Abstract
Throughout the adult life of all mammals including humans, new neurons are incorporated to the dentate gyrus of the hippocampus. During a critical window that lasts about two weeks, adult-born immature neurons are more excitable and plastic than mature ones, and they respond to a wider range of inputs. In apparent contradiction, new neurons have been shown to be crucial to solve behavioral tasks that involve the discrimination of very similar situations, which would instead require high input specificity. We propose that immature neurons are initially unspecific because their task is to identify novel elements inside a high dimensional input space. With maturation, they would specialize to represent details of these novel inputs, favoring discrimination.
Introduction
Among cortical structures, the dentate gyrus (DG) of the hippocampus presents a unique degree of plasticity conferred by the continuous production of new principal neurons, the adult-born dentate granule cells (GCs) [1–3]. Thousands of new GCs are produced every day and develop over several weeks, generating millions of new connections that modify the preexisting circuits [4]. Extensive evidence accumulated over the last decade has demonstrated that adult-born GCs can modify signal processing in the DG and that they are necessary to perform specific tasks requiring discrimination of very similar situations [5,6]. In this review we focus on the hypothesis that the functional role played by adult-born GCs depends on their developmental stage. We propose a mechanism for the involvement of new GCs in novel input discrimination based on recent electrophysiological, behavioral and computational modeling evidence.
Network Remodeling by Adult Neurogenesis
A remarkable and unique process takes place in the subgranular zone of the DG, a thin layer where neural stem cells self-amplify and give rise to new GCs that become integrated to the preexisting circuit. Most of what is known about their functional characteristics comes from research in rodents where adult-born GCs develop in living animals and their morpho-functional properties are studied in acute slices (electrophysiology) or fixed tissue sections (microscopy). Recently, the use of transgenic mice and retroviral vectors targeting dividing progenitor cells has allowed the selective expression of fluorescent reporters and light-activated channels into developing GCs to study their function, both in vitro and in vivo.
The development of adult-born GCs is remarkably slow, lasting about 6 – 7 weeks. Over this time, morphology, intrinsic electrical properties and synaptic connections evolve in parallel towards a mature neuronal phenotype [7–12]. Dendritic GABAergic synaptogenesis occurs during the second week (w2) and it is the earliest event that connects developing GCs with the circuit. The initial combination of a high input resistance and the depolarizing effect of GABA facilitates functional glutamatergic synaptogenesis, which displays a delayed onset in comparison to GABA [13–15]. At this early stage of GC development, activation of GABAergic networks upon brief exploratory behavior in an enriched environment (EE) promotes unsilencing of immature excitatory contacts, which incorporate AMPA-subtype of glutamate receptors into NMDAR-only synapses and become capable of fast transmission [16]. With time, developing GCs undergo a substantial decrease in membrane resistance that is accompanied by a switch that transforms GABA-mediated signaling from excitatory to inhibitory [8]. Around w4, GABAergic transmission is already inhibitory, but GCs continue to be functionally immature due to their membrane resistance (still higher than what is typically found in mature GCs) and lack of perisomatic GABAergic connections, resulting in a high neuronal gain. This peculiar combination of intrinsic and network properties spans from about w4 to w7, during which young (immature) GC activity is characterized by low spiking threshold and poor input specificity [17,18]. Coincidently, GCs at w4 also display enhanced activity-dependent potentiation of glutamatergic synapses that only lasts for about two weeks, suggesting extensive remodeling of input and output connections during this period [19,20]. This remodeling is likely to determine the role of each new GC in information processing. The output of young (w4) and mature (w8) GCs was recently compared using optogenetic stimulation and electrophysiological recordings in the dentate and CA3 areas [21]. While mature GCs can reliably recruit both CA3 networks and feedback inhibition onto the granule cell layer, young GCs can activate CA3 networks but exert poor recruitment of proximal feedback interneurons. Also recently, Bergami and colleagues (2015) showed that the input can be modulated by experience in an EE, producing a dramatic expansion in the number of excitatory and inhibitory neurons that synapse onto developing GCs [22]. Interestingly, sensitivity to EE is highest in GCs within w2 to w6, a window that overlaps with the developmental stages of high excitability, enhanced synaptic plasticity, and poor coupling to inhibitory loops.
Overall, at around w4 GCs undergo a transition, lasting at most until w6 to w8, during which they are very active, poorly coupled to inhibition and highly susceptible to activity-dependent synaptic modification of input and output connections. As maturation proceeds, activation of new GCs becomes input specific and their connections are stabilized.
Neurogenesis and Pattern Separation
A major challenge in the field is to understand how the network plasticity described above may contribute to information processing in the hippocampus. The structure and sparse activity of the DG suggest its involvement in pattern separation, i.e. the transformation of similar inputs into dissimilar outputs [23,24]. In a way this mechanism acts in the opposite direction of pattern completion, a critical process for the retrieval of memories during which representations are transformed, presumably by the influence of CA3 recurrent collateral connections, to make them similar to a previously stored sample [25,26]. The conflict arises during the acquisition of a new memory due to the fact that any influence from previous stored representations would introduce spurious correlations among memories, compromising their future retrieval. Computational models thus require the prevalence of pattern separation at this stage [27], which is thought to occur due to the strength of detonator mossy synapses targeting CA3 pyramidal cells [28].
In agreement with the pattern separation hypothesis, mice lacking NMDA receptors selectively in GCs show impaired fear-context discrimination for similar but not dissimilar contexts [29]. This manipulation also produces a reduced contextual modulation in the firing rate of CA3 place cells. Accordingly, GCs in rats can exhibit a particular sensitivity to small contextual variations, which is not present in their target CA3 cells [30]. Pattern separation has also been studied in the spatial domain. Lesion studies show that the DG is required to discriminate between two very proximal positions in physical space, and becomes progressively less important with increasing discrimination distance [31,32]. Similar conclusions have been reached through the local manipulation of BDNF [33]. However, the spatial response of GCs has been a somewhat controversial issue. GCs have been reported to be spatially tuned, with response fields as selective as those of CA3 place cells [34], or alternatively as bearers of multiple and unstable fields, suggesting a rather low spatial information content [30]. This difference could be explained by the recent report of two coexisting populations of putative principal cells in the DG, one spatially tuned and one with low spatial information [35,36]. Interestingly, Neunuebel and colleagues (2012) [35] provide indirect evidence pointing to the identification of the low-spatial-information group with immature GCs. This hypothesis agrees with known properties of immature GCs in vitro but has not yet been tested in vivo by means of age-tagging techniques.
Only in the last decade it has been possible to address the crucial issue of whether or not young GCs are specifically involved in behavioral pattern separation. To achieve this, animals with altered levels of neurogenesis were tested in discrimination tasks with varying levels of similarity. The manipulation of neurogenesis was attained through x-irradiation [37–39], lentiviral expression of dominant-negative Wnt protein [37], expression of proapoptotic Bax protein [38,40], deletion of NR2B-containing NMDA receptors [41] or voluntary exercise [42]. The behavioral paradigms included delayed non-matching to place in a radial arm maze [37,39], two-choice spatial discrimination in a touch-screen system [37,42] and contextual fear-discrimination learning [38– 41]. The convergence of results obtained through this combination of techniques and tests points to a crucial role of young GCs in pattern separation. Animals with ablated neurogenesis were impaired in their capability to discriminate situations with a high degree of similarity, while animals with increased neurogenesis outperformed controls. In all studies, as the task became easier by making situations more dissimilar, the differences in performance between treated and control animals tended to disappear.
These experiments have been fundamental in describing the importance of newborn GCs in hippocampal processing. However, the question of the precise developmental phase in which young GCs are crucial has remained unaddressed, partly due to the low temporal resolution of the manipulations. In all of the experiments discussed above, alteration of neurogenesis started 6-16 weeks before training and lasted throughout the testing period. For instance, Sahay and colleagues (2011) observed that performance in a contextual fear-discrimination task correlated with the expansion or reduction of the adult-born GC population [38]. The expansion was triggered 8 weeks before training, while the permanent ablation through X-ray irradiation occurred with an anticipation of 4 months. A second element in common in these experiments is that training and testing were almost simultaneous. In such a scheme, it has not been possible to assess the memory stage in which young GCs are important: task acquisition, retrieval or both. An insight on these time-related issues would be essential to understand mechanistically the proposed role of young GCs in pattern separation.
Computational models
As reviewed in the previous sections, young GCs are:
specifically involved in pattern separation,
hyper-plastic, excitable and input unspecific,
only transiently unique.
The first conceptual challenge for modelers is to solve the apparent contradiction between points A and B. The separation of very similar patterns of activity would naturally occur if young GCs coded for highly specific details of the input, yet they seem to follow the opposite strategy. Even if these points were reconciled, a second conceptual challenge, posed by point C, would remain. Why are these two populations dynamic instead of stable groups with different characteristics? Strategies based on the mere division of labor between different neurons are ubiquitous across the brain, but neurogenesis is a costly extravagance.
One line of models addresses these issues by focusing on the idea that hyper-plasticity would make young GCs code for all events occurring during their critical time window, so that all associated representations in DG and CA3 would share a common piece of code, i.e. a temporal tag [43]. This tag would help discriminating situations that were not experienced during the same period of life but would bring together memories of contemporaneous events, encoded by the same cohorts of new GCs [44]. Interestingly, simulations predict that the contribution of young GCs to the discrimination of similar contexts would be negative [45]. This prediction could be tested by recording specifically young GCs while animals face a task involving small contextual variations, such as carried out by Leutgeb and colleagues (2007) [30].
A different line of models assigns to young GCs the task of representing novel experiences, characterized by surprising inputs with different statistics than expected from the individual history of the animal. As opposed to the temporal tagging idea, distinct features of the novel phenomena would be represented by different neurons, so no common piece of code would be present in CA3 representations. Simulations show that if input statistics change over time, a strategy based on a growing internal chart obtains a better input representation than other strategies such as fixed populations of plastic neurons or partial neural turnover [46,47]. Young GCs may be unique in their firing properties because they are ready to expand the code in any necessary direction, which only becomes specific after a critical time window that involves learning and maturation (Fig. 1) [21,48,49]. Thus, although young GCs would be essential to learn a novel task, it is only in their mature stage that they would perform actual pattern separation, enabled by high input specificity and strong coupling to feedback inhibitory networks.
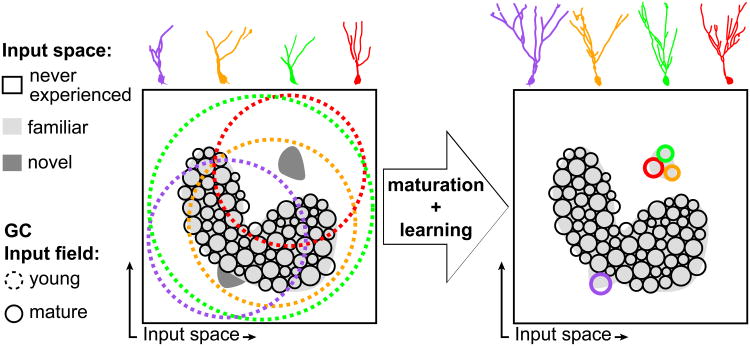
Figure 1. A conceptual model of the functional role of GCs in pattern separation across maturation.
All possible patterns of inputs to the dentate gyrus are projected into a two-dimensional space for visualization purposes. Inputs are divided into familiar (light grey), novel (dark grey) and a vast majority of never experienced input combinations (white). Every GC has an input field, a region of the input to which it is responsive, represented by solid (mature) or dashed (immature) circles. Left: The familiar input space is covered by small and non-overlapping mature input fields, so that close-by inputs are represented by different neurons, reflecting pattern separation. In contrast, immature GCs present wide and overlapping fields due to high excitability and low inhibition. Right: Through maturation, learning, and coupling to feedback inhibitory networks these GCs acquire mature input fields. This strategy allows the coverage of vast regions of unexperienced but potentially novel input by a few young GCs, which identify the small novel input regions and learn to represent their details in a highly specific way. Upper panels represent developing GCs at immature (left) and mature (right) developmental phases. Diagram adapted with permission from ref. [21]. GC, granule cell.
Conclusions
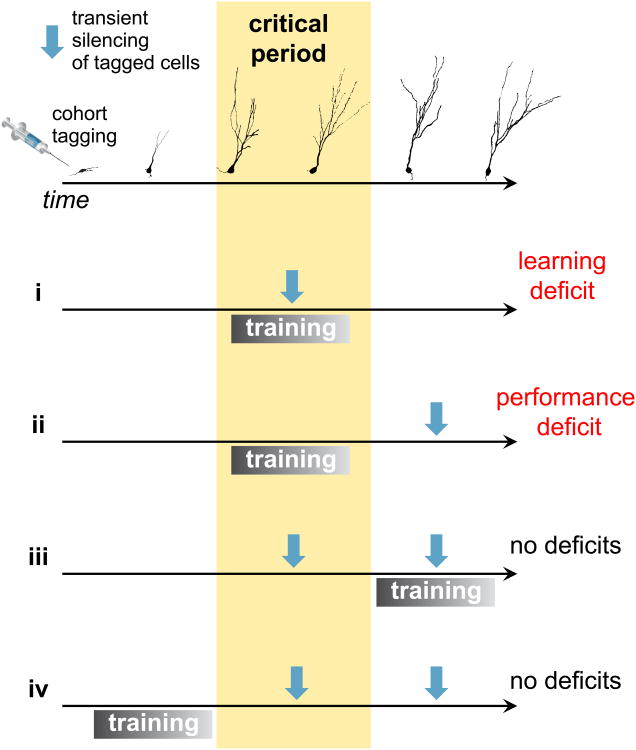
Recent years have witnessed great advances in the description of the course of new GCs incorporated to the DG. Important behavioral correlates of the deficit or surplus of young GCs were found, allowing us to construct computational models that aim to explain their role and importance inside the hippocampal machinery. Models suggest that a new generation of experiments should take into account the developmental timing and the different stages of memory processing. In an idealized experiment, a specific cohort of new GCs could be tagged to express opto- or chemogenetically activated channels, allowing for reversible and temporally-restricted silencing. The model by Wiskott and colleagues (2006) [46], further developed by Temprana and colleagues (2015) [21], predicts that not all silencing of young GCs would result in a pattern separation deficit. Instead, four different scenarios arise (Fig. 2). First, coincident training and silencing during the critical window would result in a learning deficit (i), affecting all future performance even without further silencing. The experiments discussed in the Neurogenesis and Pattern Separation section fit into this scenario. Second, if learning took place normally without silencing (ii), a performance deficit would only appear at a later stage when silencing this particular cohort of (mature) GCs. Finally, training outside the critical window of the tagged GCs (iii-iv) would result in no deficit, independently of whether silencing is applied or not.
Figure 2. Experimental testing and predictions for the conceptual model presented in Fig. 1.
In an idealized experiment, a cohort of young GCs is tagged with opto- or chemogenetically activated channels, allowing for a precise control of their silencing. A novel task requiring pattern separation is introduced. If training occurs while tagged GCs undergo their critical period, silencing these cells during training will generate a learning deficit, compromising future performance even without further silencing (i). If no silencing occurs during the training stage, future deficits in performance will appear transiently when silencing these GCs, even if fully mature (ii). If training occurs outside the critical window, no effect of silencing on performance is expected (iii and iv). The upper panel depicts the maturation process of a tagged cohort of developing GCs. GC, granule cell.
The confirmation or refutation of these predictions would increase our knowledge on the functional role of young GCs in mechanistic terms. However, other questions would remain open, such as the nature of the mechanism that recruits young GCs in a task-specific manner, perhaps following similarity criteria. Only the combined effort of computational and experimental research will allow us to further understand this information-processing aspect of neurogenesis.
Highlights.
New immature neurons are incorporated to pre-existing networks of the dentate gyrus
They are hyper-plastic, excitable, uncoupled from inhibition and input unspecific
They are crucial for tasks involving the discrimination of very similar situations
While immature they could detect novel input features in a high dimensional space
Only in their mature stage they would engage in input discrimination
Acknowledgments
E.K., S.M.Y. and A.F.S. were supported by the National Research Council (CONICET). This work was supported by grants from the Argentine Agency for the Promotion of Science and Technology (PICT2010-1798 to A.F.S. and PICT2012-0548 to E.K.), the National Institutes of Health (FIRCA R03TW008607-01 to A.F.S.), and the Howard Hughes Medical Institute (SIRS grant #55007652 to A.F.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
- 1.Christian KM, Song H, Ming GL. Functions and Dysfunctions of Adult Hippocampal Neurogenesis. Annu Rev Neurosci. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lepousez G, Nissant A, Lledo PM. Adult Neurogenesis and the Future of the Rejuvenating Brain Circuits. Neuron. 2015;86:387–401. doi: 10.1016/j.neuron.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst A, Frisén J. Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol. 2015;13:e1002045. doi: 10.1371/journal.pbio.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piatti VC, Ewell LA, Leutgeb JK. Neurogenesis in the dentate gyrus: carrying the message or dictating the tone. Front Neurosci. 2013;7:50. doi: 10.3389/fnins.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: why the dentate gyrus? Learn Mem. 2013;20:710–729. doi: 10.1101/lm.026542.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, Gage FH, Schinder AF. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:e409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mongiat LA, Esposito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunner J, Neubrandt M, Van-Weert S, Andrasi T, Kleine Borgmann FB, Jessberger S, Szabadics J. Adult-born granule cells mature through two functionally distinct states. Elife. 2014;3:e03104. doi: 10.7554/eLife.03104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markwardt SJ, Dieni CV, Wadiche JI, Overstreet-Wadiche L. Ivy/neurogliaform interneurons coordinate activity in the neurogenic niche. Nat Neurosci. 2011;14:1407–1409. doi: 10.1038/nn.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshpande A, Bergami M, Ghanem A, Conzelmann KK, Lepier A, Gotz M, Berninger B. Retrograde monosynaptic tracing reveals the temporal evolution of inputs onto new neurons in the adult dentate gyrus and olfactory bulb. Proc Natl Acad Sci U S A. 2013;110:E1152–61. doi: 10.1073/pnas.1218991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chancey JH, Adlaf EW, Sapp MC, Pugh PC, Wadiche JI, Overstreet-Wadiche LS. GABA depolarization is required for experience-dependent synapse unsilencing in adult-born neurons. J Neurosci. 2013;33:6614–6622. doi: 10.1523/JNEUROSCI.0781-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335:1238–1242. doi: 10.1126/science.1214956. This ex-vivo study shows that developing GCs can process incoming inputs before reaching maturation and display low spiking threshold and poor input specificity due to their reduced GABAergic inhibition. Upon maturation, GCs become highly input specific. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieni CV, Nietz AK, Panichi R, Wadiche JI, Overstreet-Wadiche L. Distinct determinants of sparse activation during granule cell maturation. J Neurosci. 2013;33:19131–19142. doi: 10.1523/JNEUROSCI.2289-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. The paper shows that perforant path inputs onto adult-born GCs display enhanced activity-dependent potentiation within a critical period stretching from w4 to w6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF. Delayed Coupling to Feedback Inhibition during a Critical Period for the Integration of Adult-Born Granule Cells. Neuron. 2015;85:116–130. doi: 10.1016/j.neuron.2014.11.023. This work uses an optogenetic approach to demonstrate that mature GCs activate feedback inhibition that tightly controls spiking in the granule cell layer, whereas immature GCs do not recruit or respond to such inhibition. A computational model on the role of adult neurogenesis in memory encoding is presented based on functional data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann KK, Schinder AF, Götz M, et al. A Critical Period for Experience-Dependent Remodeling of Adult-Born Neuron Connectivity. Neuron. 2015;85:710–717. doi: 10.1016/j.neuron.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 24.O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 25.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wills TJ, Lever C, Cacucci F, Burgess N, O'Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. This modeling work uses an elegant information-theory approach to demonstrate that the full potential of CA3 for memory processing cannot be exploited unless acquisition and retrieval are supported by two qualitatively distinct sources of input, linking them to known properties of perforant path and mossy fibers. [DOI] [PubMed] [Google Scholar]
- 28.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 29.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 30•.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. This study presents one of the strongest pieces of evidence in favor of the pattern separation hypothesis from a mechanistic perspective. In vivo recordings of rats exploring a set of morphs between two different environments show that individual GCs are tuned to change their firing maps in response to small contextual variations. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 32.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 33.Bekinschtein P, Kent BA, Oomen CA, Clemenson GD, Gage FH, Saksida LM, Bussey TJ. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 2013;5:759–68. doi: 10.1016/j.celrep.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 35.Neunuebel JP, Knierim JJ. Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J Neurosci. 2012;32:3848–3858. doi: 10.1523/JNEUROSCI.6038-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–427. doi: 10.1016/j.neuron.2013.11.017. This study reports simultaneous recordings from CA3 and DG during a task in which proximal and distal cues are displaced relative to one another. The authors show that CA3 cells respond to this conflictive scene with high population coherence, while populations of cells in the DG have a degraded coherence, suggestive of pattern separation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. The first behavioral study to suggest an involvement of adult hippocampal neurogenesis in pattern separation. Mice with ablated neurogenesis were specifically impaired in spatial discrimination when stimuli were presented with a small separation, in both a navigable and non-navigable tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. Using Inducible genetic expansion of the population of adult-born granule cells, the authors show that increasing adult neurogenesis is sufficient to improve performance in a specific cognitive task where two similar contexts need to be distinguished, which is indicative of enhanced pattern separation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Barrera VR, Chittajallu R, Iwamoto KS, McBain CJ, et al. Young Dentate Granule Cells Mediate Pattern Separation, whereas Old Granule Cells Facilitate Pattern Completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tronel S, Belnoue L, Grosjean N, Revest JM, Piazza PV, Koehl M, Abrous DN. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- 41.Kheirbek MA, Tannenholz L, Hen R. NR2B-Dependent Plasticity of Adult-Born Granule Cells is Necessary for Context Discrimination. J Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. In this perspective article, the temporal tagging hypothesis is proposed as a conceptual model. High plasticity in a cohort of young GCs would make all CA3 representations of events experienced within their critical window share a common piece of code, tying these memories together. [DOI] [PubMed] [Google Scholar]
- 44.Aimone JB, Wiles J, Gage FH. Computational Influence of Adult Neurogenesis on Memory Encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuneo JI, Quiroz NH, Weisz VI, Argibay PF. The computational influence of neurogenesis in the processing of spatial information in the dentate gyrus. Sci Rep. 2012;2 doi: 10.1038/srep00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: Avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. Considering the hippocampus as an auto-encoder, this modeling study compares different strategies to face the problem of unpredictable change in input statistics. Simulations show that neurogenesis outperforms other strategies such as fixed high plasticity or neural turnover. The key is that new neurons can adapt to novel features of the input, while mature ones present a fixed code that is optimized to represent all past experiences. [DOI] [PubMed] [Google Scholar]
- 47.Appleby PA, Wiskott L. Additive neurogenesis as a strategy for avoiding interference in a sparsely-coding dentate gyrus. Network. 2009;20:137–161. doi: 10.1080/09548980902993156. [DOI] [PubMed] [Google Scholar]
- 48.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rangel LM, Quinn LK, Chiba AA, Gage FH, Aimone JB. A hypothesis for temporal coding of young and mature granule cells. Front Neurosci. 2013;7 doi: 10.3389/fnins.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]