Abstract
Primitive erythropoiesis is regulated in a non cell-autonomous fashion across evolution from frogs to mammals. In Xenopus laevis, signals from the overlying ectoderm are required to induce the mesoderm to adopt an erythroid fate. Previous studies in our lab identified the transcription factor GATA2 as a key regulator of this ectodermal signal. To identify GATA2 target genes in the ectoderm required for red blood cell formation in the mesoderm, we used microarray analysis to compare gene expression in ectoderm from GATA2 depleted and wild type embryos. Our analysis identified components of the non-canonical and canonical Wnt pathways as being reciprocally up- and down-regulated downstream of GATA2 in both mesoderm and ectoderm. We show that up-regulation of canonical Wnt signaling during gastrulation blocks commitment to a hematopoietic fate while down-regulation of non-canonical Wnt signaling impairs erythroid differentiation. Our results are consistent with a model in which GATA2 contributes to inhibition of canonical Wnt signaling, thereby permitting progenitors to exit the cell cycle and commit to a hematopoietic fate. Subsequently, activation of non-canonical Wnt signaling plays a later role in enabling these progenitors to differentiate as mature red blood cells.
Keywords: GATA2, Wnt, primitive hematopoiesis, non cell-autonomous signals, Xenopus laevis
Introduction
During vertebrate hematopoiesis, stem cells differentiate along one of the various blood lineages (Drevon and Jaffredo, 2014). The first blood progenitors arise from mesodermal cells that are specified with a hematopoietic fate during gastrulation. These cells will differentiate predominantly as red blood cells (RBCs) several days later in a process termed primitive hematopoiesis. A second phase of blood development, definitive hematopoiesis, generates hematopoietic stem cells (HSCs) that give rise to progenitors of the erythroid, lymphoid and myeloid lineages.
Signals from the microenvironment, or niche, that determine the balance between quiescence, proliferation and differentiation of HSCs during adult hematopoiesis are similarly required for primitive hematopoiesis. Tissue recombination studies have shown that non-hematopoietic cells must send a signal to the nascent mesoderm during gastrulation in order for it to form blood (Belaoussoff et al., 1998; Maeno et al., 1994). In Xenopus, ectodermal cells provide this signal. When ectoderm is physically separated from the hematopoietic ventral mesoderm in Xenopus embryos, RBCs fail to form despite the normal differentiation of other mesodermal cell types (Kikkawa et al., 2001).
Although the precise molecular nature of the signal(s) transmitted by ectodermal cells during primitive blood development is unknown, it is known that bone morphogenetic proteins (BMPs) are needed to generate this signal. BMPs are required for ventral patterning of all germ layers, and RBCs fail to develop when BMP signal reception is blocked in either mesodermal or ectodermal cells (Kumano et al., 1999; Walters et al., 2001). It has been suggested that BMPs secreted from the ectoderm provide the signal that enables mesoderm to form blood (Kumano et al., 1999; Maeno, 2003; Tran et al., 2010), However, activation of the intracellular BMP signaling cascade is not sufficient to rescue erythropoiesis in isolated ventral mesoderm (Dalgin et al., 2007).
Wnt signaling is also required for primitive blood development. Wnts signal via two general pathways, termed canonical and non-canonical (Chien et al., 2009). Canonical Wnt signaling is associated with progenitor cell proliferation whereas non-canonical Wnt signaling is often necessary for exit from pluripotency and progenitor cell specification. In Xenopus, Wnt4 signals from mesodermal cells during gastrulation to activate the canonical Wnt cascade in the overlying ectoderm, and possibly also the mesoderm, and this is required for primitive blood formation (Tran et al., 2010).
The transcription factor GATA2 functions cell-autonomously in blood progenitors during erythropoiesis (Tsai et al., 1994), but is also required in ectodermal cells of Xenopus embryos for primitive blood formation (Dalgin et al., 2007). Epistasis analysis in whole embryos and explants support a model in which BMPs signal to ectodermal cells to activate transcription of GATA2, and GATA2 subsequently induces expression of a secondary signaling molecule(s) that is essential for mesoderm to form blood (Dalgin et al., 2007). The current studies explore the nature of the signals that are induced by GATA2.
Materials and Methods
Embryo culture and manipulation
Embryos were obtained, microinjected, and cultured as described (Mimoto and Christian, 2012). Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). Ectoderm or mesoderm explants were dissected with watchmakers’ forceps and cultured independently or as recombinants as described previously (Goldman et al., 2006). Ectoderm removal assays were performed by removing the vitelline coat with watchmakers' forceps, dissecting away the dorsal half of the embryo and then peeling off the ectoderm using tungsten needles or an eyebrow knife. Stage 13 embryos were incubated in 0.005% Trypsin for 1–5 minutes prior to ectoderm removal and then transferred to 0.02% trypsin inhibitor for 20 minutes prior to culture. The vitelline coat was removed from late gastrula stage embryos using watchmakers' forceps, the ventral half was removed using tungsten needles and then separated into ectodermal and mesendodermal fragments using an eyebrow knife. Transient activation of the canonical Wnt pathway was achieved by culture of whole embryos in 0.25 M LiCl in 0.5× Modified Barth’s Saline (MBS) for twenty minutes followed by 4–5 washes in 0.5× MBS. Double label in situ hybridization assays were performed as described (Harland, 1991) except that the vitelline coat was not removed prior to fixation and probes were detected using BM purple as a substrate.
Microarray Analysis
To generate samples for the microarray analysis, FOG RNA (250 pg), GATA2 RNA (250 pg) or GATA2 anti-sense morpholino oligonucleotides (MOs) (20 ng) (Dalgin et al., 2007) was injected into each cell near the animal pole of embryos at the two-cell stage. Embryos were allowed to develop to stage 10, at which point ectoderm was removed using watchmakers’ forceps and cultured to stage 12 in 0.5× NAM. Approximately 40 ectodermal explants from a single injection condition were pooled and RNA samples were generated for microarray analysis according to standard protocols provided by the manufacturer (Affymetrix). Briefly, RNA was extracted using Trizol (Invitrogen) and purified with an RNA clean-up kit (QIAGEN). RNA samples were sent to the Oregon Health & Science University Affymetrix Microarray Core facility for further processing and hybridization to the microarrays. Three biological replicates collected during three separate days of experiments were used for each of the four microarray conditions. Array probe levels were summarized using the Robust Multiarray Analysis RMA method (Irizarry et al., 2003a; Irizarry et al., 2003b), implemented in the Affymetrix package under BioConductor in the R programming language. A second level of probeset normalization was performed using the Global Rank-Invariant Set Normalization (GRSN) method (Pelz et al., 2008). Hierarchical clustering based on a Pearson’s correlation coefficient was performed as an unsupervised method to identify outliers. Based on this analysis, Uninjected #3 was identified as a significant outlier and was removed from subsequent analysis. A batch adjustment was performed using an internal method based on Distance Weighted Discrimination (DWD) (Benito et al., 2004), which was developed by our statistician, Carl Pelz. This analysis was used to correct for a batch effect from the different biological replicates, likely due to the fact that Xenopus laevis is not isogenic, creating a significant degree of background variability between the biological replicates that was unrelated to our scientific question. To facilitate analysis of a smaller sample size, the eBayes method (implemented through (Smyth, 2004) was used to identify up and down-regulated genes. Genes that showed changes +/− 1.2-fold were considered potentially significant.
Analysis of RNA
For Northern blotting, total RNA was isolated and analysis was performed as described previously (Christian et al., 1991). Bands were quantified using the NIH ImageJ software. For quantitative reverse transcription PCR (qPCR) analysis, total RNA was isolated using Trizol (Invitrogen) according to the manufacturer’s instructions, from which cDNA was generated using the AMV Reverse Transcriptase First-strand cDNA Synthesis Kit (Life Sciences, Inc.) with a poly d(T) primer. qPCR was performed using a SYBR Green-based assay (QIAGEN) and a 7900 HT Sequence Detector (ABI). Each sample was analyzed in triplicate and normalized to the housekeeping gene, ornithine decarboxylase (ODC). Forward (F) and reverse (R) primers used for PCR are listed in Table 1. Tm used was between 58 and 60°C.
Morpholinos and cDNA constructs
GATA2 (Dalgin et al., 2007) morpholino antisense oligonucleotides (MOs) were purchased from Gene Tools, LLC (Philomath, OR), along with a standard control MO. All cDNAs were subcloned into pCS2+ for RNA transcription.
Results and Discussion
Ectodermal signals required for blood differentiation in the mesoderm are transmitted by the end of gastrulation
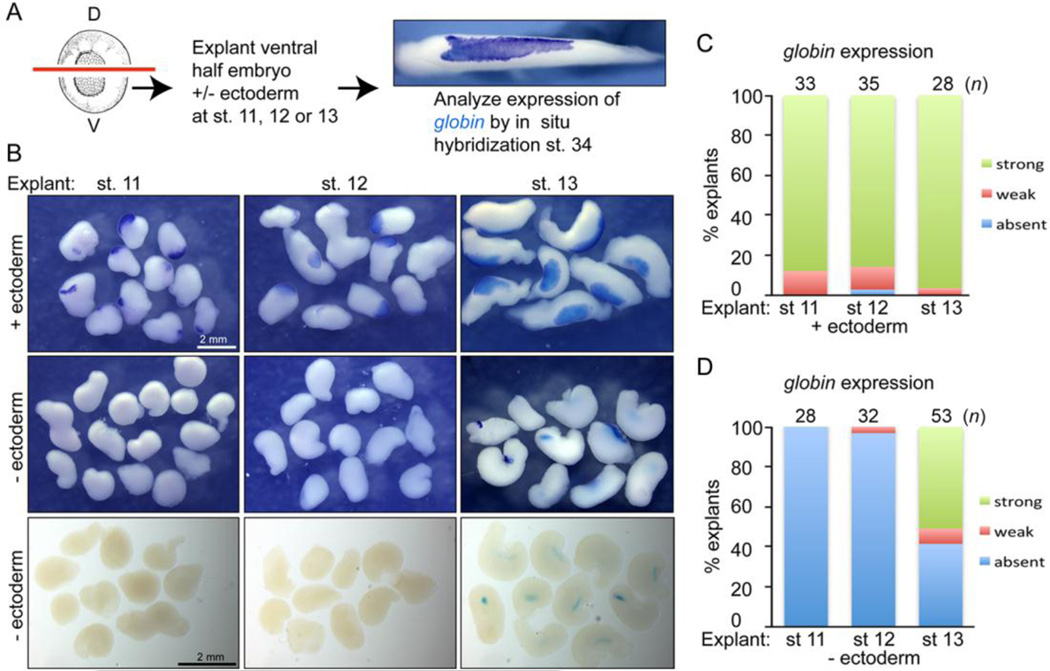
In order to identify GATA2 targets in the ectoderm that might be required for blood development, we first wanted to determine the developmental window during which ectoderm is required for primitive erythropoiesis. To do this, the ventral half of embryos, which gives rise to the majority of RBCs in the VBI (Ciau-Uitz et al., 2010; Maeno et al., 2012), was explanted and ectoderm was either retained or removed at successive stages of development, from mid-gastrulation (stage 11) through the end of gastrulation (stage 13). The ventral explants were cultured until wild type siblings reached the tailbud stage (stage 34) and then assayed by whole mount in situ hybridization (WMISH) for expression of globin, a marker of differentiated RBCs (assay illustrated in Fig. 1A). Expression of globin was detected in nearly all explants cultured in the presence of ectoderm (Fig. 1B, top row, Fig. 1C). By contrast, expression of globin was absent in ventral explants in which ectoderm was removed at stages 11 and 12 (Fig. 1B, middle and bottom rows, Fig. 1D). When ectoderm was removed at stage 13, expression of globin was detected in 50–70% of ventral explants in three independent experiments (Fig. 1B, middle and bottom rows, Fig. 1D). Whereas globin expressing mesoderm was located near the superficial surface of explants cultured in the presence of ectoderm (Fig. 1B, top row), it was present at a more internal location in explants from which the ectoderm was removed at stage 13, and was more readily visible in cleared explants (Fig. 1B, bottom row). The same result was obtained when globin expression was analyzed by qPCR (Fig. S1). These results demonstrate that the ventral mesoderm does not have the capacity to form RBCs in the absence of ectoderm through stage 12. However, by stage 13, when mesodermal cells become committed to the primitive hematopoietic lineage (Turpen et al., 1997), the requisite ectodermal signals have been transmitted and at least a subset of mesodermal cells are competent to form RBCs.
Figure 1. Signals from the ectoderm are required to induce blood formation in the mesoderm during gastrulation.
(A) Illustration of ectoderm removal assay. The ventral half of embryos was isolated and ectoderm was either retained or removed from explants at stages 11, 12 or 13. Explants were allowed to develop until intact siblings reached the tailbud stage (stage 34) and assayed for expression of globin by WMISH. (B) Photographs of representative globin stained explants mounted in PBS (top two rows) or in Murrays clear (bottom row). (C, D) Quantitation of percent of explants expressing globin when cultured in the presence (C) or absence (D) of ectoderm. Results are pooled from 2–3 independent experiments for each group.
Microarray analysis identifies ectodermal GATA2 targets
Although ectodermal GATA2 is essential for erythropoiesis (Dalgin et al., 2007), it is a transcription factor and thus cannot directly mediate a signal transmitted from ectoderm to hematopoietic mesoderm. To identify genes downstream of GATA2 that are expressed in the ectoderm during the window of development in which ectoderm is required for erythropoiesis, we used microarray analysis to compare gene expression profiles from late gastrula stage wild type and GATA2 depleted ectoderm. Expression of GATA2 was knocked down by injection of previously characterized GATA2 morpholino antisense oligonucleotides (MOs) (Dalgin et al., 2007). Ectodermal explants were dissected at the onset of gastrulation (stage 10), and cultured until sibling embryos reached the late gastrula stage (stage 12). The ectodermal explants were then harvested and RNA was isolated for hybridization to a microarray (illustrated in Fig. 2A).
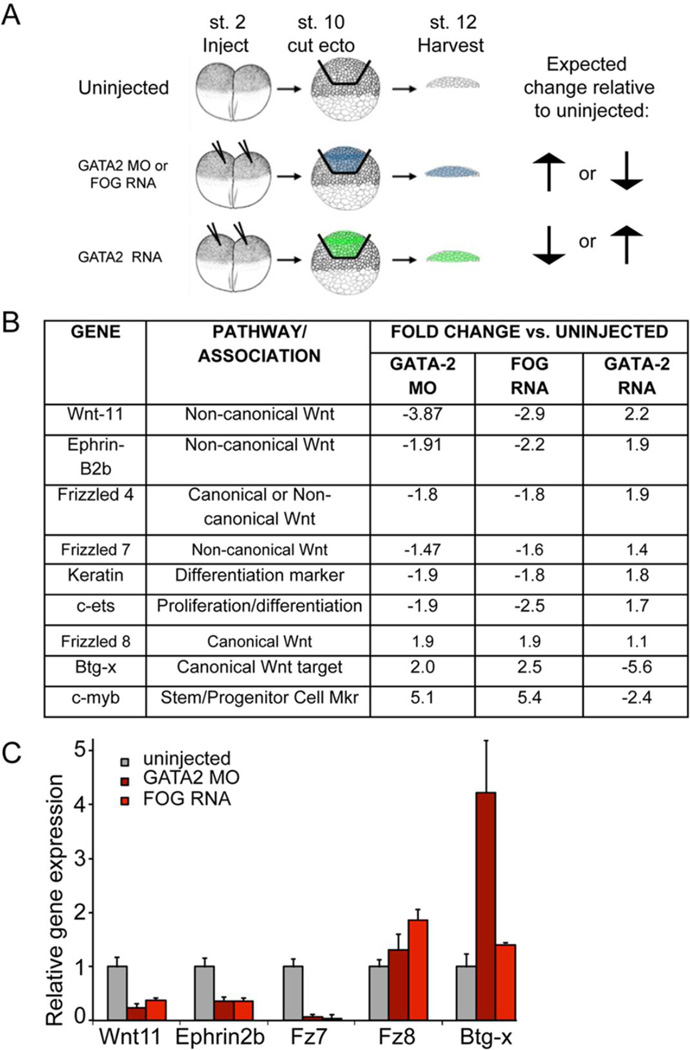
Figure 2. Microarray approach to identify ectodermal GATA2 targets required for blood formation in the mesoderm.
(A) Schematic of microarray strategy and sample acquisition. Embryos were injected at the two-cell stage with GATA2 MO (40 ng), FOG RNA (500 pg) or GATA2 RNA (500 pg) and cultured to the early gastrula stage (stage 10). Ectoderm was explanted and cultured to stage 12, at which point ectodermal explants in each group were pooled and RNA was extracted for microarray analysis. (B) Genes identified by microarray analysis that are predicted to be relevant for hematopoiesis (C) qPCR analysis of gene expression in ectodermal explants isolated at stage 10 from embryos injected with GATA2 MOs or FOG RNA and cultured to stage 12. Quantification of relative gene expression (mean +/− SD) in three independent experiments is shown.
Because Xenopus laevis is not an isogenic model system, we anticipated a high degree of background variability. We therefore used two additional experimental conditions to refine our array data and to increase the specificity of our list of candidate genes. First, we analyzed gene expression in ectoderm isolated from Friend of GATA (FOG) RNA injected embryos. We have shown that overexpression of FOG in the mesoderm dominantly interferes with GATA function, causing disruption of erythropoiesis (Mimoto and Christian, 2012). Similarly, FOG overexpression phenocopies GATA2 knockdown in the ectoderm, as expression of globin was reduced in recombinants consisting of wild type mesoderm recombined with ectoderm isolated from embryos injected with 500 pg of FOG RNA relative to wild type mesoderm recombined with control ectoderm (Fig. S2). Thus, we would predict that changes in gene expression in FOG overexpressing ectoderm would mirror those observed in GATA2 depleted ectoderm. Second, we analyzed ectoderm isolated from embryos injected with 500 pg of GATA2 mRNA. This serves as a control for non-specific effects of injection. In addition, if overexpressed GATA2 is sufficient to induce target gene expression, then genes that show parallel changes in expression in GATA2 MO and FOG RNA injected embryos would be expected to show changes in expression in the opposite direction upon overexpression of GATA2 (illustrated by arrows in Fig. 2A). We used these predicted relationships to execute a preliminary screen for candidate genes in the ectoderm that might play a role in regulating erythropoiesis. Employing these criteria, we identified 750 genes that showed a greater than 1.2-fold change and 150 genes that showed a greater than two-fold change in the predicted directions for at least two conditions. A list of candidate genes relevant to this study, along with the foldchange relative to uninjected controls, is presented in Fig. 2B. The full results of the microarray will be presented elsewhere.
Our microarray data yielded several interesting trends, which were used to guide further analysis. First, several genes associated with the non-canonical and canonical Wnt pathways appeared to be reciprocally regulated downstream of GATA2 (Fig. 2B). Specifically, genes in the non-canonical Wnt pathway were down regulated in GATA2 morphant and FOG overexpressing ectoderm, but were up regulated by GATA2 overexpression. For example, genes identified that were positively regulated by GATA2 include Wnt11 and Fz7, which signal together to activate non-canonical Wnt pathways (Yu et al., 2012), Fz4, which can activate both non-canonical and canonical Wnt pathways (Ye et al., 2009; Yu et al., 2010), and Ephrins, which activate non-canonical Wnt signaling through interaction with Disheveled (Lee et al., 2006; Tanaka et al., 2003). In contrast, several genes associated with the canonical Wnt pathway were up regulated in GATA2 morphants and in FOG overexpressing embryos but were negatively regulated by GATA2 overexpression. Genes identified that were negatively regulated by GATA2 included Fz8, which activates canonical Wnt signaling (Sheldahl et al., 1999) and Btg-x, which is a canonical Wnt target gene (Mann et al., 1999; Wessely et al., 2005).
A second observation was that c-myb, a gene associated with proliferation and progenitor maintenance, was upregulated in GATA2 morphant ectoderm. c-myb must be downregulated to promote exit from the progenitor state and allow for differentiation during definitive hematopoiesis (Bresson-Mazet et al., 2008). Induction of this progenitor cell marker raises the possibility that in the absence of GATA2, there is a defect in the ectoderm’s ability to properly differentiate. Consistent with this hypothesis, expression of the ectodermal differentiation marker, keratin, and the transcription factor c-ets, which promotes proliferation or differentiation in a context-specific manner (Sieweke et al., 1997), was downregulated in GATA2 morphant ectoderm.
To begin to validate changes in gene expression predicted by the microarray analysis, we initially used qPCR to analyze expression of a subset of target genes in ectodermal explants isolated from uninjected embryos, or from embryos injected with GATA2 MO or FOG RNA, under conditions identical to those used in the microarray. Ectoderm was explanted at stage 10 and and cultured to stage 12–13 prior to harvesting for analysis. In ectodermal explants, genes associated with non-canonical Wnt signaling (xWnt11, EphrinB2, Fz7) were repressed in both GATA2 MO and FOG RNA injected explants, whereas those associated with the canonical pathway (xFz8, x-Btgx) were activated in response to GATA2 MO or FOG RNA injection (Fig. 2C).
The finding that non-canonical Wnt pathway components and genes associated with differentiation are upregulated and canonical Wnt targets and genes associated with proliferation are downregulated by GATA2 is interesting given that these two arms of the Wnt pathway have been shown to be alternately required for different phases of development (Tian et al., 2010). Specifically, canonical Wnt pathway activation is required in multiple systems to promote progenitor cell proliferation in order to generate an adequate precursor pool to populate an organ. Subsequent activation of the non-canonical Wnt pathway is then necessary for cells to cease proliferation and become specified or differentiate with a given fate. Our previous studies suggested that ectodermal GATA2 is required for differentiation but not specification of blood (Dalgin et al., 2007), but more recent studies have shown that markers of hematopoietic specification are lost when GATA2 expression is knocked down in the entire embryo (Liu et al., 2008). Consistent with this, we find that markers of hematopoietic commitment (stem cell leukemia, scl) and differentiation (globin) are reduced at the neurula and tailbud stages, respectively, when ectodermal GATA2 expression is knocked down using antisense MOs (Dalgin et al., 2007) (Fig. S3). Taken together, our microarray results support the hypothesis that GATA2 activates non-canonical Wnt signaling, and represses canonical Wnt signaling in ectodermal cells in order to halt progenitor proliferation and enable mesodermal cells to be specified with a primitive hematopoietic fate during gastrulation.
Non-canonical Wnt pathway members are upregulated and canonical Wnt pathway members are downregulated downstream of GATA2
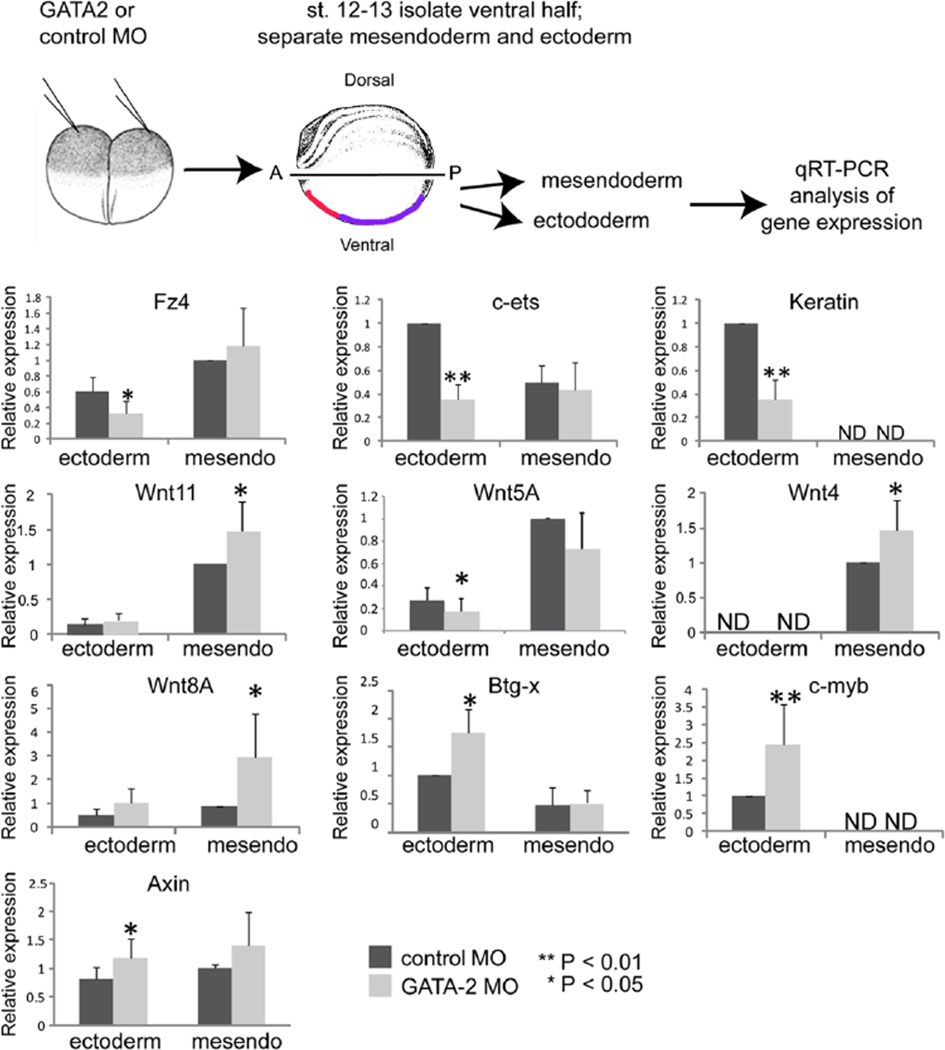
Microarray analysis provided an unbiased approach that pointed to components of the Wnt pathway as potential GATA2 target genes in explanted ectoderm that was cultured in isolation from mesoderm until late gastrula stages. However, the situation is more complex in vivo, where ectoderm and mesoderm come into contact and communicate with each other throughout gastrulation. Furthermore, GATA2 is expressed in both germ layers during gastrulation (Walmsley et al., 1994). This raises the possibility that some of the target genes identified in ectodermal cells, as well as additional Wnt pathway components that are not expressed in ectoderm and thus would not have been identified in our screen, are regulated by GATA2 in the mesoderm. We thus wanted to broaden our analysis and examine effects of GATA2 knockdown on Wnt pathway components and downstream target genes in each germ layer in the more physiological context of the intact embryo, where the mesoderm and ectoderm are in close contact and can signal to each other. To do so, we injected GATA2 MOs into both cells of 2-cell embryos, to knock down expression of GATA2 in all germ layers throughout the entire embryo. We then cultured embryos to the late gastrula stage, dissected away the ventral half of the embryo along the entire anterior-posterior axis as illustrated in Figure 3, and separated it into ectodermal and mesendodermal fragments. Expression of relevant target genes was then analyzed by qPCR in pooled ventral ectoderm or mesendoderm from GATA2 or control morphants. Importantly, by the late gastrula stage, cells originally derived from the dorsoanterior region of the early gastrula stage embryo, which will give rise to the anterior VBI (aVBI), have migrated and now reside in the anterior ventral region of the embryo (Fig. 3, red shading) while cells derived from the ventroposterior region of early gastrulae, which give rise to the posterior VBI (pVBI), now reside in the posterior ventral region (purple shading) (Bauer et al., 1994; Lane and Smith, 1999). Thus, the tissue fragments that we are analyzing include mesodermal and ectodermal cells that will give rise to or overlie, respectively, the entire anterior posterior extent of the VBI. Although we used overexpression of GATA2 and FOG as controls to eliminate false positives in our preliminary screen, these controls were not used to validate targets in whole embryos because overexpressed GATA2 may activate genes that are not relevant under physiological conditions (e.g. genes that are normally activated by other GATA family members) and overexpressed FOG most likely blocks the function of all GATAs, not just GATA2 (Mimoto and Christian, 2012).
Figure 3. GATA2 regulates expression of Wnt pathway components and target genes during gastrulation.
GATA2 or control MO (40 ng per embryo) was injected into two-cell embryos. At stage 12–13, the ventral half of the embryo was removed as illustrated and dissected into ectodermal or mesendodermal pieces. The location of cells that will give rise to the anterior VBI (red) and posterior VBI (purple) is indicated. RNA was extracted from pooled ectoderm or mesendoderm (mesendo) and gene expression analyzed by qPCR for each target gene. Quantification of relative gene expression (mean +/− SD) in at least three independent experiments is shown.
As predicted by the microarray, expression of the non-canonical Wnt receptor Fz4, and the differentiation markers c-ets and keratin was reproducibly reduced in the ectoderm of GATA2 morphants (Fig. 3). However, expression of Wnt11 was unchanged in ectodermal cells and was elevated in mesodermal cells of GATA2 morphants (Fig. 3). Because we were interested in exploring Wnt signaling more broadly, we also analyzed expression of the other major non-canonical Wnt ligand (Wnt5A) that functions during gastrulation. Expression of Wnt5A was repressed in the ectoderm of GATA2 morphants (Fig.3). Collectively, these data are consistent with the possibility that GATA2 promotes non-canonical Wnt signaling during gastrulation to drive ectodermal specification or differentiation, although further analysis of downstream pathway activation will be required to validate this conclusion.
We next examined expression of genes associated with the canonical Wnt pathway that were identified in our microarray, as well as the major canonical Wnt ligands (Wnt4, Wnt8) that are expressed primarily in mesoderm during gastrulation, and thus would not have been identified in our screen. We found that expression of Wnt4 and Wnt8 was elevated in the mesoderm of GATA2 morphants. As predicted by the microarray, we also found that expression of the Wnt target gene, Btg-x, and the marker of proliferation, c-myb, was enhanced in the ectoderm of GATA2 morphants. To further assay the status of Wnt signaling in GATA2 morphants, we analyzed expression of the direct canonical Wnt target gene, axin2, and found that it was upregulated in GATA2 morphants. Our finding that Wnt4 and Wnt8 are upregulated in mesodermal cells, while Wnt target genes and a marker of proliferation are upregulated in ectodermal cells in GATA2 morphant embryos, suggest that the mesodermally derived ligands might be signaling primarily to the ectoderm. These data support our hypothesis that GATA2 dampens canonical Wnt signaling toward the end of gastrulation, enabling progenitor cells to halt proliferation.
Notably, not all of the Wnt pathway genes that validated as GATA2 targets in ectoderm that was isolated and aged ex vivo (Fig. 2C) did so in ectoderm that was aged in vivo in the context of the intact embryo. Specifically, we did not detect statistically significant changes in expression of Wnt11, ephrinB2b, Fz7 or Fz8 in ectodermal cells isolated from intact GATA2 morphants (Fig. 3 and data not shown). These results demonstrate that cross-talk between the ectoderm and mesoderm during gastrulation, when these tissues are directly apposed, can influence gene expression in ways that cannot be predicted based on analysis of ectoderm that is aged in isolation from the mesoderm.
Hematopoietic defects in GATA2 morphants are not caused by a loss of BMP expression
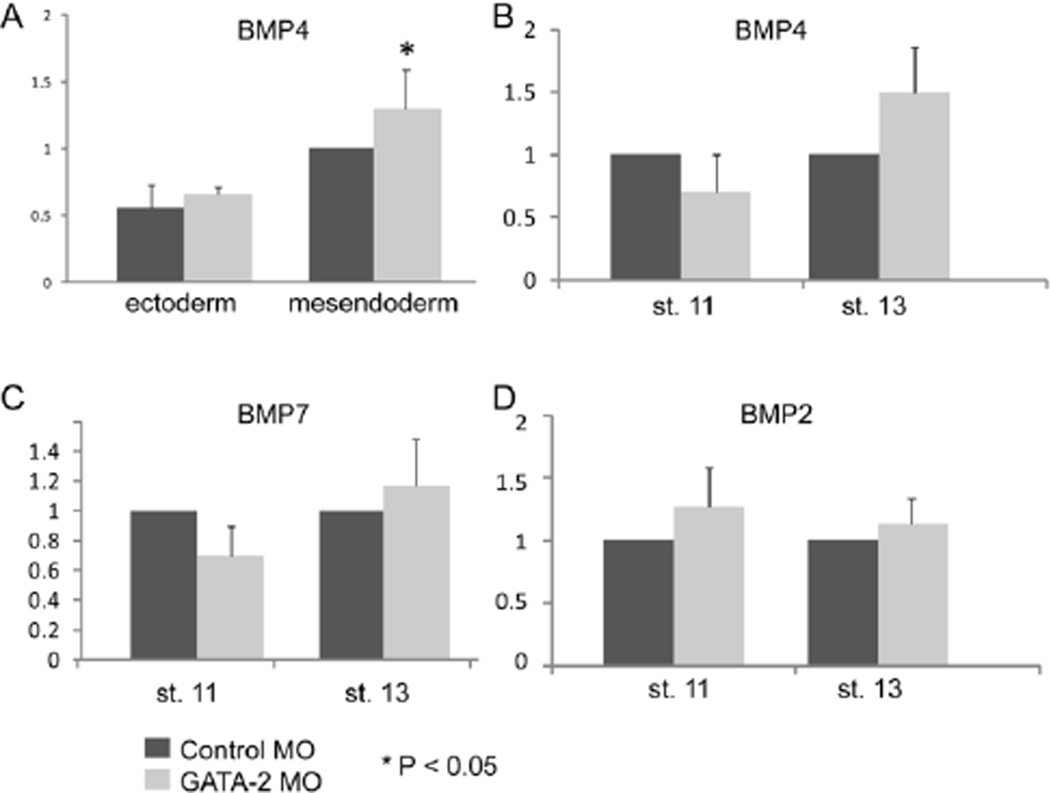
Canonical Wnt signals are required for expression of BMP ligands during gastrulation and vice versa, and both signaling pathways are required for primitive hematopoiesis. Specifically, mesodermal BMPs induce expression of canonical Wnt ligands that are necessary for hematopoietic specification (Lengerke et al., 2008). Conversely, Wnt4 signals from the mesoderm to activate the canonical Wnt pathway in ectodermal cells during gastrulation and this induces expression of Bmp4, which then signals to the mesoderm to enable it to form blood (Tran et al., 2010). We have previously shown that expression of Bmp2, Bmp4 and Bmp7 is unchanged in ectoderm that is explanted from GATA2 morphants (Dalgin et al., 2007). Given our current findings that expression of Wnt4 is elevated in mesodermal cells of GATA2 morphants (Fig. 3), and that changes in gene expression in explanted ectoderm do not always accurately reflect changes in ectodermal cells of the intact embryo, we wanted to re-examine germ layer specific interactions between GATA2 and BMP4 in the context of the intact embryo. A small increase in Bmp4 transcript levels was detected in mesendodermal, but not ectodermal cells dissected from the ventral half of GATA2 morphants (Fig. 4A). No significant differences were detected in levels of Bmp4, Bmp2 or Bmp7 in intact GATA2 morphant embryos relative to controls at stage 11 or stage 13 (Fig. 4B–D). These results confirm and extend our previously published studies (Dalgin et al., 2007) showing that hematopoietic defects in GATA2 morphants are not caused by reduced levels of Bmp transcripts.
Figure 4. GATA2 is not required for expression of Bmp2, Bmp4 or Bmp7 during gastrulation.
(A) GATA2 or control MOs (40 ng per embryo) were injected into two-cell embryos. At stage 13, the ventral half of the embryo was removed and dissected into ectodermal or mesendodermal pieces that were analyzed for expression of Bmp4 by qPCR. Quantification of relative gene expression (mean +/− SD) in four independent experiments is shown. (B–D) GATA2 or control MOs were injected into two-cell embryos and expression of Bmp4, 7, and 2 was analyzed by qPCR in whole embryos at stage 11. Quantification of relative gene expression (mean +/− SD) in three independent experiments is shown.
Upregulation of canonical Wnt signaling during gastrulation represses hematopoietic specification
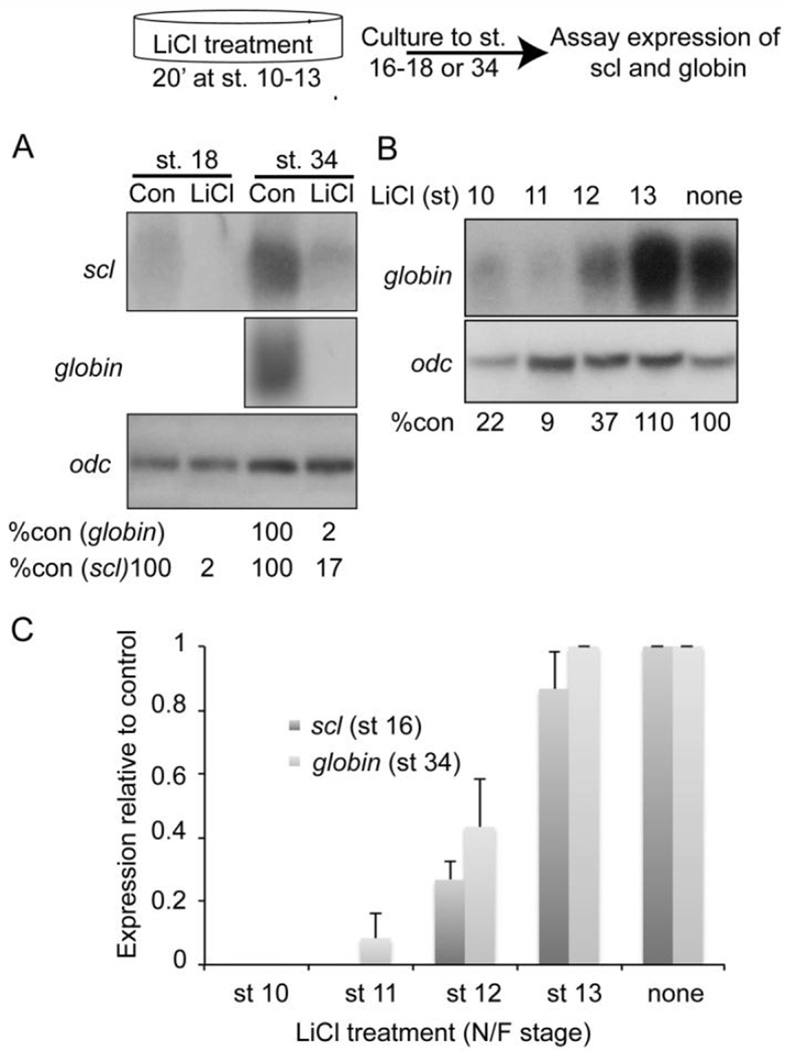
To test the hypothesis that upregulation of canonical Wnt signaling during gastrulation can block hematopoietic specification, we exposed gastrulating embryos to lithium chloride (LiCl), which stabilizes β-catenin to activate canonical Wnt signaling. Whereas hyperactivation of the canonical Wnt pathway during early cleavage stages leads to a dorsalized phenotype, activation of the same pathway during gastrulation causes dorsal mesodermal cells to adopt a more ventral fate and leads to a loss of anterior neural structures (Christian and Moon, 1993; Fredieu et al., 1997) (Fig. S4). Embryos were treated with LiCl for 20 minutes at stage 10 and then cultured to stage 18, when expression of scl was analyzed to look for defects in hematopoietic specification, or to stage 34, when expression of the RBC differentiation markers, globin and scl was examined. Expression of scl was significantly repressed at stage 18, demonstrating that specification of hematopoietic fate is disrupted by overactivation of canonical Wnt signaling. In addition, expression of globin and scl was nearly ablated at stage 34 (Fig. 5A, Fig. S4). The latter finding is consistent with previous studies showing that overexpression of zygotic Wnt8 leads to a loss of differentiated RBCs (Collavin and Kirschner, 2003; Hoppler and Moon, 1998; Kumano et al., 1999).
Figure 5. Upregulation of canonical Wnt signaling during gastrulation represses hematopoietic specification.
Schematic of approach used to transiently activate canonical Wnt signaling. Briefly, embryos at the stages specified were incubated in LiCl solution for 20 minutes, followed by several washes in 0.1× MBS culture medium. Embryos were allowed to develop to stage 16–18 or 34 and assayed for expression of globin and/or Scl. (A) Embryos were treated with LiCl at stage 10, allowed to develop to stage 18 or 34 and assayed for expression of Scl and/or globin by Northern blotting. Levels of globin or scl transcripts are normalized and reported as a percentage of globin or scl levels in untreated embryos below each lane. (B) Embryos were treated with LiCl at stages 10, 11, 12 or 13, allowed to develop to stage 34 and expression of globin was determined by Northern blotting. Levels of globin transcripts are normalized to and reported as a percentage of globin levels in untreated embryos below each lane. (C) Embryos were treated with LiCl at stages 10, 11, 12 or 13, allowed to develop to stage 16 or 34 and expression of scl or globin was determined by qPCR in three independent experiments.
Given the above data together with results from our microarray, we predicted that the developmental window during which hematopoiesis is sensitive to canonical Wnt upregulation would overlap with the requirement for ectodermal signaling. We therefore treated embryos with a single 20 minute pulse of LiCl at stages that encompass gastrulation (stages 10–13). Expression of scl or globin was analyzed at stage 16 or stage 34, respectively, using Northern blotting (Fig. 5B) and/or qPCR (Fig. 5C). Expression of globin (Fig. 5B, C) and scl (Fig. 5C) was repressed when embryos were treated with LiCl beginning at stage 10, 11 or 12, but not when embryos were exposed to LiCl at stage 13. Thus, upregulation of canonical Wnt signaling during the same developmental window in which ectoderm is required for blood formation (Fig. 1) represses hematopoietic specification.
Upregulation of zygotic canonical Wnt signaling in either ectoderm or mesoderm represses primitive hematopoiesis
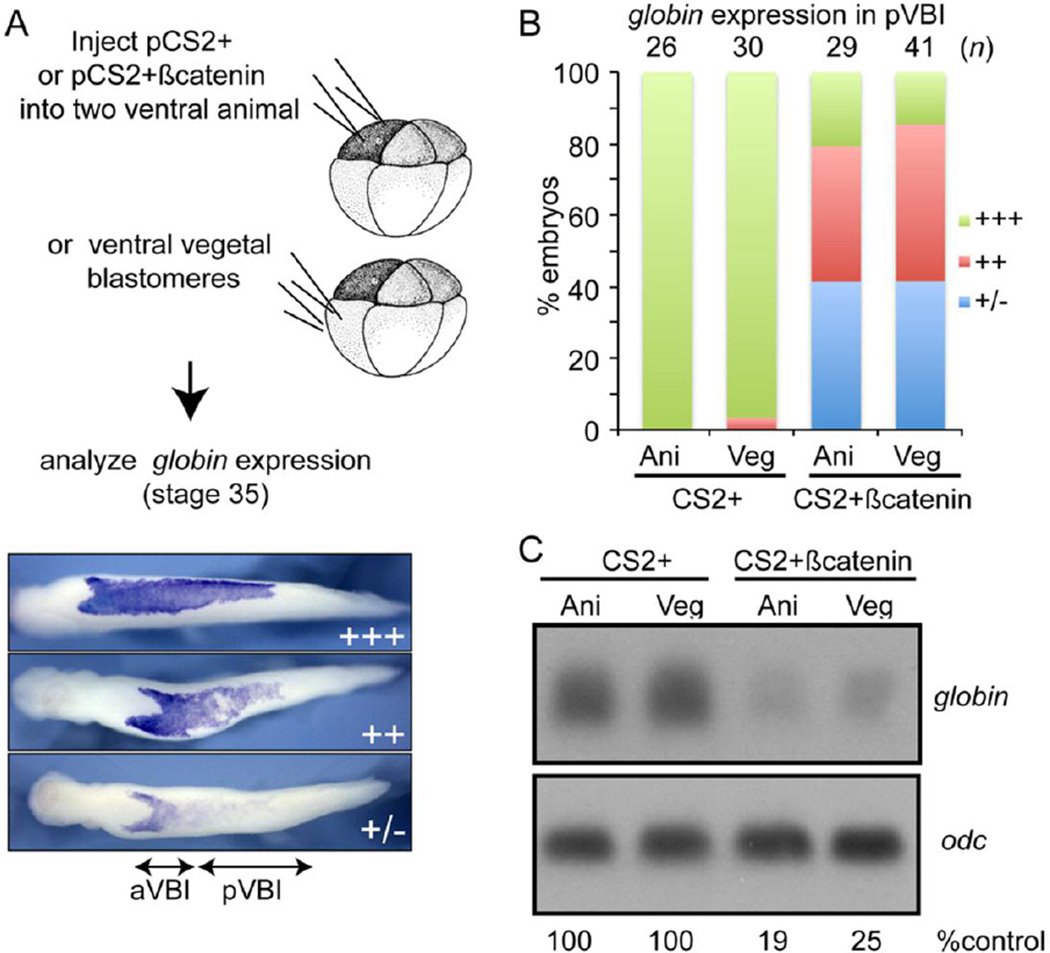
Given our finding that upregulation of canonical Wnt signaling during gastrulation represses hematopoiesis, we next wanted to know whether this inhibitory effect operates in mesodermal and/or ectodermal cells. To address this question, we activated the zygotic canonical Wnt pathway by injection of pCS2+βcatenin DNA (100 pg) into two ventral animal pole or ventral vegetal pole blastomeres of 8-cell embryos and then analyzed expression of globin at stage 35 by WMISH (illustrated in Fig. 6A). Injection of pCS2+βcatenin DNA will lead to ectopic expression of βcatenin beginning at the midblastula transition, which marks the onset of zygotic gene transcription. This strategy enables us to avoid the dorsalization of embryonic fate that occurs when canonical Wnt signaling is activated prior to the midblastula stage (Christian and Moon, 1993). Control embryos injected with only CS2+ plasmid DNA showed strong expression of globin along the entire anterior-posterior length of the VBI (Fig. 6B). By contrast, when CS2+βcatenin DNA was targeted to either animal or vegetal pole blastomeres, to activate zygotic Wnt signaling in ectoderm or mesoderm, respectively, expression of globin was moderately (++) or severely (+/−) repressed in the pVBI (Fig. 6B). Expression of globin was intact in cells of the aVBI, which is expected since these cells are derived from dorsal blastomeres (Lane and Smith, 1999) that were not targeted in our injections. A more quantitative analysis of globin expression by Northern blot confirmed that upregulation of zygotic canonical Wnt signaling in either ectodermal or mesodermal cells lead to a loss of differentiated blood cells (Fig. 6C).
Figure 6. Upregulation of zygotic canonical Wnt signaling in either ectodermal or mesodermal cells disrupts primitive erythropoiesis.
(A–B) Empty CS2+vector DNA or CS2+βcatenin DNA (100 pg per blastomere) was injected into both ventral animal pole or vegetal pole cells of 8-cell embryos as illustrated and expression of globin was analyzed by WMISH at stage 34. Globin staining in the posterior VBI (pVBI) of experimental embryos was scored as barely or not detectable (−/+), moderately decreased (++) or strong (+++) using the scale illustrated in panel A. (C) Embryos were injected as illustrated in panel A and expression of globin was determined by Northern blotting at stage 34. Levels of globin transcripts are normalized to and reported as a percentage of globin levels in CS2+ embryos below each lane. Results shown were replicated in one additional experiment.
Non-canonical Wnt signaling is required for RBC differentiation during primitive erythropoiesis
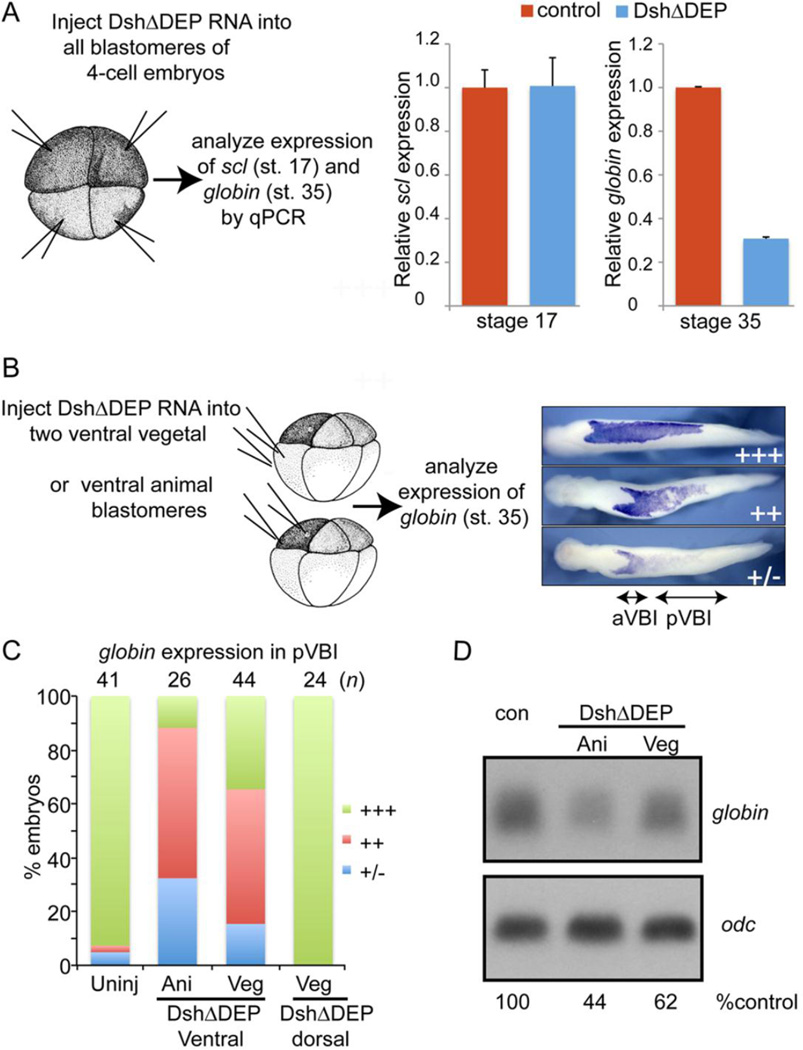
Based on our microarray data, we predicted that activation of the non-canonical Wnt pathway would be required for progenitors to commit to a hematopoietic fate. To selectively inhibit the non-canonical Wnt pathway, we used a dominant-negative version of the Wnt effector disheveled that lacks most of the DEP domain (DshΔDEP; D6), which is required for non-canonical signaling (Boutros et al., 1998). However, canonical signaling via DshΔDEP remains intact (Rothbacher et al., 2000).
In initial studies, we injected 500 pg of RNA encoding DshΔDEP into each blastomere of 4-cell embryos to inhibit non-canonical Wnt signaling throughout the embryo (illustrated in Fig. 7A). We then used qPCR to analyze expression of scl at stage 17 and expression of globin at stage 34. Expression of scl was unchanged in embryos injected with DshΔDEP RNA, relative to controls (Fig. 7A), demonstrating that noncanonical Wnt signaling is not essential for progenitors to commit to a hematopoietic fate during gastrulation. Expression of globin, however, was severely repressed in embryos injected with DshDEP RNA (Fig. 7A). Collectively, these results demonstrate that non-canonical Wnt signaling is not required for erythroid progenitors to commit to a hematopoietic fate during gastrulation, but it most likely plays a later role in enabling these progenitors to differentiate as mature RBCs.
Figure 7. Non-canonical Wnt signaling is required in ectodermal and mesodermal cells for primitive erythropoiesis.
(A) RNA encoding DshΔDEP (500 pg) was injected into each blastomere of 4-cell embryos as illustrated and expression of scl and globin was analyzed by qPCR at stage 17 and stage 35, respectively. (B-D) RNA encoding DshΔDEP (750 pg) was injected into each ventral animal pole or ventral vegetal pole blastomere of 8-cell embryos as illustrated. (C) Expression of globin was analyzed by WMISH at stage 34 and globin staining in the posterior VBI (pVBI) was scored as barely or not detectable (−/+), moderately decreased (++) or strong (+++) using the scale illustrated on the right in panel C. (D) Expression of globin was determined by Northern blotting at stage 34. Levels of globin transcripts are normalized to and reported as a percentage of globin levels in control embryos below each lane. In this blot, the control lane contains RNA from CS2+ injected embryos and is the same control shown for Fig. 6 since all samples were run on the same gel. Results were replicated in two additional experiments.
We then injected 750 pg of RNA encoding DshΔDEP into each ventral animal or vegetal pole blastomere of 8-cell embryos to preferentially inhibit non-canonical signaling in the ventral-posterior ectoderm or mesoderm, respectively (Fig. 7B). These embryos were allowed to develop to the tailbud stage (stage 35) and were assessed for expression of globin by WMISH using the scoring system illustrated in Fig. 7B. Targeting of DshΔDEP RNA to either ventral animal or vegetal pole cells, led to moderate (++) or severe (+/−) repression of globin in the pVBI of most embryos (Fig. 7C), demonstrating that non-canonical Wnt signaling is required in both ectodermal and mesodermal tissues for primitive erythropoiesis. An identical result was obtained when we used a second mutant Xenopus disheveled (Xdd1), that contains an internal deletion of the conserved PDZ/DHR domain (Sokol, 1996), to block non-canonical Wnt signaling (data not shown). As a control to show that injection of DshΔDEP can effectively block non-canonical Wnt signaling, we targeted DshΔDEP to dorsal mesodermal cells and verified that these embryos showed a shortened anterior-posterior axis, including the blood islands, (Fig. S5), due to defects in convergent extension movements. Expression of globin, however, remained intact in the pVBI of embryos expressing DshΔDEP in dorsal mesodermal cells (Fig. 7C), which is expected since these cells do not contribute to the pVBI. Analysis of globin expression by Northern blot confirmed that downregulation of non-canonical Wnt signaling in either ectodermal or mesodermal cells lead to a reduction in globin expression (Fig. 6D).
Collectively, the current data suggest that GATA2 represses canonical Wnt signaling, and may activate non-canonical Wnt signaling during gastrulation. Although canonical Wnt signaling is essential for hematopoietic specification (Tran et al., 2010) our results show that sustained canonical Wnt signaling prevents mesodermal cells from committing to a hematopoietic fate. During organ development, the relative dominance of canonical or non-canonical Wnt pathways often switches in a pattern that correlates temporally with distinct phases of progenitor proliferation and differentiation, respectively (Tian et al., 2010). In addition GATA factors play a central role in regulating this switch from canonical to non-canonical Wnt pathway activation in different organs, including the lung and heart (Afouda and Hoppler, 2011; Afouda et al., 2008; Zhang et al., 2008). Such a stage specific and biphasic role for Wnt signaling has been suggested during hematopoiesis based on analysis of in vitro differentiated embryonic stem cells (Naito et al., 2006; Tarafdar et al., 2013; Vijayaragavan et al., 2009). Specifically, canonical Wnt signaling initiates the mesodermal program and promotes primitive hematopoietic fate while non-cannonical Wnt signals are required to control exit from the pluripotent state (Tarafdar et al., 2013; Vijayaragavan et al., 2009).
It is possible that GATA2 mediated upregulation of non-canonical Wnt signaling indirectly leads to repression of canonical Wnt signaling but we find this unlikely for several reasons. First, we show that GATA2 function is required to dampen expression of two canonical Wnt ligands (Wnt8 and Wnt4), suggesting a direct effect. Second, we show that sustained activation of canonical Wnt signaling prevents progenitors from committing to a blood fate during gastrulation whereas non-canonical Wnt signaling is dispensable for this process. This suggests that the two pathways are operating sequentially and independently.
While our data suggest that GATA2 contributes to changes in Wnt signaling that are required for primitive hematopoiesis, it is unlikely that manipulation of Wnt signaling would be sufficient to rescue blood development in GATA2 morphants. Ongoing studies in our lab have identified additional GATA2 target genes that operate independent of Wnt signaling, and that are essential for hematopoiesis.
Conclusions
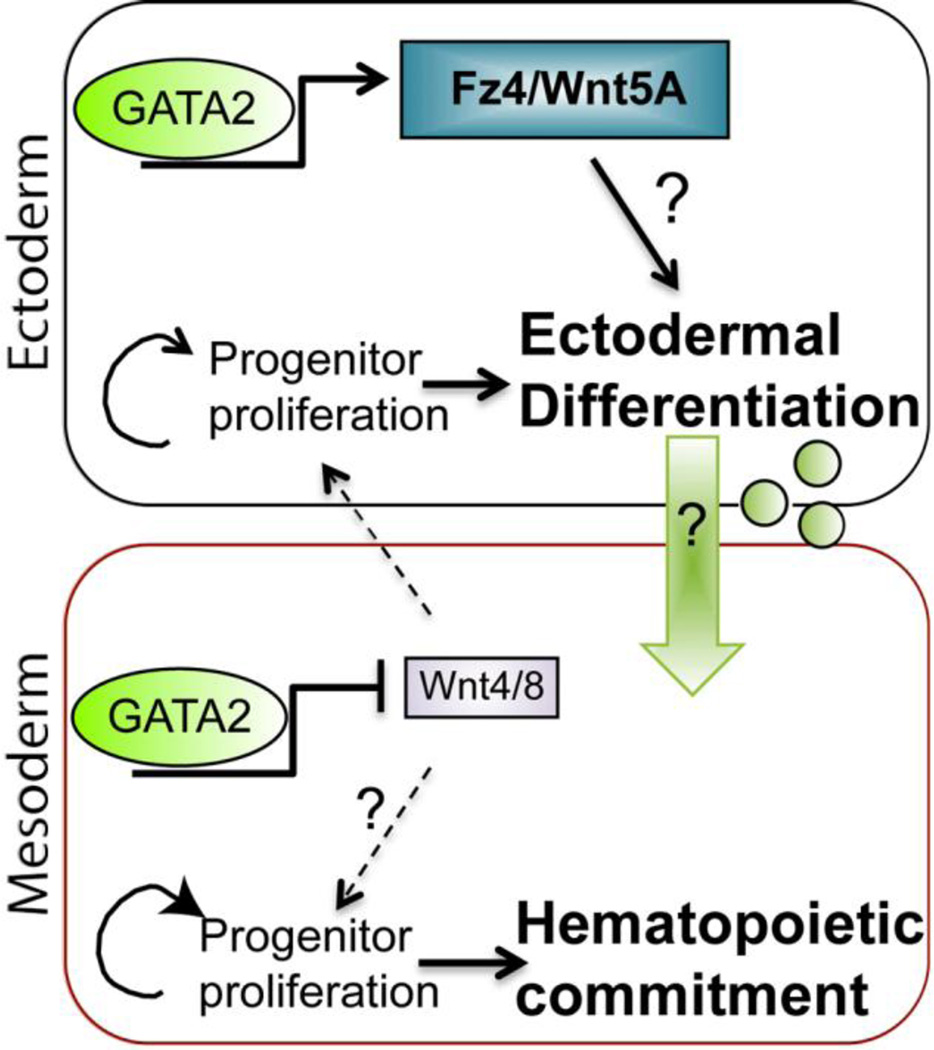
Our data show that a proper balance of canonical and non-canonical Wnt signaling is required in both mesodermal and ectodermal cells for normal primitive hematopoiesis. We propose that GATA2 is a contributing factor that promotes non-canonical Wnt signaling (through upregulation of Fz4 and Wnt5A in ectoderm) and dampens canonical Wnt signaling (through repression of Wnt4 and Wnt8 in mesoderm) toward the end of gastrulation (illustrated in Fig. 8). In Xenopus, Wnt 4 secreted from the mesoderm activates canonical Wnt signaling in ectodermal, and possibly also mesodermal, cells during gastrulation, and this is required for the induction of primitive hematopoiesis (Tran et al., 2010). Canonical Wnt signaling is also required for the maintenance of primitive hematopoiesis after this time (Tran et al., 2010). Our data suggest that there is a temporal window in the course of gastrulation during which canonical Wnt signaling must be transiently reduced in mesodermal cells to enable blood progenitors to cease proliferation and commit to a hematopoietic fate (Fig. 8, lower panel). Analysis of Wnt target genes, as well as markers of proliferation and differentiation (Fig. 3), suggest that GATA2 mediated repression of Wnt4 and Wnt8 in mesodermal cells leads to downregulation of canonical Wnt signaling, reduced progenitor proliferation and enhanced differentiation primarily in ectodermal cells. It is less obvious how ectodermal commitment or differentiation influences mesodermal blood progenitors. One possibility is that differentiation is required to generate additional, yet to be identified secondary signals that are transmitted to mesodermal cells to enable them to form blood (Fig. 8, green arrow and circles). We are continuing to analyze a number of ectodermal GATA2 target genes that are candidates for this secondary signal, and these will be described in future manuscripts.
Figure 8. Model of how GATA2 regulates the balance of Wnt signaling to promote hematopoietic commitment.
During normal primitive hematopoiesis, GATA2 induces expression of non-canonical Wnt signaling components, such as Fz4 and Wnt5A in ectodermal cells, and represses expression of the canonical Wnt ligands Wnt4 and Wnt8 in mesodermal cells. Downregulation of canonical Wnt tips the balance away from progenitor proliferation and toward commitment/differentiation. In the mesoderm, this enables progenitors to commit to a hematopoietic fate. In the ectoderm, we hypothesize that differentiation is required for cells to generate a secondary signal (green arrow and circles) that is transmitted to mesodermal cells, enabling them to commit to a blood fate.
Supplementary Material
Highlights.
Xenopus GATA2 target genes were identified using microarray analysis
GATA2 regulates components of canonical and non-canonical Wnt pathways
Upregulation of canonical Wnt signaling blocks hematopoietic commitment
Inhibition of non-canonical Wnt signaling blocks erythroid differentiation
Acknowledgements
We thank Ken Cho for the DshΔDEP plasmid. This work was supported by grants from the NIH (RO1HD067473 and RO3HD050242) to J.L.C., and by postdoctoral fellowships from the AHA to D.G. and from the NIH (T32 DK007115) to Y.S.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Afouda BA, Hoppler S. Different requirements for GATA factors in cardiogenesis are mediated by non-canonical Wnt signaling. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:649–662. doi: 10.1002/dvdy.22570. [DOI] [PubMed] [Google Scholar]
- Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. GATA transcription factors integrate Wnt signalling during heart development. Development. 2008;135:3185–3190. doi: 10.1242/dev.026443. [DOI] [PubMed] [Google Scholar]
- Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann's Organizer: analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development. 1994;120:1179–1189. doi: 10.1242/dev.120.5.1179. [DOI] [PubMed] [Google Scholar]
- Belaoussoff M, Farrington SM, Baron MH. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–5018. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- Benito M, Parker J, Du Q, Wu J, Xiang D, Perou CM, Marron JS. Adjustment of systematic microarray data biases. Bioinformatics. 2004;20:105–114. doi: 10.1093/bioinformatics/btg385. [DOI] [PubMed] [Google Scholar]
- Bresson-Mazet C, Gandrillon O, Gonin-Giraud S. Stem cell antigen 2: a new gene involved in the self-renewal of erythroid progenitors. Cell proliferation. 2008;41:726–738. doi: 10.1111/j.1365-2184.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. The Journal of investigative dermatology. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes & development. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Liu F, Patient R. Genetic control of hematopoietic development in Xenopus and zebrafish. The International journal of developmental biology. 2010;54:1139–1149. doi: 10.1387/ijdb.093055ac. [DOI] [PubMed] [Google Scholar]
- Collavin L, Kirschner MW. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development. 2003;130:805–816. doi: 10.1242/dev.00306. [DOI] [PubMed] [Google Scholar]
- Dalgin G, Goldman DC, Donley N, Ahmed R, Eide CA, Christian JL. GATA-2 functions downstream of BMPs and CaM KIV in ectodermal cells during primitive hematopoiesis. Developmental biology. 2007;310:454–469. doi: 10.1016/j.ydbio.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon C, Jaffredo T. Cell interactions and cell signaling during hematopoietic development. Experimental cell research. 2014;329:200–206. doi: 10.1016/j.yexcr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Fredieu JR, Cui Y, Maier D, Danilchik MV, Christian JL. Xwnt-8 and lithium can act upon either dorsal mesodermal or neurectodermal cells to cause a loss of forebrain in Xenopus embryos. Developmental biology. 1997;186:100–114. doi: 10.1006/dbio.1997.8566. [DOI] [PubMed] [Google Scholar]
- Goldman DC, Berg LK, Heinrich MC, Christian JL. Ectodermally derived steel/stem cell factor functions non-cell autonomously during primitive erythropoiesis in Xenopus. Blood. 2006;107:3114–3121. doi: 10.1182/blood-2005-09-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods in cell biology. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Moon RT. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mechanisms of development. 1998;71:119–129. doi: 10.1016/s0925-4773(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic acids research. 2003a;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003b;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Kikkawa M, Yamazaki M, Izutsu Y, Maeno M. Two-step induction of primitive erythrocytes in Xenopus laevis embryos: signals from the vegetal endoderm and the overlying ectoderm. The International journal of developmental biology. 2001;45:387–396. [PubMed] [Google Scholar]
- Kumano G, Belluzzi L, Smith WC. Spatial and temporal properties of ventral blood island induction in Xenopus laevis. Development. 1999;126:5327–5337. doi: 10.1242/dev.126.23.5327. [DOI] [PubMed] [Google Scholar]
- Lane MC, Smith WC. The origins of primitive blood in Xenopus: implications for axial patterning. Development. 1999;126:423–434. doi: 10.1242/dev.126.3.423. [DOI] [PubMed] [Google Scholar]
- Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nature cell biology. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JB, Zon LI, Daley GQ. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell stem cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Current biology : CB. 2008;18:1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Maeno M. Regulatory signals and tissue interactions in the early hematopoietic cell differentiation in Xenopus laevis embryo. Zoological science. 2003;20:939–946. doi: 10.2108/zsj.20.939. [DOI] [PubMed] [Google Scholar]
- Maeno M, Komiyama K, Matsuzaki Y, Hosoya J, Kurihara S, Sakata H, Izutsu Y. Distinct mechanisms control the timing of differentiation of two myeloid populations in Xenopus ventral blood islands. Development, growth & differentiation. 2012;54:187–201. doi: 10.1111/j.1440-169x.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- Maeno M, Ong RC, Xue Y, Nishimatsu S, Ueno N, Kung HF. Regulation of primary erythropoiesis in the ventral mesoderm of Xenopus gastrula embryo: evidence for the expression of a stimulatory factor(s) in animal pole tissue. Developmental biology. 1994;161:522–529. doi: 10.1006/dbio.1994.1050. [DOI] [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimoto MS, Christian JL. Friend of GATA (FOG) interacts with the nucleosome remodeling and deacetylase complex (NuRD) to support primitive erythropoiesis in Xenopus laevis. PloS one. 2012;7:e29882. doi: 10.1371/journal.pone.0029882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. Amsterdam: North Holland Publishing Co., Amsterdam; 1967. [Google Scholar]
- Pelz CR, Kulesz-Martin M, Bagby G, Sears RC. Global rank-invariant set normalization (GRSN) to reduce systematic distortions in microarray data. BMC bioinformatics. 2008;9:520. doi: 10.1186/1471-2105-9-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. The EMBO journal. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Current biology : CB. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Tekotte H, Frampton J, Graf T. MafB represses erythroid genes and differentiation through direct interaction with c-Ets-1. Leukemia. 1997;11(Suppl 3):486–488. [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical applications in genetics and molecular biology. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Current biology : CB. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kamo T, Ota S, Sugimura H. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. The EMBO journal. 2003;22:847–858. doi: 10.1093/emboj/cdg088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafdar A, Dobbin E, Corrigan P, Freeburn R, Wheadon H. Canonical Wnt signaling promotes early hematopoietic progenitor formation and erythroid specification during embryonic stem cell differentiation. PloS one. 2013;8:e81030. doi: 10.1371/journal.pone.0081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Cohen ED, Morrisey EE. The importance of Wnt signaling in cardiovascular development. Pediatric cardiology. 2010;31:342–348. doi: 10.1007/s00246-009-9606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Sekkali B, Van Imschoot G, Janssens S, Vleminckx K. Wnt/beta-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16160–16165. doi: 10.1073/pnas.1007725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Turpen JB, Kelley CM, Mead PE, Zon LI. Bipotential primitive-definitive hematopoietic progenitors in the vertebrate embryo. Immunity. 1997;7:325–334. doi: 10.1016/s1074-7613(00)80354-4. [DOI] [PubMed] [Google Scholar]
- Vijayaragavan K, Szabo E, Bosse M, Ramos-Mejia V, Moon RT, Bhatia M. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell stem cell. 2009;4:248–262. doi: 10.1016/j.stem.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley ME, Guille MJ, Bertwistle D, Smith JC, Pizzey JA, Patient RK. Negative control of Xenopus GATA-2 by activin and noggin with eventual expression in precursors of the ventral blood islands. Development. 1994;120:2519–2529. doi: 10.1242/dev.120.9.2519. [DOI] [PubMed] [Google Scholar]
- Walters MJ, Wayman GA, Christian JL. Bone morphogenetic protein function is required for terminal differentiation of the heart but not for early expression of cardiac marker genes. Mechanisms of development. 2001;100:263–273. doi: 10.1016/s0925-4773(00)00535-9. [DOI] [PubMed] [Google Scholar]
- Wessely O, Kim JI, Tran U, Fuentealba L, De Robertis EM. xBtg-x regulates Wnt/beta-Catenin signaling during early Xenopus development. Developmental biology. 2005;283:17–28. doi: 10.1016/j.ydbio.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Xing Y, Harrison SC, Kirchhausen T. Structural analysis of the interaction between Dishevelled2 and clathrin AP-2 adaptor, a critical step in noncanonical Wnt signaling. Structure. 2010;18:1311–1320. doi: 10.1016/j.str.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Ye X, Guo N, Nathans J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development. 2012;139:4383–4394. doi: 10.1242/dev.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6- Wnt pathway required for epithelial stem cell development and airway regeneration. Nature genetics. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.