Abstract
Background
Although IgE antibodies to cow's milk and wheat are common in patients with EoE, titers are low and responses to diet are not dependent on having IgE antibodies.
Objective
To better define specific IgE antibody responses to foods, focusing on those foods that appear to play a role in EoE.
Methods
Adult (n=46) and pediatric (n=51) EoE patients were recruited for skin prick testing and serum measurement (whole and diluted) of IgE specific for aeroallergens, food extracts and component allergens by ImmunoCAP. Immuno Solid-phase Allergen Chip (ISAC) analysis was also used to measure IgE to 112 allergen molecules.
Results
In adults and children, there was a higher prevalence of sensitization to food extracts by ImmunoCAP compared with skin prick testing. Using ISAC to assess the specificity of IgE antibodies to 112 allergen molecules, results for food allergens were mostly negative. In contrast, ImmunoCAP assays for specific milk allergens gave positive IgE antibody results in 31/34 sera. The correlations between specific IgE antibody to Bosidi4 or Bos d 5 and milk extract were strong (R=0.89 and R=0.76 respectively; p<0.001). The evidence that IgE to foods was directed at minor components of the extracts was further supported by measurements on diluted sera.
Conclusion
The IgE responses in cow's milk sensitized EoE patients are frequently to whey proteins Bos d 4 and Bos d 5, minor components of the extract. These IgE assays may be able to identify the proteins that are relevant to EoE even though IgE is not the primary mechanism.
Keywords: eosinophilic esophagitis, food allergy, serum IgE measurements, component resolved diagnostics
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic disease that affects children and adults. In some patients it starts in childhood and lasts into adulthood.1,2 In most patients, EoE is associated with food and aeroallergen sensitization.3-8 Furthermore, the disease typically improves or resolves with food elimination diets.5,9-12 However, symptoms do not usually occur immediately upon ingestion of problem food(s). Therefore, triggers can be difficult to identify, and the contribution of specific antigens to the disease process is not yet understood.
When evaluating EoE patients, serum IgE and skin prick testing to foods is recommended for consideration of immediate hypersensitivity; although, the role of these diagnostic modalities in planning dietary treatment is not clear.3 The relationship between skin prick testing and clinical response to diet has been described with positive predictive values for individual foods ranging from 57-96% and negative predictive values ranging from 14-65%.9 As such, in some children, resolution of symptoms has been demonstrated in patients who avoid milk and foods that are positive by skin prick and patch testing.10 However in other pediatric and adult studies, although food seems to play a causal role, skin testing has not identified the problem food(s).11-12 We have previously reported in pediatric patients that serum IgE antibody assays detect more allergic sensitization to foods than skin prick testing.8 This may also be true for adults.6
In Europe, measurements of IgE specific for purified allergens (components) have suggested that at least some food sensitization in adults with EoE is related to (birch) pollen cross-reactivity.13 In general among patients with food allergy other than EoE, sensitization to specific allergenic molecules has provided information about distinct clinical symptoms upon exposure, and the pathway for development of IgE antibodies.14-16 In addition, it has been reported that for the same clinical pathways, the prevalence of sensitization to different allergen specificities may vary regionally.17 Serum IgE to specific proteins has not been reported in patients with EoE who live in the United States. We report on a cross-sectional study using different testing modalities to detect and delineate IgE antibodies in patients with EoE. The objective of this study was to measure food and aeroallergen sensitization (IgE antibody positivity) in adult and pediatric patients recruited in parallel and to investigate serum IgE to component allergens for those foods that elimination diets suggest may be relevant to the eosinophilic inflammation.
METHODS
Adult patients (n=46) who were referred to the Allergy Clinic at The Ohio State University Wexner Medical Center for evaluation of EoE and had >15 eosinophils/high power field (hpf) documented by esophageal biopsy were recruited between September, 2010 and December, 2013. Although not all of the patients had been treated with proton pump inhibitor (PPI) for a full 8 weeks prior to biopsy, over 90% were taking a PPI at allergy evaluation and had not had resolution of symptoms. We did not exclude patients who had not been fully treated with PPI prior to the biopsy. This study was approved by the institutional review board of The Ohio State University, and all patients provided written informed consent.
Pediatric patients (n=51) were recruited from the allergy clinic at Nationwide Children's Hospital (Columbus, Ohio) during the same time period. A separate protocol for children was approved by the IRB at Nationwide Children's Hospital.
At a single study visit, subjects completed questionnaires detailing symptoms of EoE and treatment for other allergic diseases. In adults, skin prick tests were performed to multiple foods (chicken egg, cow's milk, wheat, soy, peanut, tree nuts, fish, shellfish, legumes, grains, meats, and vegetables) and a range of common aeroallergens. Tests were applied with a Greer Pick (Greer, Lenoir, NC) using standard allergen extracts. In pediatric patients, skin prick tests were performed to a panel of fifteen foods (chicken egg, cow's milk, wheat, soy, peanut, cashew, shrimp, oat, beef, chicken, pork, rice, rye, pea, corn) and a range of common aeroallergens using Sharp-Test Applicators (Panatrex, Placentia, CA). A skin wheal at least 3 mm larger than the negative diluent control was considered positive.
Total serum IgE and allergen extract-specific IgE antibodies were measured by ImmunoCAP (Thermo Fisher Scientific/Phadia, Uppsala, Sweden). The food and aeroallergen specificities tested included cow's milk, chicken egg, wheat, soy, peanut, cashew, beef, dust mite, cat, dog, mold mix, birch, rye grass, weed mix, and ragweed. Specific IgE antibodies to galactose-α-1,3-galactose (alpha-gal), MUXF3 (bromelain), Candida albicans, and Staphylococcal enterotoxins A and B were also measured by ImmunoCAP. Specific IgE antibody results ≥0.35 IU/mL were considered positive. In addition for patients who had a positive IgE antibody test to cow's milk, wheat, or peanut, IgE specific for molecular allergens (i.e. components) from these whole food extracts were also measured using ImmunoCAP (Table E1, A and B). To test for quantitative accuracy, samples with positive results to wheat, milk, or soy and enough remaining serum, were re-analyzed at serial dilutions (1:2 through 1:8) by ImmmunoCAP (See methods in online repository).
Immuno Solid-phase Allergen Chip (ISAC) analyses were performed at Johns Hopkins University to evaluate each serum for IgE antibodies to 112 allergen molecules (Thermo Fisher Scientific/Phadia, Uppsala, Sweden).18,19 ISAC testing is a multiplex assay that uses purified allergen molecules that have been immobilized in triplicate on a glass chip in a microarray format. Following chip preparation, 30 microliters of serum were applied to the chip for two hours. After a buffer wash, bound IgE antibody was detected with FITC-labeled anti-human IgE. Image acquisition of the chip was collected in a microarray fluorescence scanner and IgE antibody results were interpolated from a standard curve into semiquantitative ISAC Standardized Units (ISUs) with 0.3 ISU as the lower limit of quantitation. IgE antibody levels ranging from 0.3 to 3 were considered low, 3-15 moderate, and >15-100 high.
The frequencies of individual clinical characteristics in adult and pediatric patients were compared using the chi-square test. We analyzed the prevalence of IgE antibody results to dust mite, cat, cow's milk, wheat, peanut, and grass in comparison with those from an unselected pediatric cohort (Table E2). Total serum IgE and allergen-specific IgE antibody levels were log transformed for analysis. Geometric mean (GM) quantities of IgE antibodies to food and inhalant allergens were compared by ANOVA. Linear regression was used to compare specific IgE levels to food allergens and component allergens and cross reactive pollen aeroallergens. Statistical analyses were performed using SPSS software, version 19 (IBM, Armonk, NY).
RESULTS
Clinical characteristics and skin prick testing
As expected, our adults and children were significantly different with regard to their presenting symptoms and visual endoscopic findings. Using skin prick testing, more adults than children with EoE showed evidence of sensitization to food (63% and 39% respectively) (p=0.02). Restricted sensitization to food was only seen in a few children (5.9%) and the rate of sensitization to aeroallergens was not different among the two groups; however, a greater proportion of adults had a positive skin prick test to both food(s) and environmental allergens (p=0.003) (Table 1). In both adults and children with EoE, most of whom had multiple allergic sensitivities and other allergic diagnoses, total serum IgE was not elevated as a group. The total serum IgE was lower in adults [GM 52 IU/ml (95% CI 5.4-110)] compared with children [GM 120 IU/ml (95% CI 100-310) (p=0.01)]. A subgroup of patients (10/97, of whom 9/10 were children) had an elevated total serum IgE > 600 IU/ml. Among the group with elevated total serum IgE, a diagnosis of asthma was more common (p=0.01), and peripheral blood eosinophil counts were higher (mean 647/mm3) compared with the others (mean 209/mm3) (p=0.05).
Table I.
Clinical characteristics, skin prick tests, and endoscopy results on EoE patients.
| Clinical Feature | Adults* (n=46) | Children† (n=51) | p value* | ||
|---|---|---|---|---|---|
| Age (y), median (range) | 38 (18-64) | 11 (1.4-20) | |||
| Male gender, n and % | 28 | 61% | 34 | 67% | 0.6 |
| Allergic sensitization†, n | |||||
| None | 3 | 6.5% | 12 | 24% | 0.03‡ |
| Food only | 0 | 3 | 5.9% | ---‡ | |
| Aeroallergen only | 13 | 28% | 16 | 31% | 0.7 |
| Food and aeroallergen | 29 | 63% | 17 | 33% | 0.003 |
| Associated diseases, n | |||||
| Asthma ever | 15 | 33% | 25 | 49% | 0.1 |
| Rhinitis | 35 | 76% | 36 | 71% | 0.5 |
| Self-reported food allergy | 18 | 39% | 20 | 39% | 1.0 |
| Symptom(s), n | |||||
| Dysphagia | 38 | 83% | 26 | 51% | 0.001 |
| Food stuck | 43 | 93% | 28 | 55% | <0.001 |
| Vomiting | 8 | 17% | 28 | 55% | <0.001‡ |
| Abdominal pain | 16 | 35% | 28 | 55% | 0.05‡ |
| Reported stricture | 15 | 33% | 0 | --- | |
| Endoscopy, n | |||||
| Furrows | 17 | 37% | 37 | 73% | <0.001‡ |
| Rings | 16 | 35% | 2 | 3.9% | <0.001 |
| White plaques | 5 | 11% | 15 | 29% | 0.04‡ |
| Stricture(s) | 6 | 13% | 0 | --- | |
| Biopsy results (eos/hpf§) | 15-50€ | 20-110± | --- | ||
| Total IgE GM (95% CI) | 52 (5.4-110) | 120 (100-310) | 0.01 | ||
p value based on X2 test comparing adult and pediatric groups except as noted.
Sensitization in this table is based on having a positive skin prick test.
more common in children.
hpf: high power field.
Biopsy results for three adults were reported as “consistent with EoE”.
One child's biopsy was reported as >100.
Histories of an allergic reaction to food
At enrollment, all patients were asked whether they had ever had an “allergic” reaction to food and if yes, to what food(s). Although this self-diagnosed food allergy was reported by 18 adults (39%) and 20 children (39%) (Table 1), only 26% of the cohort reported reactions to foods that have been shown to be responsible for inflammation in EoE (i.e. milk, wheat, egg, soy, or peanut).12 Pediatric patients reported reactions to peanut or tree nut most frequently while adults most often reported seafood. Only four people reported allergic reactions to cow's milk (3 adults and 1 child), and among those patients only one had a positive test to cow's milk by skin prick or serologic evaluation. An allergic reaction to wheat was reported by two adults. In contrast with the results for cow's milk and wheat, most of the patients who reported reactions to peanut also had positive skin prick or serologic testing (11/12). Thus, typical immediate hypersensitivity reactions were only common for peanut and seafood. Of note, 12 adult patients who did not report having an immediate reaction to peanut and/or tree nut had positive skin prick testing to peanut and/or tree nut. In contrast, 9/10 children with positive skin prick test to peanut and/or tree nut were the same patients who reported a reaction or a possible reaction.
Serological assays to whole food extracts and aeroallergen extracts
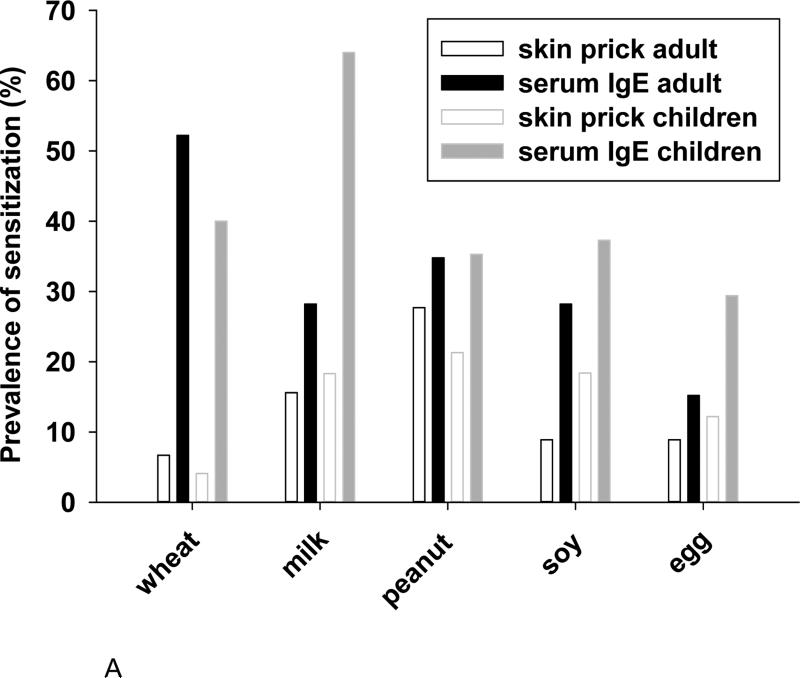
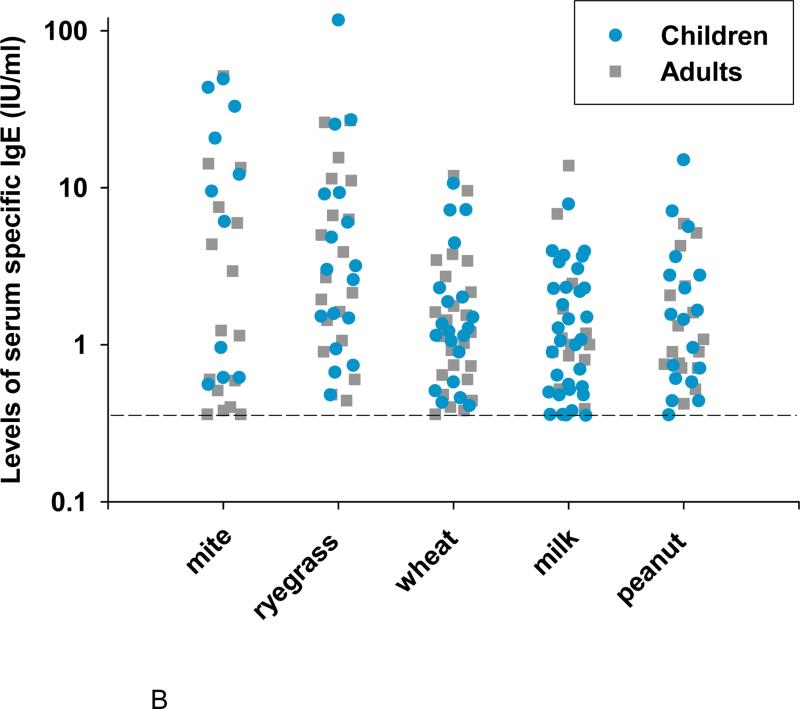
In keeping with our previously published results on a different group of children with EoE8, the serological analyses of adults and children showed a higher prevalence of sensitization to foods than did skin prick tests (Fig 1, A). The prevalence of IgE antibodies to wheat, cow's milk, peanut, and soy by serological methods was striking (See Table E2). In comparison with the pediatric patients, adults were less likely to have IgE antibodies to cow's milk; however, the frequency of sensitization to milk was still high at 28% (Fig 1, A). Concurrent sensitization to soy was found in nearly all patients with IgE antibodies specific for peanut (Fig E1). Among sensitized patients the GM levels of IgE antibodies to wheat, cow's milk, and peanut were 1.4, 1.2, and 1.6 IU/ml, respectively (Fig 1, B). There are two remarkable features of these data. First, serum IgE antibody measurements in patients with EoE showed a high prevalence of sensitization to milk, wheat, and peanut in both adult and pediatric cases. Second, the quantities of specific IgE antibodies to these foods in patients with EoE were consistently low (Fig 1, B).
Fig 1.
The prevalence of sensitization to common whole food extracts in adults and children with EoE as determined by skin prick testing or ImmunoCAP testing (A). Levels of specific IgE antibody for those aeroallergens and foods to which patients with EoE have frequent sensitization (B).
The prevalence of sensitization to one or more aeroallergen specificities was 93% in adults compared with 65% for the children with EoE. Sensitization to individual aeroallergens ranged from 12% to 61% (Table II). Aeroallergen-specific IgE levels were higher in children than adults. Geometric mean levels of specific IgE to pollens ranged from 1.85 IU/ml to 5.03 IU/ml (Table II).
Table II.
Sensitization to aeroallergens among adults and children with EoE.
| Adults (n=46) |
Children (n=51) |
|||||
|---|---|---|---|---|---|---|
| Prevalence | Quantity* | Prevalence | Quantity* | |||
| Allergen | Skin prick n=46 (%) | Serum assay n=46 (%) | (IU/ml) | Skin prick n=49 (%) | Serum assay n=48 (%) | (IU/ml) |
| Dust mite | 54.3 | 34.8 | 1.90 (1.33-8.14) | 34.7 | 25.0 | 8.46 (5.04-115) |
| Cat | 43.5 | 34.8 | 3.87 (2.73-16.0) | 22.4 | 31.3 | 9.29 (6.46-81.9) |
| Dog† | 23.9 | 36.4† | 8.16 (4.40-29.0) | 14.3 | 44.4 | 2.89 (1.82-10.0) |
| Mold‡ | 32.6 | 33.3‡ | 5.00 (2.63-16.4) | 12.2 | 33.3 | 5.35 (2.46-22.7) |
| Tree pollen | 58.7 | 30.4 | 1.85 (1.31-4.10) | 46.9 | 25.0 | 5.03 (3.13-19.9) |
| Grass pollen | 60.9 | 41.3 | 3.18 (2.39-8.81) | 40.8 | 35.4 | 3.67 (2.65-13.4) |
| Ragweed | 43.5 | 50.0 | 1.94 (1.56-5.79) | 30.6 | 31.3 | 4.93 (3.38-30.6) |
Quantity is reported as geometric mean (95% confidence interval) of the positive serum specific IgE antibody values.
Twenty-two adults and 27 children were tested for serum IgE to dog.
Twenty-one adults and 27 children were tested for serum IgE to mold mix.
Component sensitization profiles using ISAC
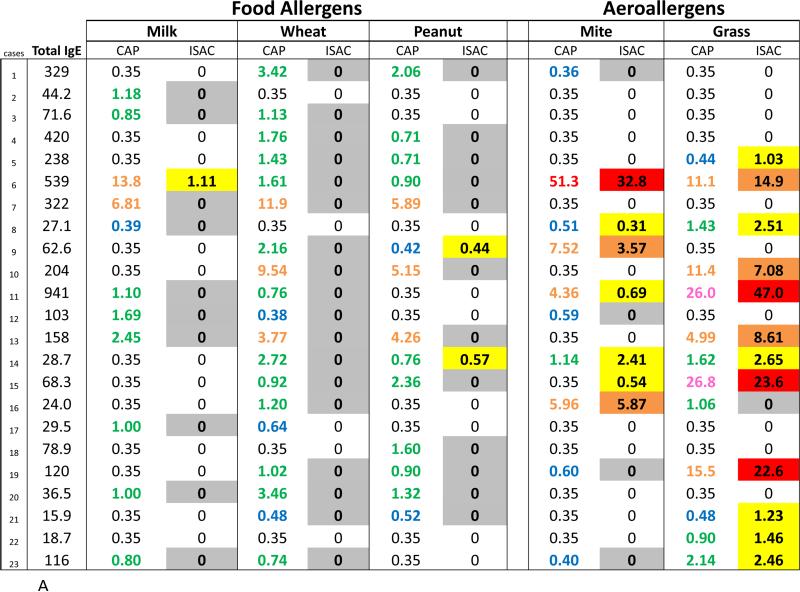
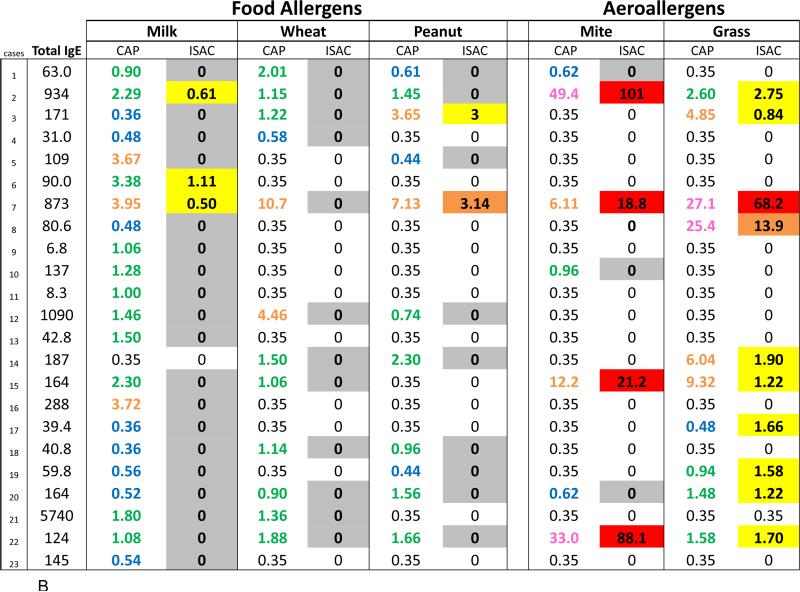
Using ISAC, specific IgE antibodies to molecular allergens were identified in the majority of sera that were positive to an aeroallergen by ImmunoCAP (Fig 2, A and B). In contrast, the ISAC results on sera with IgE antibodies specific for extracts of cow's milk, wheat, peanut, soy, and egg were largely undetectable (Table III, Fig 2, A and B). These results might be explained by the low quantities of IgE antibody to these foods and the lower analytical sensitivity of the chip ISAC microarray in comparison with the extract-based singleplex ImmunoCAP. However, considering sera with moderate IgE antibody levels >0.70 IU/ml in Fig 2, A and B, molecular allergen-specific IgE analysis was positive in 28 out of the 30 ImmunoCAP positive results for aeroallergens and only 7 out of the 71 ImmunoCAP positive results for food-specific IgE antibodies (p<0.001).
Fig 2.
Comparison of quantities of allergen extract-specific IgE measured using ImmunoCAP with semiquantitative ISAC standardized unit (ISU) levels of IgE antibody from ISAC testing for molecular allergens. Quantities of IgE antibody are distinguished by color: low levels (blue), moderate (green), high (orange), very high (pink), and extremely high (red). Ranges of ISU levels are highlighted in gray (negative with positive CAP), low (yellow), medium (orange), and high (red). A subgroup of adult (A) and pediatric (B) patients who have serum IgE antibody to whole allergen extract is shown.
Table III.
Frequency of positive results as measured by serological IgE antibody assays (ImmunoCAP) and ISAC analysis on the sera positive to extracts in adult and pediatric patients with EoE.
| ISAC molecular allergen |
|||||||
|---|---|---|---|---|---|---|---|
| Allergen extract | IgE Ab* (n=89) | ISAC† (n=90) | Number of molecules | 1st | 2nd | 3rd | 4th |
| egg | 19/89 | 4/19 | (4)‡ | Gal d 1 (0/19)§ | Gal d 2 (0/19) | Gal d 3 (2/19) | Gal d 5 (2/19) |
| milk | 42/89 | 4/42 | (5) | Bos d 4 (2/42) | Bos d 5 (1/42) | Bos d 6 (1/42) | Bos d 8 (0/42) |
| wheat | 39/89 | 0/39 | (3) | Tri a 14 (0/39) | Tri a 19 (0/39) | Tri a A_T (0/39) | - |
| soy | 28/89 | 8/28 | (3) | Gly m 4 (2/28) | Gly m 5 (5/28) | Gly m 6 (5/28) | - |
| peanut | 33/89 | 11/33 | (5) | Ara h 1 (2/33) | Ara h 2 (2/33) | Ara h 8 (4/33) | Ara h 9 (3/33) |
| dust mite | 26/87 | 18/26 | (5) | group 1 (12/18) | group 2 (11/18) | Der p 10 (5/18) | Blo t 5 (4/18) |
| cat | 31/87 | 30/31 | (3) | Fel d 1 (27/30) | Fel d 4 (10/30) | Fel d 2 (5/30) | - |
| dog | 17/46 | 9/17 | (4) | Can f 1 (8/17) | Can f 2 (6/17) | Can f 3 (2/17) | Can f 5 (10/19) |
| mold | 15/47 | 13/15 | (6) | Alt a 1 (13/15) | Asp f 1 (0/15) | Cla h 8 (1/15) | - |
| tree | 25/87 | 19/25 | (3) | Bet v 1 (15/19) | Bet v 2 (8/19) | Bet v 4 (0/12) | - |
| grass | 33/87 | 30/33 | (9) | Phl p 1 (20/30) | Phl p 4 (12/30) | Phl p 12 (9/30) | Cyn d 1 (24/30) |
| ragweed | 35/87 | 21/35 | (1) | Amb a 1 (21/35) | - | - | - |
Results are given as number positive/number of samples tested by ImmunoCAP and ISAC.
Results are given as number of sera positive to any ISAC molecule/number positive to allergen extract by ImmunoCAP.
Number of molecules tested for each allergen by ISAC
Results in parentheses are given as number positive to the specific ISAC molecule/number positive to whole allergen extract.
Molecular allergen ImmunoCAP assays
To address the concern of the lower analytical sensitivity of the ISAC, positive sera for IgE antibody to cow's milk, peanut, and wheat extracts were also tested with ImmunoCAP for the presence of IgE antibodies specific for individual allergens (Table IV, A). By ImmunoCAP, we detected specific IgE to at least one component in 78% of patients who had IgE antibodies to milk proteins (Table IV, A). Specific IgE antibodies to Bos d 4 (α-lactalbumin) and Bos d 5 (β-lactoglobulin) were the most common and had the best correlation with specific IgE to cow's milk extract (Table IV A and Fig 3). In contrast to the ISAC results, ImmunoCAP molecular component testing was positive in 58% of patients with IgE to peanut extract, and 23% of this group was sensitized to Ara h 1, Ara h 2, or Ara h 3. By contrast, >40% were sensitized to Ara h 8 [pathogenesis-related protein-10 (PR-10) family] and Ara h 9 (lipid transfer protein family). For patients who were sensitized to Ara h 8, the quantitative correlation with peanut extract specific IgE was not significant (p=0.06) (Table IV, A). However, IgE to peanut was highly correlated with specific IgE to soy, birch, and grass; and both birch and grass sensitization correlated with the presence of Ara h 8-specific IgE antibodies (Table IV, B). In the 16 patients we tested, IgE antibodies to molecular wheat allergens were detected in few sera. However, having specific IgE to wheat was highly correlated with soy and moderately correlated with grass sensitization (Table IV, B).
Table IVA.
Prevalence, levels, and correlations of IgE antibody to molecular allergens of milk, peanut and wheat assayed with individual CAP assays in sera positive for that food.
| Allergen | Prevalence | GM* (IU/ml) | Correlation† (R) | p value | |
|---|---|---|---|---|---|
| Number positive | % | ||||
| Milk‡ | 1.2 | ||||
| Bos d 4 | 19 | 45 | 1.0 | 0.89 | <0.001 |
| Bos d 5 | 23 | 59 | 0.95 | 0.76 | <0.001 |
| Bos d 8 | 16 | 40 | 0.82 | 0.73 | 0.001 |
| Bos d 6 | 9 | 23 | 1.2 | 0.56 | 0.1 |
| Peanut‡ | 1.6 | ||||
| Ara h 1 | 6 | 19 | 1.2 | N/A§ | N/A§ |
| Ara h 2 | 4 | 13 | 2.2 | N/A§ | N/A§ |
| Ara h 3 | 2 | 7.7 | 0.76 | N/A§ | N/A§ |
| Ara h 8 | 10 | 42 | 0.93 | 0.65 | 0.06 |
| Ara h 9 | 10 | 48 | 0.77 | 0.39 | 0.2 |
| Wheat‡€ | 1.4 | ||||
| Omega-5 | 0 | 0 | N/A§ | N/A§ | N/A§ |
| Tri a 14 | 3 | 19 | 1.8 | N/A§ | N/A§ |
GM is the geometric mean level of serum IgE antibody in the positive samples.
Linear regression was used to compare serum IgE antibody to molecular allergens and whole allergen extracts.
The proportion of samples tested that did not have any molecular allergens with IgE ≥0.35 was 22% for milk, 42% for peanut, and 81% for wheat.
This statistical test was not performed because there were not enough positive samples.
Only 16 samples were tested because of a lack of serum.
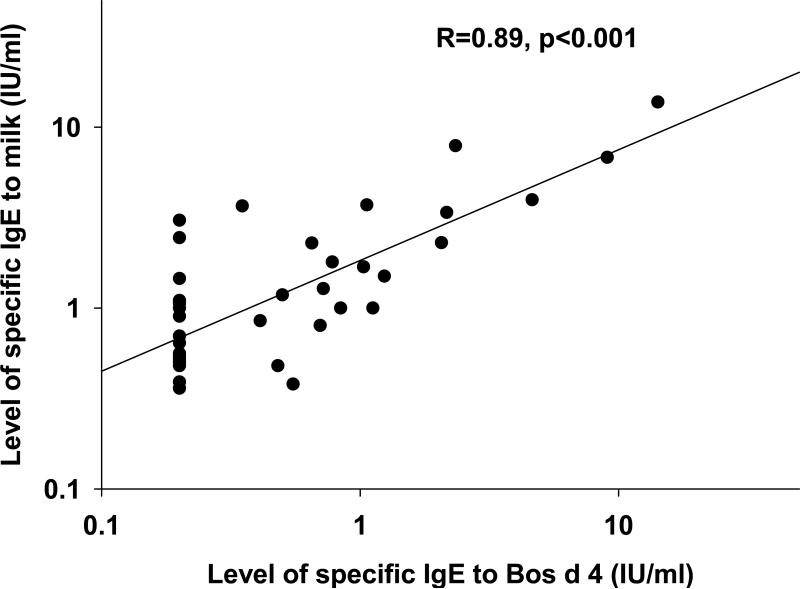
Fig 3.
The relationship between levels of IgE antibody specific for Bos d 4 (α-lactalbumin) and IgE antibody specific for cow's milk extract with each dot representing an individual patient. The regression line is shown.
Table IVB.
Prevalence, levels, and correlations of IgE antibody to food extracts or food molecular allergens by individual CAP and cross reactive pollen allergens.
| Allergen | Prevalence | GM* (IU/ml) | Correlation† (R) | p value | |
|---|---|---|---|---|---|
| Number positive | % | ||||
| Milk | 1.2 | ||||
| Soy | 18 | 46 | 1.1 | 0.091 | 0.7 |
| Birch | 12 | 32 | 2.9 | 0.30 | 0.3 |
| Grass | 18 | 42 | 4.4 | 0.27 | 0.3 |
| Peanut | 1.6 | ||||
| Soy | 29 | 83 | 1.1 | 0.74 | <0.001 |
| Birch | 19 | 58 | 3.9 | 0.71 | 0.001 |
| Grass | 21 | 64 | 3.2 | 0.80 | <0.001 |
| Wheat | 1.4 | ||||
| Soy | 27 | 61 | 1.1 | 0.76 | <0.001 |
| Birch | 23 | 53 | 3.2 | 0.33 | 0.1 |
| Grass | 25 | 58 | 4.4 | 0.43 | 0.03 |
| Soy | 1.1 | ||||
| Ara h 1 | 5 | 19 | 1.5 | N/A‡ | N/A‡ |
| Ara h 2 | 5 | 19 | 1.9 | N/A‡ | N/A‡ |
| Ara h 3 | 2 | 10 | 0.76 | N/A‡ | N/A‡ |
| Ara h 8 | 10 | 37 | 0.84 | 0.32 | 0.4 |
| Ara h 9 | 12 | 48 | 0.86 | 0.03 | 0.9 |
| Birch | 2.9 | ||||
| Ara h 1 | 3 | 17 | 0.76 | N/A‡ | N/A‡ |
| Ara h 2 | 3 | 17 | 0.85 | N/A‡ | N/A‡ |
| Ara h 3 | 2 | 13 | 0.76 | N/A‡ | N/A‡ |
| Ara h 8 | 10 | 56 | 0.92 | 0.95 | <0.001 |
| Ara h 9 | 8 | 47 | 0.75 | 0.60 | 0.1 |
| Grass | 3.4 | ||||
| Ara h 1 | 4 | 21 | 0.65 | N/A‡ | N/A‡ |
| Ara h 2 | 3 | 16 | 0.85 | N/A‡ | N/A‡ |
| Ara h 3 | 2 | 13 | 0.76 | N/A‡ | N/A‡ |
| Ara h 8 | 9 | 47 | 1.0 | 0.67 | 0.05 |
| Ara h 9 | 7 | 39 | 0.95 | 0.25 | 0.6 |
GM is the geometric mean level of serum IgE antibody in the positive samples.
Linear regression was used to compare serum IgE antibody to molecular allergens and whole allergen extracts.
This statistical test was not performed because there were not enough positive samples.
Testing for IgE Antibody on diluted serum using ImmunoCAP
The results of ISAC testing and the low levels of IgE antibodies detected by ImmunoCAP to cow's milk, wheat, or soy extracts suggested that the allergen specificities relevant for EoE might represent only a small proportion of the proteins present on the assay solid phases. Extensive unpublished experience has confirmed that under these circumstances, ImmunoCAP analysis of diluted sera will not give the expected result based on quantities detected in assays with the undiluted serum. In our study, assays with sera diluted 1:8 produced calculated results for IgE to foods that were higher than expected when corrected for dilution (Table V) (See also online supplement Table E3). In fact, 26/34 of the positive results for chicken egg, wheat, cow's milk, or soy gave apparent values more than 25% higher after 1:8 dilution. By contrast, the effect was only seen in 3/15 sera with IgE antibody specific for dust mite or cat allergen (p<0.001). Thus in many cases, the assay of undiluted serum underestimates the IgE specific for food-related proteins. (For further details see online supplement and Table E3).
Table V.
ImmunoCAP IgE antibody levels for foods or inhalants on EoE sera run undiluted or diluted 1:8.
| FOODS |
INHALANTS |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| cow's milk (f2) | wheat (f4) | soy (f14) | dust mite (d1) | cat (e1) | |||||
| undiluted measured | 1:8 (value ×8) and %* | undiluted measured | 1:8 (value ×8) and % | undiluted measured | 1:8 (value ×8) and % | undiluted measured | 1:8 (value ×8) and % | undiluted measured | 1:8 (value × 8) and % |
| 8.82 | 13.4 51% | <0.35 | nd | <0.35 | nd | 16.2 | 16.2 0% | 1.35 | 2.56 90% |
| <0.35 | nd† | <0.35 | nd | 1.47 | 1.6 9% | <0.35 | nd | <0.35 | nd |
| <0.35 | nd | <0.35 | nd | <0.35 | nd | 13.8 | 14.9 8% | 14.3 | 13.8 −3% |
| 6.12 | 37.7 516% | 0.93 | nd | 1.06 | nd | 13.9 | nes‡ | <0.35 | nd |
| 12.5 | nes | 28.3 | nes | 9.99 | 16.4 64% | 0.49 | nes | <0.35 | nd |
| 0.67 | 1.68 151% | 2.01 | 5.92 195% | 1.35 | 4.32 220% | 0.62 | nes | <0.35 | nd |
| <0.35 | nd | <0.35 | nd | <0.35 | nd | 14.8 | 15.0 2% | 5.47 | 6.24 14% |
| 0.36 | nd | 7.22 | 17.4 140% | 4.67 | nes | <0.35 | nd | 1.83 | nes |
| nes | nes | nes | nes | 6.89 | 9.36 36% | nes | nes | nes | nes |
| <0.35 | nd | <0.35 | nd | <0.35 | nd | <0.35 | nd | 8.17 | 8.72 7% |
| <0.35 | nd | <0.35 | nd | <0.35 | nd | 10.9 | 10.9 0% | <0.35 | nd |
| 7.29 | 7.52 3% | 0.72 | 1.28 78% | <0.35 | nd | 28.0 | 31.5 13% | 3.52 | 3.84 9% |
| 6.81 | 27.0 296% | 11.9 | 16.6 39% | 0.67 | 1.20 79% | <0.35 | nd | <0.35 | nd |
| <0.35 | nd | 2.16 | nes | <0.35 | nd | 6.43 | 7.20 12% | 2.56 | nes |
| <0.35 | nd | 9.54 | 20.8 118% | 2.05 | nes | <0.35 | nd | 5.58 | nes |
| 2.33 | 8.96 285% | 3.76 | 6.56 74% | 2.93 | nes | <0.35 | nd | <0.35 | nd |
| 6.78 | 6.56 −3% | 6.47 | nes | 2.68 | nes | >100 | 433.0 | >100 | 204.0 |
| <0.35 | nd | 0.66 | 1.84 179% | 6.32 | 7.68 22% | >100 | 225.0 | 12.8 | 13.0 1% |
| <0.35 | nd | 0.49 | 1.36 178% | 0.60 | 1.12 87% | 0.52 | 0.88 69% | 0.71 | nes |
| 15.8 | 48.9 209% | 0.43 | 0.88 105% | <0.35 | nd | <0.35 | nd | 1.05 | nes |
| 12.3 | 13.2 7% | 5.39 | 10.5 94% | 4.89 | 9.44 93% | <0.35 | nd | 9.41 | 26.1 177% |
% is percent increase = [(calculated result for 1:8 dilution-measured result for undiluted)/measured result for undiluted] × 100
nd is not determined.
nes is not enough serum remaining for measurement.
DISCUSSION
EoE is a chronic, allergic disorder in which most patients have sensitization to multiple allergens; however, the mechanism by which individual allergic sensitivities contribute to the disease process is not understood. Although recent studies using anti-IgE as treatment for EoE have not been successful, it is important to recognize that IgE antibody could have different roles in the disease process.20,21 Our study shows striking similarity in adult and pediatric patients with EoE for allergic sensitization as identified by skin prick testing and serological analyses and emphasizes the high prevalence of IgE antibody to cow's milk and wheat in both populations. Although our results do not show that the IgE antibodies are responsible for the inflammation in the esophagus, it seems possible that this distinct pattern of food sensitization identified by serological measurements is relevant to existing evidence that dietary intake of cow's milk and wheat plays a significant role in the inflammation of EoE.10,12
Similar to our previous report, sensitization to wheat (40%) and milk (64%) was striking in children with EoE. Interestingly, the prevalence of sensitization to milk in the current group of children was significantly higher than in the previous group that we studied (p=0.006).8 In adult patients, Roy-Ghanta and colleagues reported a similar prevalence of sensitization to wheat (39%) and milk (30%).6 We identified wheat sensitization in 52% of our adults and milk sensitization in 32%. By contrast, in population based studies, specific IgE to milk has been identified in up to 10% of samples.22 Thus, the prevalence of specific IgE to milk in adults with EoE is two to three times greater than expected (See Table E2 online).
Most reported diets for EoE (the six-food elimination diet and an amino acid based diet) exclude cow's milk and wheat.10-12 Indeed successful diets based on avoidance of foods that are positive by skin prick and patch testing have also removed milk even in cases where the results were negative.10 Studies of food reintroduction after avoidance suggest that cow's milk and wheat are the two foods that cannot be added back in to the diet without causing return of symptoms.12 During reintroduction of foods in pediatric patients, milk was identified as a problem food in 74% of cases (and wheat in 26%).12 In adults wheat or milk appeared to be a problem in 60% and 50% of patients respectively.23 We prospectively observed children treated with PPI and single food elimination of cow's milk, and we found that 64% had remission based on having esophageal count <15/hpf after 6-8 weeks of treatment.24 In that study, successful responses to cow's milk avoidance did not relate to the presence of IgE antibodies (Unpublished data).
In EoE, specific IgE antibody levels to foods are low in comparison with quantities reported for other forms of food allergy.25 This suggests that the food sensitivity in EoE may involve a more selective or restrictive immune response. It is possible that the low quantities of IgE antibody detected in the serum of EoE subjects may be a result of IgE to a quantitatively minor component of the allergenic food proteins that are coupled to the ImmunoCAP allergosorbent. An alternative hypothesis is that the low levels of IgE antibody to food result from continuous exposure to sensitizing foods. Though EoE likely results from an immune response to food antigens, patients usually cannot identify food triggers and don't self-restrict dietary intake. Continued ingestion of the relevant allergen(s) may also result in production of non-IgE antibodies (e.g. IgG) that could interfere with mast cell activation or IgE assays.26
Levels of IgE antibodies may also be low in EoE due to independent factors unrelated to the serological assay analysis. Prussin and colleagues compared T cell responses to peanut in patients with eosinophilic gastrointestinal disease (EGID) with T cell responses in patients with immediate-type food sensitivity.27 Although T cells producing IL-4 were observed in both conditions, they found a population of IL-5 producing cells that was significantly larger in patients with EGID compared with traditional peanut allergy.27 This raises the question of whether the IgE antibody is contributing to the allergic response or is simply part of the response in which the T cells are the primary cause of the inflammation that results in symptoms.
Our results from molecular allergen assays in patients with EoE suggest that serum results should be interpreted differently for each food. We tested a wide variety of milk allergen components (see Table IV, A) and the responses varied by individual. The allergens designated Bos d 4 (α-lactalbumin), Bos d 5 (β-lactoglobulin), and Bos d 6 (bovine serum albumin) are whey proteins that make up 0.7-12% of the total protein content. Bos d 8 (αs1-casein) is one of the four types of casein proteins which comprise 32-70% of cow's milk proteins. Antibody levels to the whole extract may be higher or lower depending on which allergenic components have induced the IgE antibody response for an individual patient. The correlation between Bos d 4 and milk was highly significant. Specific IgE antibodies to cow's milk did not appear to be related to cross reactive sensitization. For wheat, neither of the two available components was identified as an important allergen molecule in the 16 sera that were tested.28 Our data suggested two associations. A proportion of patients showed a strong correlation between levels of specific IgE antibody to wheat and IgE antibody to soy; for these patients, the IgE antibody to wheat may indicate a clinically relevant sensitization (see Table IV, B). The correlation between levels of IgE antibody to wheat and IgE antibody to grass pollen may well represent cross reactivity but does not define which was the primary sensitization (see Table IV, B).
For peanut, IgE antibody responses specific for Ara h 1, 2, 3, 8, or 9 were analyzed. These peanut proteins function in seed storage, lipid transfer, defense (PR-10), and as profilins. Only 23% of peanut-sensitized EoE patients had specific IgE antibody to Ara h 1, 2, and 6. None of the patients with Ara h 1 or Ara h 2 were adults. Among the pediatric patients with Ara h 1 or 2, 60% reported having immediate reactions to peanut. A high proportion of EoE patients with IgE antibody to peanut could be explained by cross-reactive IgE antibody to soy, birch, or grass. Evidence from reintroduction of food after the six-food elimination diet suggests that patients with EoE can tolerate peanut. Taken together this evidence raises the possibility that some patients with EoE who are eating peanut may not need to stop eating it simply because of a positive IgE antibody test.29,30 It also emphasizes the role of IgE antibody analysis in understanding the overlap between non-EoE and EoE food reactions in patients.
A potential limitation of our study is that we did not classify our patients as PPI responsive or PPI non-responsive. In our series, the prevalence and titers of IgE antibody to milk, wheat, and soy were not different in those who had not been treated with PPI. Other investigators have shown that there are no clinical differences, including results of pH probe measurement, between the patients with PPI responsive esophageal eosinophilia (PPI-REE) and those who do not respond.31,32 Results of in vitro studies on esophageal cell cultures from EoE patients show that response to PPIs can be separate from any effect on gastric acid secretion.33 Some experts have suggested that PPI-REE may not be a distinct group of patients.34 We are not convinced that PPI-REE should be regarded as an etiologically distinct group simply because of a difference in therapeutic response. A significant limitation is that our evaluation of molecular allergen-specific IgE antibody responses in patients with EoE is restricted to those purified reagents that were available. The major impetus for purifying food allergens has come from anaphylaxis cases. It may well be that the individual food proteins that are relevant to EoE are not the same as those that are relevant to anaphylaxis following exposure to the same food source.
In conclusion, there are two striking features of the IgE response to milk and wheat in EoE. First that the titers of serum IgE antibodies are consistently low, and second that analysis of components for milk suggests that these IgE responses are specific for relatively minor components of the milk extract. Although the IgE responses do not correlate with successful response to avoidance diets, they may nonetheless correctly identify the components that are relevant for the T cell response even in patients who have completely negative skin prick and serum IgE testing. This is especially true for milk, and our data identifies Bos d 4 and Bos d 5 as possible primary antigens. Thus analysis of IgE antibodies may be highly relevant for understanding the role of these foods in the disease through studies to identify the underlying mechanism and also the route of sensitization.
Supplementary Material
Text highlights box.
What is already known about this topic?
Dietary avoidance can often successfully treat eosinophilic esophagitis (EoE) suggesting that foods are causal. Although serum IgE antibody assays identify more positive food results than skin prick testing, neither test consistently identifies the correct foods to avoid.
What does this article add to our knowledge?
Assays on diluted sera and component analysis suggest that in patients with EoE the IgE antibodies to milk are specific for proteins that represent a small proportion of the proteins in the whole extract (α-lactalbumin and β-lactoglobulin).
How does this study impact current management guidelines?
Although IgE is not the primary mechanism, molecular allergen and extract-based IgE antibody assays for cow's milk suggest that the quantitatively minor whey components are relevant to EoE. The relevant proteins in wheat have not yet been identified.
Acknowledgments
Funding: National Institutes of Health grants K23-AI-059317 and R01-AI-20565 and a Davis Bremer Grant from The Ohio State University
Abbreviations used
- EoE
Eosinophilic esophagitis
- hpf
high power field
- PPI
proton pump inhibitor
- Alpha-gal
galactose α-1/3-galactose
- ISAC
Immuno Solid-phase Allergen Chip
- ISU
ISAC Standardized Units
- GM
geometric mean
- PR-10
pathogenesis-related protein-10
- EGID
eosinophilic gastrointestinal disease
- PPI-REE
proton-pump inhibitor responsive esophageal eosinophilia
- nd
not determined
- nes
not enough serum
- OR (95% CI)
odds ratio (95% confidence interval)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon HU, Straumann A, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–70. doi: 10.1038/ajg.2009.657. [DOI] [PubMed] [Google Scholar]
- 2.DeBrosse CW, Franciosi JP, King EC, Butz BK, Greenberg AB, Collins MH, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011;128:132–8. doi: 10.1016/j.jaci.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;12:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Orenstein S, Shalaby T, DiLorenzo C, Putnam PE, Sigurdsson L, Mousa H, et al. The spectrum of pediatric eosinophlic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000;95:1422–30. doi: 10.1111/j.1572-0241.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- 5.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109:363–8. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 6.Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2008;6:531–5. doi: 10.1016/j.cgh.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 7.Penfield JD, Lang DM, Goldblum JR, Lopez R, Falk GW. The role of allergy evaluation in adults with eosinophilic esophagitis. J Clin Gastroenterol. 2010;44:22–7. doi: 10.1097/MCG.0b013e3181a1bee5. [DOI] [PubMed] [Google Scholar]
- 8.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TAE. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2010;104:496–502. doi: 10.1016/j.anai.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:509–11. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–7. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;9:1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Kagalwalla AF, Shah A, Li BU, Sentongo TA, Ritz S, Manuel-Rubio M, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr. 2011;53:145–9. doi: 10.1097/MPG.0b013e31821cf503. [DOI] [PubMed] [Google Scholar]
- 13.Van Rhijn BD, van Ree R, Versteeg SA, Vlieg-Boerstra BJ, Sprikkelman AB, Terreehorst I, et al. Birch pollen sensitization with cross-reactivity to food allergen predominates in adults with eosinophilic esophagitis. Allergy. 2013;68:1475–81. doi: 10.1111/all.12257. [DOI] [PubMed] [Google Scholar]
- 14.Klemans RJ, Otte D, Knol M, Knol EF, Meijer Y, Gmelig-Meyling FH, et al. The diagnostic value of specific IgE to Ara h 2 to predict peanut allergy in children is comparable to a validated and updated diagnostic prediction model. J Allergy Clin Immunol. 2013;131:157–63. doi: 10.1016/j.jaci.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–5. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Cabauatan CR, Lupinek C, Scheiblhofer S, Weiss R, Focke-Tejkl M, Bhalla PL, et al. Allergen microarray detects high prevalence of asymptomatic IgE sensitizations to tropical pollen-derived carbohydrates. J Allergy Clin Immunol. 2014;133:910–14. doi: 10.1016/j.jaci.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vereda A, van Hage M, Ahlstedt S, Ibanez MD, Cuesta-Herranz J, van Odijk J, et al. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127:603–7. doi: 10.1016/j.jaci.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Gadisseur R, Chapelle JP, Cavalier E. A new tool in the field of in-vitro diagnosis of allergy: preliminary results in the comparison of ImmunoCAP 250 with the ImmunoCAP ISAC. Clin Chem Lab Med. 2011;49:277–80. doi: 10.1515/CCLM.2011.052. [DOI] [PubMed] [Google Scholar]
- 19.Patelis A, Gunnbjornsdottir M, Malinovschi A, Matsson P, Onell A, Hogman M, et al. Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol. 2012;130:397–402. doi: 10.1016/j.jaci.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 20.Rocha R, Vitor AB, Trindade E, Lima R, Tavares M, Lopes J, Dias JA. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr. 2011;170:1471–4. doi: 10.1007/s00431-011-1540-4. [DOI] [PubMed] [Google Scholar]
- 21.Clayton F, Fang JC, Gleich GJ, Lucendo A, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–9. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798–806. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults: food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–9. doi: 10.1053/j.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Kruszewski PG, Russo JM, Franciosi JP, Varni JW, Platts-Mills TA, Erwin EA. A prospective, comparative effectiveness trial of cow's milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus. doi: 10.1111/dote.12339. In press. [DOI] [PubMed] [Google Scholar]
- 25.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444–51. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 26.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIB cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5(+) and IL-5(−) Th2 responses. J Allergy Clin Immunol. 2009;124:1326–32. doi: 10.1016/j.jaci.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripathi A, Commins SP, Heymann PW, Platts-Mills TA. Diagnostic and experimental food challenges in patients with nonimmediate reactions to food. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2014.11.032. In press. [DOI] [PubMed] [Google Scholar]
- 29.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Asarnoj A, Nilsson C, Lidholm J, Galumann S, Ostblom E, Hedlin G, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130:468–72. doi: 10.1016/j.jaci.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108:1854–60. doi: 10.1038/ajg.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110–7. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Cheng E, Xiaofang H, Chunhua Y, Zhang Q, Pham TH, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. doi: 10.1371/journal.pone.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dohil R, Newbury RO, Aceves S. Transient PPI responsive esophageal eosinophilia may be a clinical sub-phenotype of pediatric eosinophilic esophagitis. Dig Dis Sci. 2012;57:1413–9. doi: 10.1007/s10620-011-1991-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.