g creatinine)*,** | ||||||
| Mean ± SE | 25th | 50th | 75th | 95th | ||
| Perchlorate | 99.6 | 3.54 ± 0.2 | 1.44 | 2.57 | 4.41 | 8.74 |
| Nitrate | 100 | 42149.54 ± 1416.8 | 27250 | 33250 | 50800 | 88325 |
| Thiocyanate | 99.3 | 1006.46 ± 65.29 | 372.25 | 672.00 | 1290.00 | 2852.50 |
| Iodide | 100 | 235.39 ± 39.40 | 89.25 | 138.50 | 217.00 | 520.25 |
Analytical limit of detection (LOD): Perchlorate = 0.05 ng/ml; Nitrate = 700 ng/ml; Thiocyanate = 20 ng/ml; Iodide = 0.2ng/ml.
Mean ± SE ng/ml creatinine = 110.3 ± 4.9
Urinary Exposure Measures
Perchlorate, nitrate, thiocyanate and iodide were detected in nearly all spot urine samples collected from women during the first half of pregnancy. Concentrations of exposure variables are described in Table 2.
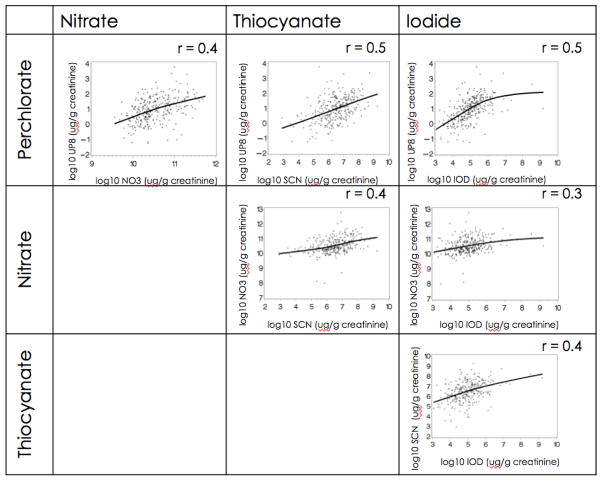
Creatinine adjusted levels of the four urinary analytes were positively and significantly correlated (Spearman’s r > 0.4, p < 0.05) (Figure 1).
Figure 1.
Spearman’s rank correlation coefficients of urinary concentrations of perchlorate, nitrate, thiocyanate and iodide (log scale and creatinine adjusted, N = 284), p < 0.001. Lines represent Loess curve.
Unadjusted associations between urinary exposure measures and thyroid function
Creatinine adjusted urinary perchlorate measures were positively associated with elevated serum TSH (Spearman’s r = 0.101, p = 0.09). Nitrate and thiocyanate were not associated with changes in serum TSH. No correlations were found between any urinary contaminant and serum Free T4.
Adjusted associations between NIS inhibitor exposure and thyroid function
Multiple regression approach
We first developed a core model including any covariates associated at p < 0.2 with log TSH and/or Free T4 in univariate analyses. The parameter estimates, standard errors and p-values of core variables predicting log TSH and Free T4 are described in Supplementary Table 2. The slope parameters for BMI and week of sample collection were positively associated with log TSH. No core variables demonstrated significant associations with Free T4 (Supplementary Table 2).
Table 3 displays the results from the multiple regression of the core model along with individual chemical contaminants; perchlorate, nitrate and thiocyanate. No significant associations were observed between urinary perchlorate, nitrate or thiocyanate and serum TSH or Free T4 controlling for potential confounding variables. Results of multiple logistic regression analyses treating TSH as a dichotomous variable comparing the highest 10% TSH values with the remaining 90% suggest increasing exposure to perchlorate was associated with a significant increase in the odds of having a serum TSH level in the highest 10th percentile (b (95% CI) = 1.78 (0.99 to 3.21, p = 0.05). No associations between any exposure and dichotomous Free T4 were found (Supplementary Table 3). No consistent patterns of interaction were identified between exposure and outcomes measures.
Table 3.
Estimated regression coefficients and Standard Error (SE) from multiple regression relating perchlorate, nitrate, and thiocyanate individually with log TSH and free T4 in maternal serum (N = 284).
| Perchlorate | Nitrate | Thiocyanate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Log TSH | b | SE | p value | b | SE | p value | b | SE | p value |
| Model – 1 natural log exposure | |||||||||
| Ln(exposure) | .073 | .063 | .248 | .011 | .076 | .886 | .033 | .055 | .548 |
| Model 2 – quartiles of exposure, reference quartile 1 (lowest exposure quartile)* | |||||||||
| Quartile 2 | .172 | .126 | .172 | −.201 | .132 | .128 | −.035 | .120 | .773 |
| Quartile 3 | −.016 | .149 | .915 | .042 | .164 | .799 | −.005 | .125 | .965 |
| Quartile 4 | .233 | .162 | .150 | −.003 | .187 | .988 | .125 | .140 | .370 |
| Quartile 4 | .178 | .112 | .112 | .028 | .119 | .816 | .139 | .112 | .217 |
| Free T4 | b | SE | p value | b | SE | p value | b | SE | p value |
| Model 1 – natural log exposure | |||||||||
| Ln(exposure) | −0.002 | 0.010 | 0.875 | 0.002 | .009 | 0.821 | −0.016 | 0.009 | 0.076 |
| Model 2 – quartiles of exposure, reference quartile 1 (lowest exposure quartile)* | |||||||||
| Quartile 2 | −0.005 | 0.022 | 0.806 | −0.004 | 0.019 | 0.825 | −0.017 | 0.021 | 0.429 |
| Quartile 3 | 0.025 | 0.026 | 0.335 | −0.009 | 0.018 | 0.625 | −0.021 | 0.022 | 0.330 |
| Quartile 4 | −0.017 | 0.028 | 0.535 | −0.030 | 0.020 | 0.125 | −0.028 | 0.024 | 0.250 |
| Model 3 – highest quartile of exposure vs. all other quartiles | |||||||||
| Quartile 4 | −0.029 | 0.019 | 0.129 | 0.016 | 0.020 | 0.442 | −0.013 | 0.019 | 0.507 |
All models adjusted for ethnicity (Hispanic vs non-Hispanic), cigarette smoke during pregnancy (yes vs no), prepregnancy BMI, week at sample collection, urinary iodide and creatinine. Sample week at collection was allowed to have a quadratic relationship with log TSH.
Range of concentrations of exposure variables within each quartile presented in Supplementary Table 4.
The inclusion of the 9 subjects with subclinical hyperthyroidism did not affect the magnitude or the direction of the associations between exposure and thyroid function for any of the multiple regression analyses.
Weighted quantile sum (WQS) regression
WQS regression was conducted on log TSH and Free T4 in separate analyses. The resulting average weights for the three chemicals and p values associated with the slope parameter for WQS are provided in Table 4. The slope associated with WQS in the analysis of TSH was positive and significant (slope = 0.12; root mean square error = 0.7, p = 0.005), suggesting an association between the mixture and TSH. The WQS suggests perchlorate was the dominant chemical (weighted at 75%) with nitrate receiving about a third of its weight (22%). The slope for WQS and free T4 was not statistically significant (p= 0.784).
Table 4.
Weighted average of 100 bootstrap weights and p value for WQS index with positive slope parameter*. The signal function was the relative test statistic in each bootstrap sample.
| Weighted average weights | p value | |||
|---|---|---|---|---|
| Perchlorate | Nitrate | Thiocyanate | ||
| Log TSH | 0.75 | 0.22 | 0.03 | 0.005 |
| FreeT4 | 0.41 | 0.57 | 0.02 | 0.784 |
All models adjusted for race/ethnicity (Hispanic vs non-Hispanic), cigarette smoke during pregnancy (yes vs. no), prepregnancy BMI, week at sample collection, urinary iodide and creatinine. Sample week at collection was allowed to have a quadratic relationship with log TSH.
DISCUSSION
The objective of this study was to examine the combined effect of urinary measures of perchlorate, thiocyanate and nitrate on TSH and Free T4 concentrations among healthy pregnant women. Because these contaminants are correlated, standard statistical approaches may be limited in their ability to detect an association between exposures and outcomes. We employed linear and logistic regression approaches to understand the associations between concentrations of each of the NIS inhibitors individually and thyroid function and a novel empirical method to address the chemicals as a complex mixture: weighted quantile sum regression (WQS) (Carrico et al., 2014). In WQS regression, we derived a single weighted index to account for the correlations between contaminants and for the correlations between each contaminant and the outcome variable. We found a significant association between the WQS index and increased TSH. Perchlorate had the largest weight in the index, indicating the largest contribution to the WQS, followed by nitrate with thiocyanate contributing the least to the WQS. Notably, in the linear regression approach, we did not find associations between individual contaminant concentrations and TSH. This is the first epidemiologic study to document a significant association between exposure to a mixture of NIS inhibitors and altered thyroid function, indicated by increased TSH among healthy pregnant euthyroid women.
There is growing evidence that the thyroid gland is particularly vulnerable to the adverse effects of exposure to endocrine disrupting chemicals (EDC) (Schmutzler et al., 2007). One target of EDC action on thyroid function may be the sodium iodide symporter (NIS) (Zoeller, 2010). The NIS transports iodide from serum into the thyroid for formation of thyroid hormones, triiodothyronine (T3) and thyroxine (T4). Experimental evidence suggests perchlorate and other NIS inhibitors compete with iodide for transport into the thyroid via NIS at the level of the thyroid gland, inhibiting both T3 and T4 production and increasing TSH production (Dohan et al., 2007; Tran et al., 2008). In the current study, we measured maternal serum free T4 and TSH during the first half of pregnancy. During pregnancy, maternal thyroid activity undergoes significant physiologic changes to allow the woman to maintain a euthyroid state. In euthyroid individuals, the increased demand on the thyroid gland during pregnancy does not result in pathology. However, it is possible that the increased burden on the thyroid coupled with exposure to thyroid disrupting chemicals make pregnancy a time of unique vulnerability. In our study, the majority of women in the cohort were enrolled at the end of the first trimester or beginning of the second trimester. Concentrations of free T4 and TSH did not differ by gestational age at sample collection.
Simultaneous to collecting blood samples for thyroid function measures, we collected maternal spot urine samples and measured concentrations of perchlorate, nitrate, thiocyanate and iodide. Concentrations of these compounds among our subjects were similar to those measured in the overall NHANES 2001–2002 cohort, a representative sample of the United States population between 2001–2002 (Blount and Valentin-Blasini, 2007). Concentrations of perchlorate among subjects in our cohort were lower than those reported among pregnant women sampled in NHANES 2003–2004 (Woodruff et al., 2011).
Using these NHANES datasets, several studies have examined associations between perchlorate exposure and thyroid function. Among non-pregnant females > 12 years of age enrolled in NHANES 2001–2002, urinary perchlorate was significantly associated with decreasing levels of total T4 (Blount et al., 2006). In the Blount (2006) study, the largest effects on thyroid function were observed in analyses restricted to women with low urinary iodine (< 100 μg/L); amongst these women, urinary perchlorate levels were positive predictors of TSH and negative predictors of total T4. The Blount (2006) study raised considerable concern about the health consequences of low-level perchlorate exposure and highlighted the need to further investigate potential effects of co-exposure to mixtures of NIS inhibitors including nitrate and thiocyanate.
Additional studies in NHANES confirmed the association observed between perchlorate and thyroid function and expanded the analyses to address the potential additive effect of co-exposure to perchlorate and thiocyanate. Using the same NHANES dataset as Blount (2006), Steinmaus (2007) found an interaction between urinary thiocyanate, perchlorate and iodide and thyroid function. Independent of perchlorate exposure, no statistically significant associations were identified between urinary thiocyanate and total T4 or TSH (Steinmaus et al., 2013). However, urinary perchlorate was independently associated with lower total T4 and the association between increasing urinary perchlorate and decreasing T4 was strongest among women with low iodine (< 100 μg/L) and high thiocyanate (>1800 μg/L). Steinmaus (2013) investigated the combined effects of perchlorate, thiocyanate, and iodine on thyroid function. Subjects were categorized into one of the three groups based on their urinary concentrations of these chemicals. Subjects with high perchlorate, high thiocyanate and low iodine had total T4 concentrations 12.9% lower (mean difference = 1.07 μg/dL, 95% confidence interval = 0.55–1.59) and free T4 concentration 7.1% lower (mean difference = 0.058, 95% confidence interval = 0.012–0.104) than subjects with low perchlorate, low thiocyanate and adequate iodine (Steinmaus et al., 2013).
A recent paper by Bruce et al (2013) reanalyzed the NHANES 2001–2002 dataset to include nitrate, and assess the potential additive relationship among urinary perchlorate, nitrate, and thiocyanate on serum thyroid parameters using a perchlorate equivalent concentration approach (PEC) (Bruce et al., 2013). This approach accounts for the different potencies of nitrate, thiocyanate, and perchlorate to inhibit the NIS. Potency factors were based on iodide uptake inhibition at the NIS following exposure each of the analytes (Tonacchera et al., 2004). Using this method, the authors found no evidence of functional thyroid abnormality associated with exposure to any single NIS inhibitor, or with the mixture of exposures.
While the literature described above characterizes the association between perchlorate and thyroid function with and without concomitant exposure to other NIS inhibiting anions in national samples, these studies exclude pregnant women, who may represent the subgroup at highest risk to thyroid disruption. The potential health risks of low-level perchlorate, nitrate, and thiocyanate exposures are most relevant to women of childbearing age and their offspring as insufficient maternal iodine during pregnancy and the immediate postpartum period results in various neurological and psychological deficits in children (Haddow et al., 1999; Pop et al., 1999; Zoeller and Rovet, 2004). Suh et al (2013) examined the independent effects of perchlorate, nitrate, and thiocyanate on serum Free T4 in NHANES focusing specifically on sensitive subpopulations including pregnant women and individuals with low urinary iodine levels. They performed an analysis combining 2001–2002 and 2007–2008 NHANES datasets. Among non-pregnant women, urinary perchlorate, nitrate and thiocyanate were individually predictive of decreased serum free T4. The inverse association between urinary NIS inhibitors and serum free T4 was not observed in pregnant women. Among the small number of pregnant women in the study (n = 44), urinary perchlorate was positively associated with free T4, in the opposite direction than anticipated from the proposed mechanism of action of perchlorate. Among these same pregnant women, no associations were observed between nitrate and/or thiocyanate and free T4. Notably, a surge in free T4 occurs in pregnant women at the end of first trimester in pregnancy to ensure adequate supply of free T4 for the fetus (Skeaff et al., 2012; Suh et al., 2013). The pregnant women were enrolled across the spectrum of gestation from a few weeks through full term. It is possible that any effect of perchlorate on free T4 may be masked by normal fluctuations of the hormone during pregnancy. Notably, pregnant women in this subset had the lowest levels of urinary perchlorate and thiocyanate compared with other groups included in the analysis. Due to small sample size and multicolinearity, authors did not address the effects of exposure to mixtures of these compounds.
In the last several years, several smaller epidemiologic studies focused specifically on the association between perchlorate exposure and thyroid function during pregnancy. Results from these smaller studies do not support the findings from the work conducted in NHANES. The first epidemiologic study to investigate the potential thyroid-related effects of perchlorate exposure took place in three cities in Northern Chile (Tellez Tellez et al., 2005). The Tellez Tellez study compared pregnant women from three cities in northern Chile, one city with high perchlorate exposure and two cities with lower perchlorate exposure. Perchlorate was measured in drinking water and in maternal spot urine samples. Exposure measured in either matrix was not associated with changes in TSH, thyroglobulin or free T4 in the mothers during gestation or neonates at birth. Women in all three Chilean cities had fairly high iodine intake (median urine iodine, 269 μg/L), compared with that in the US (139 μg/L) and the present study (173 μg/L). As dietary iodine may modulate potential thyroid inhibition (De Groef et al., 2006), a subsequent analysis of the Chilean data addressed the influence of iodine intake on the association between perchlorate and thyroid function (Gibbs, 2009). The subsequent analyses confirmed the original findings from Tellez Tellez (2005); perchlorate was not associated with TSH, thyroglobulin or free T4 after controlling for iodine status
Pearce et al (2010) addressed the role iodine intake plays on the association between perchlorate and thiocyanate exposure and thyroid function in a cross sectional investigation of iodine-deficient pregnant women in Wales and Italy. Even among these women with fairly low iodine intake (urinary iodine values less than 100 μg/liter), there were no associations between perchlorate or thiocyanate with thyroid function during early pregnancy (Pearce et al., 2010). A smaller study of thyroid function among pregnant women with fairly high levels of environmental exposure to perchlorate (2 to 3 times higher than previously reported in the United States (Blount and Valentin-Blasini, 2007), also found no association between perchlorate exposure and thyroid function (Pearce et al., 2011). No association between perchlorate exposure and thyroid function was found among pregnant women living in Greece (Pearce et al., 2012). In unadjusted analyses, inverse associations were observed between urinary perchlorate and free T4 and free T3, and positive associations were observed between urinary thiocyanate and TSH. After controlling for gestational age, maternal age, and urinary iodide, no associations were observed between exposure variables and thyroid outcomes. The Pearce study in Greece did not examine potential interactions between perchlorate and thiocyanate. A recent cohort study demonstrated an adverse effect of maternal perchlorate exposure and cognitive outcomes among offspring at 3 years of age (Taylor et al.). Across the cohort, urinary iodine levels were low (median 72 μg/liter). Authors did not find an association between perchlorate exposure and maternal thyroid function, suggesting perchlorate may affect the fetal thyroid and subsequent neurologic development without affecting maternal thyroid function (Taylor et al.).
The findings from our study consisting exclusively of pregnant women support findings from population studies controlling for pregnancy such as Blount (2006) and Steinmaus (2007, 2013), suggesting that NIS inhibitors may have an effect on maternal thyroid function. While we did not observe an effect of perchlorate alone on thyroid function, the WQS index suggested a positive association between exposure to the mixture of perchlorate, nitrate and thiocyanate and TSH. Further, the WQS index identified perchlorate as the analyte with the largest contribution to the mixture effect. Based on urinary iodide measures in a single spot urine sample, mothers enrolled in the current study did not appear iodine deficient. We controlled for urinary iodide in all analysis and it did not affect the observed associations.
The major strength of this study is the ability to examine multiple environmental exposures rather than studying a single exposure in isolation. The WQS approach allows us to examine the influence of co-exposure to perchlorate, nitrate and thiocyanate on thyroid function. While this study evidences the utility of the WQS method to address mixtures of exposures to three thyroid disrupting chemicals, future work should consider expanding the mixture to include a wide range of environmental toxicants that have been identified to interfere with thyroid function including polychlorinated biphenyls, polybrominated flame retardants, phthalates and pesticides.
There are some limitations to the current study. WQS assumes a linear relationship between the exposures in the mixture and the outcome. We performed a log-likelihood test to compare the four quartiles of each exposure variable against a ranked score. Using the F-test at 5% significance, we found no evidence of a systematic deviation of linearity. Regarding the sample collection, spot urine samples were collected for assessment of the exposures, as well as for an important covariate, urinary iodide. Studies suggest spot urine samples are an imperfect reflection of an individual’s dietary iodine status (Konig, 2011) and may be less than adequate to measure concentrations of perchlorate, nitrate and thiocyanate as all chemicals have a short biological half-life and concentrations in spot urine samples are known to be highly variable between samples (Aylward et al., 2014). In a study to investigate the temporal validity of urinary concentrations of perchlorate, nitrate, thiocyanate and iodide, Mervish et al (2012) provide evidence demonstrating fair temporal reliability in spot urine concentrations and suggest a single-spot urine sample is an adequate exposure measure in epidemiologic studies (Mervish et al, 2012). Although adjustment with urinary creatinine is standard to correct for this limitation, it may not completely eliminate the variability issues. Repeated measures of both exposure and outcome would be preferred. Additionally, in our study, we have only serum Free T4 and TSH. While the American Thyroid Association suggests free T4 and TSH are the most important measures to determine thyroid function (ATA, 2008), chemicals may directly interfere with TH action but not affect circulating TH levels or chemicals may affect other markers of thyroid function such as anti-thyroid peroxidase (TPO) antibodies via different mechanisms. While our study is not designed to investigate possible immune disorders evoked by exposure to NIH inhibitors, it is important to consider that the physiologic changes that occur during normal pregnancy induce complex endocrine and immune responses (Galofre and Davies, 2009). Exposure to environmental chemicals may further stress the thyroid system by triggering autoimmune thyroid disease (Brent, 2010). To inform practical and clinical recommendations, further studies of the effects of environmental exposures and thyroid function should include larger populations of pregnant women with samples collected at several stages of pregnancy and an expanded battery of outcomes to further assess thyroid function.
CONCLUSION
In this paper, we examine the effect of co-exposure to three moderately correlated NIS inhibiting environmental chemicals on maternal thyroid function. To do this, we apply WQS, a novel statistical methodology designed to evaluate the associations between a correlated mixture of exposures and an outcome. Results from our study reveal that the WQS index predicts TSH after controlling for potential covariates. This is the first epidemiologic study to demonstrate a significant effect of exposure to a mixture of these three highly correlated NIS inhibitors among healthy pregnant women. These results need to be confirmed in a larger study with repeated measures of exposure and outcome.
Supplementary Material
Highlights.
We measure perchlorate, nitrate, thiocyanate and iodide in 2nd trimester maternal urine samples
We measure thyroid function (TSH and Free T4) in 2nd trimester maternal blood samples
Weighted quantile sum (WQS) regression examines complex mixtures in epidemiologic studies
WQS regression identified an inverse association between co-exposure to chemicals and maternal TSH
Perchlorate had the largest weight in the WQS index, indicating it was the ‘bad actor’ of the mixture
Acknowledgments
We gratefully acknowledge the Columbia University Department of Obstetrics and Gynecology staff supervised by S. Bousleiman for screening and enrolling subjects into the original birth cohort study. We also acknowledge Xinhua Liu, Professor of Biostatistics at the Columbia University Medical Center for her original contribution to the statistical analysis and her careful review of the paper.
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) grants R00 ES020364, R21 ES016610 and P30ES023515. The findings in this article are the opinions of the authors and do not necessarily reflect the official opinion of the Centers for Disease Control and Prevention.
Footnotes
COMPETING FINANCIAL INTERESTS DECLARATION
The authors declare they have no actual or pending competing financial interests.
DISCLAIMER
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (ATSDR), A. f. T. S. a. D. R; U. S. D. o. H. a. H. Services, editor. Toxicological profile for Cyanide. Public Health Service; Atlanta, GA: 2006. [Google Scholar]
- ATA; A. T. Association, editor. Thyroid Function Test. 2008. [Google Scholar]
- Aylward LL, et al. Sources of variability in biomarker concentrations. J Toxicol Environ Health B Crit Rev. 2014;17:45–61. doi: 10.1080/10937404.2013.864250. [DOI] [PubMed] [Google Scholar]
- Billionnet C, et al. Estimating the health effects of exposure to multi-pollutant mixture. Ann Epidemiol. 2012;22:126–41. doi: 10.1016/j.annepidem.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Blount BC, et al. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect. 2006;114:1865–71. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Valentin-Blasini L. Analysis of perchlorate, thiocyanate, nitrate and iodide in human amniotic fluid using ion chromatography and electrospray tandem mass spectrometry. Anal Chim Acta. 2006;567:87–93. doi: 10.1016/j.aca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Blount BC, Valentin-Blasini L. Biomonitoring as a method for assessing exposure to perchlorate. Thyroid. 2007;17:837–41. doi: 10.1089/thy.2007.0106. [DOI] [PubMed] [Google Scholar]
- Blount BC, et al. Perchlorate exposure of the US Population, 2001–2002. J Expo Sci Environ Epidemiol. 2007;17:400–7. doi: 10.1038/sj.jes.7500535. [DOI] [PubMed] [Google Scholar]
- Brauer VF, et al. The role of thiocyanate in the etiology of goiter in an industrial metropolitan area. Eur J Endocrinol. 2006;154:229–35. doi: 10.1530/eje.1.02076. [DOI] [PubMed] [Google Scholar]
- Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755–61. doi: 10.1089/thy.2010.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce GM, et al. Urinary nitrate, thiocyanate, and perchlorate and serum thyroid endpoints based on NHANES 2001 to 2002. J Occup Environ Med. 2013;55:52–8. doi: 10.1097/JOM.0b013e31826bb774. [DOI] [PubMed] [Google Scholar]
- Burrow GN, et al. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–8. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- Cao Y, et al. Goitrogenic anions, thyroid-stimulating hormone, and thyroid hormone in infants. Environ Health Perspect. 2010;118:1332–7. doi: 10.1289/ehp.0901736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, et al. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of Agricultural, Biological and Environmental Statistics. 2014;20:100–120. doi: 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill SP, et al. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27:4094–106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- Dasgupta PK, et al. Perchlorate in the United States. Analysis of relative source contributions to the food chain. Environ Sci Technol. 2006;40:6608–14. doi: 10.1021/es061321z. [DOI] [PubMed] [Google Scholar]
- De Groef B, et al. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. 2006;155:17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- Dohan O, et al. The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc Natl Acad Sci U S A. 2007;104:20250–5. doi: 10.1073/pnas.0707207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke JV, et al. Perchlorate in dairy milk. Comparison of Japan versus the United States. Environ Sci Technol. 2007;41:88–92. doi: 10.1021/es061429e. [DOI] [PubMed] [Google Scholar]
- Galofre JC, Davies TF. Autoimmune thyroid disease in pregnancy: a review. J Womens Health (Larchmt) 2009;18:1847–56. doi: 10.1089/jwh.2008.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennings C, et al. A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ Health. 2013;12:66. doi: 10.1186/1476-069X-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JP. Comment on “Intake of iodine and perchlorate and excretion in human milk”. Environ Sci Technol. 2009;43:2654–5. doi: 10.1021/es8031538. author reply 2656–8. [DOI] [PubMed] [Google Scholar]
- Haddow JE, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Han H, Kwon H. Estimated dietary intake of thiocyanate from Brassicaceae family in Korean diet. J Toxicol Environ Health A. 2009;72:1380–7. doi: 10.1080/15287390903212709. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110(Suppl 3):337–48. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DR, et al. Estimating perchlorate exposure from food and tap water based on US biomonitoring and occurrence data. J Expo Sci Environ Epidemiol. 2011;21:395–407. doi: 10.1038/jes.2010.31. [DOI] [PubMed] [Google Scholar]
- IOM. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. 2001. pp. 258–289. [PubMed] [Google Scholar]
- Konig S. Urine molecular profiling distinguishes health and disease: new methods in diagnostics? Focus on UPLC-MS. Expert Rev Mol Diagn. 2011;11:383–91. doi: 10.1586/erm.11.13. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C, et al. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res. 1994;36:468–73. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Lau FK, et al. Urinary perchlorate as a measure of dietary and drinking water exposure in a representative sample of the United States population 2001–2008. J Expo Sci Environ Epidemiol. 2013;23:207–14. doi: 10.1038/jes.2012.108. [DOI] [PubMed] [Google Scholar]
- Murray CW, et al. US Food and Drug Administration's Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008;18:571–80. doi: 10.1038/sj.jes.7500648. [DOI] [PubMed] [Google Scholar]
- NRC. Health Implications of Perchlorate Ingestion. National Academies Press; Washington, D.C., USA: 2005. [Google Scholar]
- Ozpinar A, et al. Iodine status in Turkish populations and exposure to iodide uptake inhibitors. PLoS One. 2014;9:e88206. doi: 10.1371/journal.pone.0088206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EN, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women from Greece. Clin Endocrinol (Oxf) 2012;77:471–4. doi: 10.1111/j.1365-2265.2012.04407.x. [DOI] [PubMed] [Google Scholar]
- Pearce EN, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95:3207–15. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- Pearce EN, et al. Effect of environmental perchlorate on thyroid function in pregnant women from Cordoba, Argentina, and Los Angeles, California. Endocr Pract. 2011;17:412–7. doi: 10.4158/EP10293.OR. [DOI] [PubMed] [Google Scholar]
- Pop VJ, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- Schmutzler C, et al. Endocrine disruptors and the thyroid gland--a combined in vitro and in vivo analysis of potential new biomarkers. Environ Health Perspect. 2007;115(Suppl 1):77–83. doi: 10.1289/ehp.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeaff SA, et al. A comprehensive assessment of urinary iodine concentration and thyroid hormones in New Zealand schoolchildren: a cross-sectional study. Nutr J. 2012;11:31. doi: 10.1186/1475-2891-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagnaro-Green A. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid. 2011:21. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, et al. Combined effects of perchlorate, thiocyanate, and iodine on thyroid function in the National Health and Nutrition Examination Survey 2007–08. Environ Res. 2013;123:17–24. doi: 10.1016/j.envres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, et al. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001–2002 national health and nutrition examination survey. Environ Health Perspect. 2007;115:1333–8. doi: 10.1289/ehp.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, et al. Perchlorate in drinking water during pregnancy and neonatal thyroid hormone levels in California. J Occup Environ Med. 2010;52:1217–524. doi: 10.1097/JOM.0b013e3181fd6fa7. [DOI] [PubMed] [Google Scholar]
- Suh M, et al. The effects of perchlorate, nitrate, and thiocyanate on free thyroxine for potentially sensitive subpopulations of the 2001–2002 and 2007–2008 National Health and Nutrition Examination Surveys. J Expo Sci Environ Epidemiol. 2013 doi: 10.1038/jes.2013.67. [DOI] [PubMed] [Google Scholar]
- Tajtakova M, et al. Increased thyroid volume and frequency of thyroid disorders signs in schoolchildren from nitrate polluted area. Chemosphere. 2006;62:559–64. doi: 10.1016/j.chemosphere.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Tarone RE, et al. The epidemiology of environmental perchlorate exposure and thyroid function: a comprehensive review. J Occup Environ Med. 2010;52:653–60. doi: 10.1097/JOM.0b013e3181e31955. [DOI] [PubMed] [Google Scholar]
- Taylor PN, et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring; Data from the Controlled Antenatal Thyroid Study. J Clin Endocrinol Metab. 2014:jc20141901. doi: 10.1210/jc.2014-1901. [DOI] [PubMed] [Google Scholar]
- Tellez Tellez R, et al. Long-term environmental exposure to perchlorate through drinking water and thyroid function during pregnancy and the neonatal period. Thyroid. 2005;15:963–75. doi: 10.1089/thy.2005.15.963. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14:1012–9. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- Tran N, et al. Thyroid-stimulating hormone increases active transport of perchlorate into thyroid cells. Am J Physiol Endocrinol Metab. 2008;294:E802–6. doi: 10.1152/ajpendo.00013.2008. [DOI] [PubMed] [Google Scholar]
- Ward MH, et al. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21:389–95. doi: 10.1097/EDE.0b013e3181d6201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- Woodruff TJ, et al. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Zhang T, et al. Perchlorate and iodide in whole blood samples from infants, children, and adults in Nanchang, China. Environ Sci Technol. 2010;44:6947–53. doi: 10.1021/es101354g. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–18. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- Zoeller TR. Environmental chemicals targeting thyroid. Hormones (Athens) 2010;9:28–40. doi: 10.14310/horm.2002.1250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.