Abstract
An attentional bias to threat has been implicated in the etiology and maintenance of anxiety disorders. Recently, attention bias modification (ABM) has been shown to reduce threat biases and decrease anxiety. However, it is unclear whether ABM modifies neural activity linked to anxiety and risk. The current study examined the relationship between ABM and the error-related negativity (ERN), a putative biomarker of risk for anxiety disorders, and the relationship between the ERN and ABM-based changes in attention to threat. Fifty-nine participants completed a single-session of ABM and a flanker task to elicit the ERN—in counterbalanced order (i.e., ABM-before vs. ABM-after the ERN was measured). Results indicated that the ERN was smaller (i.e., less negative) among individuals who completed ABM-before relative to those who completed ABM-after. Furthermore, greater attentional disengagement from negative stimuli during ABM was associated with a smaller ERN among ABM-before and ABM-after participants. The present study suggests a direct relationship between the malleability of negative attention bias and the ERN. Explanations are provided for how ABM may contribute to reductions in the ERN. Overall, the present study indicates that a single-session of ABM may be related to a decrease in neural activity linked to anxiety and risk.
Keywords: attention bias modification, error-related negativity, event-related potentials, threat
Introduction
An attentional bias to threat has been implicated in the etiology and maintenance of anxiety disorders (Amir et al., 2003; MacLeod et al., 1986). Cognitive theories of anxiety have suggested that threat biases occur during early, automatic stages of information processing (Beck & Clark, 1997; Cisler & Koster, 2010; Mathews & Mackintosh, 1998; Mogg & Bradley, 1999), and this notion has received strong empirical support (Bar-Haim et al., 2007). Recently, attention bias modification (ABM) programs have been developed that aim to alter attentional bias to threat (Amir et al., 2008; MacLeod et al., 2002). In a typical ABM trial, participants are simultaneously shown a threat and non-threat stimulus (e.g., negative and neutral words) on a computer screen. The stimuli then disappear and participants must identify an attentional probe (e.g., the letter E or F) that is presented in the same location as the non-threat stimulus. In this way, ABM is designed to train individuals to disengage their attention from negative stimuli and facilitate attention toward neutral or positive stimuli.
There is growing evidence supporting the efficacy of ABM. Specifically, ABM has been shown to improve behavioral performance on a stressful task (Amir et al., 2008) and reduce anxiety (Beard et al., 2012; MacLeod & Clarke, 2015). Furthermore, ABM has been particularly effective in reducing threat biases and anxiety symptomatology in individuals with generalized anxiety disorder (GAD) (Amir & Taylor, 2012; Amir, Beard, Burns, et al., 2009), subclinical obsessive-compulsive disorder (OCD) (Najmi & Amir, 2010), and social phobia (Amir et al., 2008; Amir, Beard, Taylor, et al., 2009).
Research has primarily examined the impact of ABM on behavioral measures and anxiety symptoms. However, studies have begun to examine the neural changes that occur as a function of ABM. These studies have indicated that ABM influences attention via an effect on the prefrontal cortex (PFC; Browning et al., 2010; Clarke et al., 2014; Taylor et al., 2014), a region that plays a critical role in the regulation of attention and emotion. Therefore, it is possible that ABM may subsequently modify neural reactivity to threat that has been linked to anxiety and risk.
Two meta-analyses have supported the notion that anxious individuals are characterized by an increased neural response to errors (Cavanagh & Shackman, in press; Moser et al., 2013). Specifically, the error-related negativity (ERN) is a negative deflection in the event-related potential (ERP) that peaks at frontocentral sites approximately 50 ms following the commission of an error (Hajcak, 2012). The ERN is generated in the anterior cingulate cortex (ACC) (Dehaene et al., 1994; Ito et al., 2003; Stemmer et al., 2004), a region associated with processing signals of punishment (Shackman et al., 2011). Evidence suggests that the ERN indexes early detection of errors, which represent internal threat signals, and that variability in the ERN may index individual differences in sensitivity to potential threat (Weinberg, Riesel, et al., 2012).
In terms of its relationship with anxiety disorders, the ERN is enhanced among individuals with GAD (Weinberg et al., 2010; Weinberg, Klein, et al., 2012; Xiao et al., 2011) and OCD (Endrass et al., 2008; Gehring et al., 2000; Hajcak et al., 2008; Johannes et al., 2001). Moreover, the ERN is enhanced among individuals with a family history of OCD (Carrasco et al., 2013; Riesel et al., 2011) and childhood behavioral inhibition (McDermott et al., 2009), which are both risk factors for anxiety disorders. An increased ERN was recently shown to prospectively predict the onset of new anxiety disorders in late childhood (Meyer et al., in press). These findings support the hypothesis that the ERN may be a biomarker of risk for particular anxiety disorders (Manoach & Agam, 2013; Olvet & Hajcak, 2008).
To date, no study has examined the potential impact of ABM on the ERN. As previously mentioned, ABM targets the early, automatic stages of negative information processing (Amir et al., 2008; MacLeod et al., 2002), and this appears to be achieved via increased activation of the PFC (Browning et al., 2010; Clarke et al., 2014; Taylor et al., 2014). The ERN is posited to reflect an early warning signal of potential threat—that an error has been made (Hajcak, 2012). Insofar as ABM trains individuals to disengage attention from potential threat, ABM may impact the neural processing of errors (i.e., the ERN). Rather than examining whether ABM reduces symptoms that characterize heterogeneous disorders, this approach examines the relationship between ABM and neural activity that has been previously linked to anxiety disorders and risk. This approach would determine the degree to which specific treatments like ABM might alter particular biomarkers of risk, such as the ERN—and whether biomarkers like the ERN may be associated with the response to treatments such as ABM. Focusing on neural biomarkers rather than symptoms of heterogeneous disorders is also consistent with the Research Domain Criteria (RDoC) approach (Insel et al., 2010; Sanislow et al., 2010), which aims to understand core biobehavioral dimensions that are common across several disorders. Indeed, both attention bias to threat and the ERN are included in the sustained threat domain of the RDoC matrix (NIMH, 2011). Therefore, it is plausible that altering sustained threat at the behavioral level (i.e., modifying attention bias to threat) will be associated with variation in neural measures of sustained threat (i.e., the ERN).
The present study examined whether ABM can impact the ERN, and whether there is a relationship between the ERN and ABM-based changes in attention to threat. To this end, participants completed a single-session ABM program designed to train attention away from negative stimuli and toward positive stimuli; participants also completed a flanker task to elicit the ERN—in counterbalanced order. This design allowed us to examine two important questions. First, we could examine whether the ERN differed between participants who received ABM before versus after the ERN was measured. Thus, we could determine if the ERN was smaller among individuals who first underwent ABM relative to those who did not complete ABM before the ERN was measured. Second, we could examine the association between individual differences in attention bias measures during ABM and the ERN. Attention bias measures from dot probe tasks have been shown to demonstrate poor psychometric properties (Cisler et al., 2009; Price et al., 2015; Schmukle, 2005; Waechter & Stolz, in press; Waechter et al., 2014). Therefore, the present study calculated two different attention bias measures: average attention bias and change in attention bias (i.e., trainability). Thus, among participants who completed ABM before the ERN was measured, we could assess whether ABM-related threat bias (i.e., the degree to which individuals learn to disengage their attention from threat) was associated with a smaller ERN. Additionally, we could examine whether the ERN was associated with ABM-related threat bias in participants who completed ABM after the ERN was measured.
We had two primary hypotheses. First, a single-session of ABM has been shown to improve behavioral performance on a public speaking task (Amir et al., 2008), and multi-session ABM has decreased anxiety symptomatology in GAD (Amir & Taylor, 2012; Amir, Beard, Burns, et al., 2009) and OCD (Najmi & Amir, 2010), conditions associated with an enhanced ERN (Endrass et al., 2008; Gehring et al., 2000; Weinberg et al., 2010; Weinberg, Klein, et al., 2012). Therefore, we hypothesized that a single-session of ABM, relative to no intervention, would be associated with a smaller ERN. Second, we hypothesized that individual differences in ABM-related change in negative (but not positive) attention bias would be associated with a smaller ERN in ABM-before and ABM-after participants. This result would suggest that change in attention bias may both contribute to the group difference in the ERN (in the ABM-before participants) and that the ERN may be a predictor of change in attention bias (in the ABM-after participants).
Method
Participants
The sample included 59 undergraduates from Stony Brook University who participated for course credit. Informed consent was obtained prior to participation and the research protocol was approved by the local Institutional Review Board. Participants were randomly assigned to either complete ABM-before (n = 32) or ABM-after (n = 27) the ERN was measured. Current depression and anxiety was assessed using the Depression Anxiety Stress Scale-21 (DASS-21) (Lovibond & Lovibond, 1995). The DASS-21 is a measure of psychological distress over the last week. Items are rated on a 4-point Likert scale ranging from 0 (did not apply to me at all) to 3 (applied to me very much, or most of the time), with higher scores indicating greater symptom severity. The DASS-21 was designed in accordance with the tripartite model of depression and anxiety (Clark & Watson, 1991), and contains three 7-item subscales measuring low positive affect (DASS-Depression), physiological hyperarousal (DASS-Anxiety), and non-specific negative affect (DASS-Stress). Four participants were excluded from analyses for excessive EEG artifacts (n = 2), outlier ERN values (>2.5 standard deviations from the mean; n = 1), and outlier DASS-Depression, Anxiety, and Stress values (all > 2.5 standard deviations from the mean; n = 1), leaving a final sample of 55 participants (29 ABM-before and 26 ABM-after).
Procedure
Attention Bias Modification
The present study employed an adaptive variant of ABM that contained several modifications that differed from previous versions (Amir et al., 2008; MacLeod et al., 2002). First, the ABM program was a modified version of a Posner spatial cueing task (Posner, 1980). Specifically, participants were only shown one word above or below a fixation point that either cued them to disengage (negative words) or sustain (positive words) attention, and this was followed by a probe (the letter E or F) shown above or below the fixation point that they responded to with the computer mouse. Second, idiographic emotional stimuli (5 negative, 5 positive, and 5 neutral words) were used in the ABM program. To this end, prior to receiving the ABM instructions, participants were asked to generate five words that were neutral, positive, or negative in valence (15 words total). Third, in contrast to a typical ABM program where participants complete the same type of trial throughout the entire task, this variant contained multiple training components, and, within each participant, adjusted the criteria to improve their attentional bias over the course of training (see below for more details). Finally, the program trained multiple components of attention, including the ability to disengage attention from negative stimuli (negative bias), direct and sustain attention toward positive stimuli (positive bias), and attentional control in general (neutral bias). For each trial, one of these components was targeted and the overall ability to modif1y these different components was captured in the separate bias scores.
Each ABM trial began with a fixation cross presented in the center of the screen for 500 ms. Immediately following termination of the fixation cross, a neutral, positive, or negative word appeared either above or below the fixation cue for 500 ms. After presentation of the word, a probe (the letter E or F) appeared above or below the fixation cue. Participants were instructed to click the left mouse button for E and the right mouse button for F, and the letter stayed on the screen until a response had been registered. To aid participants in improving their attentional bias, both speed and accuracy of responses were emphasized. Attention was trained away from negative stimuli and toward positive stimuli by always presenting the probe in the opposite location of negative words and the same location as positive words. For neutral words, the probe appeared in the opposite and same location with equal frequency. Participants completed 722 trials that comprised various combinations of probe type (E or F), probe position (top or bottom), and word valence (positive, negative, or neutral).
In the adaptive ABM variant, participants advanced through levels based on their performance and the training consisted of two phases. In the first phase (i.e., levels 1–30), participants progressed based on their response accuracy, such that participants advanced to the next level after every seven accurate trials. For levels 1–10, participants were presented with a green or red fixation cross, followed by a neutral, positive, or negative word appearing above or below the fixation cross, and then the letter E or F appearing above or below the fixation cross. Participants were instructed that when the fixation cross was green, the letter would appear in the same location as the word, and when the fixation cross was red, the letter would appear in the opposite location as the word. The green fixation cross was always followed by a positive or neutral word, and the red fixation cross was always followed by a negative or neutral word. For levels 11–20, participants were told that the valence of the word could be used to predict where the cue would appear. Specifically, for positive words the letter would appear in the same location, for negative words the letter would appear in the opposite location, and for neutral words the letter would appear in either location. Furthermore, as participants progressed through levels 11–20, the color of the fixation cross began to fade to white, and toward the final trials the participant was completely reliant on the valence of the word to predict the location of the cue. For levels 21–30, the cue (E or F) was flanked by either congruent (i.e., EEEEE or FFFFF) or incongruent (i.e., EEFEE or FFEFF) letters, and participants were told to only respond to the middle letter. This required participants to increase their focus on the cue.
In the second phase (i.e., levels 31 and higher), participants continued to complete the same type of ABM trial as the end of the first phase (i.e., white fixation cross, using word valence to predict location of the cue, responding to the middle letter and not flanked letters). However, participants now advanced to the next level by improving their positive or negative bias by 1 ms relative to the cumulative bias of all preceding trials. The inclusion of levels was intended to motivate participants to continue improving their attention bias, and participants were able to view their level progression throughout the training. If participants completed 100 consecutive trials without advancing to the next level, the ABM training was automatically paused and the computer instructed participants to take a short break. At this time the participants’ attention bias scores were re-calibrated, such that they were reset to the highest level reached for positive words and the lowest level reached for negative words. This allowed the training to continue if participants had reached an attention bias which they could no longer surpass. Participants were able to self-resume the training when they were ready. Similar to previous ABM investigations (Amir et al., 2008), inaccurate response trials (e.g., probe was E and participant clicked right for F) and response latencies less than 200 ms or greater than 2000 ms were excluded from analyses. After 70 accurate trials the program calculated an idiographic mean and standard deviation for each participant and eliminated response latencies that were two standard deviations away from their mean response latency. These ranges were determined based on previous research.
We calculated two different attention bias scores for each participant. First, an average attention bias score was calculated using response latencies for valid (i.e., word appeared in same location as the probe) and invalid trials (i.e., word appeared in opposite location of the probe). For negative attention bias (RT negative invalid – RT neutral invalid), lower numbers indicated greater disengagement from negative, relative to neutral, trials. For positive attention bias (RT positive valid – RT neutral valid), higher numbers indicated greater engagement for positive, relative to neutral, trials. Second, a beta score was calculated that represented the average change in attention bias as a function of ABM level (i.e., trainability). The beta was calculated by regressing the attention bias score on ABM level, with the beta value representing the slope (i.e., rate of change). For the negative attention bias beta score a more negative value indicated greater disengagement from negative stimuli. Conversely, for the positive attention bias beta score a more positive value indicated greater engagement for positive stimuli.
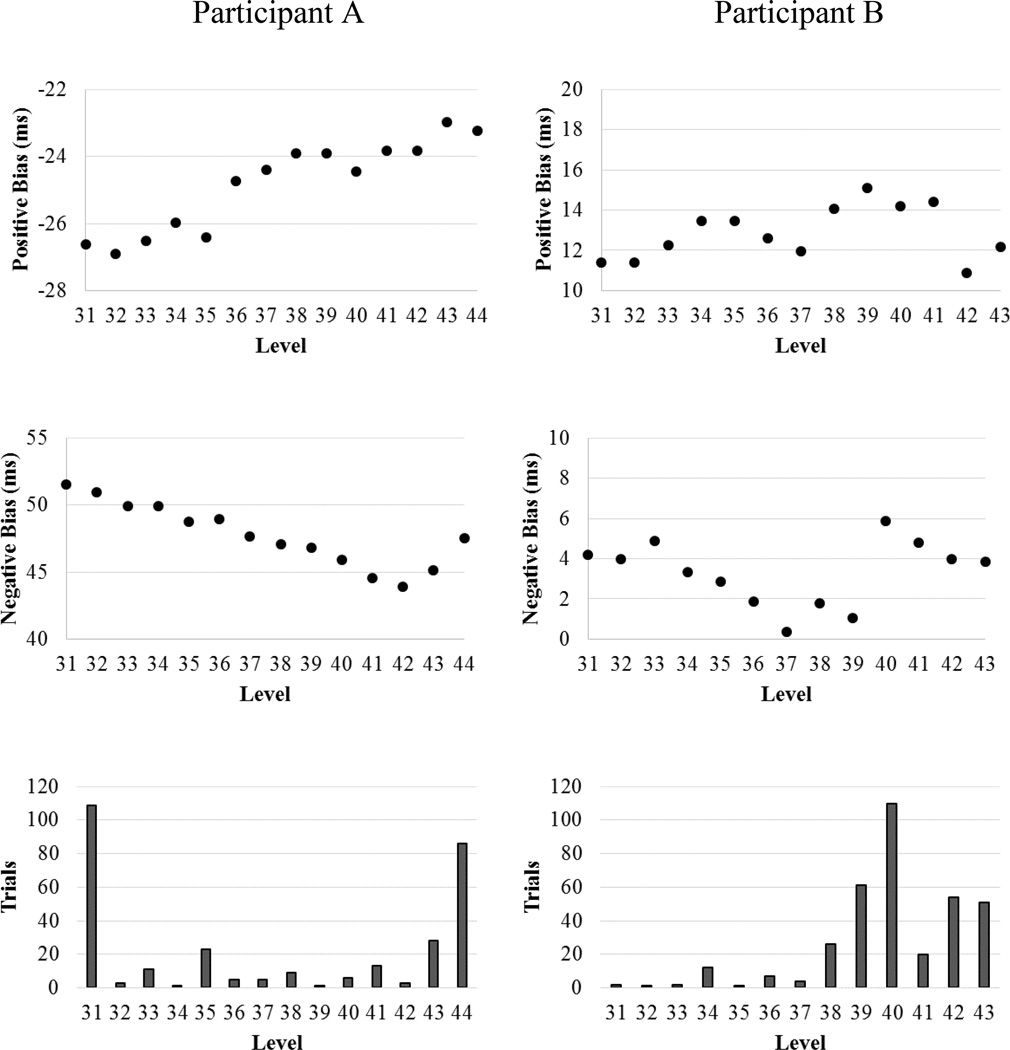
Figure 1 depicts examples of participants’ change in negative and positive attention bias as a function of ABM level. Participant A demonstrates an improved negative (i.e., values are becoming more negative) and positive (i.e., values are becoming more positive) attention bias as they progress through the ABM training. In contrast, participant B exhibits a more inconsistent pattern of change for negative and positive attention bias and thus has ‘poorer’ beta scores. Figure 1 also shows the number of trials needed for the participant to reach each successive level. Participant A required relatively few trials to advance levels, while participant B required more trials to advance levels toward the end of the training. In addition, participant B also required over 100 trials to advance from level 39 to 40, and the attention bias scores were thus re-calibrated to help the participant continue to improve their performance.
Figure 1.
Example graphical depiction of negative and positive attention bias scores as a function of level. Participant A (left column) demonstrates increased positive attention bias (middle figure; i.e., sustained attention with positive stimuli) and decreased negative attention bias (bottom figure; i.e., increased disengagement with negative stimuli) over the course of ABM training, while Participant B (right column) demonstrates minimal improvement in positive and negative attention biases. The bottom figures depit the number trials completed for each level of the ABM program.
Flanker Task
To elicit the ERN, participants completed a flanker task (Hajcak & Foti, 2008) using Presentation software (Neurobehavioral Systems Inc., Albany, CA). On each trial of the flanker task, five horizontally aligned white arrowheads were presented for 200 ms.
Participants were instructed to indicate the direction of the central arrowhead using the left or right mouse button. Half of the trials were compatible (e.g., <<<<< or >>>>>) and half were incompatible (e.g., <<><< or >><>>); trial type was randomly determined. A variable intertrial interval of 600 to 1000 ms followed the response. The arrows filled 2° of visual angle vertically and 10° horizontally, and were presented at a viewing distance of approximately 65 cm. Participants initially completed a practice block containing 20 trials, and the actual task consisted of 11 blocks of 30 trials (330 total trials).
EEG Recording and Processing
Continuous EEG was recorded using an elastic cap with 34 sintered Ag/AgCl electrode sites placed according to the international 10/20 system and two electrodes placed on the left and right mastoid. The electrooculogram was recorded from electrodes placed above and below the right eye and two placed on the outer canthus of both eyes. Data were recorded using Active Two BioSemi system (BioSemi, Amsterdam, Netherlands). The EEG was digitized with a sampling rate of 512 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 102 Hz. A common mode sense active electrode producing a monopolar (non-differential) channel was used as recording reference.
EEG data were analyzed using Brain Vision Analyzer (Brain Products, Gilching, Germany). Data were referenced offline to averaged mastoids, band-pass filtered (0.1 – 30 Hz), and corrected for blinks and horizontal eye movements (Gratton et al., 1983). Response-locked epochs with a duration of 1500 ms, including a 500 ms pre-response interval, were extracted. Epochs containing a voltage greater than 50 µV between sample points, a voltage difference of 300 µV within a segment, or a maximum voltage difference of less than 0.50 µV within 100 ms intervals were rejected. Additional artifacts were identified and removed based on visual inspection. The 500–300 ms pre-response interval was used as the baseline (Weinberg et al., 2010). Trials with response times below 200 ms and above 700 ms were excluded from averaging. Both the ERN and the negative deflection on correct trials (i.e., the correct response negativity, or CRN) were quantified as the mean amplitude between 0 and 100 ms after responses at electrode FCz, where the ERN was maximal. To isolate neural activity specific to errors, we also analyzed the difference between the ERN and CRN (i.e., ΔERN; Simons, 2010).
Results
Table 1 presents descriptive and inferential statistics for demographics and behavioral performance on the flanker task. The ABM groups did not differ in demographics, current depression, anxiety, or stress symptomatology, or the number of errors made or RT on correct or error trials, indicating comparable performance on the flanker task.
Table 1.
Demographics, ABM Bias Scores, and Flanker Task Behavior
| Group | |||
|---|---|---|---|
| ABM-before (n = 29) |
ABM-after (n = 26) |
t or χ2 | |
| Demographics | |||
| Age (years) | 20.59 (6.38) | 18.93 (0.96) | t = 1.38 |
| Sex (% Female) | 48.1% | 52.0% | χ2 = 0.01 |
| Ethnicity | χ 2 = 4.24 | ||
| Caucasian | 44.8% | 25.9% | |
| Black | 6.9% | 3.7% | |
| Hispanic | 3.4% | 14.8% | |
| Asian | 41.4% | 48.1% | |
| Other | 3.4% | 7.4% | |
| DASS-Depression | 2.34 (2.64) | 3.65 (3.30) | t = −1.64 |
| DASS-Anxiety | 2.31 (2.11) | 2.92 (2.70) | t = −0.94 |
| DASS-Stress | 4.45 (2.92) | 4.54 (3.72) | t = −0.10 |
| ABM Bias Scores | |||
| Average Bias (ms) | |||
| Positive | −26.56 (42.75) | −27.48 (34.21) | t = 0.09 |
| Negative | −1.34 (34.47) | −3.45 (22.20) | t = 0.26 |
| Change in Bias (β) | |||
| Positive | .19 (0.61) | .28 (0.66) | t = −0.49 |
| Negative | .13 (0.76) | .23 (0.74) | t = −0.48 |
| Flanker Task Behavior | |||
| Accuracy (%) | 88.73 (3.72) | 87.99 (4.62) | t = 0.66 |
| Correct RT (ms) | 387.63 (37.69) | 394.44 (31.71) | t = −0.32 |
| Error RT (ms) | 326.17 (45.08) | 329.56 (30.76) | t = −0.71 |
Note. Standard deviations are presented in parentheses. ABM = attention bias modification; DASS = Depression Anxiety Stress Scale; ms = milliseconds; RT = reaction time.
The ERN was a negative deflection in the ERP response that peaked at frontocentral electrodes approximately 50 ms after the commission of an error. A repeated-measures analysis of variance (ANOVA) confirmed that the ERP response was more negative following errors (M = 0.46, SD = 4.76) relative to correct responses (M = 7.56, SD = 4.15), F(1, 54) = 125.43, p < .001, ηp2 = .70.
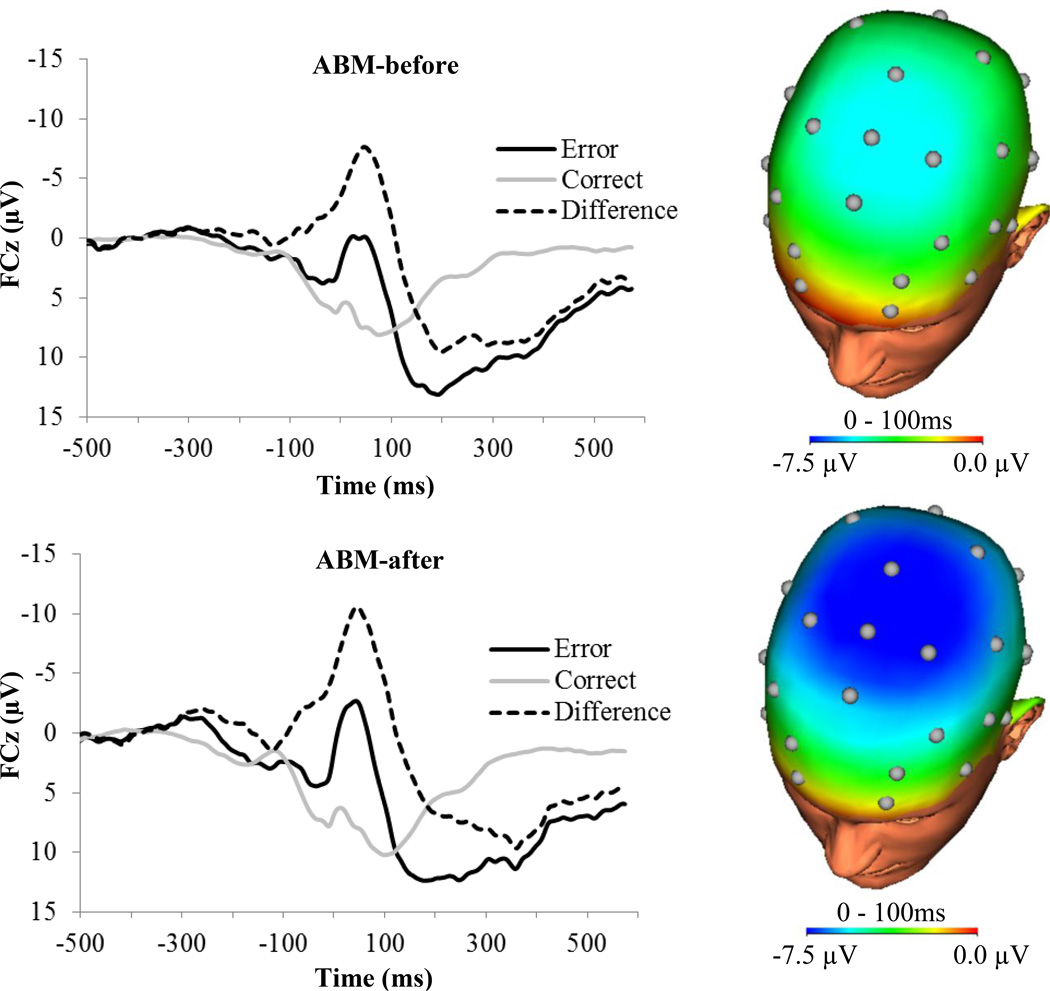
As shown in Figure 2, results indicated that ABM-before (M = −5.82, SD = 4.17), relative to ABM-after (M = −8.53, SD = 4.92), participants had a significantly smaller (i.e., less negative) ΔERN, F(1, 53) = 4.87, p < .05, ηp2 = .08. These results suggest that completing the ABM program prior to the flanker task was associated with less error-related brain activity.
Figure 2.
ERP waveforms and head maps for ABM-before (top) and ABM-after (bottom) participants. The difference waveforms and head maps represent error minus correct trials. Head maps display the average activity between 0–100 ms after response. ABM = attention bias modification; ERP = event-related potential.
Next, we examined whether individual differences in bias scores were associated with the ΔERN.1 In the analysis of ABM bias scores, two additional participants (1 ABM-before and 1 ABM-after) were excluded due to insufficient advancement through ABM training needed to calculate bias scores. Average positive and negative attention bias and positive attention bias beta scores were normally distributed. However, negative attention bias beta scores demonstrated a bimodal distribution, and participants were generally split into those who were “successful” vs. “unsuccessful” in modifying their negative attention bias over the course of the ABM program. Therefore, Pearson’s correlations were conducted to examine the relationship between average positive and negative attention bias and change in positive attention bias and the ΔERN. Spearman’s ρ correlations were conducted in order to examine relationships between change in negative attention bias and the ΔERN.
Across all participants neither average negative nor positive attention bias was associated with the ΔERN (ps > .77). However, change in negative attention bias (i.e., beta score) was negatively associated with the ΔERN, ρ(53) = −31, p < .05, such that greater attentional disengagement from negative stimuli over the course of ABM training was associated with smaller error-related brain activity.2 When examined separately, the association between change in negative attention bias and the ΔERN approached significance in the ABM-before, ρ(28) = −.29, p < .13, and ABM-after participants, ρ(26) = −.32, p < .11. Change in positive attention bias was not associated with the ΔERN (ps > .15).
Discussion
The present study examined the association between ABM and the ERN. Results indicated that the ERN was smaller among participants who first completed ABM. Furthermore, individual differences in change in negative attention bias were associated with a smaller ERN across all participants. In other words, those individuals who were most able to learn to disengage their attention from negative stimuli were characterized by a smaller (i.e., less negative) ERN. This study provides novel data on two fronts. First, results suggest that the ERN may be modified using ABM—even during a single session. However, future research is needed to determine whether changes in the ERN mediate anxiety-related outcomes in ABM. Second, results also suggest that the ERN is associated with ABM success. Therefore, the ERN may be both a mechanism and predictor of ABM-related changes in attention bias toward threat.
ABM trains individuals to automatically disengage attention from threat-relevant information, and fMRI studies have indicated that ABM increases PFC activation during subsequent emotional processing (Browning et al., 2010; Taylor et al., 2014). It is possible that ABM-related training improves top-down regulation of affective responding to threat, which includes error commission. Indeed, the ERN has been shown to be sensitive to the motivational salience of errors, such that the ERN is enhanced when errors are punished (Riesel et al., 2012), performance is evaluated (Hajcak et al., 2005; Kim et al., 2005), or accuracy is emphasized over speed (Falkenstein et al., 2000; Gehring et al., 1993). Thus, by increasing PFC activation, ABM may down-regulate the neural response to errors, reflected in a smaller ERN.
It is important to consider these findings in the context of several important limitations and alternative interpretations. First, the present study used a between-subjects design and did not include a within-subjects assessment of the ERN (i.e., pre-test/post-test design). Therefore, the smaller ERN in the ABM-before group may have been due to their treatment expectations (i.e., believing that completing ABM would make them feel less anxious) and not necessarily change in attention bias. Moreover, groups differed in terms of when the ERN was collected (i.e., early vs. late) during the experimental session. Prolonged task engagement and mental fatigue have both been shown to reduce the ERN (Boksem et al., 2006; Lorist et al., 2005; Scheffers et al., 1999); although in all these studies the ERN reduction was accompanied by impoverished behavioral performance. In the present study, ABM did not impact behavioral measures on the flankers task (i.e., RT or number of errors), suggesting comparable task engagement. Second, there was no placebo control condition and it is possible that the smaller ERN in the ABM-before participants may have been due to completing a cognitive task in general. Future studies should test the impact of ABM versus a comparable cognitive control condition on the ERN. Third, attention bias scores have been shown to demonstrate poor psychometric properties, including internal consistency and test-retest reliability (Cisler et al., 2009; Schmukle, 2005; Waechter & Stolz, in press; Waechter et al., 2014), and this may have contributed to the different pattern of results for average attention bias (i.e., no relationship) vs. change in negative bias (i.e., a negative correlation) in relation to the ERN. These findings require replication given the many issues associated with the measurement of attention bias and the present study’s use of a single-session of ABM. Future studies might also consider using neural measures of attention bias (e.g., N2pc), which have demonstrated better psychometric properties relative to behavioral measures (Kappenman, Farrens, et al., 2014; Kappenman, MacNamara, et al., 2014). Fourth, the present study was conducted in college undergraduates, and it is unclear if the results will generalize to other populations (e.g., children). Furthermore, our attempt to motivate participants through the advancement of attention bias ‘levels’ may not have been particularly effective in a non-treatment seeking sample, and it is possible this approach may be more useful in clinical populations (e.g., anxiety disorders). Fifth, the association between change in negative attention bias and the ERN was present in the entire sample and only approached significance when examined separately in the ABM-before and ABM-after participants. However, this was likely due to decreased power after splitting the sample in half. Finally, it is possible that the relationship between the ERN and change in negative attention bias in both groups may simply reflect the same phenomenon: individuals with a larger (i.e., more negative) ERN are not able to change their attention bias toward threat. This would be consistent with previous studies suggesting that slowed threat extinction may be an etiological factor in the development of anxiety (Hermann et al., 2002; Lissek et al., 2005; Sehlmeyer et al., 2011).
The present study has important implications for the conceptualization of the ERN as a biomarker. Specifically, the ERN has been proposed to be a trait-like neural indicator of threat sensitivity (Weinberg, Riesel, et al., 2012). Indeed, the ERN has been shown to be stable across two weeks (Olvet & Hajcak, 2009) and two years (Weinberg & Hajcak, 2011), associated with several genotypes (Manoach & Agam, 2013), and is moderately heritable (Anokhin et al., 2008). In addition, the ERN is enhanced among first-degree relatives of individuals with OCD (Carrasco et al., 2013; Riesel et al., 2011), and prospectively predicts the onset of anxiety disorders (Meyer et al., in press). These results support the ERN as a trait-like risk factor for anxiety disorders (Olvet & Hajcak, 2008).
However, the ERN has also been shown to be sensitive to state effects. For example, the ERN is attenuated by alcohol (Ridderinkhof et al., 2002) and psychotropic medication (De Bruijn et al., 2004; de Bruijn et al., 2006; Zirnheld et al., 2004) and enhanced by amphetamines (De Bruijn et al., 2004), caffeine (Tieges et al., 2004), stimulants (Riba et al., 2005), and contextual threat manipulations (Jackson et al., 2015; Riesel et al., 2012). Interestingly, both cognitive-behavioral therapy and mindfulness-based cognitive therapy have not impacted the ERN (Hajcak et al., 2008; Schoenberg et al., 2014), and the present study is one of the first to suggest modulation of the ERN using a behavioral intervention that targets a core mechanism of dysfunction in anxiety disorders. These results are consistent with previous studies indicating that ABM impacts vulnerability to anxiety (Amir et al., 2008; MacLeod et al., 2002), and highlights the ERN as a potential modifiable biomarker of risk for anxiety disorders.
In conclusion, the present study suggests a close relationship between the ERN and the malleability of negative attention biases—such that improvement in negative attention bias may attenuate the ERN, and that the ERN may be associated with how much negative attention bias changes in response to ABM. Future studies are needed to determine whether ABM reduces ERN in clinical populations, and if there is a dose-dependent relationship between ABM and the ERN. Finally, prospective studies are needed to determine whether ABM-related changes in ERN mediate the impact of ABM on symptom improvement, and whether these changes remain stable over time. Overall, the present study adds to a growing literature indicating that ABM may be able to modify neural measures of attentional processing (Eldar & Bar-Haim, 2010; Suway et al., 2013).
Acknowledgements
N.A. is the co-founder of a company that markets attention bias modification programs; however, the specific implementation described in this article is not available for purchase through the company. This project was supported by the National Institutes of Health grant R01MH087623 awarded to N.A.
Footnotes
The negative attention bias beta scores were negatively associated with the initial negative bias (calculated after level 10) at a trend level, r(53) = −.27, p < .06, such that a greater initial negative attention bias was associated with a greater decrease in negative attention bias across training. In contrast, the positive attention bias beta score was not associated with initial positive attention bias, r(53) = −.17, ns. Both negative and positive attention bias beta scores were negatively associated with neutral attention bias beta scores, r(53) = −.41, p < .01, r(53) = −32, p < .05, respectively. Importantly, none of the initial attention bias scores (neutral, positive, or negative) or change in neutral attention bias were associated with the ΔERN (ps > .25).
We also examined change in attention bias using a simple difference score that subtracted average bias during the first half of trials from that during the second half of trials. The positive and negative attention bias difference scores were normally distributed, and Pearson’s correlations were conducted to examine their relationship with the ΔERN. For the negative attention bias difference score a more negative value indicated greater disengagement from negative stimuli, and for positive attention bias difference scores a more positive value indicated greater engagement for positive stimuli. Results indicated the negative attention bias difference score was negatively associated with the ΔERN at a trend level, r(53) = −.23, p < .10, but the positive attention bias difference score was not associated with the ΔERN, r(53) = .08, ns. Thus, the attention bias difference scores produced a similar pattern of results, although only at trend level significance, as the beta scores.
References
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118:28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, Chen X. Attention training in individuals with generalized social phobia: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77:961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: Facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41:1325–1335. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Amir N, Taylor CT. Combining computerized home-based treatments for generalized anxiety disorder: An attention modification program and cognitive behavioral therapy. Behavior Therapy. 2012;43:546–559. doi: 10.1016/j.beth.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. Journal of Abnormal Psychology. 2008;117:860–868. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43:724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Clark DA. An information processing model of anxiety: Automatic and strategic processes. Behaviour Research and Therapy. 1997;35:49–58. doi: 10.1016/s0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Boksem MAS, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biological Psychology. 2006;72:123–132. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry. 2010;67:919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depression and Anxiety. 2013;30:39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology, Paris. 2014 doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: A critical review. Cognitive Therapy and Research. 2009;33:221–234. doi: 10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clarke PJF, Browning M, Hammond G, Notebaert L, Macleod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: Evidence from transcranial direct current stimulation. Biological Psychiatry. 2014;76:946–952. doi: 10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- De Bruijn ERA, Hulstijn W, Verkes RJ, Ruigt GSF, Sabbe BGC. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology. 2004;177:151–160. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- De Bruijn ERA, Sabbe BGC, Hulstijn W, Ruigt GSF, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Research. 2006;1105:122–129. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. 2010;40:667–677. doi: 10.1017/S0033291709990766. [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46:1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G. What we’ve learned from mistakes: insights from error-related brain activity. Current Directions in Psychological Science. 2012;21:101–106. [Google Scholar]
- Hajcak G, Foti D. Errors are aversive: defensive motivation and the error-related negativity. Psychological Science. 2008;19:103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hermann C, Ziegler S, Birbaumer N, Flor H. Psychophysiological and subjective indicators of aversive Pavlovian conditioning in generalized social phobia. Biological Psychiatry. 2002;52:328–337. doi: 10.1016/s0006-3223(02)01385-9. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, Proudfit GH. In an uncertain world, errors are more aversive: Evidence from the ERN. Emotion. 2015;15:12–16. doi: 10.1037/emo0000020. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, Dietrich DE. Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Research - Neuroimaging. 2001;108:101–110. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Farrens JL, Luck SJ, Proudfit GH. Behavioral and ERP measures of attentional bias to threat in the dot-probe task: poor reliability and lack of correlation with anxiety. Frontiers in Psychology. 2014;5:1–9. doi: 10.3389/fpsyg.2014.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, MacNamara A, Proudfit GH. Electrocortical evidence for rapid allocation of attention to threat in the dot-probe task. Social Cognitive and Affective Neuroscience. 2015;10:577–583. doi: 10.1093/scan/nsu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T. Error-related negativity in children: effect of an observer. Developmental Neuropsychology. 2005;28:871–883. doi: 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Amir N. Preliminary study of attention training to threat and neutral faces on anxious reactivity to a social stressor in social anxiety. Cognitive Therapy and Research. 2010;34:263–271. [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Boksem MAS, Ridderinkhof KR. Impaired cognitive control and reduced cingulate activity during mental fatigue. Cognitive Brain Research. 2005;24:199–205. doi: 10.1016/j.cogbrainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. Psychology Foundation of Australia. 1995;56 [Google Scholar]
- Macleod C, Clarke PJF. The attentional bias modification approach to anxiety intervention. Clinical Psychological Science. 2015;3:58–78. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986 doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Manoach DS, Agam Y. Neural markers of errors as endophenotypes in neuropsychiatric disorders. Frontiers in Human Neuroscience. 2013;7:350. doi: 10.3389/fnhum.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22:539–560. [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Proudfit GH, Torpey DC, Kujawa A, Klein DN. Increased error-related brain activity in children predicts the onset of anxiety disorders three years later. Journal of Abnormal Psychology. in press doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective attention and anxiety: A cognitive-motivational perspective. Handbook of Cognition and Emotion. 1999:145–170. [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in Human Neuroscience. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmi S, Amir N. The effect of attention training on a behavioral test of contamination fears in individuals with subclinical obsessive-compulsive symptoms. Journal of Abnormal Psychology. 2010;119:136–142. doi: 10.1037/a0017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH. NIMH Research Domain Criteria (RDoC) 2011 Retrieved from http://www.nimh.nih.gov/research-priorities/rdoc/nimh-research-domain-criteria-rdoc.shtml.
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Research. 2009;1284:89–99. doi: 10.1016/j.brainres.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, Dahl RE, Amir N. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment. 2015;27:365–376. doi: 10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Morte A, Münte TF, Barbanoj MJ. Noradrenergic stimulation enhances human action monitoring. Journal of Neuroscience. 2005;25:4370–4374. doi: 10.1523/JNEUROSCI.4437-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GPH. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. American Journal of Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49:239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Humphrey DG, Stanny RR, Kramer AF, Coles MG. Error-related processing during a period of extended wakefulness. Psychophysiology. 1999;36:149–157. [PubMed] [Google Scholar]
- Schmukle SC. Unreliability of the dot probe task. European Journal of Personality. 2005;19:595–605. [Google Scholar]
- Schoenberg PLA, Hepark S, Kan CC, Barendregt HP, Buitelaar JK, Speckens AEM. Effects of mindfulness-based cognitive therapy on neurophysiological correlates of performance monitoring in adult attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2014;125:1407–1416. doi: 10.1016/j.clinph.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Pfleiderer B, Konrad C. Neural correlates of trait anxiety in fear extinction. Psychological Medicine. 2011;41:789–798. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews: Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RF. The way of our errors: Theme and variations. Psychophysiology. 2010;47:1–14. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Stemmer B, Segalowitz SJ, Witzke W, Schönle PW. Error detection in patients with lesions to the medial prefrontal cortex: An ERP study. Neuropsychologia. 2004;42:118–130. doi: 10.1016/s0028-3932(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Suway JG, White LK, Vanderwert RE, Bar-Haim Y, Pine DS, Fox NA. Modification of threat-processing in non-anxious individuals: A preliminary behavioral and ERP study. Journal of Behavior Therapy and Experimental Psychiatry. 2013;44:285–292. doi: 10.1016/j.jbtep.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CT, Aupperle RL, Flagan T, Simmons AN, Amir N, Stein MB, Paulus MP. Neural correlates of a computerized attention modification program in anxious subjects. Social Cognitive and Affective Neuroscience. 2014;9:1379–1387. doi: 10.1093/scan/nst128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieges Z, Richard Ridderinkhof K, Snel J, Kok A. Caffeine strengthens action monitoring: Evidence from the error-related negativity. Cognitive Brain Research. 2004;21:87–93. doi: 10.1016/j.cogbrainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Waechter S, Nelson AL, Wright C, Hyatt A, Oakman J. Measuring attentional bias to threat: Reliability of dot probe and eye movement indices. Cognitive Therapy and Research. 2014;38:313–333. [Google Scholar]
- Waechter S, Stolz JA. Trait anxiety, state anxiety, and attentional bias to threat: Assessing the psychometric properties of response time measures. Cognitive Therapy and Research. in press [Google Scholar]
- Weinberg A, Hajcak G. Longer term test-retest reliability of error-related brain activity. Psychophysiology. 2011;48:1420–1425. doi: 10.1111/j.1469-8986.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology. 2012;121:885–896. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Hajcak G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion. 2012;36:85–100. [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, Fromson JA. Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll Ca, Kieffaber PD, O’Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience. 2004;16:1098–1112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]