Abstract
Background
The health of post-menopausal women Veterans is a neglected area of study. A stronger empirical evidence base is needed, and would inform the provision of health care for the nearly 1 million U.S. women Veterans currently 50 years of age or older. To this end, the present work compares salient health outcomes and risk of all-cause mortality among Veteran and non-Veteran participants of the Women’s Health Initiative (WHI).
Methods
This study features prospective analysis of long-term health outcomes and mortality risk (average follow-up 8 years) among the 3,706 women Veterans and 141,009 non-Veterans who participated in the WHI Observational Study or Clinical Trials. Outcome measurements included confirmed incident cases of cardiovascular disease (CVD), cancer, diabetes, hip fractures, and all-cause mortality.
Results
We identified 17,968 cases of CVD; 19,152 cases of cancer; 18,718 cases of diabetes; 2,817 cases of hip fracture; and 13,747 deaths. In Cox regression models adjusted for age, sociodemographic, and health risk factors, Veteran status was associated with significantly increased risk of all-cause mortality (HR: 1.13; 95% CI: 1.03 – 1.23), but not with risk of CVD (HR: 1.00; CI 95%: 0.90 – 1.11); cancer (HR: 1.04; 95% CI: 0.95 – 1.14); hip fracture (HR: 1.16; 95% CI: 0.94 – 1.43); or diabetes (HR: 1.00; 95% CI: 0.89 – 1.1).
Conclusions
Women Veterans’ post-menopausal health, particularly risk for all-cause mortality, warrants further consideration. In particular, efforts to identify and address modifiable risk factors associated with all-cause mortality are needed.
Keywords: Women, Veterans, Mortality, Postmenopausal Health
INTRODUCTION
Over the past several decades, considerable empirical attention has been directed to the associations between military service, health, and mortality risk (Kang & Bullman, 1996; McLaughlin, Nielson & Waller, 2008), with a steadily increasing focus on women (Cypel & Kang, 2008; Dalager, Kang & Thomas, 1995; Kang, Cypel & Kilbourne, 2014; Thomas, Kang & Dalager, 1991; Vajdic, Stavrou, Ward, Falster & Pearson, 2014; Waller & McGuire, 2011; Yi, 2013), whose representation in the armed forces has grown dramatically over this time period (U.S. Department of Veterans Affairs, 2011). Though this literature provides a strong foundation, it is limited in size and scope, with a predominant focus on women Veterans during their early or mid-life years (c.f., Dalager, Kang & Thomas, 1995). Given that there are nearly 1 million women Veterans who are 50 years of age or older, increased research attention to health and mortality risk in women Veterans’ post-menopausal years is warranted.
Prior population-based studies have consistently documented decreased risk of morbidity and all-cause mortality among Veterans, including women, relative to the general population (Cypel & Kang, 2008; Dalager, Kang & Thomas, 1995; Kang, Cypel & Kilbourne, 2014; Thomas, Kang & Dalager, 1991; Vajdic, Stavrou, Ward, Falster & Pearson, 2014; Waller & McGuire, 2011; Yi, 2013). This “healthy soldier effect,” typically documented in young to middle-aged Veterans, is commonly ascribed to the health and fitness standards associated with military selection, as well as the increased commitment to physical fitness among military populations, and the continuous access to health care that military and Veteran populations enjoy (c.f., Kang & Bullman, 1996; McLaughlin, Nielson & Waller 2008). A very limited literature examining health and mortality risk in older Veterans suggests that the “healthy soldier effect” attenuates with time (Liu, Engle, Kang, & Cowan, 2005; London & Wilmoth, 2010; Wilmoth, London & Parker, 2010). Some research, in fact, characterizes a health “cross-over” effect among older Veterans (i.e., age 70 or above), who, despite many years of good health, evidence greater mortality risk and accelerated health decline, relative to non-Veterans (Liu, Engle, Kang & Cowan, 2005; London & Wilmoth, 2010).
This health “cross-over” is thought to reflect the latent, cumulative, or synergistic effects of military health risks, high prevalence health risk behaviors (e.g., smoking), and the long term health consequences of military specific exposures (e.g., trauma from warzone deployment, military sexual trauma, combat) (London & Wilmoth, 2010). Moreover, it provides a useful framework with which to conceptualize the possibility of a more distal association between military service- -which typically concludes in early adulthood- -and health in older adulthood (London & Wilmoth, 2010). While this paradoxical effect would be expected to generalize to women, research evaluating the healthy soldier effect among older women Veteran populations is all but non-existent.
Given the substantial (and growing) population of women Veterans living in the U.S. today, research designed to characterize their post-menopausal health and mortality risks is warranted. The Clinical Trials and Observational Study of the Women’s Health Initiative (WHI) program (The Women’s Health Initiative Study Group, 1998) are well positioned to address this literature gap. To this end, ours is the first study to evaluate whether women Veteran participants in WHI (n = 3,706) have the same risk for key post-menopausal health conditions: cardiovascular disease (CVD), cancer, diabetes, hip fracture, and all-cause mortality, as the non-Veteran participants (n = 141,009).
MATERIAL AND METHODS
Overview of the Women’s Health Initiative
The Women’s Health Initiative (WHI) includes a set of three National Institute of Health (NIH) sponsored clinical trials (CT) and an observational study (OS) designed to identify factors associated with the development of heart disease, cancer, and fracture in post-menopausal women (within WHI menopause was defined as: no vaginal bleeding for 6 months if 55+, 12 months for 50- to 54-year-olds, prior hysterectomy, or use of postmenopausal hormones) who were aged 50–79 at WHI baseline, between 1993 and 1998 (The Women’s Health Initiative Study Group, 1998).
Participants were recruited from 1993–1998 by 40 clinical centers around the country, which helped to ensure racial/ethnic, geographic and sociodemographic diversity among the study participants. Original study endpoints for the OS and CT were in 2005. Extension Studies are currently collecting follow-up data through 2015. The present work includes follow-up data through 2011, facilitating evaluation of long-term health outcomes over more than 20 consecutive years.
Institutional review boards at all participating clinical centers reviewed and approved study procedures. All participants provided written informed consent at baseline and again at enrollment in the Extension Studies. Detailed accounts of the WHI recruitment procedures, study design, and methodology have been previously published (Curb, et al, 2003; Hays, Hunt, & Hubbell, 2003; The Women’s Health Initiative Study Group, 1998).
WHI Data Collection and Adjudication Procedures
At baseline, participants completed self-report questionnaires designed to gather information related to WHI participants’ socio-demographic, medical, and lifestyle characteristics. They also underwent a brief clinical exam that included height, weight, and blood pressure measurements. WHI study follow-up involved completion of annual, mailed, follow-up questionnaires and regular physical examinations.
Health conditions identified through these methods were confirmed via local (physicians from the local/regional WHI Clinical Centers who review participants medical record and study related medical documents to assign a diagnosis) and central adjudication (i.e., physicians at the WHI Clinical Coordinating Center and the NIH review and confirm the diagnosis). To minimize the potential for bias, local and central physician adjudicators were restricted in their access to participants’ research record such that they were not exposed to any information that could result in unblinding (c.f., Curb et al., 2003).
Outcome Ascertainment
Morbidity-related outcomes, including incident cardiovascular disease, malignant cancer, diabetes and hip fracture were identified by patient self-report via annual study follow-up questionnaires or detected during regularly scheduled medical examinations that were incorporated into the WHI follow-up procedures. All morbidity outcomes were centrally adjudicated, with the exception of diabetes, which was confirmed, centrally, whenever possible.
Incident cardiovascular disease (CVD) was identified by patient self-report in annual, mailed follow-up questionnaires, or during the regularly scheduled medical examinations that were incorporated into the WHI follow-up procedures. CVD outcomes included cases with medically adjudicated diagnoses of coronary heart disease, stroke, congestive heart failure, angina, peripheral vascular disease, and coronary revascularization.
Incident cancer was defined as incident cases of invasive or in situ cancers (except non-melanoma skin cancers) which were confirmed by local and central physician adjudication of pathology reports, and then coded according to SEER standards of cancer classification, using the second edition of the International Classification of Diseases, Oncology (ICD-0–2) (Cunningham et al., 1992; Van Holten, Van Holten & Muir, 1990). All confirmed new incident cases were classified as cancer outcomes. This included “second primary” cancer diagnoses but not cancer recurrences or instances of premalignant disease.
Incident diabetes was defined by health care provider diagnosis of diabetes treated with anti-diabetic medications (oral hypoglycemic agents or insulin) (Curb et al., 2003; deBoer et al., 2008; Margolis, et al., 2008). Data were gathered by patient self-report, and confirmed, when possible, by local and central adjudication which included medical record review and laboratory data. Prior work confirms the accuracy of self-reported (treated) diabetes in WHI (Margolis et al., 2008).
Hip fractures included treated (inpatient or outpatient) events, fracture diagnoses were adjudicated (central review) and confirmed by examination of radiographic reports.
All-cause mortality
Deaths (all causes) were identified by a variety of sources including annual medical record review, obituary searches, as well as from information provided by WHI participants’ proxy informants, and confirmed via the National Death Index Plus (Bilgrad, 1997), the “gold standard” for ascertainment of mortality outcomes in epidemiologic studies (Cowper, Kubal, Maynard, & Hynes, 2002; Doody, Hays & Bilgrad, 2001; Sohn, Arnold, Maynard, & Hynes, 2006). In addition, throughout the study observation period, routine efforts were made to match participants who were “lost to follow-up” to the National Death Index Plus, ensuring that data capture for all-cause mortality was as complete as possible.
Variables
Exposure: (Veteran status)
Participants who at baseline responded affirmatively to the question: “Have you ever served in the armed forces?” were classified as “Veterans,” all others as “non-Veterans.”
Covariates were variables, measured at WHI baseline, that were a priori thought to vary by Veteran status and/or confer risk for the outcomes of interest (Bass, French, Bradham, & Rubenstein, 2007; Cummings et al, 1995; Hopper & Seeman, 1994; Mendis, 2010; U.S. Department of Health and Human Services, 1986; van Melle, et al, 2004, Zhang et al., 2010). Demographic characteristics including age, race/ethnicity, education and cigarette smoking were assessed by self-report at study baseline. Body mass index and hypertension were clinically measured at baseline, and depressive symptomology was assessed by self-report at study baseline using the 8-item Burnam scale (Burnam, Wells & Leake, 1988) derived from the Center for Epidemiologic Studies Depression Scale (CESD) (Radloff, 1977). A noteworthy difference of the Burnam Scale, relative to other common screening measures for depression, is that the scoring algorithm dictates that individual items on the Burnam Scale are logistically weighted such that the algorithm yields a composite score with values that can range from 0 – 1, with a cutpoint of 0.06, (Burnam et al.,1988) used to signify presence of current depression.
In addition, several variables which conferred specific risk to post-menopausal bone health or fracture risk were utilized exclusively in models evaluating hip fracture outcomes. These included: self-reported baseline measures of physical activity, measured in metabolic minutes, parental hip fracture, personal history of fracture at ≥ 55 years, alcohol use (i.e., drinks per week), physical functioning (Rand SF-36 Physical Functioning Scale),(Bohannon & DePasquale, 2010), comorbid medical conditions, osteoporosis, current use of bisphosphonates, corticosteroids and psychoactive medications, which may impact bone health and/or contribute to fall risk), current or prior use of hormone replacement therapy, and calcium and vitamin D intake(Robbins et al., 2007; Williams, Weiss, Ure, Ballard, & Daling, 1982). Self-reported health (Ware & Sherbourne, 1992) and self-reported prior hip fracture were covariates used exclusively in mortality outcome models.
Other Descriptors
Baseline prevalence of self-reported CVD, cancer, diabetes, and hip fracture were included as descriptive variables and to subset cohort risk sets for analysis of respective incident events.
Statistical Analysis Plan
Descriptive statistics were used to characterize all participants with respect to demographic characteristics, baseline health risks and baseline prevalent CVD, diabetes, cancer and hip fracture. Formal tests of significance were not conducted for descriptive analyses. The large study population would be expected to produce many significant associations, potentially encouraging undue emphasis on statistically significant--rather than clinically meaningful-- differences.
Cox proportional hazards models estimated the association between Veteran status and incident CVD, cancer, diabetes, hip-fracture, and all-cause mortality in models that were sequentially adjusted for baseline age (continuous) (Model 1), and then additionally for socio-demographic factors (e.g., race/ethnicity, education), mental health (i.e., depression) and health-related (i.e., hypertension, BMI) confounders associated with all outcomes (Model 2).
Hip fracture models included all covariates in Model 2 and were then further adjusted for several additional variables with specific relevance to bone health or fracture risk (Model 3). These included baseline: alcohol use, psychoactive medication, self-reported health, number of chronic conditions, physical functioning, bisphosphonate use, corticosteroid use, parental hip-fracture, other fracture after age 55, total baseline calcium and vitamin D intake, and hormone therapy use. Mortality models included all covariates in Model 2, and were then further adjusted for alcohol use, physical activity, self-reported health, baseline prevalent number of chronic conditions, and hip fracture (Model 4).
Kaplan-Meier estimates were used to graphically view the annualized incidence of each disease outcome by Veteran status. The proportionality assumption was examined graphically and through evaluation of interaction terms between Veteran status and time on health outcomes. The interaction of Veteran status and age on health outcomes was also examined. As neither of these interaction terms was significant, they were omitted from the final models. Exclusions to each outcome model (CVD, cancer, diabetes, hip fracture, all-cause mortality) were made due to participants’ missing covariate values or baseline prevalent disease for each model’s specific outcome and participants were censored at either their last follow-up visit known to be without the outcome of interest, or death, whichever was first. Sensitivity analyses, applied to all models, utilized multiple imputations to address subject loss associated with missing covariate values.
All statistical tests were two-sided. Analyses were conducted in SAS version 9.3 for Windows (SAS Inc., Cary, NC) and STATA version 10 for Windows (STATA Inc., College Station, TX).
RESULTS
Participant Characteristics
Approximately 3% (n = 3,706) of the 144,715 participants were Veterans. Women Veterans were older than non-Veterans (mean age at WHI baseline = 67.1 vs. 63.2) (Table 1). Moreover the age distribution was quite different between groups, with nearly 50% of Veteran participants, vs. 22% of non-Veteran participants, being aged 70–79 at WHI baseline. Relative to non-Veterans, women Veteran participants were also more likely to be Caucasian (87.1% of Veterans vs. 82.4% of non-Veterans), to have completed college (46.8% of Veterans vs. 39.5% of non-Veterans), to smoke (or have smoked) cigarettes, and to have diagnosed hypertension, osteoporosis, and diminished physical functioning at baseline relative to their non-Veteran peers (Table 1).
Table 1.
Participant Characteristics at WHI Baseline
| Variable | Veteran N = 3,706 |
Non-Veteran N = 141,009 |
||||
|---|---|---|---|---|---|---|
| Demographics | M | (SD) | M | (SD) | ||
| Age * | 67.1 | (8.0) | 63.3 | (7.2) | ||
| N | % | N | % | |||
| 50–59 years | 782 | 21.1 | 46,072 | 32.7 | ||
| 60–69 years | 1,077 | 29.1 | 64,328 | 45.6 | ||
| 70–79 years | 1,847 | 49.8 | 30,609 | 21.7 | ||
| Missing | 0 | 0 | ||||
| Race/Ethnicity | N | % | N | % | ||
| White | 3,229 | 87.1 | 116,161 | 82.4 | ||
| Black or African American | 263 | 7.1 | 12,736 | 9.0 | ||
| Asian or Pacific Islander | 45 | 1.2 | 3,957 | 2.8 | ||
| Hispanic/Latino | 85 | 2.3 | 5,532 | 3.9 | ||
| American Indian or Alaskan Native | 24 | 0.7 | 597 | 0.4 | ||
| Other | 47 | 1.3 | 1,656 | 1.2 | ||
| Missing | 13 | 0.4 | 370 | 0.3 | ||
| Education | N | % | N | % | ||
| College degree or higher | 1,733 | 46.8 | 55,735 | 39.5 | ||
| Missing | 14 | 0.4 | 915 | 0.7 | ||
| Depression | M | Rangeπ | (SD) | M | Rangeπ | (SD) |
| Depressive Symptoms | 0.04 | 0–.94 | (0.12) | 0.0 4 | 0–.96 | (0.13) |
| Missing | 89 | 2.4 | 3,485 | 2.5 | ||
| Body Mass Index | N | % | N | % | ||
| Overweight (25.0 – 29.9) | 1,319 | 35.6 | 48,285 | 34.2 | ||
| Obese (> 30) | 1,062 | 28.7 | 41,764 | 29.6 | ||
| Missing | 39 | 1.1 | 1,292 | 0.9 | ||
| Physical Activity (MET Min/Week) | N | % | N | % | ||
| < 100 MET | 781 | 21.1 | 31,045 | 22.0 | ||
| 100–499 MET | 973 | 26.3 | 38,885 | 27.6 | ||
| 500 – 1200 MET | 1,093 | 29.5 | 40,263 | 28.6 | ||
| >1200 MET | 850 | 22.9 | 30,417 | 21.6 | ||
| Missing | 9 | 0.2 | 399 | 0.3 | ||
| Hypertension | N | % | N | % | ||
| 1,361 | 36.7 | 47,471 | 33.7 | |||
| Missing | 38 | 1.0 | 1,161 | 0.8 | ||
| Self-Reported Health | N | % | N | % | ||
| Excellent | 630 | 17 | 23,972 | 17 | ||
| Very Good | 1,594 | 43 | 57,814 | 41 | ||
| Good | 1,112 | 30 | 44,981 | 32 | ||
| Fair | 323 | 8.7 | 11,704 | 8.3 | ||
| Poor | 22 | 0.6 | 1,128 | 0 .7 | ||
| Missing | 25 | 0.7 | 1,410 | 1.0 | ||
| Smoking | N | % | N | % | ||
| Never | 1,625 | 43.9 | 71,190 | 50.5 | ||
| Past | 1,687 | 45.5 | 58,543 | 41.5 | ||
| Current | 317 | 8.6 | 9,469 | 6.7 | ||
| *Pack Years | M | (SD) | M | (SD) | ||
| 12.6 | (20.4) | 9.4 | (17.5) | |||
| Missing | 180 | 4.9 | 5,127 | 3.6% | ||
| Alcohol Use | M | (SD) | M | (SD) | ||
| Drinks Per Week | 2.52 | (5.0) | 2.38 | (4.9) | ||
| Missing | 11 | 0.3 | 249 | 0.2 | ||
| Comorbidities | M | (SD) | M | (SD) | ||
| Count | 2.02 | 1.1 | 1.80 | 1.4 | ||
| Missing | 595 | 16.1 | 19,966 | 14.2 | ||
| Physical Function | N | % | N | % | ||
| # with Score > 90 | 1,123 | 30.3 | 51,534 | 36.6 | ||
| Missing | 82 | 2.2 | 2,571 | 1.8 | ||
| Parent Broke Hip > age 40 | N | % | N | % | ||
| 1,286 | 34.7 | 51,667 | 36.6 | |||
| Missing | 341 | 9.2 | 11,264 | 8.0 | ||
| Fracture ≥ age 55 | N | % | N | % | ||
| 3 | 0.1 | 62 | 0.0 | |||
| Missing | 139 | 3.8 | 2,580 | 1.8 | ||
| Osteoporosis | N | % | N | % | ||
| 348 | 9.4 | 10,795 | 7.7 | |||
| Missing | 44 | 1.2 | 1,810 | 1.3 | ||
| Total Baseline Calcium Intake, mg | N | % | N | % | ||
| < 600 | 686 | 18.5 | 30,053 | 21.3 | ||
| 600–1200 | 1,401 | 37.8 | 53,032 | 37.6 | ||
| >1200 | 1,610 | 43.4 | 57,715 | 40.9 | ||
| Missing | 9 | 0.2 | 209 | 0.2 | ||
| Total Baseline Vitamin D Supplement Use, IU | N | % | N | % | ||
| < 400 | 3,042 | 82.1 | 118,645 | 84.1 | ||
| 400–600 | 561 | 15.1 | 18,501 | 13.1 | ||
| ➢ 600 | 94 | 2.5 | 3,654 | 2.6 | ||
| Missing | 9 | 0.2 | 209 | 0.2 | ||
| Medication Use^ | ||||||
| Psychoactive Med Use+ | N | % | N | % | ||
| # and % with current use | 448 | 12.1 | 17,522 | 14 | ||
| Bisphosphonates | N | % | N | % | ||
| # and % with current use | 78 | 2.1 | 2,879 | 2.0 | ||
| Corticosteroid | N | % | N | % | ||
| # and % with current use | 44 | 1.2 | 995 | 0.7 | ||
| Hormone Therapy Use | N | % | N | % | ||
| Never Used | 1,625 | 43.9 | 61,228 | 43.4 | ||
| Past User | 721 | 19.5 | 22,127 | 15.7 | ||
| Current User | 1,356 | 36.6 | 57,530 | 40.8 | ||
| Missing | 4 | 0.1 | 124 | 0.1 | ||
| WHI Component~ | N | % | N | % | ||
| Hormone Therapy Trial | 647 | 17.5 | 22,707 | 16.1 | ||
| Diet Modification Trial | 935 | 25.2 | 39,628 | 28.1 | ||
| Calcium and Vitamin D Trial | 764 | 20.6 | 30,049 | 21.3 | ||
| Observational Study | 2,291 | 61.8 | 85,034 | 60.3 | ||
Note. All variables presented are included as study covariates.
Age is included as a linear variable (years);
missing data on medication use is not available, participants were asked to endorse use, absence of data point signals non-use of medications,
psychoactive medication use includes antipsychotic, antiepileptic, anxiolytic, hypnotic and antidepressant drug use, for smoking we included Pack Years, taking into account the number of years smoked and the average number of cigarettes smoked daily, in the model.
The Burnam Scale for Depression utilizes a scoring algorithm that permits scores from 0–1 with a cutpoint of 0.06 signifiying probable current depression.
Participants could enroll in more than one study component, including more than one of the WHI related trials. As such, sums of the percent of participants across the WHI components exceed 100%,
Age stratified analyses revealed that among younger participants, aged 50–59 and 60–69 at WHI baseline, (reflecting participant ages between the years 1993–1998), women Veterans had lower baseline prevalence of CVD and cancer than non-Veterans. However, this difference reversed for participants aged 70+ (Table 2). Patterns of baseline prevalence for diabetes and hip fracture were similar, though less pronounced.
Table 2.
Self-Reported Baseline Prevalence of Disease by Veteran Status and Age Decade
| Variable | Veteran N = 3,706 |
Non-Veteran N = 141,009 |
||
|---|---|---|---|---|
| CVD | N | % | N | % |
| All | 798 | 21.9 | 24,679 | 17.7 |
| 50–59 | 106 | 2.9 | 5,665 | 4.1 |
| 60–69 | 226 | 6.2 | 11,506 | 8.3 |
| 70+ | 466 | 12.8 | 7,508 | 5.4 |
| Cancer | N | % | N | % |
| All | 476 | 13.0 | 13,437 | 9.6 |
| 50–59 | 64 | 1.7 | 3,491 | 2.5 |
| 60–69 | 122 | 3.3 | 6,049 | 4.3 |
| 70+ | 290 | 7.9 | 3,897 | 2.8 |
| Diabetes | N | % | N | % |
| All | 215 | 5.8 | 8,409 | 6.0 |
| 50–59 | 24 | 0.7 | 2,114 | 1.5 |
| 60–69 | 80 | 2.2 | 4,148 | 2.9 |
| 70+ | 111 | 3.0 | 2,147 | 1.5 |
| Hip Fracture | ||||
| All | 29 | 0.8 | 751 | 0.5 |
| 50–59 | 0 | -- | 19 | 0.0 |
| 60–69 | 2 | 0.1 | 308 | 0.2 |
| 70+ | 27 | 0.7 | 424 | 0.3 |
Main Outcomes
Table 3 presents number of events, person years, annualized incidence and results of Cox proportional models, sequentially adjusted for age, sociodemographic and health risk factors for each outcome of interest. In fully adjusted models (Model 2), Veteran status was not associated with risk of CVD (HR: 1.0; 95% CI: 0.90 – 1.11), total cancer (HR: 1.04; 95% CI: 0.95 – 1.14), or diabetes (HR: 1.00; 95% CI: 0.89 – 1.12).
Table 3.
Association of Veteran Status and Key Health Outcomes
| Primary Analyses | Sensitivity Analyses | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No of Events | Person Years | Annualized % | HR | (95%CI) | HR | (95% CI) | |
| Imputation for Missing Covariates | |||||||
| CVD | |||||||
|
| |||||||
| V | 609 | 27,265 | 2.23% | Model 1 | 1.00 (0.90 – 1.11) | ||
|
|
|
||||||
| NV | 17,359 | 1,063,671 | 1.63% | Model 2 | 1.00 (0.90 – 1.11) | ||
|
| |||||||
| Cancer | |||||||
|
| |||||||
| V | 580 | 27,445 | 2.11% | Model 1 | 1.09 (0.99 – 1.20) | ||
|
|
|||||||
| NV | 18,572 | 1,065,566 | 1.74% | Model 2 | 1.04 (0.95 – 1.14) | ||
|
| |||||||
| Diabetes | |||||||
|
| |||||||
| V | 461 | 25,340 | 1.82% | Model 1 | 0.98 (0.87 – 1.11) | ||
|
|
|||||||
| NV | 18,257 | 973,482 | 1.89% | Model 2 | 1.00 (0.89 – 1.12) | ||
|
| |||||||
| Hip Fracture | |||||||
|
| |||||||
| V | 143 | 28,582 | 0.50% | Model 1 | 1.24 (1.01 – 1.53) | Model 1 | 1.24 (1.01 – 1.53) |
|
|
|||||||
| NV | 2,674 | 1,105,542 | 0.24% | Model 2 | 1.18 (0.96 – 1.45) | Model 2 | 1.20 (1.02 – 1.43) |
| Model 3 | 1.16 (0.94 – 1.43) | Model 3 | 1.18 (0.99 – 1.40) | ||||
|
| |||||||
| All-Cause Mortality | |||||||
|
| |||||||
| V | 573 | 28,830 | 1.99% | Model 1 | 1.20 (1.09 – 1.31) | Model 1 | 1.20 (1.09 – 1.31) |
|
|
|||||||
| NV | 13,174 | 1,110,373 | 1.19% | Model 2 | 1.16 (1.05 – 1.27) | Model 2 | 1.13 (1.04 – 1.23) |
| Model 4 | 1.13 (1.03 – 1.25) | Model 4 | 1.12 (1.02 – 1.22) | ||||
Notes. V = Veterans, NV = Non Veterans. Non-Veterans are the Reference Group; Hazard Ratios < 1 indicate that Veterans are less likely than non-Veterans to have that condition. Annualized percentage is defined as cases per 100 person-years.
Model 1: Adjusted for age. Model 2: adjusted for age, race/ethnicity, education, WHI Component and, for CT participants, randomization assignment, smoking, BMI, hypertension, depression. Model 3: adjusted for all variables in Model 2 plus alcohol, psychoactive medication, self-reported health, number of chronic conditions, physical activity, physical functioning, bisphosphonates, corticosteroids, parental hip-fracture, other fracture after age 55, total baseline calcium and vitamin D intake, and hormone therapy use. Model 4: adjusted for all variables in Model 2 plus alcohol use, physical activity, self-reported health, baseline prevalent number of chronic conditions, and hip fracture.
Age adjusted models (Model 1) suggested a slightly elevated risk of hip fracture among Veteran women relative to non-Veteran controls (Table 3), however, this association was not robust to further adjustment for sociodemographic and health risk factors (Model 2) (HR: 1.16; 95% CI: 0.94 – 1.43), or in models that accounted for confounders specific to bone health or fracture risk (Model 3) (HR: 1.16; 95% CI: 0.94 – 1.43) (Table 3).
Relative to non-Veteran controls, Veteran women had significantly increased risk of all-cause mortality in age adjusted analyses (Model 1). Findings were robust in fully adjusted models that accounted for age, sociodemographic and health risk factors along with alcohol use, self-reported health, chronic health conditions and prior hip fracture (Model 4): (HR: 1.13; 95% CI: 1.03 – 1.25).
Sensitivity Analyses
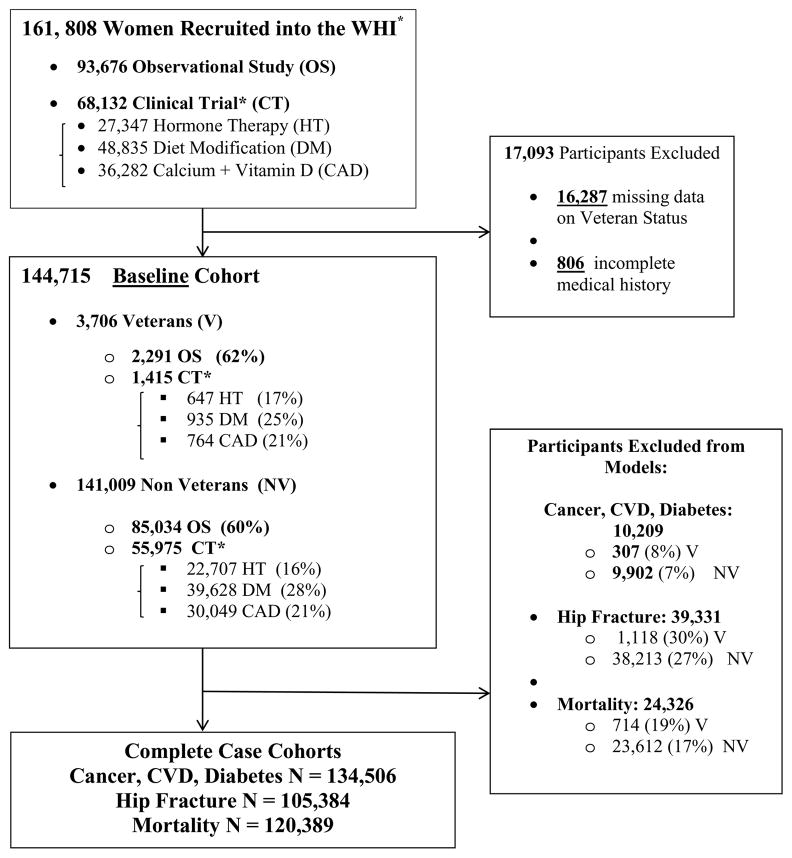
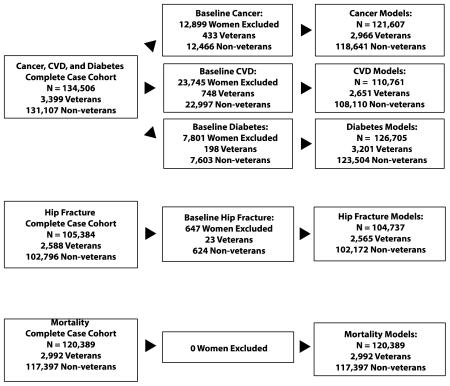
Loss of participants due to missing values on salient covariates was substantial, and particularly problematic in the hip fracture models which featured a large number of covariates (Figure 1 and Supplementary Figure 2 in Appendix B). To address this, we conducted sensitivity analyses using multiple imputations for missing values. For all outcomes, imputation-based results agreed almost entirely, in terms of significance and direction, with our main outcomes. Results of significant models (i.e., hip fracture, all-cause mortality) are presented in Table 3.
Figure 1. Cohort Composition.
*Note that clinical trial participants could enroll in more than one of the three trials; therefore, the size of the total clinical trial study population is smaller than the sum of the specific study population for each of the constituent trials.
DISCUSSION
Among the 144,715 post-menopausal WHI participants included in our study, Veteran status conferred a consistent risk to all-cause mortality that was independent of age. Veteran status was not statistically associated with risk for morbidity outcomes, however, the low incidence of hip fracture and total cancer cases among study participants may have limited power to detect small, but important, differences. Moreover, the nature and direction of these outcomes suggest a need for further investigation of women Veterans’ post-menopausal bone health and cancer risk. All findings were robust to adjustment for socio-demographic and health risk factors and held in sensitivity analyses with more inclusive cohorts.
Some key results of the present study seem to contrast with much of the prior literature on the “healthy soldier effect” (Cypel & Kang, 2008; Dalager, Kang & Thomas, 1995; Kang, et al., 2014; MacFarlane et al., 2003; MacIntyre et al., 1978; McBride, Cox, Broughton & Tong, 2013; McLaughlin, Nielsen & Waller, 2008; Thomas, Kang & Dalager, 1991; Vajdic, Stavrou, Ward, Falster & Pearson, 2014; Waller & McGuire, 2011; Yi, 2013). Indeed, several prior studies suggest similar, but more commonly deficient, risk of cancer-related morbidity or mortality among women Veterans relative to the general public (Dalager, Kang, & Thomas, 1995; MacFarlane, Vajdic, Stavrou, Ward, Falster, & Pearson, 2014). Thomas et al. (1991) found lower than expected rates of all-cause mortality among 4,600 women Veterans deployed to Vietnam relative to the general population (standardized mortality ratios or SMR: 0.82; 95% CI: 0.69–0.97) over a 13-year observation period. Similarly, a 40-year follow-up study of approximately 12,000 women who served during Vietnam found decreased risk of all-cause mortality among deployed women who served in theater relative to the general population, reporting SMRs of 0.85 (95% CI: 0.80 – 0.91) (Kang et al., 2014).
Unique aspects of the WHI study methodology and population may offer some important clues regarding the discrepancy of our findings from those of the broader literature. First, we compared mortality risk in Veteran and non-Veteran women who were health-eligible for inclusion in the clinical trials and/or observational studies of WHI. This likely attenuated the healthy soldier bias (Bross & Bross, 1987) that may be present in studies that benchmark health risks in Veterans- -who are selected into military service in part based upon their good health- -against the general population for whom no such selection bias is present.
Second, 50% of women Veteran participants, relative to about 22% of non-Veteran participants, were aged 70+ at study enrollment. Outcome models were age adjusted; nevertheless, the asymmetric age distribution of the study population may suggest that results disproportionately reflect mortality risk among the large group of older women Veterans in WHI. In this light, findings may be consistent with prior literature which implies that the healthy soldier effect may attenuate with time. Indeed, per the healthy soldier effect, heightened mortality risk would be expected among older women Veterans relative to their non-Veteran peers - - who should have experienced a period of heightened risk an earlier age. Underscoring this point, several prior studies characterize a Veteran/non-Veteran health crossover in older adulthood (> age 70) (Wilmoth, London, & Parker, 2010; London & Wilmoth, 2010). Our study was not specifically designed to compare health trajectories throughout the post-menopausal period, and we cannot confirm the presence or absence of such an effect with our present analyses. However, we note that the pattern of baseline disease prevalence (Table 2) may prefigure the true presence of a cross over effect, offering some important context in which to interpret the observed pattern of mortality risk in Veterans. Further confirmatory research, including population based studies designed to prospectively examine the possibility of such a Veteran/non-Veteran health cross-over, would deepen our understanding of women Veterans post-menopausal health trajectories.
In addition, given that the largest subgroup of Veteran participants, those aged 70–79 at baseline, are age consistent with military service during WWII (Washington, Bean-Mayberry, Hamilton, Cordasco, & Yano, 2013)- -a time when women’s military occupational roles and associated occupational health exposures were quite distinct from those of subsequent generations of military women (Treadwell, 1954)- - it may be important to consider that military service era, independently or synergistically with the effects of age, may also contribute to variability in health and mortality risk.
Further, salient differences in health risk and health risk behaviors may have contributed to women Veterans’ heightened risk of all-cause mortality. For example, though models adjusted for cigarette smoking, this may not have fully attenuated the effects of Veteran women’s greater prevalence and longer duration (pack years) of smoking. Finally, though WHI offers no contextual information about women’s prior military service, given the age and likely period of military service for WHI participants, it is reasonable to assume that many Veteran women were exposed to military specific occupational hazards (i.e., deployment overseas, warzone exposure, military sexual trauma) which would also be expected to contribute to heightened mortality risk.
Limitations include our study’s observational design which precludes causal inferences about the association of military service and women’s long- term health. Participants do not represent a population-based sample, which may limit the generalizability of our findings. However, study participants were recruited nationally and reflect the considerable racial, ethnic, geographic and socioeconomic diversity of the general U.S. population. In addition, generalizability may be limited because nearly half of the women Veteran participants in WHI are age consistent with military service during WWII, and the military occupational roles and health related exposures of these women may be quite different from those of women from subsequent military generations. Further, Veteran status was determined by participants’ self-report, but not confirmed with review of military records. However, we note that WHI’s method of assessing Veteran status by self-report is consistent with strategies used in several other large scale observational studies (e.g., Hoerster et al., 2012; Koepsell, Reiber, & Simmons, 2002). As WHI offers no contextual information about participants’ military service, we are unable to evaluate how factors such as military generation (era served), length of service, occupation or role within the military, warzone deployment, exposure to military sexual trauma or other military occupational health risks might impact variability in Veteran participants’ health or mortality risk. Finally, data on lifetime prevalence of mental health conditions are not available within WHI and mental health outcomes were not a focus of the WHI clinical trials or observational study. Though variability in Veterans’ and non-Veterans’ prevalence and severity of mental health problems (e.g., traumatic stress disorders) would be expected, and may contribute to differential morbidity and mortality risk, we are unable to account for these factors within the present study. Despite these limitations, our study has many strengths, which include the use of a large, racially, ethnically and geographically diverse group of post-menopausal women, a strong rate of participant retention, the inclusion of variables on many known health confounders and prospective attainment of adjudicated health outcomes, in which women Veterans and non-Veterans were identically followed, over a multi-decade observational follow-up period.
Implications for Practice and/or Policy
Our findings offer several important implications pertinent to clinical health care practice and policy for women Veterans. First, this study highlights heightened post-menopausal risk of all-cause mortality among women Veterans, relative to their non-Veteran peers, illuminating the potential salience of prior military service as a factor in determining women’s life-long health. As such, this work may help to increase awareness of the unique health care needs of older, post-menopausal women Veterans among the health care providers who care for this population of women.
Second, these findings underscore the importance of efforts to identify and address modifiable health and mortality risk factors among women Veterans. Our descriptive findings (Table 2) related to older (age 70+) women Veteran’s heightened baseline prevalence of cancer and CVD may offer an important clue in this regard. Moreover, the nature and direction of our cancer outcome models (Table 3) may suggest that further study of women Veterans’ post-menopausal risk for cancer would be valuable and informative. Epidemiologic research on the incidence and prevalence of specific types of cancer among women Veterans is quite scant. However, the recent HealthViews study (Kang et al., 2014) suggests a heightened prevalence of brain and pancreatic cancers among women who served as military nurses in the Vietnam theater, and Zhu and colleagues (2009) found significantly heightened risk for breast cancer among contemporary military and Veteran populations. These may represent logical and important ‘next steps’ of inquiry related to women Veterans’ cancer risk.
Third, women Veterans’ 9% baseline prevalence of osteoporosis (Table 1), coupled with the nature and direction of the hip fracture outcomes, suggest that increased attention to matters of bone health among post-menopausal women Veterans may be important. Specifically, these findings underscore the importance of screening for osteoporosis, evaluating fall risk, and identifying (and intervening with) other factors that may contribute to fracture risk among post-menopausal women Veterans. Finally, given the heightened baseline prevalence of both smoking and hypertension- -factors which may impact bone health and fracture risk- - further investigation of these factors as potential mediators of Veteran women’s risk for hip fractures is also warranted.
Conclusions
This study is among the first large scale efforts to investigate health and mortality risks among post-menopausal women Veterans. While our study population may represent a select group of women Veterans whose post-menopausal health trajectories may not be fully generalizable, this work provides a much needed empirical foundation for the study of post-menopausal health and mortality risk in women Veterans. It is our hope that this work will encourage further research efforts that will further deepen our understanding of this unique population of women.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Dr. Weitlauf’s contribution to this work was supported in part by the Department of Veterans Affairs, Office of Research and Development, Health Services Research and Development Service Career Development Award [CD206035 2 to J.W.] To the extent that the funding agencies associated with this project played no role (beyond financial support) in the development or execution of the study described, all investigators (authors) are independent from the sources of study funding.
Biographies
Julie C. Weitlauf, Ph.D., is Director of the Women’s Mental Health and Aging Core of the VISN 21 MIRECC at the VA Palo Alto Health Care System, and Clinical Associate Professor (Affiliated) of Psychiatry and Behavioral Sciences at Stanford.
Andrea Z. LaCroix, Ph.D., is Professor and Chief of Epidemiology and Director of the Women’s Health Center of Excellence within the Department of Family Medicine and Public Health at the University of California, San Diego.
Chloe E. Bird, Ph.D., is a Senior Sociologist at RAND, with particular expertise in women’s cardiovascular health. She also serves as a Professor within the Pardee RAND Graduate School, and as Editor-in-Chief of Women’s Health Issues.
Nancy Fugate Woods, Ph.D,, R.N., F.A.A.N., is a Professor of Biobehavioral Nursing, and Dean Emeritus of the University of Washington School of Nursing.
Donna L. Washington, M.D., M.P.H., is the Director of the Office of Health Equity/QUERI Partnered Evaluation Center, Greater Los Angeles VA Health Care System, and Professor of Medicine, at the University of California, Los Angeles, David Geffen School of Medicine.
Jodie G. Katon, Ph.D., M.S., is a Health Sciences Research Specialist within the VA Puget Sound Health Care System and a Senior Epidemiologic Consultant within the VA Office of Patient Care, Women’s Health Services.
Michael J. LaMonte, Ph.D., M.P.H., is Research Associate Professor of Epidemiology within the Department of Epidemiology and Environmental Health of the School of Public Health and Health Professions of the State University of New York at Buffalo.
Mary K. Goldstein, M.D., M.S. in HSR, is Director of the Palo Alto Geriatrics Research Education and Clinical Center (GRECC) within the VA Palo Alto Health Care System and Professor of Medicine within the Center for Primary Care and Outcomes Research at Stanford University School of Medicine.
Shari S. Bassuk, Sc.D., is an epidemiologist with the Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School.
Gloria E. Sarto M.D., Ph.D., is Professor Emeritus of Obstetrics and Gynecology at the University of Wisconsin-Madison, has served on several boards/committees within the Institute of Medicine, and was involved in the development of NIH’s Office of Women’s Health.
Marcia L. Stefanick Ph.D. is Professor of Medicine (Stanford Prevention Research Center), and of Obstetrics & Gynecology at Stanford University School of Medicine. She is also the Director of the Stanford Women and Sex Differences in Medicine (WSDM) Center.
Appendix A: LONG LIST OF WHI INVESTIGATORS
Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, Nancy Geller.
Clinical Coordinating Center
(Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, Charles Kooperberg, Barbara Cochrane, Julie Hunt, Marian Neuhouser, Lesley Tinker, Susan Heckbert, Alex Reiner.
Regional Centers
(Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson, Kathryn M. Rexrode, Brian Walsh, J. Michael Gaziano, Maria Bueche; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard, Lucile Adams-Campbell, Lawrence Lessin, Cheryl Iglesia, Brian Walitt, Amy Park; (The Ohio State University, Columbus, OH) Rebecca Jackson, Randall Harris, Electra Paskett, W. Jerry Mysiw, Michael Blumenfeld; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick, Mark A. Hlatky, Manisha Desai, Jean Tang, Stacy T. Sims; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson, Tamsen Bassford, Cheryl Ritenbaugh, Zhao Chen, Marcia Ko; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende, Maurizio Trevisan, Ellen Smit, Amy Millen, Michael LaMonte; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher, Michael Perri, Andrew Kaunitz, R. Stan Williams, Yvonne Brinson; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace, James Torner, Susan Johnson, Linda Snetselaar, Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller, Jane Cauley, N. Carole Milas; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson, Suzanne Satterfield, Rongling Li, Stephanie Connelly, Fran Tylavsky; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, Michelle Naughton.
Women’s Health Initiative Memory Study
(Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker, Stephen Rapp, Claudine Legault, Mark Espeland, Laura Coker, Michelle Naughton.
Former Principal Investigators and Project Officers
(Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar, Aleksandar Rajkovic, Jennifer Hays, John Foreyt; (Brown University, Providence, RI) Charles B. Eaton, Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence S. Phillips, Nelson Watts, Sally McNagny, Dallas Hall,; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley A.A. Beresford, Maureen Henderson; (George Washington University, Washington, DC) Lisa Martin, Judith Hsia, Valery Miller; (Harbor-UCLA Research and Education Institute, Torrance, CA) Rowan Chlebowski (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc, Yvonne Michael, Evelyn Whitlock, Cheryl Ritenbaugh, Barbara Valanis; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan, Robert Hiatt; (National Cancer Institute, Bethesda, MD) Carolyn Clifford; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Linda Pottern; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn, Philip Greenland; (Rush University Medical Center, Chicago, IL) Lynda Powell, William Elliott, Henry Black; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane, Iris Granek; (University at Buffalo, Buffalo, NY) Maurizio Trevisan; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis, Albert Oberman;
Appendix B: Participant Denominators for Outcome Models (Analytic Cohort)
Footnotes
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Drs. Marcia Stefanick and Andrea LaCroix had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julie C. Weitlauf, Email: wjulie1@stanford.edu.
Andrea Z. LaCroix, Email: alacroix@ucsd.edu.
Chloe E. Bird, Email: chloe@rand.org.
Nancy F. Woods, Email: nfwoods@u.washington.edu.
Donna L. Washington, Email: donna.washington@va.gov.
Jodie G. Katon, Email: jodie.katon@va.gov.
Michael J. LaMonte, Email: mlamonte@buffalo.edu.
Mary K. Goldstein, Email: mary.goldstein@va.gov.
Shari S. Bassuk, Email: sbassuk@rics.bwh.harvard.edu.
Gloria Sarto, Email: gsarto@wisc.edu.
Marcia L. Stefanick, Email: stefanick@stanford.edu.
References
- Bass E, French DD, Bradham DD, Rubenstein LZ. Risk-adjusted mortality rates of elderly Veterans with hip fractures. Annals of Epidemiology. 2007;17(7):514–9. doi: 10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bilgrad R. National Death Index User’s Manual. National Center for Health Statistics, Centers for Disease Control and Prevention (U.S.); Hyattsville, MD: 1997. [Google Scholar]
- Bohannon RW, DePasquale L. Physical Functioning Scale of the Short-Form (SF) 36: Internal consistency and validity with older adults. Journal of Geriatric Physical Therapy. 2010;33(1):16–18. doi: 10.1097/JPT.0b013e3181d0735e. [DOI] [PubMed] [Google Scholar]
- Bross ID, Bross NS. Do atomic Veterans have excess cancer? New results correcting for the healthy soldier bias. American Journal of Epidemiology. 1987;126:1042–1050. doi: 10.1093/oxfordjournals.aje.a114743. [DOI] [PubMed] [Google Scholar]
- Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Medical Care. 1988;26(8):775– 789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Annals of Epidemiology. 2002;12(7):462–8. doi: 10.1016/S1047-2797(01)00285-X. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. The New England Journal of Medicine. 1995;332(12):767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Ries L, Hankey B, Seiffert J, Lyles B, Shambaugh E, Percy C, Van Holten V, editors. The SEER Program Code Manual (Revised Edition) Bethesda, M.D: Cancer Statistics Branch, Surveillance Program, Division of Cancer Prevention and Control, National Cancer Institute; 1992. [Google Scholar]
- Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Neveitt M, Johnson KC, Proulx-Burns L, Pastore L, Critqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Annals of Epidemiology. 2003:S122–S128. doi: 10.1016/S1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- Cypel Y, Kang H. Mortality patterns among women Vietnam-era Veterans: Results of a retrospective cohort study. Annals of Epidemiology. 2008;18:244–252. doi: 10.1016/j.annepidem.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Dalager NA, Kang HK, Thomas TL. Cancer mortality patterns among women who served in the military: the Vietnam experience. Journal of Occupational and Environmental Medicine. 1995;37(3):298–305. doi: 10.1097/00043764-199503000-00005. [DOI] [PubMed] [Google Scholar]
- de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, Mason JE, Margolis KL, Siscovick DS, Weiss NS Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care. 2008;31(4):701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody MM, Hayes HM, Bilgrad R. Comparability of national death index plus and standard procedures for determining causes of death in epidemiologic studies. Annals of Epidemiology. 2001;11(1):46–50. doi: 10.1016/S1047-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Hays J, Hunt J, Hubbell FA, Anderson GL, Limacher M, Allen C, Roussouw JE. The Women’s Health Initiative Recruitment Methods and Results. Annals of Epidemiology. 2003;13:S18–S77. doi: 10.1016/S1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U.S. military, veteran, and civilian men. American Journal of Preventive Medicine. 2012;43:483–489. doi: 10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Hopper JL, Seeman E. The bone density of female twins discordant for tobacco use. The New England Journal of Medicine. 1994;330:387–392. doi: 10.1056/NEJM199402103300603. [DOI] [PubMed] [Google Scholar]
- Kang HK, Bullman TA. Mortality among U.S. veterans of the Persian Gulf War. The New England Journal of Medicine. 1996;335:1498–504. doi: 10.1056/NEJM199611143352006. [DOI] [PubMed] [Google Scholar]
- Kang HK, Cypel Y, Kilbourne AM, Magruder KM, Serpi T, Collins JF, Frayne SM, Furey J, Huang GD, Kimerling R, Reinhard MJ, Schumacher K, Spiro A., 3rd Mortality Study of Female US Vietnam Era Veterans, 1965–2010. American Journal of Epidemiology. 2014;179(6):721–30. doi: 10.1093/aje/kwt319. [DOI] [PubMed] [Google Scholar]
- Koepsell T, Reiber G, Simmons KW. Behavioral risk factors and use of preventive services among veterans in Washington State. Preventive Medicine. 2002;35:557–562. doi: 10.1006/pmed.2002.1121. [DOI] [PubMed] [Google Scholar]
- Liu X, Engle C, Kang H, Cowan D. The effect of Veteran status on mortality among older Americans and its pathways. Population Research and Policy Review. 2005;24:573–592. doi: 10.1007/s11113-005-5056-3. [DOI] [Google Scholar]
- London AS, Wilmoth JM. Military service and (dis)continuity in the life course: Evidence on disadvantage and mortality from the Health and Retirement Study and Study of Assets and Health among the Oldest-old. Research on Aging. 2010;28:135–159. [Google Scholar]
- MacFarlane GJ, Biggs AM, Maconochie N, Hotopf M, Doyle P, Lunt M. Incidence of cancer among UK Gulf war Veterans: Cohort study. British Medical Journal. 2003;327(13) doi: 10.1136/bmj.327.7428.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre NR, Mitchell RE, Oberman A, Harlan WR, Graybiel A, Johnson E. Longevity in military pilots: 37-year follow-up of the Navy’s 1,000 Aviators. Aviation, Space, and Environmental Medicine. 1978;49(9):1120–2. [PubMed] [Google Scholar]
- Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LT, Phillips LS Women Health Initiative Investigators. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clinical Trials. 5(3):240–247. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D, Cox B, Broughton J, Tong D. The mortality and cancer experience on New Zealand Vietnam war Veterans: A cohort study. British Medical Journal. 2013:e003379. doi: 10.1136/bmjopen-2013-003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin R, Nielsen L, Waller M. An evaluation of the effect of military service on mortality: Quantifying the healthy soldier effect. Annals of Epidemiology. 2008;18:928–936. doi: 10.1016/j.annepidem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Mendis S. The contribution of the Framingham Heart Study to the prevention of cardiovascular disease: a global perspective. Progressive Cardiovascular Disease. 2010;53(1):10–4. doi: 10.1016/j.pcad.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurements. 1977;1:385–401. [Google Scholar]
- Robbins J, Aragaki AK, Kooperberg C, Watts N, Wactawski-Wende J, Jackson RD, LeBoff MS, Lewis CE, Chen Z, Stefanick ML, Cauley J. Factors Associated With 5-Year Risk of Hip Fracture in Postmenopausal Women. Journal of the American Medical Association. 2007;298(20):2389–98. doi: 10.1097/01.ogx.0000310358.61870.8c. [DOI] [PubMed] [Google Scholar]
- Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Population Health Metrics. 2006 Apr 10;4(2) doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Controlled Clinical Trials. 1998 Feb;19(1):61–109. doi: 10.1016/S0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Thomas TL, Kang HK, Dalager NA. Mortality among women Vietnam Veterans, 1973–1987. American Journal of Epidemiology. 1991;134(9):973– 80. doi: 10.1093/oxfordjournals.aje.a116182. [DOI] [PubMed] [Google Scholar]
- Treadwell ME. The United States Army in World War II. Special Studies: The Women’s Army Corps. Center of Military History, U.S. Army; Washington DC: 1954. Library of Congress Catalog Card Number 53-61563 First Printed 1954-CMH Pub 11-8. [Google Scholar]
- U.S. Department of Health and Human Services. Smoking and Health: A National Status Report. Vol. 1986. Washington, DC: US Government Printing Office; 1986. DHHS publication PHS 87-8396. [Google Scholar]
- U.S. Department of Veterans Affairs, National Center for Veteran Analysis and Statistics. Table 6L: VetPop2011. 2011 Retrieved from http:/www.va.gov/vetdata/
- Vajdic CM, Stavrou EP, Ward RL, Falster MO, Pearson SA. Minimal excess risk of cancer and reduced risk of death from cancer in Australian Department of Veterans’ Affairs clients: A record linkage study. Austrailian and New Zealand Journal of Public Health. 2014;38(1):30–4. doi: 10.1111/1753-6405.12168. [DOI] [PubMed] [Google Scholar]
- Van Holten PC, Van Holten V, Muir C, editors. International Classification of Diseases for Oncology. 2. Geneva: World Health Organization; 1990. [Google Scholar]
- van Melle LP, De Jonge P, Spijkerman TA, Tijssen JGP, Ormel J, van Veldhuisen DJ, van den Brink RHS, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosomatic Medicine. 2004;66:814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Waller M, McGuire ACL. Changes over time in the “healthy soldier effect”. Population Health Metrics. 2011;9:7. doi: 10.1186/1478-7954-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington DL, Bean-Mayberry B, Hamilton AB, Cordasco KM, Yano EM. Women Veterans’ healthcare delivery preferences and use by military service era: Findings from the National Survey of Women Veterans. J Gen Intern Med. 2013;28(Suppl 2):S571–6. doi: 10.1007/s11606-012-2323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Weiss NS, Ure CL, Ballard J, Daling JR. Effect of weight, smoking and estrogen use on the risk of hip and forearm fractures in postmenopausal women. Obstetrics and Gynecology. 1982;60:695–699. [PubMed] [Google Scholar]
- Wilmoth JM, London AS, Parker WM. Military service and men’s health trajectories in later life. The Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 2010;65B(6):744–755. doi: 10.1093/geronb/gbq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SW. Cancer Incidence in Korean Vietnam Veterans During 1992–2003: The Korean Veterans Health Study. Journal of Preventive Medicine and Public Health. 2013;46:309–318. doi: 10.3961/jpmph.2013.46.6.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Cardiovascular diseases in American women. Nutrition, Metabolism and Cardiovascular Disease. 2010 Jul;20(6):386–93. doi: 10.1016/j.numecd.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Devesa SS, Wu H, Zahm SH, Ismail J, Anderson WF, Peoples G, Maxwell LG, Granger E, Potter JF, McGlynn KA. Cancer Incidence in the U.S. Military Population: Comparison with Rates from the SEER Program. Cancer Epidemiol Biomarkers Prev. 2009 Jun;18(6):1740–1745. doi: 10.1158/1055-9965.EPI-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]