Abstract
Organogenesis is orchestrated by cell and tissue interactions mediated by molecular signals. Identification of relevant signals, and the tissues that generate and receive them, are important goals of developmental research. Here, we demonstrate that Retinoic Acid (RA) is a critical signaling molecule important for morphogenesis of mammalian submandibular salivary glands (SMG). By examining late stage RA deficient embryos of Rdh10 mutant mice we show that SMG development requires RA in a dose-dependent manner. Additionally, we find that active RA signaling occurs in SMG tissues, arising earlier than any other known marker of SMG development and persisting throughout gland morphogenesis. At the initial bud stage of development, we find RA production occurs in SMG mesenchyme, while RA signaling occurs in epithelium. We also demonstrate active RA signaling occurs in glands cultured ex vivo, and treatment with an inhibitor of RA signaling blocks growth and branching. Together these data identify RA signaling as a direct regulator of SMG organogenesis.
Keywords: Salivary, Submandibular, Retinoic Acid, Rdh10, retinol dehydrogenase, organogenesis, branching, Vitamin A
INTRODUCTION
Saliva lubricates the mouth, aiding in speech, swallowing and digestion, neutralizing acids damaging to teeth, and protecting oral tissues. In mammals saliva is produced in large part by three salivary gland pairs - the submandibular, sublingual, and parotid - the formation and function of which have been well described in recent reviews (Holmberg and Hoffman, 2014; Knosp et al., 2012; Tucker, 2007)). Formation of salivary glands during embryogenesis is an active area research, being important both to the field of developmental biology, and also because knowledge of organogenesis can serve as a paradigm for regeneration of adult organs (reviewed in (Patel and Hoffman, 2014)).
Each salivary gland is composed of numerous sac-like acini, where saliva is produced. These are connected to the oral cavity via a branching network of ducts. The acini and ducts, which are epithelial, are surrounded by nerve fibers, blood vessels, fibroblasts, and immune cells, all within the context of extracellular matrix. Current knowledge about formation of mammalian salivary glands is based largely upon studies of organogenesis of the SMG in mouse. Mouse mutations that cause defects in embryonic formation of salivary glands have been instrumental in identifying genes critical for gland formation. Ex vivo culture experiments of explanted mouse SMG tissues have yielded information about molecules and tissue interactions that regulate gland formation.
Development of the mouse SMG occurs as a progressive series of events, many of which involve interactions between different tissue types. Early in gland organogenesis, at embryonic day 10.5 (E10.5), a domain of epithelium within the developing mandible gains the ability to induce gland formation when combined with underlying pharyngeal arch mesenchyme (Wells et al., 2013). Genes or molecules responsible for the instructive signal are not known, nor have any markers of this early E10.5 pre-SMG territory been identified. At E11.5, the epithelium thickens to form a placode in the oral epithelium, a morphogenic event that coincides with a switch in instructive capacity from the epithelium to the mesenchyme (Wells et al., 2013). The placode epithelium subsequently invaginates into the underlying mesenchyme forming an initial bud by E12.5, which then branches to become a pseudoglandular structure by E13.5. The epithelium continues to grow and branch, a process dependent upon signals from the mesenchyme (Kratochwil, 1969; Kusakabe et al., 1985; Tucker, 2007; Wells et al., 2013), resulting in a highly branched tree-like structure of epithelial acini and ducts by E17.5. In addition to signals between epithelium and mesenchyme, there are also important are interactions between the SMG epithelium and the submandibular parasympathetic ganglion and nerve that are critical for development of the ganglion, and for maintaining epithelial progenitor cells in an undifferentiated state (Knosp et al., 2015; Knox et al., 2013; Knox et al., 2010).
Retinoic acid (RA) is a diffusible small hormone-like molecule generated by a two-step enzymatic oxidation of dietary Vitamin A (Fig. 1 A) (reviewed in (Duester, 2008; Niederreither and Dolle, 2008)). Vitamin A, also known as all-trans-retinol, is first converted into the metabolic intermediate all-trans-retinal (atRAL) via retinol dehydrogenase 10 (RDH10) (Sandell et al., 2007). The intermediate atRAL is subsequently converted into the active product RA via enzymes of the aldehyde dehydrogenase 1A (ALDH1A) family. The initial oxidative reaction is reversible, with the reverse conversion of the intermediate atRAL into the precursor all-trans-retinol, being carried out by the enzyme dehydrogenase/reductase (SDR family) member 3 (DHRS3) (Billings et al., 2013; Feng et al., 2010). Canonical RA signaling involves regulation of gene expression by binding of RA as a ligand to nuclear receptor transcription factors known as RA receptors (RAR) (reviewed in (Mark et al., 2009)), but RA has a diverse array of functions in addition to canonical activity (Al Tanoury et al., 2013).
Figure 1. Deficient production of RA in Rdh10trex/trex embryos impairs development of the SMG.
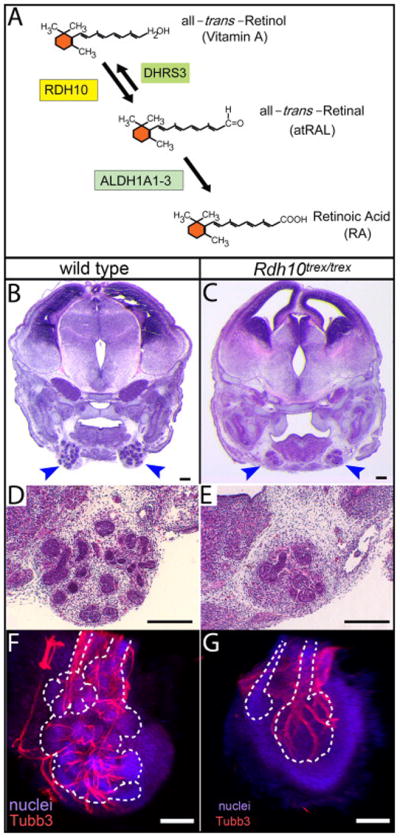
(A) Vitamin A is converted into RA via two sequential enzymatic reactions. The first reaction, conversion of all-trans-Retinol (Vitamin A) to all-trans-Retinal (atRAL) is mediated within an embryo by RDH10. The first reaction is reversible, with the opposite reaction being mediated by DHRS3. The second reaction, the irreversible conversion of the intermediate atRAL to RA, is mediated by three aldehyde dehydrogenases, ALDH1A1, ALDH1A2, and ALDH1A3. Lack of RDH10 function results in severe RA deficiency and embryonic lethality usually prior to E11.5 (Sandell et al., 2007). (B–E) SMG development is impaired in Rdh10trex/trex embryos rescued to survive to E14.5 by maternal dietary supplementation with a minimal dose (40 μg) of the intermediate atRAL. Frontal paraffin sections through SMG of wild type and mutant embryos were stained with Hematoxylin and Eosin. SMG of 40 μg atRAL-rescued Rdh10trex/trex embryos (C, E) were substantially smaller than those of wild type littermates (B, D). Blue arrowheads indicate SMG. (F, G) Parasympathetic SMG ganglion and nerve development occurs in Rdh10trex/trex mutant embryos. Whole mount SMG from wild type (F) and Rdh10trex/trex mutant (G) embryos from 40 μg atRAL litters were immunostained for βIII neuronal tubulin (Tubb3), to reveal neurons of the parasympathetic ganglion and nerve, and with fluorescent nuclear stain Red Dot, to reveal all nuclei. For each gland a z-stack of confocal images was collapsed to form a single projection image. White dotted lines represent outline of gland epithelium visualized based on density of nuclear stain. Scale bars = 100 μm.
Vitamin A, through its active metabolite RA, is crucial for regulating an astonishing variety of biological process, beginning in early embryogenesis and on through adulthood (reviewed in (Clagett-Dame and Knutson, 2011)). During embryogenesis, RA signaling is critical for proper development of ears, eyes, palate, heart, lungs, kidneys, the central nervous system, and other important organs and structures, and, for some processes, the cellular and molecular function of RA has been analyzed in depth. In contrast, the influence of RA on salivary gland morphogenesis has not been investigated in any detail, although some data suggests it plays a role. Lineage analysis using a Cre driver responsive to RA signaling indicates that salivary gland tissues experience RA signaling at some stage of their development (Dollé et al., 2010). Compound mutants with double or triple Rar mutations have been noted to have defects of salivary gland development (Lohnes et al., 1995; Lohnes et al., 1994). Induction of Vitamin A deficiency by dietary restriction during the latter half of gestation in rats has also been shown to cause defects in salivary gland development (See et al., 2008). The fact that genetic or nutritional RA deficiency disrupts salivary gland development indicates that sufficient RA signaling is required for gland organogenesis. However, these studies provide no information about whether salivary gland formation is regulated by RA directly, or if salivary gland phenotypes result as an indirect consequence of a requirement for RA in a different process, for example, owing to the well-established essential role for RA in patterning the pharyngeal arches (reviewed in (Mark et al., 2004)). The lack of investigation of RA in salivary gland development is likely due to the fact that RA has many essential functions early in development, making it difficult to examine the requirement for RA in morphogenesis of tissues and organs that form during the latter half of gestation.
In this study, using Rdh10 mutant mice, we have investigated the role of RA in morphogenesis of the SMG. Owing to the position of the RDH10-catalyzed oxidation within the sequence of Vitamin A metabolic reactions, and to the reversible nature of the RDH10 catalyzed step, late stage RA deficient Rdh10−/− mutant embryos may be generated by supplementing the maternal diet with the metabolic intermediate atRAL at early stages of development (Rhinn et al., 2011). Our analysis of atRAL supplemented Rdh10−/− embryos reveals that RA is required for development of the SMG. Moreover, we show that growth of SMG epithelium is sensitive to RA in a dose-dependent manner. We also demonstrate that RA signaling is active in SMG tissues throughout the time course of their development. Interestingly, we find that RA signaling is present in mandibular tissues at E10.5, prior to gland initiation, in domains approximating the area where future SMG development will begin. Additionally, we observe that RA signaling occurs in ex vivo cultured tissues, and that treatment of cultured glands with a chemical inhibitor of RA signal blocks their growth and branching morphogenesis. Together these data identify that RA signaling directly regulates growth and branching of SMG epithelium in vivo and in vitro.
MATERIALS and METHODS
Mice
The Rdh10trex and Rdh10βgeo mutant mouse lines used in this study have been previously described (Sandell et al., 2012; Sandell et al., 2007). The RARE-lacZ reporter mice used to visualize RA activity, originally generated by Janet Rossant (Rossant et al., 1991), were obtained from Jackson laboratories (official name, Tg(RARE-Hspa1b/lacZ)12Jrt). The day of the vaginal plug was considered E0.5. All experiments involving mice were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Louisville.
Maternal dietary supplementation with atRAL
For modulating the dose of RA in Rdh10trex/trex mutant embryos, supplemental atRAL was administered in the diet of pregnant female mice by mixing a suspension of all-trans-retinal (Sigma, R2500) with powdered mouse chow. Three different doses of atRAL supplementation were utilized in this study: 40 μg atRAL/gram maternal food/day from E7.5 through E11.5; 80 μg atRAL/gram maternal food/day from E7.5 through E11.5, and 300 μg atRAL/gram maternal food/day from E7.5 through E11.5. In each case, supplemental atRAL was administered on gestational days E7.5 through E11.5, replaced fresh each day, with the final dose of atRAL supplemented chow being removed on E12.5.
SMG ex vivo culture
SMG gland rudiments containing submandibular and sublingual salivary glands were dissected from mouse embryos at E12.5 or E13.5. SMG rudiments were cultured at the medium air/interface on filter disks (Whatman Nucleopore, 13mm, 0.1 μm pore size: VWR) supported at surface of medium by gaskets made from Syglard elastomer. Medium was DMEM/F12 with 100U/ml penicillin, 100 μg/ml streptomycin, 150 μg/ml Vitamin C, and 50 μg/ml transferrin. Gland rudiments were cultured in a humidified incubator at 37°C with 5%CO2/ 95% air. For experimental treatment with pan-RAR inhibitor, a stock solution of 1 mM BMS 493 (Tocris, #3509) in DMSO was added to medium for final concentration of 2 μM BMS 493 in culture medium. An equivalent volume of DMSO was added to medium for control samples.
Stain for RARE-lacZ or Rdh10βgeo reporter activity
RARE-lacZ or Rdh10βgeo reporter β-galactosidase activity was assayed by fixing whole embryo or tissue specimens in 2% Paraformaldehyde / 0.2% glutaraldehyde for 45–90 minutes on ice. Following fixation, specimens were rinsed and incubated 30 minutes at room temperature in Rinse Solution A: 5mM EGTA / 2 mM MgCl2 / PBS pH 7.3 (lab mixed or purchased from Millipore). Specimens were then rinsed and incubated 15 minutes at 37°C in pre-warmed Rinse Solution B: 2 mM MgCl2 / 0.01% Sodium deoxycholate / 0.02% NP40 / PBS pH 7.3 (lab mixed or purchased from Millipore). Stain Base solution: 0.5mM K3Fe(CN)6 / 0.5mM K4Fe(CN)6 / 2 mM MgCl2 / 0.01% Sodium deoxycholate / 0.02% NP40 / PBS pH 7.3 (lab mixed or purchased from Millipore), was pre-warmed to 37°C prior to addition of the reaction substrate X-gal. When specimens were fixed, rinsed, and ready to be stained, X-gal (Sigma-Aldrich B4252, suspended at 40mg/ml in Dimethyl Formamide) was added to Stain Base Solution to a final concentration of 1mg/ml. Specimens were incubated in stain solution overnight at 37°C in the dark. After staining, specimens were post-fixed in 4% Paraformaldehyde overnight at 4°C. For section staining of RARElacZ embryos, stained whole mount specimens were equilibrated to paraffin using minimal incubation times in Neo-Clear xylene substitute and embedded. After sectioning, slides were de-paraffinized and counterstained briefly with Nuclear Fast Red. For section staining of Rdh10βgeo embryos, unstained embryos were embedded in OCT compound (Tissue-TEK), cryosectioned, and stained for β-galactosidase activity. Stained sections on slides were post-fixed in 4% Paraformaldehyde 1 hour at room temperature, then counterstained briefly with Nuclear Fast Red.
Immunostain
Whole mount SMG tissues were immunostained as previously described (Sandell et al., 2014). Primary antibodies used were anti-β-glactosidase (Abcam ab9361) 1:500, anti-E-cadherin (BD Biosciences #610182) 1:50, anti-Neuronal Class III β-Tubulin (Covance, PRB0435-P) 1:1000. Fluorescently conjugated secondary antibodies Alexafluor 488, AlexaFluor 546 (Invitrogen), or Dynalight 488 (Abcam) were used at 1:300. Nuclei were stained with RedDot 2 (Biotium).
Analysis of SMG epithelial volume
Confocal z-stacks were rendered with the 3D image processing software IMARIS (Bitplane AG). Surface rendering of fluorescence signal was performed to quantitate volume enclosed by surface.
RESULTS
RA produced by RDH10-mediated metabolism of Vitamin A is required for development of SMG
As part of a comprehensive characterization of the requirement for RDH10-mediated metabolism of Vitamin A in embryonic development we have examined the phenotype of Rdh10−/− mutant embryos at multiple stages of gestation. Analysis of SMG growth was performed on embryos carrying the Rdh10trex mutant allele. In order to obtain Rdh10trex/trex mutant embryos E14.5 and older, the metabolic intermediate atRAL was supplemented to maternal food at a dose of 40 μg atRAL/gram maternal food/day from E7.5 through E11.5, henceforth “40 μg atRAL”. This low dose of atRAL was empirically determined to rescue a subset of embryos past the early embryonic lethality, yet leaving embryos very RA deficient. Under these atRAL supplementation conditions Rdh10−/− mutant embryos are recovered at E14.5 at ~50% the expected frequency (Table. S1). All mutant embryos recovered under these conditions exhibit strong RA deficiency phenotypes of orofacial malformations and small forelimbs.
Embryos partially rescued by maternal supplementation with 40 μg atRAL were collected at E14.5. Histological sectioning and staining reveals that the SMG of Rdh10trex/trex are much smaller than the SMG of wild type littermates (Fig. 1 B–E). The smaller size of the SMG in Rdh10trex/trex mutant embryos, is not an indirect consequence of reduced head size, as overall head size is similar in wild type and mutant embryos (Fig. 1 B–C). Heterozygous Rdh10trex/+ embryos were not distinguishable from homozygous wild type Rdh10+/+. The observation that SMG development is impaired in Rdh10trex/trex mutant embryos, which are deficient in production of RA, is consistent with previous descriptions of mouse embryos with compound mutations in multiple RAR genes, and in rat embryos with nutritional Vitamin A deficiency (Lohnes et al., 1995; Lohnes et al., 1994; See et al., 2008). In each case RA deficiency causes defects in salivary gland development. Our data additionally demonstrate that the RA needed for SMG development is produced by activity of RDH10.
Although the SMG of Rdh10trex/trex embryos were smaller than wild type littermates, their morphology appeared grossly normal. All histologically identifiable tissues were detected in Rdh10trex/trex mutant SMG, including branched epithelium, surrounding mesenchyme, and neurons. The parasympathetic ganglion and nerve of the SMG, which is known to be important for gland development, was evident in the Rdh10trex/trex SMG in histological sections. However, to assess the 3-dimensional morphology of the SMG parasympathetic ganglion and nerve in Rdh10trex/trex mutant embryos, whole mount glands from 40 μg atRAL supplemented litters were immunostained for βIII neuronal tubulin (Tubb3). Whole mount immunostaining revealed that the submandibular parasympathetic nerve of Rdh10trex/trex embryos is smaller, corresponding to the reduced size of the gland, but appears otherwise morphologically normal (Fig. 1 F–G). The relatively normal morphology of the parasympathetic nerve in Rdh10trex/trex glands suggests that failure of neurogenesis is not the primary defect in underdevelopment of the RA deficient SMG.
RA effect on SMG development is dose-dependent
We then investigated whether developmental growth of SMG is sensitive to RA in a dose-dependent manner. To address the issue of RA dosage, we examined embryos supplemented with two different doses of atRAL. Because it is production of the intermediate atRAL that is blocked in embryos lacking RDH10 function (Fig. 1A), RA levels may be modulated in Rdh10trex/trex mutant embryos by varying the amount of atRAL supplemented to the maternal diet. RA levels in mutant embryos can be varied from severely deficient up to nearly normal wild type levels. The amount and distribution of RA signaling can be inferred by the pattern activity of the RARE-lacZ reporter transgene, which drives expression lacZ in response to canonical RA signaling through RAR receptors (Rossant et al., 1991).
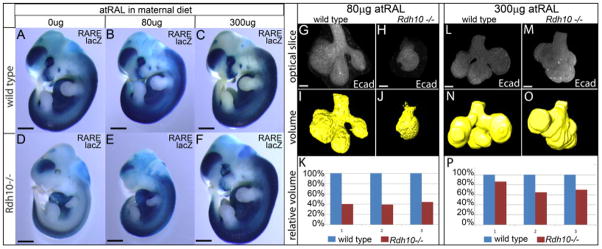
To examine embryos with different levels of RA signaling, pregnant females were supplemented with either 80 μg atRAL/g food/day E7.5 through E11.5, or with 300 μg atRAL/gram food/day E7.5 through E11.5. Because RA production is maintained in homeostatic balance via feedback regulation (D’Aniello and Waxman, 2015; Sandell et al., 2012), and because of the reversible nature of the Retinol/Retinal conversion reaction, supplementation with atRAL at these dosage levels does not markedly disturb normal RA signaling in wild type embryos (Fig. 2 A–C). In contrast to wild type embryos for which RA signaling remains relatively consistent at different doses of atRAL, supplementation, Rdh10trex/trex mutant embryos have graded levels of RA signaling from very deficient up to nearly wild type (Fig. 2 D–F). Rdh10trex/trex mutant embryos from unsupplemented litters have very little RA signaling (Fig. 2 D). In these embryos the small amount of RA activity is localized primarily within the trunk, and is not detected with developing orofacial tissues. Supplementation of the maternal diet with 80 μg atRAL yields Rdh10trex/trex mutant embryos with increased RA (Fig. 2 E) relative to unsupplemented embryos (Fig. 2 D), but still much lower than that of wild type littermates (Fig. 2 B). At this level, RA signaling is rescued preferentially within the trunk of the embryo, with developing orofacial tissues remaining very RA deficient. A more complete rescue of RA signaling is observed at the 300μg atRAL level. At this dose Rdh10trex/trex mutant embryos have RA signaling in a pattern nearly indistinguishable from that of wild type littermates (Fig. 2 C, F).
Figure 2. RA impacts developmental growth of SMG in a dose-dependent manner.
(A–F) Addition of different amounts of atRAL to the maternal diet yields Rdh10trex/trex mutant embryos with different levels of RA deficiency. Level of RA signaling is detected in E11.5 RARE-LacZ transgenic embryos by staining for β-galactosidase activity. Supplementation of the maternal diet with up to 300 μg atRAL/g food/day does not markedly perturb RA signaling in wild type embryos (B, C) relative to embryos developing without supplementation (A). Without atRAL supplementation Rdh10trex/trex embryos have severely reduced RA signaling activity (D) relative to wild type littermates (A). At 80 μg atRAL RA signaling is partially rescued in Rdh10trex/trex mutant embryos, with RA signal being rescued preferentially in the trunk of the embryo while orofacial RA signal remains deficient (E). At 300 μg atRAL supplementation, Rdh10trex/trex mutant embryos exhibit near complete rescue of RA signaling in the trunk and craniofacial structures (F). In these embryos RA signaling is similar in level and distribution to that of wild type littermates (C). (G–P) Size of Rdh10trex/trex SMG epithelium is smaller than wild type littermates as measured by 3-dimensional volume of E-cadherin signal. E-cadherin signal was collected as individual optical sections of whole mount SMG from wild type and Rdh10trex/trex embryos (G,H,L,M). For each z-stack data set, an epithelial volume was computationally rendered as a surface, either by automated signal detection (I–J), or manually (N–O), using IMARIS image analysis software. At the 80 μg supplementation dose the epithelium of Rdh10trex/trex mutant SMG are 40% the volume of their wild type littermates (K). At the 300 μg supplementation dose the epithelium of Rdh10trex/trex mutant SMG are ~70% the volume of their wild type littermates (P). For each condition n=3 litters, 1 gland/litter. Black scale bar is 1mm (A–F). White scale bars are 40μm (G,H,L,M).
In order to determine whether RA dose impacts developmental growth of SMG, we quantified the growth of SMG epithelium of Rdh10trex/trex mutant glands relative wild type littermates from 80 μg atRAL and from 300 μg atRAL supplementation litters. Embryos from atRAL supplemented dams were collected at E13.5 when SMG are at the pseudoglandular stage of development. SMG were isolated and whole mount specimens were immunostained for E-cadherin to reveal gland epithelium. Stained glands were then cleared and imaged by confocal microscopy (Fig. 2 G,H,L,M). Z-stacks of optical sections were collected through the entire gland epithelium. E-cadherin signal was then computationally rendered as surface volume using IMARIS image analysis software in order to assess the amount of gland epithelium in each specimen (Fig. 2 I,J,N,O). For calculation of epithelial volume primary ducts were included at a uniform length of ~40 μm.
Analysis of SMG epithelial volume from embryos rescued with 80 μg atRAL reveals that glands from Rdh10trex/trex mutant embryos are reproducibly underdeveloped relative to the glands of wild type littermates. With 80 μg atRAL, Rdh10trex/trex SMG were approximately 40% the size of wild type littermates (Fig. 2 K). The volume analysis reveals that, in addition to smaller size, mutant SMG have less branching morphogenesis. SMG from wild type embryos were branched with an average of four distinct end buds, as expected for the E13.5 pseudoglandular stage of development. In contrast, the corresponding SMG from Rdh10trex/trex embryos had only a single endbud.
Increased rescue of RA signaling resulted in improved growth and development of gland epithelium. At the 300 μg level of atRAL supplementation the epithelium of Rdh10trex/trex mutant SMG was approximately 70% that of wild type littermates (Fig. 2 P). In addition to the better rescue of epithelial volume, the higher level of atRAL supplementation resulted in improved rescue of branching morphogenesis. Rdh10trex/trex mutant SMG from 300 μg atRAL litters had more branches than those of the lower 80 μg atRAL rescue litters (Fig. 2O), although the branches were not as elongated as those of wild type SMG (Fig. 2 N) and clefts between branches were not as deep. Taken together, these data demonstrate that the influence of RA on SMG development is dose-dependent.
RA signaling in SMG tissues begins prior to initiation and continues throughout development
The observation that RA deficiency impairs SMG development prompted us to examine whether RA signaling was active in SMG tissues over the time course of development of the glands. Wild type embryos carrying the RARE-lacZ reporter were collected at multiple stages of gestation, prior to, and over the time course of, SMG morphogenesis. Glands or mandible tissues were isolated from embryos from E9.5 to E16.5 and were stained for β-galactosidase activity to identify the location of active RA signaling.
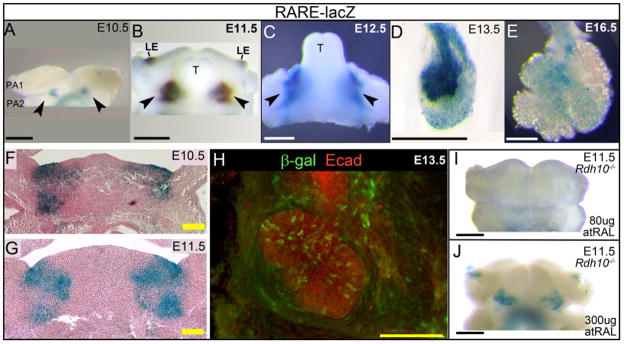
Analysis of RARE-lacZ activity at multiple gestational ages reveals that RA signaling activity is present in developing SMG tissues throughout morphogenesis of the glands (Fig. 3 A–E). Robust RA signaling is clearly evident within isolated glands at E13.5 (Fig. 3 D), corresponding to pseudo-glandular SMG that have begun branching morphogenesis. At this stage strong β-galactosidase activity is apparent within the gland epithelium. Scattered cells positive for RA signal are detected also within the surrounding mesenchyme. Analysis of mandibles isolated from earlier stage embryos reveals that RA signaling is present at E12.5 (Fig. 3 C), the stage when SMG initial bud formation has occurred by invagination of the oral epithelium into the underlying mesenchyme. At this stage RA signaling is observed within developing mandibles in two domains corresponding to the locations of SMG initial buds, one on each side of the base of the tongue.
Figure 3. RA signaling activity is present in SMG tissues throughout development of the gland.
RARE-lacZ reporter signal reveals active RA signaling in embryo tissues. (A–E) Staining for β-galactosidase activity reveals RA signaling is present throughout SMG development. (A) Two domains of active RA signaling are present on the interior region of the developing mandible at E10.5. (B) At E11.5, as the tongue begins to elevate on the interior surface of the mandible, a domain of active RA signal is visible on either side of the base of the tongue. (C) At E12.5, strong RA signal is visible at the base of the tongue in the position where the SMG initial bud has formed. (D) At E13.5, strong RA signal is visible in an isolated gland. (E) RA signaling can be detected in isolated SMG at E16.5. (F,G) Frontal paraffin section of stained mandibles reveals that RA signaling in E10.5 (F) and E11.5 (G) mandibles occurs within epithelial and mesenchymal tissues. (H) Immunostaining whole mount E13.5 SMG for β-galactosidase protein demonstrates RA signaling is present within individual cells of gland epithelium. (I, J) Domains of RA signaling in E11.5 mandibles of Rdh10trex/trex mutant embryos are modulated by atRAL supplementation. With 80μg atRAL supplementation, mandibles of Rdh10trex/trex mutant embryos lack RA signaling (I). With 300μg atRAL supplementation, RA signaling in mandibular domains is partially rescued (J). Wild type controls corresponding to (I,J) are shown in Supplemental Figure S1. Black scale bars, white scale bars = 500 μm. Yellow scale bar = 100 μm, Black arrowheads point to mandibular domains of active RA signal. LE, lateral edge of oral cavity; PA1, pharyngeal arch 1; PA2, pharyngeal arch 2; T, tongue.
Looking at earlier stages of development, we observe RA signaling in the mandible at E11.5 (Fig. 3B), the stage when SMG development can first be detected morphologically as a thickening of the oral epithelium on each side of the future base of tongue. At this stage we observe RA signaling on the interior face of the developing mandible as two domains corresponding to two sites of SMG initiation. RA signaling is also detected in the lateral edge of the oral cavity at this stage. A previous study using a Cre driver responsive to RA has shown that, at E12.5, RA signaling at the lateral edges of the oral cavity lineage marks a domain of cells that partially overlaps with the lateral edges of molar tooth buds (Dollé et al., 2010).
In order to determine the earliest onset of RA signaling activity in presumptive SMG tissues we examined RARE-lacZ expression in developing mandibles at E9.5 and E10.5, prior to any overt morphological development of the SMG. At E9.5, no RA signaling is detected in the mandible (not shown). At E10.5, two domains of RA signal become visible on the interior surface of the future mandible (Fig. 3 A). The two RA signaling domains at E10.5 encompass a region of pharyngeal arch 1, pharyngeal arch 2, and the pouch between them on the inner surface of the developing mandible. Analysis of frontal sections through the RARE-lacZ positive regions of E10.5 and E11.5 mandibles indicates that the RA signaling domains at E10.5 and E11.5 include both epithelial and mesenchymal tissues (Fig. 3 F, G). The two RA positive domains appear to correspond to those detected later at E11.5 and E12.5, although the developmental relationship between the E10.5 domains and those of later stages can only be demonstrated cell lineage analysis.
In addition to marking early developing SMG tissues, RA signaling persists later in SMG morphogenesis as well. At E16.5 scattered cells positive for RA signaling are detected within isolated glands (Fig. 3 E). At this canalicular/terminal bud stage of SMG development, a smaller fraction of cells are positive for RA signaling, suggesting that the peak of RA signaling occurs at earlier stages of development.
Enzymatic staining for β-galactosidase to detect RARE-lacZ transgene activity is an extremely sensitive assay for canonical RA signaling. However, fluorescent immunostaining for β-galactosidase protein and imaging by confocal microscopy can yield a more accurate picture of the distribution of cells with RA signaling. In order to precisely define the identity and location of cells with active RA signaling we co-immunostained E13.5 whole mount SMG for β-galactosidase protein, to visualize RA signaling, along with E-cadherin, to visualize gland epithelium. Confocal imaging of immunostained glands revealed that RA signaling occurs in a mosaic pattern in scattered cells of the epithelium of pseudoglandular SMG (Fig. 3 H). Cells positive for β-galactosidase are present in the branching endbuds, and in the primary duct. Scattered cells positive for β-galactosidase are also present within the gland mesenchyme, particularly within a region that appears as a “collar” around the primary duct.
We also assessed the level and distribution of RA signaling in mandibular tissues of Rdh10trex/trex embryos rescued at different levels of atRAL supplementation. Rdh10trex/trex and wild type littermate embryos carrying the RARE-lacZ transgene were rescued by maternal dietary supplementation of atRAL as described previously. Embryos were collected at E11.5 and mandibles stained for RA activity. At 80 μg atRAL, the E11.5 Rdh10trex/trex mandible has no evidence of RA signal (Fig. 3 I). At 300μg atRAL two domains of RA signaling were detected in the Rdh10trex/trex mandible (Fig. 3 J). RA signaling was detected wild type littermates at each dose (Supplemental Fig. S1). The pattern and level of RA signaling detected in Rdh10trex/trex mandibles is consistent with that observed for whole embryos. In each case 80 μg atRAL treatment provides only poor rescue of RA signaling to orofacial tissues, while 300μg atRAL provides a more complete rescue.
Initiation of SMG development in unsupplemented Rdh10−/− embryos
The presence of RA signaling in the region of SMG initiation in E11.5 embryos, and in E10.5 mandibles preceding SMG formation, suggests the possibility that RA signaling may be critical for promoting the initiation of SMG development. Our dose analysis experiment demonstrates that development of SMG initiates in embryos supplemented with atRAL. However, it is possible that the initiation of gland growth observed in supplemented embryos occurred because the supplemental atRAL yielded sufficient RA signal for initiation. We therefore investigated whether initiation of SMG development occurs in Rdh10trex/trex mutant embryos not supplemented with atRAL.
Without supplementation, most Rdh10−/− mutant embryos die prior to E11.5 or E12.5 (Rhinn et al., 2011; Sandell et al., 2012; Sandell et al., 2007). However, the phenotype of Rdh10−/− mutant embryos is variable, and E12.5 embryos can be recovered at low frequency in the absence of atRAL supplementation. Histological examination of a rare unsupplemented E12.5 Rdh10trex/trex mutant embryo reveals evidence of initiation of SMG development (Fig. 4 A, B). At the base of the tongue the oral epithelium is clearly invaginated into the underlying mesenchyme. The epithelial invagination appears abnormal, lacking a distinct bulbous endbud and primary duct. The presence of an epithelial invagination in an unsupplemented mutant embryo suggests that RA signaling is not necessary for initiation of SMG development. However, the interpretation must be made with great caution, as it is possible that a rare surviving E12.5 Rdh10trex/trex mutant embryo is an outlier in terms of RA deficiency, and that the RA status of the embryo that enabled survival to E12.5 was, likewise, sufficient to promote SMG initiation.
Figure 4. SMG initiation occurs in uncommon Rdh10−/− embryos surviving to E12.5 without atRAL supplementation.
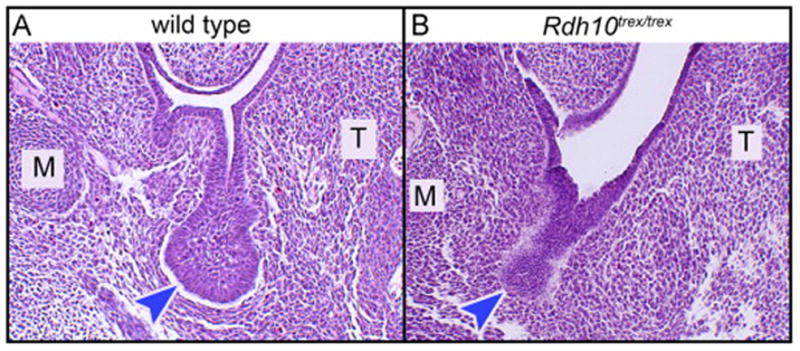
In the absence of atRAL supplementation few Rdh10−/− embryos survive past E11.5. Rare example of surviving E12.5 Rdh10−/− mutant embryo has evidence of initiation of SMG morphogenesis. (A) SMG of wild type embryo at the E12.5 stage of development is an initial bud with open primary duct lumen connecting to the oral surface. Initial bulbous endbud is distinctly wider than primary duct. (B) SMG of mutant embryo appears as a thick dense epithelial invagination into the mesenchyme. Primary “duct” has no lumen, “endbud” is approximately same width as “duct”. M, Meckel’s cartilage; T, tongue; blue arrowheads indicate terminal endbud, or endbud-like region, of SMG epithelial invagination.
RA production occurs in mesenchyme but RA signaling occurs primarily in epithelium of initial bud
We next sought to define the specific locations of RA production and the resulting distribution of RA signaling activity within developing SMG. To visualize the expression pattern of Rdh10, which is the major enzyme responsible for the initial step of metabolism Vitamin A into atRAL, we utilized Rdh10βgeo, a lacZ knock-out reporter allele of Rdh10 (Sandell et al., 2012). Heterozygous Rdh10βgeo/+ embryos, which are phenotypically wild type, were examined at E12.5 by staining frozen sections for β-galactosidase activity. The staining pattern of Rdh10βgeo reporter at the E12.5 revealed that Rdh10 is expressed exclusively within the gland mesenchyme at the initial bud stage of development (Fig. 5 A). No signal was detected within the gland epithelium. These data indicate that the initial step of RA production in the SMG occurs within the mesenchyme
Figure 5. RA production occurs in mesenchyme but RA signaling activity occurs in epithelium of initial bud .
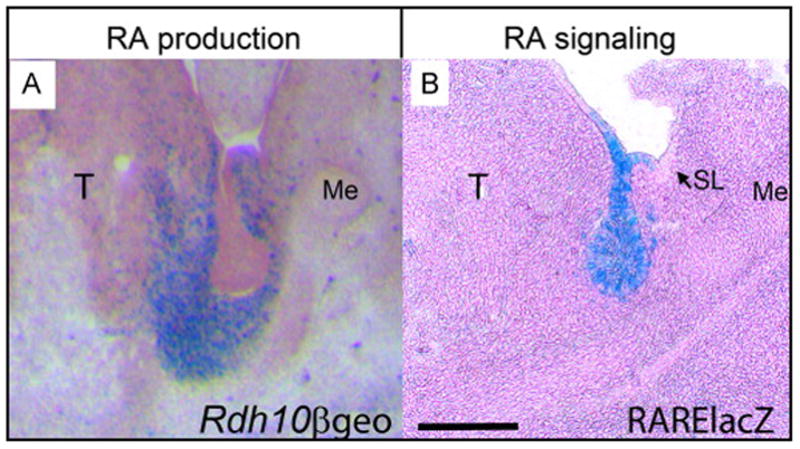
(A) Expression of Rdh10, indicated by Rdh10βgeo reporter activity, occurs in SMG mesenchyme at E12.5. Frozen sections were stained for β-galactosidase and lightly counterstained with nuclear fast red. (B) RA signaling activity, indicated by RARE-lacZ reporter, occurs primarily in SMG epithelium. A few scattered cells of mesenchyme are also positive for RA signaling, but sublingual gland epithelium is negative. Whole mount mandibles were stained for β-galactosidase, paraffin sectioned, and lightly counterstained with nuclear fast red. Scale bar = 200μm. Me, Meckel’s cartilage; SL, sublingual gland; T, tongue;
In order to compare the tissue localization of RA signaling with that of RA production we evaluated the distribution of RA signaling in E12.5 initial bud SMG using the RARE-lacZ reporter transgene. Whole mount specimens consisting of E12.5 tongue and associated SMG were stained for β-galactosidase activity followed by paraffin sectioning. RARE-lacZ reporter activity indicates that RA signaling occurs very robustly and almost exclusively within the epithelium of the E12.5 initial bud SMG (Fig. 5 B). In addition to the RA activity within the epithelium, a few scattered RARE-lacZ positive cells are also detected within the surrounding mesenchyme. Interestingly, the RA signaling activity in gland epithelium is specific for the SMG and is not detected within the epithelium of the adjacent sublingual gland. Taken together these data reveal that RA production occurs within the mesenchymal cells of the initial bud SMG, but RA activity occurs primarily within the epithelial cells.
RA signaling persists in SMG cultured ex vivo
The data presented above demonstrate that RA is required for developmental growth of the SMG. However, the fact that many laboratories have successfully cultured SMG ex vivo without added RA or serum raises the possibility that RA is not essential for SMG growth. We sought to reconcile our observations, that RA is needed for SMG development in vivo, with the ex vivo experimental model wherein SMG can be grown in medium lacking Vitamin A or RA. Because SMG freshly isolated from an embryo have robust RA signaling (Fig. 3 D), we postulated that retinoids and RA signaling might be present in gland tissues cultured ex vivo despite the absence of RA in the medium. To investigate whether RA signaling persists in cultured glands we examined expression of the RARE-lacZ transgene in SMG cultured on medium with no added RA or serum. At the start of culture freshly isolated E13.5 SMG exhibit robust RA activity (Fig. 6 A). After 48 hours in culture RA signaling can still be detected, occurring in scattered individual cells throughout the gland (Fig. 6 B). At this stage RA signal appears to coincide with epithelial tissue of the gland (Fig 6 B, B′). After 3 days of ex vivo culture RA signaling is still clearly detectable (Fig. 6 C). At the 3 day stage, RA signal appears to coincide with the location of non-epithelial tissues surrounding the main duct (Fig 6 C, C′).
Figure 6. RA signaling occurs in SMG tissues in ex vivo culture.
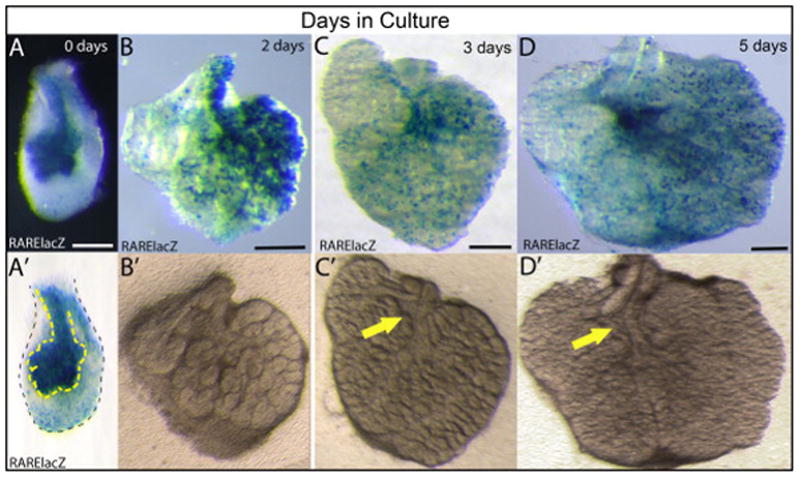
RARE-lacZ staining reveals active RA signaling in cultured SMG tissues. E13.5 SMG cultured 0–5 days ex vivo. (A, A′) When E13.5 SMG are initially isolated and placed in a culture dish, RA signaling is clearly evident, particularly in the area corresponding to epithelium. (A′) Outline of epithelium and mesenchyme. (B) RA signaling persists in E13.5 SMG cultured for 48 hours. (B′) Gland shown in (B) imaged prior to staining allows visualization of epithelium. (C) RA signaling remains in E13.5 SMG cultured for 3 days. (C′) Gland shown in (C) imaged prior to staining allows visualization of epithelium. (D) RA signaling remains in E13.5 SMG cultured for 5 days. (D′) Gland shown in (D) imaged prior to staining allows visualization of epithelium. Yellow arrows indicate position of non-epithelial tissue surrounding main duct. Scale bars = 200 μm.
One caveat to lacZ stain analyses is that β-galactosidase protein is known to be very stable, with a half-life estimated at 24–48 hours (Gonda et al., 1989; McCutcheon et al., 2010). Thus, over a 2 or 3 day time period, which is the standard duration of SMG culture experiments, β-galactosidase staining activity could potentially result, not from active RA signaling in the cultured specimens, but from residual β-galactosidase that was produced within the animal prior to culture. In order to discern if the RARE-lacZ β-galactosidase detected in cultured SMG indicates active RA signaling, we assayed RARE-lacZ activity from SMG that were cultured for 5 days, a duration substantially longer than the half-life of β-galactosidase. Surprisingly, strong RA signaling is clearly evident in SMG cultured for 5 days ex vivo (Fig. 6 D) Again, as at the 3 day stage, the distribution of RA signal appears to coincide with the location of non-epithelial tissues surrounding the main duct (Fig. 6 D, D′). These data demonstrate that retinoids present within the freshly isolated tissue persist in cultured glands and contribute to active RA signaling despite the lack of Vitamin A or RA in the medium.
RA signaling regulates growth and branching of ex vivo cultured SMG
Having established that active RA signaling occurs in ex vivo cultured SMG, we next investigated whether the RA signaling in cultured gland tissues is relevant to their growth. To that end we cultured pseudoglandular SMG in the presence or absence of the pan-RAR inhibitor BMS 493. SMG were isolated from E13.0–E13.5 embryos. Right and left glands from individual embryos were cultured as matched pairs, one on medium containing 2 μM BMS 493, the other on control on medium containing vehicle (DMSO). Glands were cultured for 72 hours with daily changes of medium.
After 48 hours in culture, the epithelial growth and branching morphology SMG grown on BMS 493-containing medium was clearly impaired relative to contralateral glands grown on control medium (Fig. 7 A–B). Glands grown on control medium had substantially more epithelium with more branches and endbuds than BMS 493-treated glands. After 48 hours culture, control glands had an average of 29 endbuds, while BMS 493-treated glands had an average of 3 endbuds (Fig. 7 E). The impaired growth and branching morphogenesis of glands cultured with BMS 493 was clearly evident following immunostain for E-cadherin (Fig.7 C–D). BMS 493-treated glands had shallower clefts and fewer branches and endbuds than controls. The endbuds of BMS-treated glands were fewer in number, but larger in size. Similar differences in growth and branching morphogenesis of BMS 493 treated SMG and controls were visible at the 24 hour time point, and following 72 hours of culture. The abnormal growth of SMG cultured in the presence of the pan-RAR inhibitor BMS 493 demonstrates that the RA signaling persisting in ex vivo cultured glands is relevant for their growth. Moreover, because perturbation of RA signaling impacts growth of isolated glands, the data reveal that RA signaling impacts gland growth directly and not merely as an indirect consequence of mandibular patterning.
Figure 7. Treatment with the RAR inhibitor BMS 493 disrupts growth and branching morphogenesis in ex vivo cultured SMG.
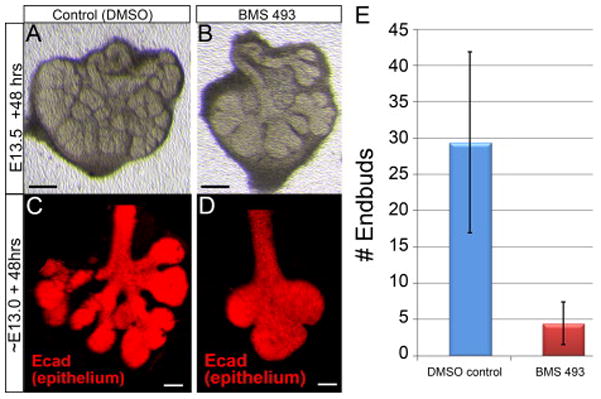
(A–B) Transmitted light images of two SMG isolated from a single embryo at E13.5 cultured ex vivo for 48 hours. SMG cultured on medium containing 2 μM BMS 493 (B) had less epithelial growth with fewer branches and endbuds relative to the contralateral gland grown on control medium (A). (C–D) SMG isolated at E13.0 and cultured ex vivo for 48 hours and stained with E-cadherin to reveal epithelium. BMS 493 – treated glands had fewer endbuds and shallower clefts (D) than contralateral gland grown on control medium (C). (E) Quantitation of branching morphogenesis by counting endbuds of E13.5 SMG cultured 48 hours. SMG grown on control medium had an average of 29 endbuds. Contralateral glands grown on medium containing BMS 493 had an average of 3 endbuds. N = 10 gland pairs. Error bars = Standard Deviation. Black scale bars = 200μm, white scale bars = 50 μm.
DISCUSSION
RA signaling is one of the key pathways that regulate many aspects of embryonic development. Despite the importance of RA, and the need for understanding salivary gland biology, the role of RA signaling in salivary gland morphogenesis has largely been overlooked.
In this study we demonstrate that RA signaling is a critical pathway important for embryonic organogenesis of the SMG. We show that RA signaling is active within developing SMG tissues from E11.5–E16.5 (Fig. 3 B–E), and that RA signal marks two domains within the developing mandible as early as E10.5, prior to any overt morphogenesis of the gland (Fig. 3 A). We also show that SMG development is impaired in Rdh10−/− mutant embryos (Fig. 1 B–E), demonstrating not only that RA is required for SMG development in vivo, but also that the RA necessary for SMG development is produced by the activity of RDH10. By examining Rdh10−/− mutant embryos supplemented with different doses of the metabolic intermediate atRAL, we demonstrate that developmental growth of SMG in vivo is influenced by RA in a dose-dependent manner (Fig. 2 A–P). By comparing the activity of reporters of Rdh10 expression versus RA signaling we show that the first step of RA production occurs within initial bud mesenchyme, while the resulting RA signaling occurs within the epithelium (Fig. 5 A–B). We demonstrate that SMG grown in culture have active RA signaling, persistent from an in vivo source (Fig. 6 A–E), and show that treatment of cultured glands with the RAR inhibitor BMS 493 impairs epithelial growth and branching morphogenesis (Fig. 7 A–E). These findings demonstrate that production of RA by RDH10-mediated metabolism of Vitamin A regulates SMG development during embryogenesis and does so by direct action on gland tissues.
Previous studies have suggested that salivary gland morphogenesis is regulated by RA. Although no salivary gland phenotype has been reported for single Rar mutations, mice with compound double or triple Rar mutations have been noted to have defects of salivary gland development (Lohnes et al., 1995; Lohnes et al., 1994). Rat embryos developing with late stage nutritional Vitamin A deficiency exhibit defects in salivary gland development (See et al., 2008). In humans, exposure to excessive RA in utero has been associated with salivary gland aplasia (Adam et al., 2007). These observations indicate that salivary gland development is disrupted if RA signaling is insufficient or excessive. However, they do not reveal whether RA signaling regulates embryonic gland tissues directly, or if the influence of RA is indirect, for example, owing to its well-established role in patterning the pharyngeal arches (reviewed in (Mark et al., 2004)). Our data demonstrate that properly regulated RA signaling is necessary for SMG morphogenesis, and that RA is produced in, and acts upon, SMG tissues directly.
The presence and distribution of active RA signaling within developing salivary glands has not been previously examined in any detail. Lineage tracing analysis with an RA responsive RARE Cre driver shows strong labeling of epithelium of an unspecified salivary gland at E17.5, indicating that RA signaling was active in salivary epithelium at, or prior to, E17.5 (Dollé et al., 2010). Several genes involved in RA production or RA signaling, including Rdh10, Aldh1a family members, and Rar family members, have been shown to be expressed in salivary gland tissues at E14.5 (Dolle et al., 1990; Visel et al., 2004). However, our study is the first to demonstrate that active RA signaling in SMG tissues begins much earlier and continues over the time course of gland development. We document that RA signaling occurs in two domains of the mandible at E10.5, beginning as two small patches that appear to overlap with, or correspond to, the area where the bilaterally paired SMG will subsequently develop. RA signal increases through the initial bud stage at E12.5, peaking at the pseudoglandular stage of development at E13.5, and then persists in dispersed cells through at least the E16.5 stage of gland development. The pattern of reporter staining indicates that RA signaling is present and active during SMG initiation, as well as during early epithelial growth, and branching morphogenesis. The presence of RA signaling at E10.5 in the area of the mandible near or overlapping where the SMG will subsequently develop is particularly intriguing, as the E10.5 stage is when the mandibular epithelium gains the capacity to induce gland formation in combination with non-gland mesenchyme (Wells et al., 2013). Despite the developmental importance of this early event, no other molecule has been identified that marks or overlaps with the future SMG domain at this early stage.
Development of SMG involves dynamic interactions between epithelium and mesenchyme (Kratochwil, 1969; Kusakabe et al., 1985; Tucker, 2007; Wells et al., 2013). Our observation that the E12.5 SMG mesenchyme expresses Rdh10, while SMG epithelium at that stage experiences active RA signaling (Fig. 5 A–B) suggests that diffusion of the metabolic product RA or the intermediate atRAL away from the site of synthesis in the mesenchyme into the adjacent epithelium may be one means of interaction between these two tissue types.
Ex vivo organ culture of SMG has been extensively used as an experimental model system for the study of SMG organogenesis. Our data demonstrate that, somewhat surprisingly, RA signaling is active in cultured glands despite the lack of Vitamin A or RA within the culture medium (Fig. 6 A–D). Although the scattered RA-positive cells observed in cultured glands have a distribution that is reminiscent of the pattern seen in freshly isolated glands from corresponding staged embryos (Fig. 6 C and 3 E), we do not know if the RA is present the cultured glands accurately reflects the RA signaling pattern that occurs in vivo. In vivo, disturbances of RA signaling levels are buffered by feedback regulation of RA production, RA signaling, and RA degradation, and can yield paradoxical consequences in RA regulation (D’Aniello et al., 2013; D’Aniello and Waxman, 2015; Lee et al., 2012; Sandell et al., 2012). Thus, it is possible that cultured glands, lacking the Vitamin A precursor normally available in circulating serum, experience feedback regulatory changes in RA homeostasis relative to glands in vivo, possibly increasing transcription of RAR receptors or decreasing transcription of RA degrading enzymes. Similarly, owing to the feedback mechanisms that mediate homeostatic regulation of RA signaling, the effect of treatment with RAR inhibitor may be more complex than a simple block of RA signaling. Nonetheless, our observation that treatment with BMS 493 impairs growth of glands in culture demonstrates a direct link between RA signaling and gland morphogenesis (Fig. 7 A–D).
Our study demonstrates that Vitamin A metabolism and RA signaling are critical for embryonic development of the SMG. However, the importance of RA in salivary gland biology is likely not limited to their developmental growth in the embryo. RA may play a role in post-natal salivary gland maintenance and regeneration as well. Studies of salivary gland function in rat indicate that Vitamin A metabolism / RA production are relevant to salivary gland health and function after birth (Anzano et al., 1981; Anzano et al., 1980; Horn et al., 1996; Johansson et al., 1989; Trowbridge, 1969). Radiation-induced salivary gland damage is exacerbated by Vitamin A deficiency (Funegård et al., 1991). In humans, chronic nutritional deficiency in children in India, where Vitamin A deficiency is prevalent, is associated with reduced saliva secretion (Johansson et al., 1994).
In summary, we have shown that RDH10-mediated metabolism of Vitamin A generates RA that activates RA signaling within the developing SMG, and that the RA signaling directly regulates embryonic growth and morphogenesis of the gland.
Supplementary Material
Highlights.
Retinoic Acid is important for mammalian submandibular salivary gland organogenesis.
Retinoic Acid signaling is active in salivary tissue throughout gland morphogenesis.
Retinoic Acid is produced in gland mesenchyme, while signaling occurs in epithelium.
Retinoic Acid signaling regulates growth and branching of glands grown ex vivo.
Acknowledgments
Research in the Sandell laboratory is supported by the University of Louisville School of Dentistry, and by P20 RR017702 to Dr. Robert M. Greene, Birth Defects Center, University of Louisville, Louisville, KY. The Microscopy Suite at the Cardiovascular Innovation Institute in Louisville, KY is supported by GM103507.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam MP, Abramowsky CR, Brady AN, Coleman K, Todd NW. Rhabdomyomatous hamartomata of the pharyngeal region with bilateral microtia and aural atresia: A new association? Birth Defects Res. Part A: Clin Mol Teratol. 2007;79:242–248. doi: 10.1002/bdra.20338. [DOI] [PubMed] [Google Scholar]
- Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects: Thematic Review Series: Fat-Soluble Vitamins: Vitamin A. J Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzano MA, Lamb AJ, Olson JA. Impaired salivary gland secretory function following the induction of rapid, synchronous vitamin A deficiency in rats. J Nutr. 1981;111:496–504. doi: 10.1093/jn/111.3.496. [DOI] [PubMed] [Google Scholar]
- Anzano MA, Olson JA, Lamb AJ. Morphologic alterations in the trachea and the salivary gland following the induction of rapid synchronous vitamin A deficiency in rats. Am J Pathol. 1980;98:717–732. [PMC free article] [PubMed] [Google Scholar]
- Billings SE, Pierzchalski K, Butler Tjaden NE, Pang X-Y, Trainor PA, Kane MA, Moise AR. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. The FASEB Journal. 2013 doi: 10.1096/fj.13-227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett-Dame M, Knutson D. Vitamin a in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aniello E, Rydeen AB, Anderson JL, Mandal A, Waxman JS. Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid. PLoS Genet. 2013;9:1–13. doi: 10.1371/journal.pgen.1003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aniello E, Waxman JS. Input overload: Contributions of retinoic acid signaling feedback mechanisms to heart development and teratogenesis. Dev Dyn. 2015;244:513–523. doi: 10.1002/dvdy.24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P, Fraulob V, Gallego-Llamas J, Vermot J, Niederreither K. Fate of retinoic acid–activated embryonic cell lineages. Dev Dyn. 2010;239:3260–3274. doi: 10.1002/dvdy.22479. [DOI] [PubMed] [Google Scholar]
- Dolle P, Ruberte E, Leroy P, Morriss-Kay G, Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990;110:1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hernandez RE, Waxman JS, Yelon D, Moens CB. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev Biol. 2010;338:1–14. doi: 10.1016/j.ydbio.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funegård U, Johansson I, Franzén L, Ericson T. Acute Radiation Effects on Saliva Composition in Rats with Different Vitamin a levels in Serum. Acta Oncol. 1991;30:975–980. doi: 10.3109/02841869109088252. [DOI] [PubMed] [Google Scholar]
- Gonda DK, Bachmair A, Wunning I, Tobias JW, Lane WS, Varshavsky A. Universality and structure of the N-end rule. J Biol Chem. 1989;264:16700–16712. [PubMed] [Google Scholar]
- Holmberg KV, Hoffman MP. Anatomy, biogenesis and regeneration of salivary glands. Monogr Oral Sci. 2014;24:1–13. doi: 10.1159/000358776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn VJ, Redman RS, Ambudkar IS. Response of rat salivary glands to mastication of pelleted vitamin A-deficient diet. Arch Oral Biol. 1996;41:769–777. doi: 10.1016/s0003-9969(96)00069-6. [DOI] [PubMed] [Google Scholar]
- Johansson I, Lenander-Lumikari M, Saellstrom AK. Saliva composition in Indian children with chronic protein-energy malnutrition. J Dent Res. 1994;73:11–19. doi: 10.1177/00220345940730010101. [DOI] [PubMed] [Google Scholar]
- Johansson I, Lumikari M, Ericson T. Effect of a moderate vitamin A deficiency on saliva secretion rate and some salivary glycoproteins in adult rat. Scand J Dent Res. 1989;97:263–267. doi: 10.1111/j.1600-0722.1989.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Knosp WM, Knox SM, Hoffman MP. Salivary gland organogenesis. Wiley Interdisciplinary Reviews: Developmental Biology. 2012;1:69–82. doi: 10.1002/wdev.4. [DOI] [PubMed] [Google Scholar]
- Knosp WM, Knox SM, Lombaert IM, Haddox CL, Patel VN, Hoffman MP. Submandibular parasympathetic gangliogenesis requires sprouty-dependent Wnt signals from epithelial progenitors. Dev Cell. 2015;32:667–677. doi: 10.1016/j.devcel.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IMA, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, Hoffman MP. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SM, Lombaert IMA, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic Innervation Maintains Epithelial Progenitor Cells During Salivary Organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71. doi: 10.1016/0012-1606(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Kusakabe M, Sakakura T, Sano M, Nishizuka Y. A pituitary-salivary mixed gland induced by tissue recombination of embryonic pituitary epithelium and embryonic submandibular gland mesenchyme in mice. Dev Biol. 1985;110:382–391. doi: 10.1016/0012-1606(85)90097-1. [DOI] [PubMed] [Google Scholar]
- Lee LMY, Leung CY, Tang WWC, Choi HL, Leung YC, McCaffery PJ, Wang CC, Woolf AS, Shum ASW. A paradoxical teratogenic mechanism for retinoic acid. Proceedings of the National Academy of Sciences. 2012;109:13668–13673. doi: 10.1073/pnas.1200872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dollé P, Decimo D, LeMeur M, Dierich A, Gorry P, Chambon P. Developmental roles of the retinoic acid receptors. The Journal of Steroid Biochemistry and Molecular Biology. 1995;53:475–486. doi: 10.1016/0960-0760(95)00094-g. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Retinoic acid signalling in the development of branchial arches. Curr Opin Genet Dev. 2004;14:591–598. doi: 10.1016/j.gde.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon S, Jones K, Cumming S, Kemp R, Ireland-Zecchini H, Saunders J, Houghton C, Howard L, Winton D. Characterization of a heat resistant SZ-glucosidase as a new reporter in cells and mice. BMC Biol. 2010;8:89. doi: 10.1186/1741-7007-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol. 2014;25–26:52–60. doi: 10.1016/j.semcdb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Schuhbaur B, Niederreither K, Dolle P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc Natl Acad Sci U S A. 2011;108:16687–16692. doi: 10.1073/pnas.1103877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Goguere V. Expression of retinoic acid response element-hsp lacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Sandell LL, Butler Tjaden NE, Barlow AJ, Trainor PA. Cochleovestibular nerve development is integrated with migratory neural crest cells. Dev Biol. 2014;385:200–210. doi: 10.1016/j.ydbio.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Lynn ML, Inman KE, McDowell W, Trainor PA. RDH10 Oxidation of Vitamin A Is a Critical Control Step in Synthesis of Retinoic Acid during Mouse Embryogenesis. PLoS ONE. 2012;7:e30698. doi: 10.1371/journal.pone.0030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See AWM, Kaiser ME, White JC, Clagett-Dame M. A nutritional model of late embryonic vitamin A deficiency produces defects in organogenesis at a high penetrance and reveals new roles for the vitamin in skeletal development. Dev Biol. 2008;316:171–190. doi: 10.1016/j.ydbio.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Trowbridge HO. Salivary gland changes in vitamin-A-deficient rats. Arch Oral Biol. 1969;14:891. doi: 10.1016/0003-9969(69)90267-2. IN812. [DOI] [PubMed] [Google Scholar]
- Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18:237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KL, Gaete M, Matalova E, Deutsch D, Rice D, Tucker AS. Dynamic relationship of the epithelium and mesenchyme during salivary gland initiation: the role of Fgf10. Biology Open. 2013 doi: 10.1242/bio.20135306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.