Abstract
Adjuvant endocrine therapy is a mainstay of treatment for patients with endocrine-responsive early breast cancer. Questions remain concerning which patients should receive what type of endocrine therapy and for how long. Several factors have been considered as potential indicators to predict benefit of endocrine therapy, including patient factors, clinico-pathological factors and multigene assays. To date, factors associated with risk of recurrence have been the most widely adopted to influence treatment selection. The International Breast Cancer Study Group (IBCSG)-led adjuvant endocrine therapy trials BIG 1–98, for postmenopausal women, and SOFT and TEXT, for premenopausal women, can shed light on the role for risk of recurrence in identifying who should receive which type of adjuvant endocrine therapy and for how long.
Keywords: adjuvant therapy, aromatase inhibitor, postmenopausal, premenopausal, tamoxifen
Benefit of Adjuvant Endocrine Therapy for Postmenopausal Women
In adjuvant endocrine therapy trials, the superior efficacy of aromatase inhibitors versus tamoxifen for postmenopausal women with endocrine-responsive early breast cancer has been declared on the basis of a relative efficacy measure, the hazard ratio. For additional clinical insight, the trial reports have included Kaplan-Meier estimates of the primary endpoint at 5 years since randomization. In the Breast International Group (BIG) 1–98 trial, 5 years of letrozole resulted in an 18% relative reduction in a disease-free survival event versus 5 years of tamoxifen (hazard ratio 0.82, 95% confidence interval (CI) 0.71–0.95; p=0.0007), which translated into an absolute improvement in disease-free survival of 2.9% at 5 years, from 81.1% to 84.0% [1].
The trials have been rich sources for investigations searching for predictive markers of differential relative responsiveness to one treatment or the other, based on extensive clinical databases and banked formalin-fixed tumor samples. There have been many such investigations, considering the patient environment, histopathologic factors, and expression of a single gene, or multiple genes in a signature. In BIG 1–98, for example, central assessment of estrogen receptor, progesterone receptor and HER2 status were evaluable for 74% of patients randomized to the monotherapy treatment groups. HER2-positive disease was prognostic—associated with poorer disease-free survival in these endocrine therapy-treated women (note that these patients were treated prior to the availability of trastuzumab for HER2-positive tumors)—but it was not a predictive marker of differential responsiveness to letrozole versus tamoxifen [2]. The subgroups had hazard ratios (0.72 and 0.62) that were not statistically different from one another according to a formal test of treatment-by-HER2 interaction in a Cox proportional hazards model. Yet because of the poorer prognosis with HER2-positive disease, the estimated absolute improvement in 4-year disease-free survival for letrozole versus tamoxifen of 9% for HER2+ disease, was much greater than the 4% observed for HER2-negative disease.
Despite the efforts to identify predictive markers, clinical practice guidelines for adjuvant endocrine therapy primarily point to the risk of recurrence for decision-making, indicating a strong reliance on the absolute treatment benefit. The 2013 St. Gallen consensus [3], for example, referred to a preference to start patients who are at high risk of recurrence with an aromatase inhibitor upfront. When this preference was first introduced in the 2009 St. Gallen consensus [4], we assessed whether risk of recurrence predicted for differential magnitude of letrozole effectiveness compared with tamoxifen, when used either as monotherapy or a sequence of the two agents [5]. Risk of recurrence was defined as a composite risk score that was calculated from a Cox proportional hazards model including standard clinico-pathologic prognostic factors. Using the Subpopulation Treatment Effect Pattern Plot (STEPP; 6) method to investigate the predictive value of continuous-value markers on absolute treatment effects (Kaplan-Meier estimates of 5-year disease-free survival), three patterns of treatment effects were revealed: patients with highest-risk scores did best when treated with 5 years of letrozole; any of the three letrozole-containing regimens was acceptable for those patients in an intermediate risk range; whereas patients with lowest-risk scores did similarly well with letrozole monotherapy, a sequence of letrozole and tamoxifen, or tamoxifen monotherapy. Thus, in BIG 1–98 we confirmed that the principle of composite assessment of risk, analogous to the clinical practice of integrating multiple risk factors when physicians and patients are deciding on the best adjuvant endocrine treatment for the individual patient, informed treatment selection and supported the choice of 5 years of letrozole for patients at the highest risk for recurrence within the first 5 years.
Building on this work, we considered two additional clinical scenarios: the role for risk of recurrence as a factor for adjuvant endocrine treatment selection for premenopausal women; and the role for risk of recurrence—as assessed at initiation of endocrine therapy—as a factor for decision-making about extended adjuvant therapy.
Benefit of Adjuvant Endocrine Therapy for Premenopausal Women
The TEXT and SOFT complementary tailored treatment trials investigated the roles of aromatase inhibitors and of ovarian suppression for treatment of premenopausal women [7–9]. Combining data from TEXT and SOFT, the results showed that adjuvant treatment with the aromatase inhibitor exemestane plus ovarian function suppression significantly reduced recurrence as compared with tamoxifen plus ovarian function suppression [8]. Additionally in SOFT, results showed that adding ovarian suppression to tamoxifen, versus tamoxifen alone, did not provide a significant benefit in the overall study population [9].
The use of an aromatase inhibitor with ovarian suppression provided an absolute improvement in 5-year breast cancer-free interval, versus tamoxifen with or without ovarian function suppression, that was as large as the absolute improvement at 5 years of approximately 3% observed in randomized trials of aromatase inhibitor versus tamoxifen for postmenopausal women [1]. However the necessity of using ovarian function suppression with the aromatase inhibitor in premenopausal women brings additional side effects and costs that must be weighed against the benefits for an individual patient.
In TEXT and SOFT, the use of adjuvant chemotherapy was by physician and patient choice, and trial results were also presented separately according to chemotherapy use in order to better inform clinical decision-making. In SOFT patients who did not receive chemotherapy and in those SOFT patients who had received prior chemotherapy, the absolute improvements in 5-year breast cancer-free interval with exemestane plus ovarian function suppression versus tamoxifen alone were 1.3% and 7.7%, respectively [9]. Chemotherapy use was a reasonable approximation for risk of recurrence, for example, node-positive disease was much more frequent in the two chemotherapy cohorts of TEXT and SOFT (66% and 57%, respectively), as compared with the TEXT and SOFT no-chemotherapy cohorts (21% and 9%, respectively). Using the same approach as in BIG 1–98—calculating a composite risk score for all patients in TEXT and SOFT based on multiple standard clinico-pathologic risk factors and comparing treatments using the STEPP method—provided additional insight into how the magnitude of benefit of aromatase inhibitor plus ovarian function suppression versus tamoxifen with or without ovarian function suppression increased as the continuum of risk scores increased. The analysis supported the role for risk of recurrence in treatment selection for premenopausal women with endocrine-responsive disease.
Benefit of Extended Adjuvant Endocrine Therapy
Long-term follow-up of the postmenopausal adjuvant endocrine therapy trials allows investigation into which patients should receive extended adjuvant endocrine therapy. Recent research has indicated that traditional clinico-pathologic prognostic features, and multi-marker or multigene assays, may predict those women at higher, or very low, risk of late distant recurrence [10].
Corresponding to clinical decision-making, the statistical analyses to investigate recurrence beyond 5 years may be framed by re-defining the population as those women who were alive and cancer-free at 5 years after the initiation of endocrine therapy. In this context, the question was asked in the BIG 1–98 trial of whether the initial risk of recurrence—as assessed prior to the initiation of adjuvant endocrine therapy—was informative beyond 5 years for decision-making about extended adjuvant therapy.
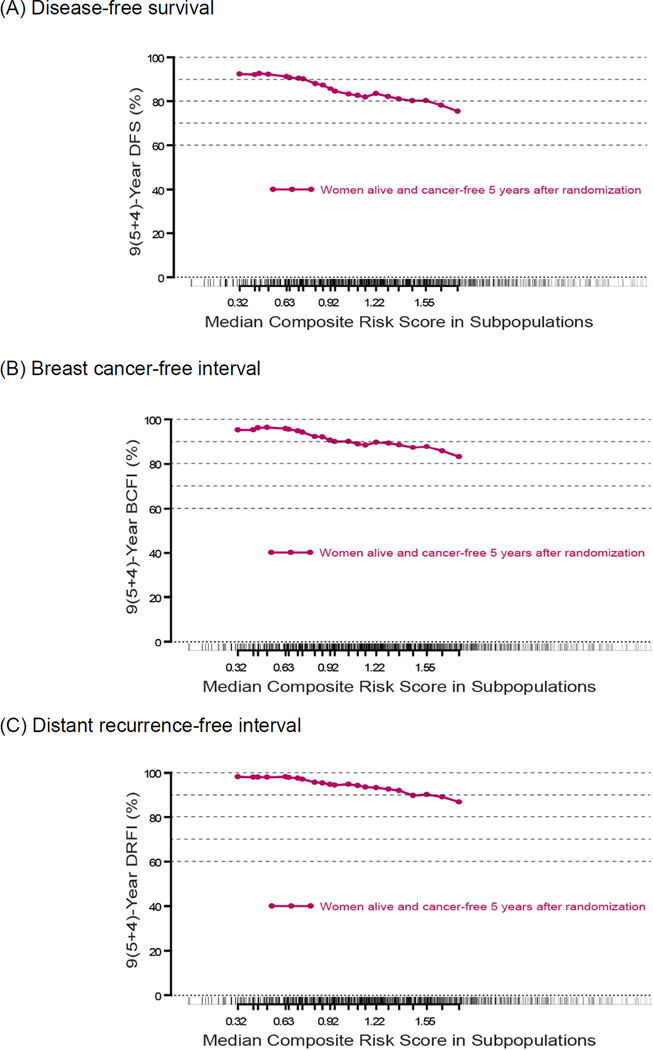
Using the composite risk score previously analyzed [5] in the population of trial patients who were alive and cancer-free at 5 years since randomization, the STEPP method focused on disease outcomes 4 years later, or 9 years after randomization and initiation of adjuvant endocrine therapy in the trial. In this population, the composite risk score from endocrine therapy initiation remained prognostic for disease-free survival 4 years later (i.e., 9 years from therapy initiation; Figure 1A). Considering that 9 years later, women in BIG 1–98 were on average 70 years of age, the disease-free survival endpoint—which includes second (non-breast) malignancies and deaths without prior cancer event—may not be the best endpoint for clinical decision-making beyond 5 years. The results were consistent for the endpoints of breast cancer-free interval (which includes distant, local-regional recurrences and contralateral breast cancer) and of distant recurrence-free interval (Figure 1B, 1C). With each endpoint, the prognostic value of the initial risk score was evident at 9 years, and the pattern of outcomes corresponded with the same three zones of treatment effects previously noted for 5-year outcome [5].
Figure 1.
Subpopulation Treatment Effect Pattern Plot (STEPP) of outcomes at 9 years after randomization in BIG 1–98 trial patients who were alive and cancer-free at 5 years after randomization and initiation of adjuvant endocrine therapy, according to a composite risk score [5]. Outcomes are (A) disease-free survival; (B) breast cancer-free interval; and (C) distant recurrence-free interval.
In BIG 1–98 we also investigated whether the prior endocrine therapy regimen—letrozole or tamoxifen alone or in sequence, corresponding to 5, 3, 2 or 0 years of prior letrozole—might influence decisions about extended endocrine therapy There was no evident pattern for distant recurrence at 9 years according to prior therapy, that might be useful for decision-making about extended therapy. Thus we confmed that in women who were alive and cancer-free after 5 years of adjuvant endocrine therapy, the initial risk assessment at time of therapy initiation was still informative about her risk of recurrence beyond 5 years, and her potential to benefit from extending treatment.
And for women who are premenopausal at initiation of endocrine therapy, the long-term follow-up of the TEXT and SOFT trials will be critical to improve decision-making for these women.
ACKNOWLEDGMENT
The IBCSG Statistical Center is partially supported by US National Cancer Institute grant CA075362. Pathology material collection and translational research in the BIG 1–98, TEXT and SOFT trials were supported by the International Breast Cancer Study Group, Novartis, Pfizer, Breast Cancer Research Foundation, Susan G. Komen for the Cure (KG080081), and the US National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Láng I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jacobsen EH, Price KN, Goldhirsch A. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: Update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen BB, Regan MM, Lykkesfeldt AE, Dell’Orto P, Del Curto B, Henriksen KL, Mastropasqua MG, Price KN, Méry E, Lacroix-Triki M, Braye S, Altermatt HJ, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Gusterson BA, Thürlimann B, Coates AS, Viale G for the BIG 1–98 Collaborative and the International Breast Cancer Study Groups. Adjuvant letrozole vs. tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1–98 randomised trial. Lancet Oncol. 2009;9:23–28. doi: 10.1016/S1470-2045(07)70386-8. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ Panel members. Thresholds for therapies: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viale G, Regan MM, Dell’Orto P, Mastropasqua MG, Maiorano E, Rasmussen BB, MacGrogan G, Forbes JF, Paridaens RJ, Colleoni M, Láng I, Thürlimann B, Mouridsen H, Mauriac L, Gelber RD, Price KN, Goldhirsch A, Gusterson BA, Coates AS. Which patients benefit most from adjuvant aromatase inhibitors? Results using a composite measure of prognostic risk in the BIG 1–98 randomised trial. Ann Oncol. 2011;22:2201–2207. doi: 10.1093/annonc/mdq738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazar AA, Cole BF, Bonetti M, Gelber RD. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: Subpopulation Treatment Effect Pattern Plot. J Clin Oncol. 2010;28:4539–4544. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan MM, Pagani O, Fleming GF, Walley BA, Price KN, Rabaglio M, Maibach R, Ruepp B, Coates AS, Goldhirsch A, Colleoni M, Gelber RD, Francis PA International Breast Cancer Study Group and the SOFT and TEXT investigators. Adjuvant treatment of premenopausal women with endocrine-responsive early breast cancer: Design of the TEXT and SOFT trials. Breast. 2013;22:1094–1100. doi: 10.1016/j.breast.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, Gomez HL, Tondini C, Burstein HJ, Perez EA, Ciruelos E, Stearns V, Bonnefoi HR, Martino S, Geyer CE, Jr, Pinotti G, Puglisi F, Crivellari D, Ruhstaller T, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Bernhard J, Luo W, Ribi K, Viale G, Coates AS, Gelber RD, Goldhirsch A, Francis PA TEXT and SOFT Investigators and the International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, Martino S, Davidson NE, Geyer CE, Jr, Walley BA, Coleman R, Kerbrat P, Buchholz S, Ingle JN, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Colleoni M, Viale G, Coates AS, Goldhirsch A, Gelber RD SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sestak I, Cuzick J, Dowsett M, Lopez-Knowles E, Filipits M, Dubsky P, Cowens JW, Ferree S, Schaper C, Fesl C, Gnant M. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian Breast and Colorectal Cancer Study Group 8 and Arimidex, Tamoxifen Alone or in Combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2015;33:916–922. doi: 10.1200/JCO.2014.55.6894. [DOI] [PubMed] [Google Scholar]