Abstract
Background
Common variable immunodeficiency may be complicated by interstitial lung disease, leading to worsened morbidity and mortality in some. While immunomodulatory treatment has efficacy, choice of patient, duration of treatment, and long-term follow-up are not available. Interstitial lung disease appears stable in certain instances, so it is not known whether all patients will develop progressive disease or require immunomodulatory therapy.
Objective
This study aims to determine if all common variable immunodeficiency patients with interstitial lung disease have physiological worsening, and if clinical and/or laboratory parameters may correlate with disease progression.
Methods
Retrospective review of medical records at Mount Sinai Medical Center in New York was conducted for referred patients with common variable immunodeficiency, CT scan-confirmed interstitial lung disease, and periodic pulmonary function testing covering 20 or more months prior to immunomodulatory therapy. Fifteen patients were identified from the retrospective review and included in this study.
Results
Nine of the 15 common variable immunodeficiency patients had physiological worsening of interstitial lung disease adapted from consensus guidelines, associated with significant reductions in FEV1, FVC, and DLCO. Those with progressive lung disease also had significantly lower mean IgG levels, greater increases and highest levels of serum IgM, and more significant thrombocytopenia.
Conclusion
Interstitial lung disease resulted in physiological worsening in many, but not all subjects, and was associated with suboptimal IgG replacement. Those with worsening pulmonary function tests, elevated IgM, and severe thrombocytopenic episodes appear to be at highest risk for progressive disease. Such patients may benefit from immunomodulatory treatment.
Keywords: common variable immunodeficiency, CVID, granulomatous interstitial lung disease, GLILD, interstitial lung disease, pulmonary function testing
INTRODUCTION
Despite being the most common symptomatic primary immunodeficiency, common variable immunodeficiency (CVID) remains an enigma, with surprisingly little understood about its predisposing genetic and immunologic factors.1, 2 CVID is clinically defined by marked reduction of serum immunoglobulin (Ig) levels coupled with impaired antibody responses to immunization and/or infection.3 While the frequency and severity of infections in CVID patients have significantly improved with the usage of IgG replacement therapy, non-infectious complications of CVID have emerged as the predominant source of morbidity and mortality.4–7 Non-infectious complications of CVID include autoimmune cytopenias, gastrointestinal inflammatory disease and malabsorption, lung disease, liver disease, and lymphoma, among others.8, 9 The reasons for developing these non-infectious complications, and why only a subset of CVID patients manifest them, are not known.
Chronic lung disease is among the most common complications of CVID, effecting approximately 30–60% of patients, depending upon the cohort.6, 7, 10 Bronchiectasis is likely the most common chronic lung disease in these patients, found in as many of 50% of those with CVID.11 Interstitial lung disease (ILD) also occurs frequently in CVID, and worsens mortality to a greater extent than bronchiectasis.7, 12 While the true incidence of this complication is not clear because large studies have not distinguished ILD from other forms of chronic lung disease, we found CT evidence of ILD in 39 of 61 CVID patients examined at our tertiary care center, suggesting that the frequency of this complication may be underestimated.13 The etiology of ILD in CVID is unclear and it is notable that bronchiectasis does not progress into ILD in many cases nor is it a prerequisite for ILD development.11, 13, 14 However, this does not rule out infection as a cause of ILD and an association with human herpesvirus 8 has been reported.15 Alternatively, ILD may be a consequence of generalized benign lymphoproliferation inherent to a subset of CVID patients,16 as correlations of ILD with persistent lymphadenopathy and splenomegaly have been reported.6, 12, 13, 17, 18 Along these lines, biopsies of CVID ILD demonstrate benign lymphoproliferative pathology (such as follicular bronchiolitis, lymphocytic interstitial pneumonitis, and nodular lymphoid hyperplasia) in nearly all cases.12, 19, 20 Intrinsic immune dysregulation, perhaps in the absence of infection, can result in ILD that is pathologically similar to that seen CVID. This is demonstrated by the characteristic lymphoproliferative ILD seen in patients with monogenic immune dysregulation disorders such as cytotoxic T lymphocyte-associated protein-4 (CTLA-4) deficiency and signal transducer and activator of transcription 3 (STAT3) gain-of-function mutations as well as autoimmune diseases like Sjogren’s Syndrome.21–24
While IgG replacement therapy alone is not believed to be effective in most cases, immunosuppression with cyclophosphamide, cyclosporine, or combination therapy with azathioprine and rituximab have shown efficacy as treatment for CVID ILD.25–27 However, immunosuppressive therapy carries risks of infection and malignancy, which may be of greater concern in immunocompromised patients.28, 29 As there are reports that CVID ILD is not progressive in some instances, not all cases of CVID ILD may warrant aggressive and potentially risky immunosuppression.14, 30 Moreover, CVID ILD is characterized by heterogeneous pathology, consisting of differing proportions of pulmonary lymphoid hyperplasia, organizing pneumonia, and granulomatous inflammation in individual patients, resulting in the general classification of granulomatous-lymphocytic interstitial lung disease (GLILD).13, 30–32 This variable constituency of pathology amongst individual patients may differentially influence the rate of progression and severity of disease. In this study, we culled CVID patients with CT evidence of ILD who had periodic pulmonary function tests (PFTs) over the course of 20 or more months prior to any immunomodulatory treatment to find clinical and laboratory parameters that might be useful for identifying those at greatest risk for progression of ILD.
METHODS
Study Design
This study was conducted via retrospective review of electronic medical records from Mount Sinai Hospital in New York. Electronic medical records are available for patient encounters or submitted data from January 2003 until present, and are linked with printed records that predate the electronic transition as well as test results and progress notes from referring providers. Patients with the International Classification of Diseases, Ninth Revision Code for CVID (279.06) who had a CT scan of the chest and at least two measurements of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) conducted 20 or more months apart found in their medical record were included in this study. In many cases PFTs were done by referring physicians, with additional testing conducted at our institution on subsequent occasions. PFTs and other patient data obtained prior to 6 months of IgG replacement therapy or after immunomodulatory treatment with azathioprine, cyclosporine, cyclophosphamide, mercaptopurine, or rituximab were excluded. These records were screened to confirm that the diagnostic criteria of CVID were met based on markedly low IgG and IgA and/or IgM levels (IgG ≤ 400 mg/dL, IgA < 45 mg/dL, IgM < 35 mg/dL), poor response to vaccines, and exclusion of other causes of hypogammaglobulinemia.33 Chest CT scans were required to show evidence of ground glass opacity and/or > 4 nodules of at least 1 mm in diameter for patients to be included. All 15 patients that met these study criteria were included in the final analysis and were lifelong non-smokers. This study was approved by the institutional review board of the Icahn School of Medicine at Mount Sinai and was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Data Collection
Patient age, sex, medication history, PFTs, laboratory values (IgG, IgA, IgM, complete blood count with differential, lymphocyte subsets, alkaline phosphatase), chest CT radiologist report, and pathologist report of lung biopsy, were derived from the medical record. Additionally, history of autoimmune hemolytic anemia (AIHA) or immune thrombocytopenic purpura (ITP), lymphadenopathy and/or splenomegaly, hepatitis or nodular regenerative hyperplasia, enteropathy, and/or malignancy was determined by review of medical notes and radiology reports.
Statistical Analysis
Associations between stable or progressive ILD and categorical clinical parameters were assessed by Fisher exact tests. Differences in continuous laboratory values between the two groups were analyzed using nonparametric Mann Whitney tests using a significance cut-off of P = 0.05 for each test.
RESULTS
More rapidly progressive ILD occurs in a subset of CVID patients
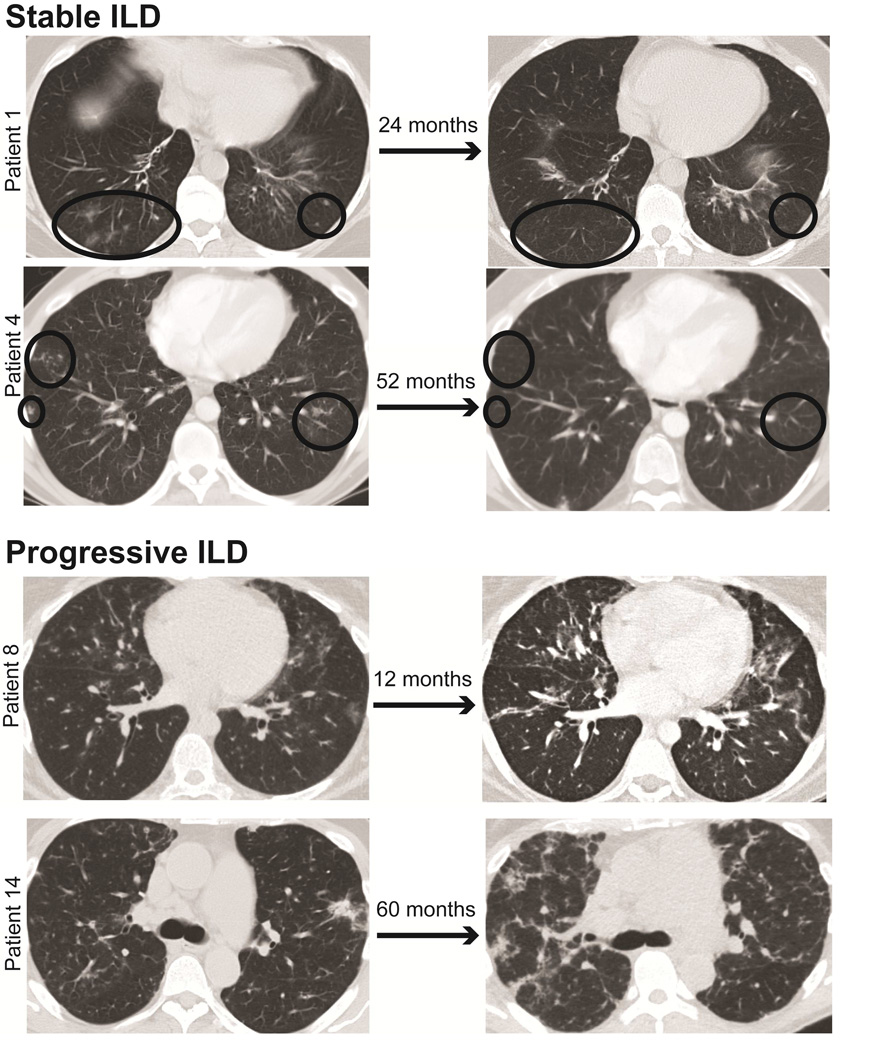
To objectively assess whether ILD resulted in physiological worsening in our cohort of CVID patients, we performed a retrospective study of those with CT-confirmed ILD and PFTs over 20 or more months after at least 6 months of IgG replacement therapy and prior to intervention with immunomodulatory therapy. Of the 15 CVID patients meeting the inclusion criteria, 9 were found to have significant physiological decline over the follow-up period by a definition adapted from consensus guidelines of absolute decline in FVC percent predicted ≥ 10 or absolute decline in DLCO percent predicted ≥ 15 for idiopathic pulmonary fibrosis, also extrapolated to other forms of ILD.34, 35 Decline in FVC percent predicted ≥ 10% avoids typical variability of testing because repeat measurement of FVC differs by less than 10% in 95% of normal subjects. CVID patients with physiological worsening by these criteria were denoted as having progressive ILD for the purpose of this study, in contrast to the 6 patients with waxing-and-waning CT findings and more stable PFTs denoted as stable ILD (Figure 1). The individual characteristics of all patients in this study are included in Table I.
Figure 1.
CT chest comparison of CVID patients with stable or progressive ILD. CVID patients with stable ILD show waxing-and-waning CT findings over the course of years (demarcated with black circles), while those with progressive ILD demonstrate steady and diffuse worsening of interstitial disease.
Table I.
Patient Characteristics
| Age | Sex | IgG (mg/ dL) |
IgA (mg/ dL) |
IgM (mg/ dL) |
CT evidence of ILD | CT evidence of bronchiectasis |
Lung Biopsy |
Pathology Diagnosis |
Other Complications |
Medications | % Change in FVC |
% Change in DLCO |
Follow-up (months) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stable ILD | ||||||||||||||
| 1 | 43 | F | <60 | <5 | 34 | Patchy areas of groundglass and solid nodules in right middle lobe and both lower lobes |
yes | ND | ND | borderline splenomegaly |
Inhaled budesonide/formoterol, prophylactic azithromycin |
+3 | +33 | 21 |
| 2 | 44 | M | 39 | 44 | 21 | Multiple ill-defined nodules throughout both lungs, lower lobe predominance |
no | VATS | FB and nodular LH with rare ill- defined granulomas |
ITP, lymphadenopathy |
Inhaled fluticasone/salmeterol, prophylactic ciprofloxacin |
−7 | −5 | 22 |
| 3 | 45 | F | 9 | <7 | 15 | Patchy centrilobular nodularity throughout both lungs |
yes | EB | FB | marginal zone B cell lymphoma (8 years after CVID diagnosis), splenomegaly |
Inhaled budesonide, prophylactic azithromycin |
+24 | ND | 144 |
| 4 | 45 | M | 338 | 25 | 35 | Numerous subcentimeter ground-glass infiltrates in both lower lobe areas |
no | VATS | Mixed lymphocytic and granulomatous infiltrate with patchy OP |
ITP, lymphadenopathy, splenomegaly |
Inhaled budesonide/formoterol, prophylactic azithromycin |
0 | −2 | 20 |
| 5 | 65 | F | 174 | 39 | 17 | Scattered areas of ground glass opacification seen diffusely within both lungs with superimposed nodular opacities |
yes | VATS | BIP with fibrosis, LH, OP, and occasional poorly formed granulomas |
hepato- splenomegaly, enteropathy |
none | −9 | −8 | 79 |
| 6 | 68 | F | 321 | 4 | 4 | Extensive bilateral lower lobe infiltrates and scattered bilateral nodularity |
yes | EB | Chronic inflammation with organizing interstitial pneumonitis, no granulomas identified |
splenomegaly | Inhaled budesonide/formoterol, prophylactic azithromycin |
+12 | +31 | 20 |
| Progressive ILD | ||||||||||||||
| 7 | 26 | M | 349 | 0 | 15 | Multiple small nodules with ill- |
no | ND | ND | ITP, lymphadenopathy, |
none | −26 | −51 | 63 |
| defined borders throughout the lungs bilaterally with areas of ground glass opacification |
splenomegaly | |||||||||||||
| 8 | 31 | F | 312 | 16 | 17 | Innumerable nodular densities are noted through both lungs with basilar predominance |
yes | VATS | atypical nodular lymphoid infiltrate with poorly formed granulomas |
ITP, lymphadenopathy, splenomegaly |
Inhaled beclomethasone, prophylactic azithromycin |
−13 | ND | 45 |
| 9 | 32 | F | 217 | <7 | 9 | Widespread nodules, areas of airspace consolidation, and groundglass opacities throughout both lungs |
yes | EB | NA, non-malignant reactive inflammation by report | ITP, lymphadenopathy, splenomegaly, pancytopenia |
Inhaled budesonide/formoterol, prophylactic azithromycin |
−19 | ND | 34 |
| 10 | 35 | M | 400 | 6 | 26 | Patchy nodular infiltrates in both lungs |
no | EB | LIP | ITP, hepato- splenomegaly, lymphadenopathy, NRH |
none | −40 | ND | 80 |
| 11 | 45 | M | 107 | <15 | <4 | Multiple scattered nodular infiltrates, most are peribronchial but are pleural based in some instances |
no | ND | ND | ITP, AIHA, hepato- splenomegaly, lymphadenopathy, LH of parotid gland |
Inhaled budesonide/formoterol, prophylactic azithromycin |
−22 | −16 | 70 |
| 12 | 48 | F | 30 | 6 | 7 | Nodular and irregular consolidative densities throughout the lungs with peripheral predominance |
yes | ND | ND | ITP, splenomegaly, lymphadenopathy |
Inhaled budesonide/formoterol, prophylactic azithromycin |
−18 | −14 | 50 |
| 13 | 48 | M | 113 | <5 | 6 | Focal consolidations and nodules throughout both lungs |
no | EB | Reactive lymphoid tissue and few poorly formed granulomas |
Interface hepatitis, hepato- splenomegaly, lymphadenopathy, LH of parotid gland |
Inhaled budesonide/formoterol, prophylactic azithromycin |
−1 | −37 | 24 |
| 14 | 58 | M | 57 | 4 | 6 | Reticulonodular changes seen throughout the lung fields with patchy areas of more dense consolidation |
no | ND | ND | ITP, AIHA, hepato- splenomegaly, NRH, prostate cancer |
Inhaled budesonide/formoterol, prophylactic azithromycin |
−64 | −17 (from months 28 to 71) |
71 |
| 15 | 61 | M | 120 | 2 | 3 | ILD is present that is significantly worse at the lung bases, extensive subpleural interstitial change in both the upper and lower lobes |
yes | ND | ND | ITP, hepato- splenomegaly, lymphadenopathy, NRH |
none | −28 | −35 | 48 |
AIHA, autoimmune hemolytic anemia; BIP, brochiolocentric interstitial pneumonia; EB, endobronchial biopsy; FB, follicular bronchiolitis; ITP, immune thrombocytopenic purpura; ILD, interstitial lung disease; LH, lymphoid hyperplasia; LIP, lymphocytic interstitial pneumonia; NA, report not available; ND, not done; NRH, nodular regenerative hyperplasia; OP, organizing pneumonia; VATS, video-assisted thoracoscopic surgery
Interestingly, CVID patients with progressive ILD had significantly higher initial FEV1 (P = 0.03) (Table II). Though not statistically significant, progressive ILD patients also demonstrated higher initial FVC (P = 0.07), lower initial platelet count (P = 0.09), and more frequent histories of ITP (P = 0.09) and lymphadenopathy (P = 0.09). Moreover, nodular regenerative hyperplasia and chronic non-infectious hepatitis only occurred in those with progressive ILD. Notably, there was no difference among those with stable or progressive ILD for age, sex, presence of bronchiectasis, inhaled corticosteroid or prophylactic antibiotic use, initial diffusion capacity of the lung for carbon monoxide (DLCO), IgG level at diagnosis, or peripheral isotype-switched memory B cells. Likewise, pathologic diagnoses and the presence of granulomas in biopsies did not differentiate those with progressive versus stable ILD.
Table II.
Comparison of Initial Clinical and Laboratory Characteristics
| Progressive (n = 9) | Stable (n = 6) | Pvalue | |
|---|---|---|---|
|
Median Age (years) |
45 | 45 | 0.38 |
|
Female (%) |
3 (33) | 4 (67) | 0.32 |
|
CT-confirmed Bronchiectasis (%) |
4 (44) | 4 (67) | 0.61 |
|
History of ITP (%) |
8 (89) | 2 (33) | 0.09 |
|
History of Splenomegaly (%) |
9 (100) | 5 (83) | 0.40 |
|
History of Lymphadenopathy (%) |
8 (89) | 2 (33) | 0.09 |
|
History of NRH or Chronic Non-Infectious Hepatitis (%) |
4 (44) | 0 (0) | 0.10 |
|
Inhaled Corticosteroid Usage (%) |
6 (67) | 5 (83) | 0.60 |
|
Prophylactic Antibiotic Usage (%) |
6 (67) | 5 (83) | 0.60 |
|
Initial FEV1 (% of predicted) 25% – 75% Percentile |
84.5 – 101.5 | 68.5 – 88.5 | 0.03 |
|
Initial FVC (% of predicted) 25% – 75% Percentile |
85.5 – 102.5 | 74.3 – 86.5 | 0.07 |
|
Initial FEV1/FVC (%) 25% – 75% Percentile |
78.0 – 92.5 | 75.0 – 83.8 | 0.12 |
|
Initial DLCO (% of predicted) 25% – 75% Percentile |
61.5 – 89.8 | 51.0 – 98.0 | 1.0 |
|
Initial IgG (mg/dL) 25% – 75% Percentile |
82 – 330.5 | 31.5 – 525.3 | 0.96 |
|
Initial IgA (mg/dL) 25% – 75% Percentile |
3.0 – 10.0 | 4.0 – 40.3 | 0.19 |
|
Initial IgM (mg/dL) 25% – 75% Percentile |
5.0 – 16.0 | 12.25 – 34.3 | 0.10 |
|
Initial CD19+ B cells (cells/µL) 25% – 75% Percentile |
3.5 – 92 | 25 – 468 | 0.26 |
|
Initial CD27+IgM- B cells (% of CD19+ B cells) 25% – 75% Percentile |
0.44 – 1.1 | 0.3 – 2.0 | 0.95 |
|
Initial CD27+IgM+ B cells (% of CD19+ B cells) 25% - 75% Percentile |
11.6 – 28.3 | 1.7 – 34.7 | 0.41 |
|
Initial CD4+ T cells (cells/µL) 25% - 75% Percentile |
277 – 617 | 436 – 787 | 0.44 |
|
Initial CD8+ T cells (cells/µL) 25% – 75% Percentile |
115 – 364 | 141 – 566 | 0.52 |
|
Initial CD4:CD8 Ratio 25% – 75% Percentile |
0.95 – 4.8 | 1.1 – 3.3 | 0.79 |
|
Initial NK Cells (cells/µL) 25% – 75% Percentile |
14 – 163 | 24 – 149 | 0.70 |
|
Initial Neutrophils (cells/µL) 25% – 75% Percentile |
1595 – 3950 | 2890 – 4100 | 0.20 |
|
Initial Lymphocytes (cells/µL) 25% – 75% Percentile |
565 – 1400 | 700 – 1860 | 0.74 |
|
Initial Monocytes (cells/µL) 25% – 75% Percentile |
200 – 445 | 319 – 500 | 0.28 |
|
Initial Platelets (cells/µL) 25% – 75% Percentile |
74 – 165 | 139 – 229 | 0.09 |
|
Initial Hemoglobin (G/DL) 25% – 75% Percentile |
12.2 – 15.2 | 11 – 16 | 0.94 |
|
Initial Alkaline Phosphatase (U/L) 25% – 75% Percentile |
74 – 148 | 61 – 170 | 0.59 |
DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ITP, immune thrombocytopenic purpura; NRH, nodular regenerative hyperplasia
Changes in pulmonary function over time in patients with CVID ILD
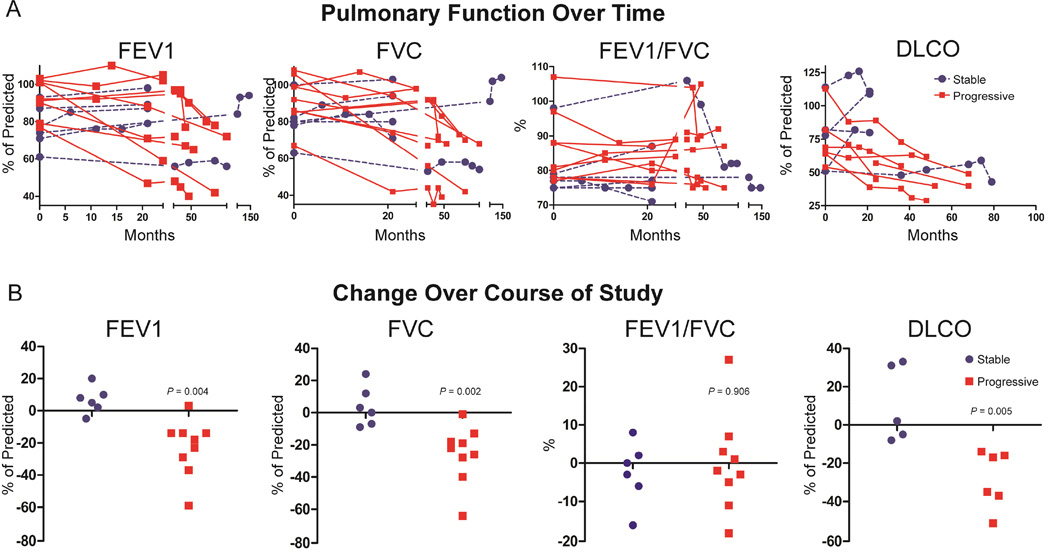
All CVID patients with progressive ILD had a decline in FVC over the course of the study, while steady FVC values persisted in those with stable ILD, even markedly improving in two cases during the course of the study (Figure 2A). Similarly, FEV1 and DLCO steadily decreased in CVID patients with progressive ILD, but remained unchanged or improved in those with stable ILD. Changes in FEV1/FVC ratio over time did not notably diverge between those with stable or progressive ILD. Decreases in DLCO preceded significant decreases in FVC in 2 patients (patient 7 and patient 13). Patient 7 had a decrease in DLCO from 113% of predicted to 88% while FVC was virtually unchanged over the first 26 months (99% of predicted to 98%). Patient 12 did not have had worsening of FVC (99% of predicted initially to 98% at end of study), but DLCO decreased from 82% to 45% of predicted. There were no instances where FVC significantly decreased without concurrent reduction of DLCO (when measured).
Figure 2.
A. Changes in PFTs over time. Those with progressive ILD have precipitous drops in FEV1, FVC, and DLCO during the course of the study. B. Progressive ILD was associated with significantly greater decreases in FEV1, FVC, and DLCO. CVID patients with stable ILD are denoted by blue circles, progressive ILD are red squares.
Demonstrating that CVID subjects can be delineated into two ILD subsets based upon PFT criteria of physiological decline, a distinct group with progressive ILD was identified with significantly larger decreases in percent of predicted FVC, FEV1, and DLCO (P = 0.002, P = 0.004, and P = 0.005, respectively) (Figure 2B). There was no significantly different change in FEV1/FVC between CVID patients with stable or progressive ILD. Together these data suggest that there are distinct subsets of CVID ILD patients with either stable or progressively worsening PFTs defined by decline in FVC, FEV1, and/or DLCO.
Characteristics of laboratory tests over time in patients with CVID ILD
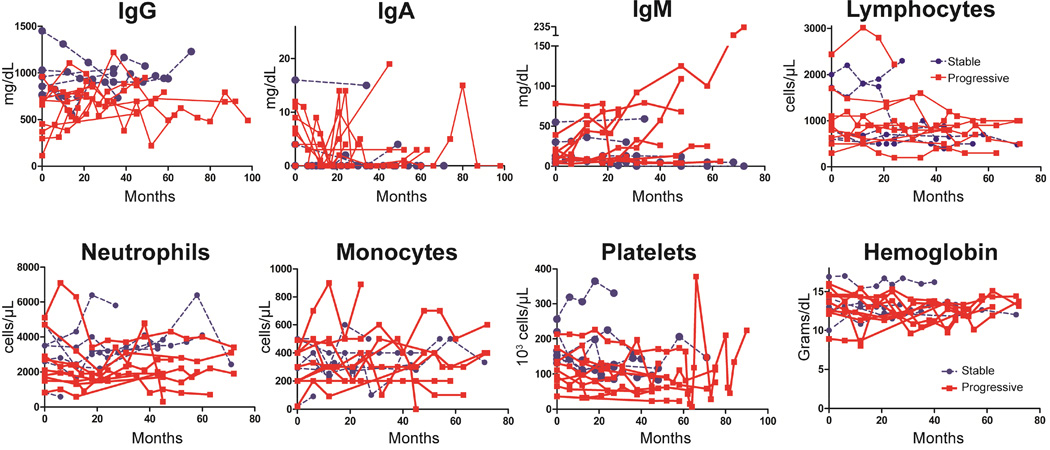
Serum IgM increases of > 20 mg/dL were observed across some time points for 5 of the 9 progressive ILD patients during the course of the study, but remained unchanged in all of those with stable ILD (Figure 3). Other laboratory values tested did not demonstrate any divergent trends over time between the two groups. All patients were on IgG replacement therapy during the course of the study, yet those with progressive ILD had a significantly lower mean serum IgG levels than those with stable ILD (P = 0.012) (Figure 4A). Lower serum IgG levels were found in progressive ILD patients despite the fact that there was no significant difference in IgG replacement dosage (Figure 4B). The ratio of serum IgG to IgG dosage was significantly lower in those with progressive ILD (P = 0.0026), demonstrating that these patients require higher dosages of IgG replacement than stable ILD patients to achieve similar serum IgG levels (Figure 4C). Over the course of the study, peak levels of serum IgM level were significantly higher in patients with progressive ILD compared to those with stable ILD (P = 0.02) (Figure 4D). Changes in leukocyte subsets and platelets (Figure 4E) as well as hemoglobin and serum IgA (data not shown) did not statistically differ between the two groups.
Figure 3.
Changes in laboratory parameters over time. Serum IgM levels steadily climb in many with progressive ILD, but remain unchanged in those with stable ILD. CVID patients with stable ILD are denoted by blue circles, progressive ILD are red squares.
Figure 4.
A. The mean IgG level during the course of the study was significantly lower in those with progressive ILD. B. The peak level of serum IgM was significantly higher in patients with progressive ILD. C. Changes in laboratory parameters over time for other laboratory parameters. CVID patients with stable ILD are denoted by blue circles, progressive ILD are red squares.
As we found lower mean IgG, higher IgM, and lower platelet values to be associated with physiologic worsening of CVID ILD, we looked more closely to determine whether specific values for these parameters were found more commonly in patients with progressive disease. Mean serum IgG levels greater than 700 mg/dL or 1000 mg/dL, typical goals of IgG replacement for conventional and complicated CVID patients respectively, were significantly more common in those with stable disease (Table III). Additionally, serum IgM levels greater than the median value of all serum IgM measurements (13 mg/dL) were measured in significantly more patients with progressive ILD, and patients with stable ILD did not have recorded IgM levels above 60 mg/dL while more than half of those with progressive ILD did. Similarly, thrombocytopenic episodes with platelet counts less than the median of all study measurements (112 ×l03/µL) were found in significantly more progressive ILD patients and platelet counts never dropped below 80 × 103/µL in those with stable ILD during course of the study.
Table III.
Specific Laboratory Values in CVID ILD
| Lab Value | Stable ILD (n = 6) |
Progressive ILD (n = 9) |
Pvalue |
|---|---|---|---|
| Mean IgG (mg/dL) | |||
| IgG > 700 | 6 (100%) | 3 (33%) | 0.03 |
| IgG > 1000 | 4 (67%) | 0 (0%) | 0.01 |
| Highest IgM (mg/dL) | |||
| > 13 (median) | 2 (33%) | 9 (100%) | 0.01 |
| > 60 | 0 (0%) | 5 (56%) | 0.04 |
| Lowest Plt (103/µL) | |||
| < 112 (median) | 3 (50%) | 9 (100%) | 0.04 |
| < 80 | 0 (0%) | 9 (100%) | 0.002 |
Plt, platelet
DISCUSSION
A major goal of this study was to examine whether physiological worsening was observed in all patients in this CVID ILD cohort. While most patients did have progressive ILD, 6 of 15 subjects did not meet PFT criteria of physiological worsening over the course of 20 or more months. While this does not exclude the possibility that patients with stable ILD can worsen at a later time, the fact that two subjects did not have significant physiological worsening over more than 6 years of follow-up (up to 12 years in one case) suggests that ILD is not necessarily progressive in all CVID patients. This is consistent with anecdotal reports of others who base treatment decisions upon decline in PFTs rather than radiologic findings alone.30 Those with stable ILD had waxing-and-waning CT chest findings, a phenomenon that has been observed before in CVID.17 Yet, our results suggest that ILD is progressive in many, and likely the majority, of CVID patients. Prior work has reported progressive worsening of PFTs in CVID patients, however these studies did not independently evaluate the impact of ILD relative to other chronic lung conditions, such as bronchiectasis.36, 37 We also found that decreases in DLCO preceded drops in FVC in 2 of the patients with progressive ILD, while drops in FVC never preceded reductions in DLCO. These data are consistent with prior reports that DLCO is useful in screening and monitoring of CVID lung disease.14, 19, 38 In addition to small study size, our report is limited by referral bias, as our medical center is frequently referred refractory patients with worsening clinical status. Additional studies are necessary to corroborate our findings.
Interestingly, we found that CVID patients with progressive ILD had significantly higher initial FEV1 and FVC values. There are a few potential explanations for this observation. It is possible that those with stable ILD had progressive ILD prior to the initiation of this study. Subsequently, the development of fixed parenchymal changes and fibrosis may limit further worsening of PFTs. However, fibrosis was not apparent on the initial chest CT scans of these patients and there were no notable differences in the radiologist reports of CT scans between those with chronic or progressive ILD. Additionally, patients with progressive ILD had steadily worsening PFTs through the duration of study without any apparent plateau of decline, thus we did not find evidence that ILD progression spontaneously halts in CVID. Alternatively, differences in age may have influenced initial PFT values. While there was no significant difference in median age between the two groups, about half of the progressive ILD subjects, but none of those with stable ILD, were under the age of 40. Additionally, bronchiectasis could have also contributed to the lower initial PFTs, as bronchiectasis was found in 67% of stable ILD patients but only 44% of those with progressive ILD, and may have been more severe in those patients.
While thrombocytopenia could seem unlikely to be related to ILD, multiple studies have found that these two complications are strongly associated in CVID.18, 39 Thrombocytopenia may be due to splenic sequestration as splenomegaly was found in nearly all patients included in this study. Alternatively, autoimmune cytopenias, most commonly manifesting as ITP, may be driven by the same dysregulated lymphoproliferation that leads to ILD.40 Notably, the concurrence of autoimmune cytopenias and lymphoproliferation is a characteristic of other primary immunodeficiencies with clinical presentations that can be quite similar to CVID, such as autoimmune lymphoproliferative syndrome and CTLA-4 deficiency.21, 22, 41 Higher serum IgM appears to be a marker for lymphoproliferative complications in CVID,6 and we have previously found higher levels of IgM to distinguish CVID patients with ILD from those without ILD.13 Our small study suggests that serum IgM increases of at least 20 mg/dL in individual patients or IgM levels above the median for subjects in this study (13 mg/dL) may be indicators for those at risk of ILD progression, but larger studies are necessary to confirm these findings. It should be noted that the normal range of serum IgM in healthy adults is approximately 40–300 mg/dL, thus what is referred to as “high” for CVID patients may still be below the normal range.
A predominant pathological feature of CVID ILD are ectopic pulmonary B cell follicles containing germinal centers that act as active centers of cellular proliferation.19 It may be within these proliferating follicles that B cell responses are activated, with IgM being the default Ig produced by CVID B cells with limited ability to produce other isotypes. Thus, the elevated serum IgM seen in CVID patients with progressive ILD may be indicative of the inflammatory activation within these ectopic follicles that could drive both ILD and autoimmune cytopenias. Indeed, benign lymphoproliferation has been previously linked with ITP.40, 42 More extensive study of this residual IgM production in CVID is needed to determine whether it is simply a biomarker of lymphoproliferation and/or inflammation or whether IgM-producing B cells are directly impacting disease.
We found that CVID patients with progressive ILD had lower mean IgG levels than those with chronic ILD. It is impossible to conclude from this retrospective study that such a difference in serum IgG could contribute to ILD progression. However, it is notable that there was no significant difference in IgG replacement dosage between the two groups. Rather, our data suggests that those with progressive ILD require higher dosages of IgG replacement, as the ratio of serum IgG to IgG replacement dosage was significantly lower in these patients. CVID patients with GI disease causing extensive IgG loss or those with inflammation markedly increasing catabolism may require higher IgG replacement dosages. We note here that increasing IgG dosage requirements in a CVID patient with ILD may be indicative that the lung disease is progressive. While the exact reason that lower IgG replacement dosages were achieved in those with progressive disease is not known, it seems prudent to ensure customary serum IgG levels are achieved. We suggest that serum IgG levels should be maintained at least 700 mg/dL in patients with CVTD ILD, with a goal of 1000 mg/dL strongly considered, as 4 of 6 patients with stable ILD, but no patients with progressive ILD, had mean IgG values > 1000 mg/dL. This is similar to the result from a study of 24 adult CVID patients that found IgG replacement greater than 600 mg/dL to be protective against pulmonary physiological worsening.37 However, that study did not differentiate the etiology of chronic lung disease, so the benefit of IgG replacement at that dosage may have been due treatment of bronchiectasis rather than ILD.
It is clear through our experience, and that of others, that while IgG replacement may improve some cases of CVID ILD, the progression of ILD is not altered by this therapy in many instances. Immunosuppressive therapies including corticosteroids, cyclosporine, infliximab, and rituximab have been utilized in such patients, but results with these agents are variable.25, 26, 43, 44 Combination immunotherapy with azathioprine and rituximab may hold the most promise in treatment of CVID ILD, as this regimen was efficacious in a case series of 7 patients.27 However, the usage of immunosuppressive therapy in immunocompromised patients may heighten the risk of infection or malignancy.28, 29 As we found that CD4+ T cell counts below 700 cells/µL raises the risk of bronchiectasis in CVTD patients at our medical center, the usage of immunosuppression to treat CVTD lung disease may not be trivial decision.13 This current report is a retrospective study of relatively small size from one clinical center, so treatment guidelines for CVID ILD cannot be derived from its results. Larger prospective studies are needed to confirm risk factors of disease progression and determine which patients should be treated with immunomodulatory therapy. As there are currently no guidelines for the treatment of CVID ILD, the results of this current study help to inform decisions in CVTD ILD management by highlighting that vigilant IgG replacement is a necessity, PFTs are useful for identifying those with progressive ILD and should include DLCO, and severe thrombocytopenia and elevated IgM levels can be clues to those at greatest risk of physiologic worsening.
What is already known about this topic?
Interstitial lung disease frequently complicates common variable immunodeficiency, worsening morbidity and mortality. Immunomodulatory treatment has efficacy in small studies, but adverse effects due to immunosuppression necessitate the identification of patients most appropriate for such therapy.
What does this article add to our knowledge?
Interstitial lung disease is progressive in many cases of common variable immunodeficiency. In our cohort, those most likely to have physiologic worsening had history of severe thrombocytopenia as well lower IgG and higher IgM levels.
How does this study impact current management guidelines?
Worsening pulmonary function, increased IgG replacement dosage requirements, severe thrombocytopenia, and elevated serum IgM can be clues to those at greatest risk of physiologic worsening and identify patients for which immunomodulatory therapy may be indicated.
ACKNOWLEDGEMENTS
We gratefully acknowledge Lin Radigan for flow cytometry measurement of peripheral B cell subsets and Laureen Ojalvo for valuable discussion.
Supported by the Jeffrey Modell Foundation, Clinical Immunology Society/Baxter Senior Fellowship Award, Thrasher Research Fund Early Career Award, National Institutes of Health grants AI 048693 and AI 061093, and the David S. Gottesman Immunology Chair.
ABBREVIATIONS
- AIHA
autoimmune hemolytic anemia
- CVID
common variable immunodeficiency
- DLCO
diffusion capacity of the lung for carbon monoxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GLILD
granulomatous-lymphocytic interstitial lung disease
- Ig
immunoglobulin
- ILD
interstitial lung disease
- ITP
immune thrombocytopenic purpura
- PFTs
pulmonary function tests
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Yong PF, Thaventhiran JE, Grimbacher B. “A rose is a rose is a rose,” but CVID is Not CVID common variable immune deficiency (CVID), what do we know in 2011? Adv Immunol. 2011;111:47–107. doi: 10.1016/B978-0-12-385991-4.00002-7. [DOI] [PubMed] [Google Scholar]
- 2.Keller MD, Jyonouchi S. Chipping away at a mountain: genomic studies in common variable immunodeficiency. Autoimmun Rev. 2013;12:687–689. doi: 10.1016/j.autrev.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129:1425–1426. doi: 10.1016/j.jaci.2012.03.025. e3. [DOI] [PubMed] [Google Scholar]
- 4.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109:1001–1004. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 5.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin Immunol. 2010;137:21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 7.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematology Am Soc Hematol Educ Program. 2012;2012:301–305. doi: 10.1182/asheducation-2012.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1:545–556. doi: 10.1016/j.jaip.2013.09.015. quiz 57. [DOI] [PubMed] [Google Scholar]
- 10.Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–316. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 11.Touw CM, van de Vens AA, de Jong PA, Terheggen-Lagro S, Beek E, Sanders EA, et al. Detection of pulmonary complications in common variable immunodeficiency. Pediatr Allergy Immunol. 2010;21:793–805. doi: 10.1111/j.1399-3038.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 12.Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114:415–421. doi: 10.1016/j.jaci.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 13.Maglione PJ, Overbey JR, Radigan L, Bagiella E, Cunningham-Rundles C. Pulmonary radiologic findings in common variable immunodeficiency: clinical and immunological correlations. Ann Allergy Asthma Immunol. 2014;113:452–459. doi: 10.1016/j.anai.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kainulainen L, Varpula M, Liippo K, Svedstrom E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 1999;104:1031–1036. doi: 10.1016/s0091-6749(99)70085-0. [DOI] [PubMed] [Google Scholar]
- 15.Wheat WH, Cool CD, Morimoto Y, Rai PR, Kirkpatrick CH, Lindenbaum BA, et al. Possible role of human herpesvirus 8 in the lymphoproliferative disorders in common variable immunodeficiency. J Exp Med. 2005;202:479–484. doi: 10.1084/jem.20050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva SP, Resnick E, Lucas M, Lortan J, Patel S, Cunningham-Rundles C, et al. Lymphoid proliferations of indeterminate malignant potential arising in adults with common variable immunodeficiency disorders: unusual case studies and immunohistological review in the light of possible causative events. J Clin Immunol. 2011;31:784–791. doi: 10.1007/s10875-011-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torigian DA, LaRosa DF, Levinson AI, Litzky LA, Miller WT., Jr Granulomatous-lymphocytic interstitial lung disease associated with common variable immunodeficiency: CT findings. J Thorac Imaging. 2008;23:162–169. doi: 10.1097/RTI.0b013e318166d32f. [DOI] [PubMed] [Google Scholar]
- 18.Bondioni MP, Soresina A, Lougaris V, Gatta D, Plebani A, Maroldi R. Common variable immunodeficiency: computed tomography evaluation of bronchopulmonary changes including nodular lesions in 40 patients Correlation with clinical and immunological data. J Comput Assist Tomogr. 2010;34:395–401. doi: 10.1097/RCT.0b013e3181cad9da. [DOI] [PubMed] [Google Scholar]
- 19.Maglione PJ, Ko HM, Beasley MB, Strauchen JA, Cunningham-Rundles C. Tertiary lymphoid neogenesis is a component of pulmonary lymphoid hyperplasia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;133:535–542. doi: 10.1016/j.jaci.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrillo J, Restrepo CS, Rosado de Christenson M, Ojeda Leon P, Lucia Rivera A, Koss MN. Lymphoproliferative lung disorders: a radiologic-pathologic overview Part I: Reactive disorders. Semin Ultrasound CT MR. 2013;34:525–534. doi: 10.1053/j.sult.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubry MC. Pulmonary Pathology: LC22-1 NON-NEOPLASTIC PULMONARY LYMPHOID PROLIFERATIONS. Pathology. 2014;46(Suppl 2):S36–S37. [Google Scholar]
- 25.Boursiquot JN, Gerard L, Malphettes M, Fieschi C, Galicier L, Boutboul D, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol. 2013;33:84–95. doi: 10.1007/s10875-012-9778-9. [DOI] [PubMed] [Google Scholar]
- 26.Davies CW, Juniper MC, Gray W, Gleeson FV, Chapel HM, Davies RJ. Lymphoid interstitial pneumonitis associated with common variable hypogammaglobulinaemia treated with cyclosporin A. Thorax. 2000;55:88–90. doi: 10.1136/thorax.55.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chase NM, Verbsky JW, Hintermeyer MK, Waukau JK, Tomita-Mitchell A, Casper JT, et al. Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID) J Clin Immunol. 2013;33:30–39. doi: 10.1007/s10875-012-9755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chien SH, Liu CJ, Hong YC, Teng CJ, Hu YW, Shen CC, et al. Use of azathioprine for graft-vs-host disease is the major risk for development of secondary malignancies after haematopoietic stem cell transplantation: a nationwide population-based study. Br J Cancer. 2015;112:177–184. doi: 10.1038/bjc.2014.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasse A, Kayser G, Warnatz K. Common variable immunodeficiency-associated granulomatous and interstitial lung disease. Curr Opin Pulm Med. 2013;19:503–509. doi: 10.1097/MCP.0b013e3283642c47. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez PerezS ER. Granulomatous lymphocytic interstitial lung disease. Immunol Allergy Clin North Am. 2012;32:621–632. doi: 10.1016/j.iac.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Verbsky JW, Routes JM. Sarcoidosis and common variable immunodeficiency: similarities and differences. Semin Respir Crit Care Med. 2014;35:330–335. doi: 10.1055/s-0034-1376862. [DOI] [PubMed] [Google Scholar]
- 33.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 34.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiely PD, Chua F. Interstitial lung disease in inflammatory myopathies: clinical phenotypes and prognosis. Curr Rheumatol Rep. 2013;15:359. doi: 10.1007/s11926-013-0359-6. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Stirling RG, Paul E, Hore-Lacy F, Thompson BR, Douglass JA. Longitudinal decline in lung function in patients with primary immunoglobulin deficiencies. J Allergy Clin Immunol. 2011;127:1414–1417. doi: 10.1016/j.jaci.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 37.de Gracia J, Vendrell M, Alvarez A, Pallisa E, Rodrigo MJ, de la Rosa D, et al. Immunoglobulin therapy to control lung damage in patients with common variable immunodeficiency. Int Immunopharmacol. 2004;4:745–753. doi: 10.1016/j.intimp.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Bouvry D, Mouthon L, Brillet PY, Kambouchner M, Ducroix JP, Cottin V, et al. Granulomatosis-associated common variable immunodeficiency disorder: a case-control study versus sarcoidosis. Eur Respir J. 2013;41:115–122. doi: 10.1183/09031936.00189011. [DOI] [PubMed] [Google Scholar]
- 39.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 40.Daridon C, Loddenkemper C, Spieckermann S, Kuhl AA, Salama A, Burmester GR, et al. Splenic proliferative lymphoid nodules distinct from germinal centers are sites of autoantigen stimulation in immune thrombocytopenia. Blood. 2012;120:5021–5031. doi: 10.1182/blood-2012-04-424648. [DOI] [PubMed] [Google Scholar]
- 41.Rensing-Ehl A, Warnatz K, Fuchs S, Schlesier M, Salzer U, Draeger R, et al. Clinical and immunological overlap between autoimmune lymphoproliferative syndrome and common variable immunodeficiency. Clin Immunol. 2010;137:357–365. doi: 10.1016/j.clim.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Franchini M, Vescovi PP, Garofano M, Veneri D. Helicobacter pylori-associated idiopathic thrombocytopenic purpura: a narrative review. Semin Thromb Hemost. 2012;38:463–468. doi: 10.1055/s-0032-1305781. [DOI] [PubMed] [Google Scholar]
- 43.Kohler PF, Cook RD, Brown WR, Manguso RL. Common variable hypogammaglobulinemia with T-cell nodular lymphoid interstitial pneumonitis and B-cell nodular lymphoid hyperplasia: different lymphocyte populations with a similar response to prednisone therapy. J Allergy Clin Immunol. 1982;70:299–305. doi: 10.1016/0091-6749(82)90066-5. [DOI] [PubMed] [Google Scholar]
- 44.Malbran A, Juri MC, Fernandez RomeroS DS. Common variable immunodeficiency and granulomatosis treated with infliximab. Clin Immunol. 2010;134:359–360. doi: 10.1016/j.clim.2009.11.014. [DOI] [PubMed] [Google Scholar]