Abstract
Objective
Adverse outcomes associated with chronic depressive symptoms are of clinical importance. The objective was to identify subgroups of older adults based on their trajectories of depressive symptoms over a ten-year period, and determine if these subgroups predicted oral health outcomes.
Methods
Secondary analysis of data from the Health and Retirement Survey. The sample was 944 adults age 65+ who participated in the oral health module in 2008. Depressive symptoms were measured with a modified version of the Center for Epidemiologic Studies-Depression (CES-D) scale. Latent class trajectory analysis was used to identify distinct subgroups of elders based on their CES-D scores from 1998–2008. Group membership was used to predict self-rated oral health, overall mouth condition (problems with bleeding gums, gum sensitivity, and food avoidance), and edentulism in 2008.
Results
Three distinct subgroups were identified using zero inflated Poisson regression models: (a) minimal depressive symptoms over the study period (43%), (b) low but generally stable level of depressive symptoms (41%), and (c) moderate symptoms and higher CES-D scores than the other groups over the 10 years (16%). Controlling for demographic and health variables and edentulism status, having a trajectory of moderate symptoms was associated with poorer mouth condition (p<0.0001) and poorer self-rated oral health (p=0.0003) compared to those with minimal symptoms. Having low levels of depressive symptoms was not significantly associated with these two outcomes. Group membership was not significantly associated with the probability of edentulism.
Conclusions
Chronic moderate depressive symptoms are associated with poorer oral health in older adults.
Keywords: depressive symptoms, oral health, edentulism, latent class trajectory analysis
Introduction
Clinicians treating older adults are aware of the adverse consequences of depressive symptoms. Late life depressive symptoms are linked to functional decline, cognitive decline, increased mortality, poorer cardiovascular health, and decreased social functioning (Blazer, 2003). Treating the symptoms in some cases can reverse the outcomes, or at least stabilize decline, yet many older adults experience a chronic course of depressive symptoms.
Little is known about oral health outcomes associated with chronic depressive symptoms. Older adults with depressive symptoms may be less likely to make self-care, including oral hygiene and preventive dental care, a priority. Late life depression and depressive symptoms may be associated with poor nutrition, diminished salivary flow, distorted taste, a higher oral lactobacillus count, dental decay, advanced periodontal disease, and oral discomfort (D'Mello, 2003; Friedlander and Norman, 2002). Poor oral health has its own adverse consequences. Poor mouth conditions, such as bleeding gums, for example, may lead to incident cardiovascular disease (Buhlin et al., 2003). Problems with oral pain and discomfort and tooth loss may lead to problems with nutrition (Nowjack-Raymer and Sheiham, 2003; Poisson et al., 2014) and subsequent frailty (Fried et al., 2001), which can be associated with depression (Blazer, 2003).
Most studies examining the association between depressive symptoms and oral health have been cross-sectional, and among younger and middle-aged adults. Depressive symptoms have been linked to poorer self-rated oral health (Finlayson et al., 2010) and oral health related quality of life (de Andrade et al., 2012; Hassel et al., 2011). In the National Survey of American Life, 12.8% of the adults who had fair or poor self-rated oral health had experienced major depression in the previous 12 months compared to 4.4% of those with good, very good, or excellent self-rated oral health. Controlling for demographic variables, other psychosocial stressors, and psychosocial resources, adults with major depression were twice as likely to have fair or poor self-rated oral health (Finlayson et al., 2010). Adults with current depression are more likely to have tooth loss (Okoro et al., 2012). Numerous cross-sectional studies have found an association between depressive symptoms and increased periodontal disease (Ng and Leung, 2006; Saletu et al., 2005), although others have not found an association (Solis et al., 2004).
There have been fewer studies from older populations. Among older adults, individuals with more depressive symptoms have reported oral discomfort (dry mouth, mouth pain, mouth swelling) and worse oral health-related quality of life (Kressin et al., 2002; Saintrain et al., 2013). While associations between depressive symptoms and periodontitis among younger adults are generally significant, in a sample of 701 older adults, depressive symptoms were associated with heart attack, stroke, high blood pressure, cardiovascular disease, chronic pain, osteoarthritis and osteoporosis but not periodontitis. Persons with depressive symptoms, however, had a higher self-reported risk score for future tooth loss (Persson et al., 2003). There is very limited understanding of how chronic depressive symptoms are related to later oral health outcomes in the older population.
Over time, the presence or absence of depressive symptoms can be dynamic. Trajectories of depressive symptoms can reveal much information about the course of clinically significant symptoms. That is, trajectories can be superior to cross-sectional measures of depression which may reflect transitory affective states due to life events. Distinct courses of depressive symptoms in the general community-dwelling older populations have been identified (Liang et al., 2011). It is not known whether these distinct population subgroups based on their trajectories of depressive symptoms have different oral health outcomes.
The purpose of these analyses was 1) to identify distinct classes of older adults based on their trajectories of depressive symptom scores over ten years; and 2) to examine trajectory class membership as a predictor of oral health at the end of the study period. We hypothesized that multiple trajectories of depressive symptoms exist, which differ in their levels and rates of change. In addition, we hypothesized that these trajectories of depressive symptoms would be associated with oral health, mouth condition, and dentate status. For example, that a trajectory of a higher level of chronic depressive symptoms would be associated with poorer oral health (i.e., self-rated oral health, mouth condition, and dentate status).
Methods
Study Sample
The data were taken from six waves (1998–2008) of the Health and Retirement Survey (HRS) conducted in the U.S. using a national representative probability sample of older adults, with interviews every two years. Detailed information about the HRS can be found on its website (http://hrsonline.isr.umich.edu). An oral health module was included in the 2008 survey and administered to a subsample of the HRS participants. A total of 944 adults who completed the oral health questions and were age 65 or older in 2008 comprised the study sample for the analyses presented here. Depressive symptoms of interest were those measured over the ten-year period preceding the oral health module.
Study Variables
Depressive symptoms were measured using a modified version of the Center for Epidemiologic Studies – Depression (CES-D) scale (Radloff, 1977). Eight CES-D symptoms were included in the HRS: felt depressed, felt everything was an effort, sleep was restless, felt happy, felt lonely, enjoyed life, felt sad, and could not get going. Each symptom was coded 1 if present much of the time in the previous week and 0 if not. The symptoms were summed with items in the positive direction reverse coded for a possible range of 0–8. The modified CES-D scores used were those in 1998, 2000, 2002, 2004, 2006, and 2008
Oral health was measured in three ways: Self-rated oral health asked respondents to describe the condition of their mouth and teeth using the categories of poor, fair, good or very good. The possible range of responses was 1–4, with higher scores reflecting better self-reported oral health. A composite score measuring problems with oral health was determined by summing the responses to three questions. Respondents were asked how often in the past year 1) they had avoided particular foods because of problems with their teeth, mouth, or dentures; 2) their gums had been sensitive to hot, cold, or sweets; and 3) they had their gums bleed when they brushed their teeth. The range of responses for the composite score was 3 to 15, with higher scores reflecting less often (better oral health). Edentulism was measured by self-report. Respondents were first asked if they had lost more than two natural permanent teeth (Respondents who answered no were coded as dentate). Respondents who answered yes to losing more than two permanent teeth were then asked if they had lost all teeth from their upper jaw and lower jaw. Respondents who had lost all teeth in both the upper and lower jaw were coded as edentulous. Responses were coded 1=No teeth and 0=one or more teeth.
Covariates known to be associated with depression and potentially oral health (Hybels et al., 2001) were used at their 2008 value at the time of the oral health module. Demographic variables included age (range 65–100), sex, race (coded as White Y=1/N=0, Black Y=1/N=0, and Other Y=1/N=0), Hispanic ethnicity coded as Y=1/N=0, years of education (range 0–17), and annual household income in thousands (range 0 to 818). Health variables included the number of functional difficulties (range 0–12) measured as difficulties with walking, dressing, bathing, eating, getting in/out of bed, using the toilet, reading a map, using the telephone, handling money, taking medication, shopping, and preparing meals. Responses were coded as 0=no difficulty and 1=some difficulty or unable to do. Self-rated health was assessed by asking respondents to rate their health as excellent, very good, good, fair, or poor. Responses were coded from 1 to 5 with higher scores indicating poorer self-rated health. The total number of health conditions was measured by asking about the presence of seven chronic conditions: high blood pressure, diabetes, cancer, heart disease, stroke, lung disease, and arthritis. The number of conditions was summed, for a possible range of 0 to 7. Cognition was measured using a brief cognitive screening measure that assessed immediate and delayed recall, serial 7's, backwards count, orientation to time, object naming, and naming of the president and vice president. Scores could range from 0 to 35, with higher scores indicating stronger cognitive functioning.
Statistical Analysis
We first identified distinct subgroups of participants based on their trajectories of CES-D scores over the ten-year period using group-based semi-parametric mixture models. While the range of CES-D scores was 0–8, there were a disproportionate number of zero scores, so we estimated zero inflated Poisson regression (ZIP) models. The trajectory groups were estimated from the CES-D scores alone with time as the only independent variable, with no covariates added that could influence class membership. We first tested a single trajectory model, allowing for both quadratic and cubic terms to model the function of time in the Poisson distribution. We removed non-significant higher order terms, and determined our best fitting single trajectory model. We then estimated the best fitting model with two, three, and then four trajectories following the same process. The models were compared using Bayesian Information Criteria (BIC) statistics and posterior probabilities.
In our second step we examined the association between trajectory class membership from our best fitting model (as noted below this model had three trajectory classes) and the three measures of oral health, controlling for age, race, ethnicity, sex, years of education, income, health conditions, functional difficulties, self-reported physical health, and cognition. In the models examining the association between trajectory group and both the composite score reflecting mouth condition as well as self-rated oral health, we also controlled for dentate status. Variables were entered in a hierarchical manner. Model 1 included depression group only. Demographic variables were added in Model 2, health variables were added in Model 3, and dentate status was added in Model 4. To minimize the number of participants lost due to missing data, we imputed values for the oral health measures, CES-D score, and the covariates as necessary using three imputations. We adopted an inclusive analysis strategy that included a number of auxiliary variables in addition to the predictors in our models in imputing missing values (Enders, 2010; Schafer, 1997).
We then averaged the regression coefficients and adjusted the standard errors based on three imputed data files (Rubin, 1987). All analyses were conducted using SAS Version 9.3 (2011). The latent class trajectories were estimated using SAS Proc Traj (Jones and Nagin, 2007).
Results
The characteristics of the sample are shown in Table 1. Our sample was predominantly female, non-Hispanic, and white, with a mean age of 75 years. The median income was approximately $34,000. On average, the sample was healthy, with few functional limitations and good cognitive functioning. Oral health variables showed the sample had fair to good oral health. About one-fifth were edentulous.
Table 1.
Characteristics of the sample in 2008 (n=944)
| Sample Characteristic | |
|---|---|
| Dental Variables | |
| No. Edentulous (%) | 204 (21.6%) |
| Mean Self-Rated Oral Health (sd) | 2.9 (1.1) |
| Mean Score for Mouth Condition (sd) | 12.9 (2.1) |
| Demographic Variables | |
| No. Female (%) | 549 (58.2%) |
| No. White (%) | 799 (84.6%) |
| No. Black (%) | 111 (11.8%) |
| No. Other Race (%) | 34 (3.6%) |
| No. Hispanic (%) | 74 (7.8%) |
| Mean age (sd) | 75.0 (7.2) |
| Mean years of education (sd) | 12.4 (3.1) |
| Mean income in thousands (sd) | 55.1 (76.2) |
| Health Variables | |
| Mean number of functional limitations (sd) | 0.9 (1.8) |
| Mean number of health conditions (sd) | 2.3 (1.4) |
| Mean total cognition score (sd) | 21.8 (5.3) |
| Mean CES-D Score (sd) | 1.3 (1.9) |
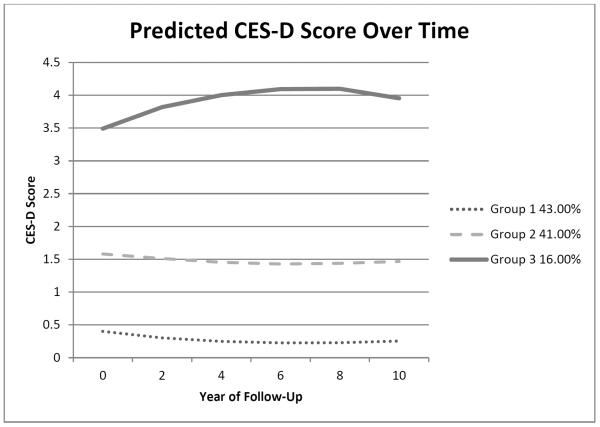
A model with three trajectory classes fit the data best. The mean trajectories for the classes are shown in Figure 1. A total of 43% of the sample was in the group with minimal depressive symptoms (Group 1). This group had lower CES-D scores in 1998 compared to the other two groups, saw a decrease in CES-D score, and then remained stable with minimal symptoms over the ten-year period. A total of 41% of the sample was in the group with low depressive symptoms (Group 2), who on average saw a slight decrease in CES-D score after 1998 and then had depressive symptoms at a low level over the ten years. Sixteen percent of the sample was in a group who had moderate symptom levels throughout the survey period (Group 3). Within this class, the CES-D score did not significantly change over time. Those participants in the class with moderate depressive symptoms had scores around a score of 4 over the ten-year period. The HRS documentation shows that a score of 4 or more on the modified CES-D scale is comparable to a score of 16 or greater on the traditional CES-D scale, the cut-point suggested as indicating `clinically significant depressive symptoms' (Survey Research Center, 2000).
Figure 1.
Predicted values of CES-D score by trajectory class over 10 years (n=944)
The results of the three trajectory classes estimated by the ZIP model are shown in Table 2. The parameter estimates represent the effect on the logged count of depressive symptoms from which we can derive the predicted count. The predicted CES-D score at baseline for those with minimal depressive symptoms (Group 1) would be 0.48, while the predicted scores for those with low-level depressive symptoms and those with moderate depressive symptoms (Groups 2 and 3) would be 1.91 and 4.22 respectively. The effect of time for those with minimal and low level symptoms (Groups 1 and 2) was significant and nonlinear – decreasing and then slightly increasing. The effect of time was linear and slightly increasing for those with moderate depressive symptoms (Group 3). The nonsignificant higher order term for time was removed for this group. In the second part of the model, the coefficients estimate the likelihood of a CES-D score of zero across all classes beyond that predicted by the count model. Higher order terms were not significant.
Table 2.
Change in CES-D scores over time predicted by trajectory groups estimated from a zero inflated Poisson regression model with three trajectory classes of depressive symptoms (n=944)
| Group | Parameter | Estimate | Exp (Estimate) | Standard Error | p-value |
|---|---|---|---|---|---|
| Parameter Estimates for Count Portion | |||||
| 1 | Intercept | −0.726 | 0.48 | 0.115 | <0.0001 |
| Time | −0.208 | 0.81 | 0.054 | 0.0001 | |
| Time2 | 0.016 | 1.02 | 0.005 | 0.0025 | |
| 2 | Intercept | 0.648 | 1.91 | 0.059 | <0.0001 |
| Time | −0.068 | 0.93 | 0.025 | 0.0055 | |
| Time2 | 0.006 | 1.01 | 0.002 | 0.0131 | |
| 3 | Intercept | 1.440 | 4.22 | 0.037 | <0.0001 |
| Time | 0.010 | 1.01 | 0.005 | 0.0611 | |
| Zero Inflation Parameter Estimates | |||||
| Intercept | −1.564 | 0.21 | 0.171 | <0.0001 | |
| Time | −0.302 | 0.74 | 0.101 | 0.0029 | |
| Time2 | 0.029 | 1.03 | 0.010 | 0.0034 | |
While the linear coefficient for the group with moderate symptoms (Group 3) shown in Table 2 appears inconsistent with Figure 1 (that is, the predicted score change is linear but the figure shows an increase then leveling off), this reflects the fact that the Poisson model which is linear in the logged counts is, by definition, nonlinear in the counts. The posterior probabilities for the three groups showed a good separation of classes. For the 411 persons assigned to the group with minimal symptoms (Class 1), the mean posterior probability for that class was 0.93 (SD=0.11; range 0.51 to 1.00). For the 389 persons assigned to the group with low levels of symptoms (Class 2), the mean posterior probability was 0.89 (SD=0.14; range 0.50 to 1.00). For the 144 persons assigned to the group with moderate symptoms (Class 3), the mean posterior probability was 0.92 (SD=0.14; range 0.54 to 1.00).
In Table 3 we show the results of the trajectory classes predicting mouth condition using linear regression. Model 1(M1) is uncontrolled, and shows both low (p=0.0075) and moderate (p<0.0001) depressive symptoms are significantly associated with mouth condition. For those with low symptom scores, there was a 0.43 decrease in the mouth condition composite score, while for those with moderate symptoms there was a 1.75 decrease in score relative to those with minimal symptoms. These associations remained significant when age, sex, race, ethnicity, education, and income were controlled (M2). When health conditions, functional difficulties, self-reported physical health, and cognition were controlled (M3), having low symptoms was not associated with mouth condition. Being in the group with moderate symptoms, however, was significant and remained so after dentate status was also controlled (M4). As shown in Model 4, those with moderate symptoms had a composite score 1.04 units lower than those with minimal symptoms. Being Hispanic and having more health conditions and functional limitations were also associated with poorer mouth condition. Being older and having more years of education were associated with fewer problems with the mouth and teeth. Those who were edentulous had fewer problems with the mouth and teeth.
Table 3.
Ten year trajectory groups of depressive symptoms predicting mouth condition using linear regression analyses (n=944)
| Parameter | M1 Estimate | Std Error | p-value | M2 Estimate | Std Error | p-value | M3 Estimate | Std Error | p-value | M4 Estimate | Std Error | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 13.3312 | 0.1053 | <0.0001 | 12.6319 | 0.8111 | <0.0001 | 11.7700 | 0.9761 | <0.0001 | 11.5032 | 0.9752 | <0.0001 |
| Depression Group 2 | −0.4286 | 0.1592 | 0.0075 | −0.3292 | 0.1648 | 0.0467 | −0.1464 | 0.1689 | 0.3868 | −0.1609 | 0.1680 | 0.3390 |
| Depression Group 3 | −1.7544 | 0.2015 | <0.0001 | −1.4838 | 0.2124 | <0.0001 | −1.0225 | 0.2233 | <0.0001 | −1.0374 | 0.2213 | <0.0001 |
| Age in 2008 | 0.0019 | 0.0095 | 0.8445 | 0.0233 | 0.0104 | 0.0258 | 0.0225 | 0.0103 | 0.0298 | |||
| Female (1=yes, 0=no) | −0.1878 | 0.1382 | 0.1752 | −0.2180 | 0.1372 | 0.1132 | −0.2298 | 0.1366 | 0.0936 | |||
| Black (1=yes, 0=no) | −0.2838 | 0.2137 | 0.1852 | −0.1434 | 0.2177 | 0.5106 | −0.1309 | 0.2177 | 0.5479 | |||
| Hispanic (1=yes, 0=no) | −0.7555 | 0.2773 | 0.0068 | −0.7503 | 0.2742 | 0.0066 | −0.7321 | 0.2729 | 0.0077 | |||
| Other Race (1=yes, 0=no) | 0.1904 | 0.3762 | 0.6131 | 0.2349 | 0.3707 | 0.5268 | 0.1616 | 0.3696 | 0.6623 | |||
| Years of Education (0–17) | 0.0556 | 0.0242 | 0.0224 | 0.0390 | 0.0252 | 0.1233 | 0.0539 | 0.0255 | 0.0357 | |||
| Income in 2008 (1000s) | −0.0003 | 0.0009 | 0.7683 | −0.0008 | 0.0009 | 0.3973 | −0.0006 | 0.0009 | 0.5315 | |||
| Total No. of Health Conditions in 2008 | −0.1792 | 0.0521 | 0.0007 | −0.1956 | 0.0521 | 0.0002 | ||||||
| Functional Difficulties in 2008 (high=more difficulties) | −0.2190 | 0.0428 | <0.0001 | −0.2259 | 0.0426 | <0.0001 | ||||||
| Self-Reported Physical Health (higher=poorer) | −0.0209 | 0.0744 | 0.7792 | −0.0197 | 0.0740 | 0.7903 | ||||||
| Total Cognition Score in 2008 | 0.0003 | 0.0157 | 0.9864 | 0.0033 | 0.0156 | 0.8344 | ||||||
| Edentulous (1=yes, 0=no) | 0.5517 | 0.1684 | 0.0012 |
Table 4 shows a similar pattern for self-rated oral health. Having low (p=0.0003) or moderate (p<0.0001) depressive symptoms was associated with poorer self-rated oral health in uncontrolled analyses (M1), and when controlling for demographic variables (M2). When health variables were added to the model (M3), only the group with moderate symptoms was significantly associated with self-rated oral health. Those with moderate symptoms had a 0.34 unit decrease in self-reported oral health (p=0.0004). When dentate status was added to the model (M4), having moderate symptoms was still significantly associated with poorer self-perceived oral health (p=0.0003). Being Hispanic, having more functional limitations, and reporting poorer self-rated physical health were associated with poorer self-rated oral health. Being female, having more years of education and being dentate were associated with higher self-reported oral health.
Table 4.
Ten year trajectory groups of depressive symptoms predicting self-rated oral health using linear regression analyses (n=944)
| Parameter | M1 Estimate | Std Error | p-value | M2 Estimate | Std Error | p-value | M3 Estimate | Std Error | p-value | M4 Estimate | Std Error | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 3.1518 | 0.0435 | <0.0001 | 2.8973 | 0.3368 | <0.0001 | 3.2317 | 0.4063 | <0.0001 | 3.0739 | 0.4038 | <0.0001 |
| Depression Group 2 | −0.2338 | 0.0636 | 0.0003 | −0.1806 | 0.0638 | 0.0049 | −0.0815 | 0.0649 | 0.2103 | −0.0891 | 0.0642 | 0.1664 |
| Depression Group 3 | −0.6773 | 0.0862 | <0.0001 | −0.5214 | 0.0887 | <0.0001 | −0.3329 | 0.0936 | 0.0004 | −0.3404 | 0.0934 | 0.0003 |
| Age in 2008 | −0.0031 | 0.0039 | 0.4380 | 0.0005 | 0.0043 | 0.9135 | 0.0001 | 0.0043 | 0.9831 | |||
| Female (1=yes, 0=no) | 0.1522 | 0.0574 | 0.0085 | 0.1262 | 0.0571 | 0.0279 | 0.1187 | 0.0566 | 0.0368 | |||
| Black (1=yes, 0=no) | −0.1523 | 0.0887 | 0.0871 | −0.0972 | 0.0904 | 0.2831 | −0.0935 | 0.0898 | 0.2986 | |||
| Hispanic (1=yes, 0=no) | −0.5543 | 0.1152 | <0.0001 | −0.4971 | 0.1141 | <0.0001 | −0.4870 | 0.1130 | <0.0001 | |||
| Other Race (1=yes, 0=no) | −0.0489 | 0.1563 | 0.7545 | −0.0610 | 0.1543 | 0.6927 | −0.1036 | 0.1530 | 0.4990 | |||
| Years of Education (0–17) | 0.0313 | 0.0100 | 0.0019 | 0.0267 | 0.0105 | 0.0113 | 0.0353 | 0.0105 | 0.0009 | |||
| Income in 2008 (1000s) | 0.0004 | 0.0004 | 0.2621 | 0.0002 | 0.0004 | 0.6174 | 0.0003 | 0.0004 | 0.4343 | |||
| Total No. of Health Conditions in 2008 | 0.0067 | 0.0219 | 0.7588 | −0.0029 | 0.0217 | 0.8955 | ||||||
| Functional Difficulties in 2008 (high=more difficulties) | −0.0386 | 0.0176 | 0.0294 | −0.0427 | 0.0175 | 0.0153 | ||||||
| Self-Reported Physical Health (higher=poorer) | −0.1640 | 0.0310 | <0.0001 | −0.1631 | 0.0307 | <0.0001 | ||||||
| Total Cognition Score in 2008 | −0.0045 | 0.0065 | 0.4935 | −0.0028 | 0.0065 | 0.6691 | ||||||
| Edentulous (1=yes, 0=no) | 0.3190 | 0.0695 | <0.0001 |
Table 5 shows the relationship between the trajectory groups and the probability of being edentulous. Neither having low (p=0.5290) nor moderate (p=0.7985) depressive symptoms was associated with dentate status in controlled analyses. Having more years of education, and higher income was associated with a lower probability of being edentulous, while having more health conditions was associated with a higher probability.
Table 5.
Ten year trajectory groups of depressive symptoms predicting edentulism using logistic regression analyses (n=943)
| Parameter | M1 Estimate | Std Error | p-value | Odds Ratio | M2 Estimate | Std Error | p-value | Odds Ratio | M3 Estimate | Std Error | p-value | Odds ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | −1.7179 | 0.1422 | <0.0001 | −0.5686 | 1.0009 | 0.5704 | 0.4199 | 1.2438 | 0.7359 | |||
| Depression Group 2 | 0.5848 | 0.1856 | 0.0018 | 1.80 | 0.2563 | 0.2007 | 0.2026 | 1.29 | 0.1318 | 0.2091 | 0.5290 | 1.14 |
| Depression Group 3 | 0.9893 | 0.2444 | 0.0001 | 2.69 | 0.3615 | 0.2672 | 0.1771 | 1.44 | 0.0767 | 0.3002 | 0.7985 | 1.08 |
| Age in 2008 | 0.0187 | 0.0116 | 0.1073 | 1.02 | 0.0053 | 0.0130 | 0.6849 | 1.01 | ||||
| Female (1=yes, 0=no) | 0.0552 | 0.1805 | 0.7598 | 1.06 | 0.1184 | 0.1846 | 0.5217 | 1.13 | ||||
| Black (1=yes, 0=no) | 0.0351 | 0.2535 | 0.8901 | 1.04 | −0.1148 | 0.2663 | 0.6667 | 0.89 | ||||
| Hispanic (1=yes, 0=no) | −0.3071 | 0.3239 | 0.3439 | 0.74 | −0.2765 | 0.3302 | 0.4031 | 0.76 | ||||
| Other Race (1=yes, 0=no) | 0.7345 | 0.4125 | 0.0759 | 2.09 | 0.6385 | 0.4231 | 0.1323 | 1.89 | ||||
| Years of Education (0–17) | −0.1635 | 0.0312 | <0.0001 | 0.85 | −0.1478 | 0.0334 | <0.0001 | 0.86 | ||||
| Income in 2008 (1000s) | −0.0098 | 0.0031 | 0.0019 | 0.99 | −0.0088 | 0.0031 | 0.0047 | 0.99 | ||||
| Total No. of Health Conditions in 2008 | 0.1993 | 0.0657 | 0.0026 | 1.22 | ||||||||
| Functional Difficulties in 2008 (high=more difficulties) | 0.0500 | 0.0489 | 0.3081 | 1.05 | ||||||||
| Self-Reported Physical Health (higher=poorer) | −0.0122 | 0.0938 | 0.8969 | 0.99 | ||||||||
| Total Cognition Score in 2008 | −0.0297 | 0.0198 | 0.1359 | 0.97 |
While our ten-year trajectories of depressive symptoms predicted self-rated oral health and mouth condition in 2008, we noted that the trajectories were relatively flat. We estimated separate models adjusting for CES-D score in 2008 as well as trajectory class. Those with chronic moderate depressive symptoms (Group 3) had significantly poorer mouth condition after adjusting for current CES-D score and demographic and health covariates. Those with moderate symptoms also had poorer self-rated oral health controlling for current CES-D score, but the associations were due in part to the demographic and health variables measured in 2008. These findings suggest the trajectory classes, although relatively flat, provide information beyond that due to a cross-sectional measure of depressive symptoms.
Discussion
We report the longitudinal association between depressive symptoms and oral health among older adults. We identified three distinct subgroups of older adults based on their counts of depressive symptoms over ten years. Our best fitting model, among those age 65+ who had remained in the sample for ten years prior to the oral health module, replicated earlier findings of heterogeneity among community-dwelling elders with respect to the experience of depressive symptoms over time, but had fewer trajectory classes than observed among those age 50+ who were in at least one wave of the HRS (Liang et al., 2011).
While it is unlikely that many of these elders with chronic depressive symptoms met criteria for major depression, they may have met criteria for subthreshold or minor depression. It is known that subthreshold levels can be similar to more symptomatic depression in their associations with demographic, social and physical health variables (Hybels et al., 2001). Depressive symptoms at a subthreshold level appear to have oral health consequences as well. We also note that while these classes differentiate primarily on severity (number of symptoms), the slopes indicated that those with lower CES-D scores at baseline actually saw a slight decrease in CES-D score (the nonlinear terms for time were significant) and had fewer symptoms over time, while those with higher CES-D scores at baseline essentially remained at a moderate level (the linear term was not significant). Identifying these distinct patterns which reflect initial level and rates of change is one advantage of trajectory models over a single measure of CES-D score.
Clinicians treating older adults are justifiably concerned with adverse consequences associated with late life depressive symptoms. Oral health outcomes have received little attention in this older population, yet poor oral health can be associated with decreased psychological well-being as well as poorer physical health (de Andrade et al., 2012; Hassel et al., 2011). Being in a group with moderate CES-D scores over time was associated with both poorer mouth condition and poorer self-rated oral health. Being in a group with low depressive symptomatology, however, was not associated with poorer oral health when health variables were controlled. We did not observe a longitudinal association between depressive symptoms and the probability of edentulism, yet tooth loss is the result of poor oral health. Edentulism may be less time sensitive than self-reported oral health and mouth conditions. It is also possible that risk factors for edentulism occur prior to late life or that a longer period of follow-up would be necessary to observe an association.
These findings suggest chronic levels of clinically significant depressive symptoms are associated with poor oral health outcomes. These findings are clinically important for all those treating older adults because poor oral health can also lead to adverse outcomes. Depressive symptoms may affect oral health through a number of biological pathways. For example, depression may contribute to disease (in this case poor oral health) through immune dysregulation. Depression can stimulate the production of pro-inflammatory cytokines that can influence conditions such as periodontal disease (Kiecolt-Glaser and Glaser, 2002). Use of some antidepressants is linked with hyposalivation which can lead to poor oral health conditions and diseases including mouth dryness, a burning sensation, periodontal disease, progressive dental caries, and oral functioning difficulties (Peeters et al., 1998). Non-biological pathways may include differences in dental hygiene practices and dental services use. For example, in a sample of younger adults, depressive symptoms were associated with lower tooth brushing frequency and lower frequency of dental visits (Knuuttila et al., 2006). In another study, adults with current depression were less likely to use dental services in the past year than those without the disorder (Okoro et al., 2012).
Our study has limitations. Depressive symptoms may affect the reporting of oral health. For example, in a cross-sectional study of older adults in Brazil, negative self-perception of oral health was associated with depressive symptoms, but not poor actual oral health (Mesas et al., 2008). While we examined the trajectories of depressive symptoms over the ten-year period prior to the oral health module, it is possible some problems with mouth conditions and tooth loss preceded the collection of the depressive symptom information. Our sample included only those HRS respondents who participated in the oral health module in 2008. We do not have oral health measures from other waves. The effect between depressive symptoms and oral health may be mediated through measured variables such as physical health and functioning as well as through unmeasured latent variables such as poor nutrition and poor self-care. If mediation is present, our results would have underestimated the effect of depression group in these analyses. Depressive symptom trajectories have been shown to be affected by both mortality and attrition (Liang et al., 2011). Information on antidepressant use or other treatment was not available in the HRS. Finally, depressive symptoms were measured using a modified version of the CES-D, and it is not known how these relationships may differ in the presence of late life DSM major depression.
The strengths of the study, however, outweigh any limitations. The HRS is a large representative sample of community-dwelling older adults residing in the U.S. followed biannually over time. The availability of multiple measures of depressive symptoms allowed us to plot patterns of depressive symptoms over ten years prior to the oral health assessment in 2008. Latent class trajectory analysis allowed the identification of distinct subgroups based on their trajectories rather than assuming one trajectory of depressive symptoms with random variation around the mean over time. The large sample provided an acceptable number of persons in each trajectory class. While the trajectories were estimated using CES-D score alone, these trajectories were derived from latent variables. That is, the trajectories were derived from unmeasured variables as well as CES-D score and reflect underlying changes in variables such as health and cognition and depression treatment. Previous research has shown that there is moderate agreement between the trajectories derived using CES-D score alone and trajectories derived including time-varying covariates as well (Liang et al., 2011). Finally, the analyses focused on older adults, a population subgroup at higher risk for poorer oral health.
Conclusion
Chronic depressive symptoms can lead to poor oral health outcomes in older adults. Clinicians treating older adults with moderate levels of depressive symptoms may want to discuss with patients the need for healthy behavior including good oral health care.
Key Points.
Chronic moderate levels of depressive symptoms among older adults are associated with poorer self-rated oral health and overall mouth condition including problems with bleeding gums, gum sensitivity, and food avoidance.
Clinicians treating older adults with moderate levels of depressive symptoms may want to discuss with patients the need for healthy behavior including good oral health care.
Acknowledgements
This research was supported by NIH grants R01DE019110 and R03MH095917. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. These findings were presented at the 2014 Annual Scientific Meeting of the Gerontological Society of America. The authors would like to acknowledge the statistical assistance received from Dr. Carl Pieper at Duke University.
Footnotes
Conflict of Interest: None.
References
- Blazer DG. Depression in late life: Review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58A:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Buhlin K, Gustafsson A, Hakansson J, Klinge B. Self-reported oral health, dental care habits and cardiovascular disease in an adult Swedish population. Oral Health and Preventive Dentistry. 2003;1:291–299. [PubMed] [Google Scholar]
- D'Mello D. Are your patients depressed? Implications for dental practice. Journal of the Michigan Dental Association. 2003;85:26–32. [PubMed] [Google Scholar]
- de Andrade F, Lebrao M, Santos J, Teixeira D, Duarte Y. Relationship between oral health-related quality of life, oral health, socioeconomic, and general health factors in elderly Brazilians. J Am Geriatr Soc. 2012;60:1755–1760. doi: 10.1111/j.1532-5415.2012.04104.x. [DOI] [PubMed] [Google Scholar]
- Enders C. Applied Missing Data Analysis. Guilford Press; New York: 2010. [Google Scholar]
- Finlayson T, Williams D, Siefert K, Jackson J, Nowjack-Raymer R. Oral health disparities and psychosocial correlates of self-rated oral health in the National Survey of American Life. Am J Public Health. 2010;100:S246–S255. doi: 10.2105/AJPH.2009.167783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CN, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Friedlander A, Norman D. Late-life depression: Psychopathology, medical interventions, and dental implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:404–412. doi: 10.1067/moe.2002.122434. [DOI] [PubMed] [Google Scholar]
- Hassel A, Danner D, Schmitt M, Notschke I, Rammelsberg P, Wahl H. Oral health-related quality of life is linked with subjective well-being and depression in early old age. Clin Oral Invest. 2011;15:691–697. doi: 10.1007/s00784-010-0437-3. [DOI] [PubMed] [Google Scholar]
- Hybels CF, Blazer DG, Pieper CF. Toward a threshold for subthreshold depression: An analysis of correlates of depression by severity of symptoms using data from an elderly community sample. The Gerontologist. 2001;41:357–365. doi: 10.1093/geront/41.3.357. [DOI] [PubMed] [Google Scholar]
- Jones B, Nagin D. Advances in group-based trajectory modeling and a SAS procedure for estimating them. Sociol Methods Res. 2007;35:542–571. [Google Scholar]
- Kiecolt-Glaser J, Glaser R. Depression and immune function: Central pathways to morbidity and mortality. J Psychosom Res. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Knuuttila A, Ylostalo P, Joukamaa M. Symptoms of depression and anxiety in relation to dental health behavior and self-perceived dental treatment need. Eur J Oral Sci. 2006;114:109–114. doi: 10.1111/j.1600-0722.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- Kressin N, Spiro A, Atchison K, Kazis L. Is depressive symptomology associated with worse oral functioning and well-being among older adults? J Public Health Dent. 2002;62:5–12. doi: 10.1111/j.1752-7325.2002.tb03414.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Xu X, Quinones A, Bennett J, Ye W. Mulitple trajectories of depressive symptoms in middle and late life: Racial/ethnic variations. Psychol Aging. 2011;26:761–777. doi: 10.1037/a0023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesas A, de Andrade S, Cabrera M. Factors associated with negative self-perception of oral health among elderly people in a Brazilian community. Gerodontology. 2008;25:49–56. doi: 10.1111/j.1741-2358.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- Ng S, Leung W. A community study on the relationship between stress, coping, affective dispositions and periodontal attachment loss. Community Dent Oral Epidemiol. 2006;34:252–266. doi: 10.1111/j.1600-0528.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Nowjack-Raymer R, Sheiham A. Association of edentulism and diet and nutrition in US adults. J Dent Res. 2003;82:123–126. doi: 10.1177/154405910308200209. [DOI] [PubMed] [Google Scholar]
- Okoro C, Strine T, Eke P, Dhingra S, Balluz L. The association between depression and anxiety and use of oral health services and tooth loss. Community Dent Oral Epidemiol. 2012;40:134–144. doi: 10.1111/j.1600-0528.2011.00637.x. [DOI] [PubMed] [Google Scholar]
- Peeters F, deVries M, Vissink A. Risks for oral health with the use of antidepressants. Gen Hosp Psychiatry. 1998;20:150–154. doi: 10.1016/s0163-8343(98)00017-6. [DOI] [PubMed] [Google Scholar]
- Persson G, Perrson R, MacEntee C, Wyatt C, Hollender L, Kiyak H. Periodontitis and perceived risk for periodontitis in elders with evidence of depression. J Clin Periodontol. 2003;30:691–696. doi: 10.1034/j.1600-051x.2003.00360.x. [DOI] [PubMed] [Google Scholar]
- Poisson P, Laffond T, Campos S, Dupuis V, Bourdel-Marchasson I. Relationships between oral health, dysphagia and undernutrition in hospitalised elderly patients. Gerodontology. 2014 doi: 10.1111/ger.12123. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- Rubin B. Mulitiple imputation for nonresponse in surveys. Wiley; New York: 1987. [Google Scholar]
- Saintrain MdL, Guimaraes A, de Almeida P, Vieira A. Depression symptoms and oral discomfort in elderly adults. J Am Geriatr Soc. 2013;61:651–652. doi: 10.1111/jgs.12181. [DOI] [PubMed] [Google Scholar]
- Saletu A, Pirker-Fruhauf H, Saletu F, Linzmayer L, Anderer P, Matejka M. Controlled clinical and psychometric studies on the relation between periodontitis and depressed mood. J Clin Periodontol. 2005;32:1219–1225. doi: 10.1111/j.1600-051X.2005.00855.x. [DOI] [PubMed] [Google Scholar]
- Schafer J. Analysis of incomplete multivariate data. Chapman & Hall; London: 1997. [Google Scholar]
- Solis A, Lotufo R, Pannuti C, Brunheiro E, Marques A, Loyufo-Neto F. Association of periodontal disease to anxiety and depression symptoms, and psychosocial stress factors. J Clin Periodontol. 2004;31:633–638. doi: 10.1111/j.1600-051X.2004.00538.x. [DOI] [PubMed] [Google Scholar]
- Survey Research Center . Documentation of Affective Functioning Measures in the Health and Retirement Study. University of Michigan; 2000. [Google Scholar]