Abstract
Hearing impairment is more prevalent in individuals with Autism Spectrum Disorders (ASD) than the general population, and although ASD is not caused by hearing impairment, symptoms may be made worse by difficulty hearing. Sixty participants with ASD and 16 typically developing peers ages 5-18 underwent a comprehensive screening of communication abilities (expressive and receptive language, speech articulation, phonological processing, and vocal emotion recognition) and audiology (pure tone audiometry, uncomfortable loudness level, tympanometry, acoustic reflexes, distortion product otoacoustic emissions, and auditory brainstem response). The ASD group had a higher rate (55%) of abnormal findings on at least one measure of audiological functioning than typically developing peers (14.9%) or the general population estimate (6%). The presence of sound sensitivity in at least one ear was also considerably higher for the ASD group (37%) compared to the typically developing participants (0%) or general population estimates (8-15%). When participants with ASD were divided into groups with and without evidence of abnormal audiology, there were no significant group differences in communication; however, when the relationship between hearing thresholds and communication was examined, thresholds at middle range frequencies (2000Hz) were in fact significantly related to performance on all measures of speech articulation and language. These results suggest that classifying hearing as normal versus abnormal may not be sufficient to understand its association with ASD symptoms and that treatment studies for mild/subclinical hearing loss may be worthwhile for children with ASD.
Rates of hearing impairment in individuals with Autism Spectrum Disorders (ASD) are higher than those reported in the general population. Although ASD is not caused by hearing impairment, it may exacerbate symptomatology. Participants with ASD (N=60) and typically developing peers (N=16) aged 5-18 years underwent a comprehensive audiological screening (pure tone audiometry, uncomfortable loudness level, tympanometry, acoustic reflexes, distortion product otoacoustic emissions, and auditory brainstem response) and assessment of communication abilities (expressive/receptive language, articulation, phonological awareness, and vocal affect recognition). Incidence of abnormal findings on at least one measure of audiological functioning was higher for the ASD group (55%) than controls (14.9%) or the general population estimate (6%). The presence of sound sensitivity was also considerably higher for the ASD group (37%) compared to controls (0%) or general population estimates (8-15%). When participants with ASD were dichotomized into groups with and without evidence of clinical audiological abnormality, no significant differences were identified on measures of communication; however, results of correlational analyses indicated that variability in hearing thresholds at middle range frequencies (2000Hz) was significantly related to performance on all measures of speech articulation and language after correction for multiple comparisons (r=-0.48 to r=-0.53, p<0.0045). These findings suggest that dichotomized classification of clinical audiology may not be sufficient to understand the role of subclinical hearing loss in ASD symptomatology and that treatment studies for mild/subclinical hearing loss in this population may be worthwhile.
Introduction
Abnormalities in auditory processing have been shown to contribute to functional deficits in individuals with Autism Spectrum Disorders (ASDs; Edgar et al., 2013; Kasai et al., 2005; Kuhl et al., 2005; Rojas et al, 2011; Wilson et al., 2007); however, the relative impact of peripheral versus central auditory dysfunction remains unclear (Hitoglou et al., 2010). Reported rates of hearing impairment in individuals with ASD have ranged from 0-100% (Gillberg, Rosenhall, & Johansson, 1983; Grillon, Courchesne, & Akshoomoff, 1989; Kielinen et al., 2004; Rosenblum et al., 1980; Rosenhall et al., 1999; Taylor, Rosenblatt, & Linschoten, 1892); although studies are not immediately comparable due to differences in sample criteria and definitions of hearing impairment. Klin (1993) has suggested that the etiological contribution of peripheral auditory dysfunction in ASD is too often dismissed because there are qualitative differences in social functioning and communication in individuals with an ASD versus those with hearing impairment in the absence of an ASD. For example, in a study based on a large clinical sample of children diagnosed with hearing impairment, it was reported that only 5.3% were diagnosed with autism (Jure, Rapin, & Tuchman, 1991), so it is clear that hearing loss in and of itself is not causative of autism. However, there is evidence that hearing-impaired children and adolescents show deficits common to those in ASD, such as emotion recognition (Dyck, Farrugia, & Shochet, et al., 2004; Most & Michaelis, 2012). In fact, Bachara, Raphael and Phelan (1980) found that emotion recognition ability was related to the onset of deafness, with adolescents with post-lingual onset of hearing loss performing superior to those with earlier onset or congenital deafness. This finding of emotion recognition deficits in individuals with pre-lingual onset sensorineural hearing loss has been replicated in subsequent research as well (Most & Aviner, 2009; Most & Michaelis, 2012). This association between later onset hearing loss and better outcomes in emotion recognition suggests that there may be a sensory developmental component to skill acquisition in this social cognitive domain. If so, it may be important to carefully evaluate for potential peripheral auditory dysfunction in individuals on the autism spectrum, for whom deficits in emotion recognition are common. Consideration of peripheral hearing abnormalities in a developmental context might be particularly useful in this population, especially since auditory distortion may adversely impact maturation of other systems as well. Indeed, it has been shown that early auditory training can impact maturation of prelinguistic acoustic mapping (Benasich et al., 2014). If providing additional auditory experience through training can result in facilitation of prelinguistic maturation, it is reasonable to expect that reduced or distorted auditory experience may have an opposite effect on plasticity, such as an adverse impact on maturation of systems that rely on processing auditory information to develop (e.g., language or vocal affect recognition). Thus, it is a fundamentally flawed assumption that because autism is not caused by hearing impairment, hearing impairment does not contribute to or exacerbate ASD symptomatology.
Estimated prevalence of hearing loss in the general population is 0.14% (Center for Disease Control and Prevention, 2009); however, the available literature on peripheral hearing dysfunction in ASD samples generally indicates a higher prevalence, especially when multiple measures derived from a complete audiological assessment are examined concurrently (Jure et al., 1991; Rosenhall et al., 1999; Tas et al., 2007). Frequent ear infections are also common in ASD and have been associated with peripheral hearing impairments (Hitoglou et al., 2010). For example, elevated rates of abnormal tympanometry have been reported in studies of children with ASD. Smith, McConnell, & Walter et al. (1985) reported abnormal mean impedance measures in both ears for 100% of participants diagnosed with an ASD (n=14) when taken on five occasions over a 10-week period. In a study examining children with autism and learning disability, respectively, abnormal impedance was documented in both groups, although greater negative pressure with a more frequently bilateral presentation was reported in the children with autism (Smith, Miller, & Steward et al., 1988).
Comprehensive Audiological Examination in ASD
In neurotypical children, assessment of hearing is most commonly based on threshold of audibility and tests of subjective audiometry (e.g., reported thresholds for pure tones or speech) are typically considered adequate methods for assessing hearing loss; however, they are subject to participant cooperation, task comprehension, and behavioral response in order to provide a valid estimate of hearing function. Thus, it is recommended that they are conducted in the context of a larger audiological evaluation which incorporates measures of objective audiometry as well. Because children diagnosed with ASD can present challenges in obtaining a valid estimate of hearing, much of the literature in this area is limited to reports of a subset of audiological measures, often collected for a purpose other than assessment of hearing. For example, the majority of the data on peripheral auditory processing in individuals with ASD come from studies of auditory brainstem response (ABR), subsequent to the hypothesis that sensory disturbance in ASD may be related to brainstem dysfunction (Ornitz, 1985). In a review of the 11 ABR studies available at the time, Klin (1993) concluded that evidence did not support the brainstem hypothesis in autism; however, these studies did indicate increased prevalence of “peripheral auditory abnormalities.” Rosenhall et al. (1999) have questioned the validity of these conclusions regarding hearing loss due to the fact that the reviewed studies aimed to measure ABR rather than hearing per se. The authors also raised concerns with small sample sizes and exclusion criteria, pointing out that several studies excluded children with severe hearing loss. More recent studies have supported previous findings indicating abnormal ABR in individuals with ASD, including longer wave (Thivierge et al., 1990; Wong & Wong, 1991) and inter-peak latencies (IPL; Tas et al., 2007; Thivierge et al., 1990; Wong & Wong, 1991).
In a study by Jure, Rapin and Tuchman (1991), a chart review was conducted on 46 children diagnosed with autism who were identified from a sample of 1150 hearing-impaired children. Thirty seven children had both pure tone audiometry (PTA) and auditory evoked response (AER), four had PTA only, and five had AER. Hearing loss was identified as mild (25-44 dB HL) in one participant, moderate (45-69 dB HL) in 8 participants, severe (70-89 dB HL) in 14 participants, and profound (≥90 dB HL) in 23 participants. However, a portion of the sample (n=387) was obtained from a school that does not accept children with mild to moderate hearing loss, and thus, the prevalence of mild to moderate hearing impairment may be underestimated in this study.
Other studies of audiometry in ASD have also identified increased rates of hearing loss compared to population estimates. For example, Hayes and Gordon (1977) reported hearing loss in three of the 16 children with autism assessed for pure tone hearing thresholds, although the authors did not define their criteria for hearing loss, making it difficult to compare results to other studies. Smith, McConnell, and Walter et al. (1985) reported hearing loss defined by speech thresholds >80 dB in two out of 14 individuals diagnosed with an ASD. Such audiometric assessment can be difficult to assess in children with autism, as results are dependent on task comprehension and compliance. For this reason, other methods of assessing peripheral hearing have been reported in studies of ASD. For example, measurement of acoustic stapedial reflex thresholds does not require any behavioral response from the examinee. Abnormally high acoustic stapedius muscle reflex thresholds (>95 dB) have been reported in 12 of 16 children diagnosed with autism in a study by Hayes and Gordon (1977). Further, only three additional participants had thresholds lower than 90dB at any of the frequencies tested. These results are suggestive of increased rates of severe hearing impairment in the ASD population; however, mild to moderate hearing loss can still result in a normal threshold. Thus, these numbers may underestimate the prevalence of mild or moderate hearing loss in individuals with ASD.
Otoacoustic emissions also can be measured from passive participants. Tas, Yagiz, and Tas, et al. (2007) assessed transiently evoked otoacoustic emissions (TEOAE) in 30 children with autism (ages 2-7 years) and 12 typically developing peers of similar age. In the autistic group emissions were reportedly not present in either ear for three participants, and not present in one ear for two additional participants. Emissions were present in both ears for all participants in the control group. Khalfa et al. (2001) identified two separate abnormalities in a study of TEOAE in autism. First, results suggested that there was a decrease in TEOAE amplitudes with age for children with autism but not controls, possibly suggesting a premature deterioration of outer hair cell function. Data from this study also suggested that there was an asymmetrical suppression effect with right ear suppression greater than left in the autism group. This asymmetry was not detected in the control group, suggesting that auditory filtering may be differentially lateralized in ASD.
Hyperacusis, defined as unusual intolerance of ordinary environmental sounds (American Speech-Language-Hearing Association, 2008), is another frequently reported phenomenon in individuals with ASD (Rimland & Edelson, 1995). In a review of the literature examining hyperacusis in ASD, Stiegler and Davis (2010) reported that while there was evidence of hyperacusis in some individuals with ASD based on behavioral response, no evidence of physiological contributions to sound sensitivity have been identified. The authors note that the presence of sound sensitivity may be related to more complex cortical phenomena rather than peripheral auditory dysfunction. In a study by Rosenhall et al. (1999), the prevalence of hyperacusis was estimated at 18% of the 111 participants who were reliably tested via ABR, indicating a significant presence of sound sensitivity among the ASD population.
The majority of these measures are inadequate to assess hearing on their own, however, particularly with respect to mild and moderate hearing loss. Thus, studies examining multiple measures of audiological functioning concurrently may provide better estimates of rates of hearing loss in the ASD population. For example, Rosenhall et al. (1999) reported audiological evaluation results, including audiometry, tympanometry, and hyperacusis via ABR in 199 children and adolescents diagnosed with Autistic Disorder. Hyperacusis was also assessed in a comparison group (n=57). Audiological assessment was routinely administered as part of a comprehensive evaluation in the clinic from which this sample was drawn, although not all measures were administered to all participants. Mild to moderate hearing loss, defined as hearing loss (HL) of 20-40 dB or high frequency hearing loss, was reported in 10 out of 126 individuals with autism as measured by PTA. Further, two participants diagnosed with ASD had pronounced hearing loss (40-70 dB HL) in one ear, with mild hearing loss in the contralateral ear. Seven out of the entire 199 participants diagnosed with ASD demonstrated pronounced (n=3) to profound hearing loss (>70 dB HL) or deafness in both ears (n=4). Thirty-eight additional children (not included in the 19 already reported) of the 162 who were examined with otomicroscopy or tested with typanometry had serous otitis media, 24 of whom demonstrated conductive hearing loss from 20-40 dB. As reported above, hyperacusis was reported in 18% of the 111 participants who were reliably tested via ABR. None of the participants in the comparison group demonstrated hyperacusis; however, they were not assessed for hearing loss.
Rumsey et al. (1984) also reported audiological examination results, including tympanometry, speech and pure tone thresholds, ART, and ABR, in a sample of 25 participants diagnosed with an ASD. Tympanometry and ART were normal in 64% of participants who could be tested, with abnormal results in one or both ears for six participants. Thresholds were normal for twenty of the 24 participants who could be evaluated for pure tone and speech thresholds, and mild bilateral hearing impairment (25 to 40 dB HL) was reported in the remaining four participants. The control group for this study excluded children with abnormal audiological examination results for tympanometry, hearing threshold, and ART, and thus, group comparison on these measures was not possible. However, for ABR, shorter Wave III latencies and shorter Wave I to III transmission times were reported for the ASD group relative to controls.
Other studies have reported no audiological abnormalities in ASD compared to controls. For example, Gravel et al. (2006) examined PTA, ART, TEOAE, and DPOAE in a sample of 40 children diagnosed with autism and 40 typically developing age-matched peers. No group differences were observed between groups for hearing thresholds, acoustic reflex thresholds, or otoacoustic emissions. In addition, the proportion of children in each group with hearing thresholds ≤ 0 dB HL did not differ, suggesting that the rate of hyperacusis was not increased in the autism sample.
Thus, while generally suggestive of increased rate of peripheral hearing dysfunction in the ASD population, the extant literature is lacking a comprehensive study of peripheral audiological functioning and its impact on audible communication in individuals with ASD compared to a control group. Thus, this study aimed to examine group differences in rates of failure on a battery of audiological screening measures in children and adolescents diagnosed with ASD compared to a control group. A second aim was to test (1) whether there were group differences between participants with and without audiological abnormalities on measures of audible communication, and (2) whether the degree of hearing loss or loudness sensitivity was related to these communication abilities.
Methods
Participants
Participants were 76 children and adolescents (N=60 for the ASD group and N=16 for the control group), ages 5-18, who underwent a comprehensive audiological screening as part of an NIH funded study of language functioning in ASD. Data were collected at an academic medical center in the Midwestern United States and an academic research institute in the Southwestern United States. Participant characteristics are reported in Table 1. Assessments of general intellectual abilities and communication were conducted by a neuropsychology team and audiological screening was performed by a licensed audiologist or a trained technician. Assignment to diagnostic groups was confirmed through consensus diagnosis from the neuropsychology team under the supervision of a licensed clinical neuropsychologist. Scores on the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 1989) and the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & LeCouteur, 1994), along with information obtained on a neuropsychological history questionnaire were integrated to inform consensus diagnosis. All participants in the ASD group met ADOS and ADI-R cutoff scores for ASD as well as DSM-IV-TR (2000) criteria for Autistic Disorder (n=46), Asperger's Disorder (n=10), or Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS; n=4). All participants in the control group scored below cutoffs on these measures. Participants of all language and cognitive abilities were included in the study in an attempt to obtain a representative sample of individuals with ASD. This resulted in an inability to obtain some measures from some of the lower functioning participants; however, an attempt was made to collect data from all participants who could demonstrate task comprehension and be reliably tested. For all groups, the presence of serous otitis media at the time of testing, Fragile-X, Tuberous Sclerosis or any co-morbid neurological condition other than epilepsy was exclusionary. Participants demonstrating hearing loss related to serous otitis media (SOM) were retested when possible. If retesting was not possible then those participants were excluded (N=1 for controls; N=2 for ASD group).
Table 1.
Group Characteristics (M ± SD [range])
| ASD Group | Control Group | Statistics | |
|---|---|---|---|
| Age | 10.78 ± 3.41 [5.50-18.50] | 13.58 ± 3.63 [7.58-18.92] | t(74) = 2.88* |
| VCI | 84.14 ± 23.76 [45-142] | 115.13 ± 15.19 [93-150] | t(42.48) = 5.87**† |
| PRI | 91.18 ± 20.41 [53-133] | 108.19 ± 13.77 [86-131] | t(39.68) = 3.68**† |
| FSIQ | 81.68 ± 21.97 [46-136] | 110.75 ± 13.41 [93-144] | t(44.55) = 6.06**† |
| Ethnicity (N) | |||
| Caucasian | 39 | 7 | |
| African American | 4 | 0 | |
| Hispanic | 6 | 5 | |
| Asian | 3 | 1 | |
| Other | 2 | 2 | |
| Multiracial | 6 | 1 | |
| Male : Female | 48:12 | 11:5 |
p < .01
p ≤ .001
Degrees of freedom values corrected for inequality of variances between groups
Measures
Diagnostic assessment
The ADI-R is an extensive diagnostic interview designed to elicit the range of information that is relevant to the diagnosis of autism (Lord, Rutter, & LeCouteur, 1994). Psychometric studies of the ADI-R have indicated good discriminant validity (Rutter, LeCouteur, & Lord, 2003) and test-retest reliability ranging from .93-.97 (Lord et al, 1993, 1994). The ADOS (Lord et al. 1989) is a semi-structured observational tool used to quantify social and communicative behavior in relation to autism symptomatology. In a study of classification accuracy of the ADOS compared to consensus clinical diagnosis the ADOS effectively differentiated autism from non-spectrum disorders with specificities of .93–1.0 (Lord et al., 2000).
Intelligence and Communication
Full Scale Intelligence Quotient (FSIQ) was assessed with the age-appropriate Wechsler test, including either the Wechsler Intelligence Scale for Children-IV (WISC-IV; Wechsler 2003), the Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III; Wechsler 2002), or the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler 1997). Language ability was assessed by the Clinical Evaluation of Language Fundamentals-4 (Semel, Wiig & Secord, 2003; CELF-4), a comprehensive language battery, to derive a Receptive, Expressive, and an overall age-scaled language quotient based on a normative sample. The ability to judge emotional content in speech was assessed through the Adult and Child Paralanguage subtests of the Diagnostic Analysis of Nonverbal Accuracy-2 (Nowicki & Duke, 1994; DANVA-2). The DANVA-2 is a computer-administered test that measures the ability to identify emotional content in the same semantically neutral statement (i.e., I'm going out of the room now, but I'll be back later) spoken with different emotional inflections (i.e., happy, sad, angry, or fearful) for 24 stimuli in each condition. Reliability studies of the DANVA-2 paralanguage subscale has resulted in a Chronbach's alpha coefficient of 0.77 and retest reliability of r = 0.74 at four weeks post testing (Nowicki & Carton, 1993; Nowicki & Duke, 1994). Age scaled standard scores were derived based on the total number of errors for each subtest. Phonological processing was assessed via the Comprehensive Test of Phonological Processing (CTOPP; Wagner, R.K., Torgeson & Rashotte, 1999). The Phonological Awareness Index quantifies the participant's comprehension of the phonological structure of spoken words. A standard score index was derived based on a normative sample. Finally, speech articulation was evaluated using the Sounds-in-Words section of the Goldman Fristoe-2: Test of Articulation (Goldman & Fristoe, 2000; GFTA-2). For this test the examinee identifies objects from pictures and errors made on specified sounds within the word trials are totaled. Age-scaled standard scores are then derived from total articulation errors based on a normative sample.
Audiology
Pure Tone Audiometry (PTA) and Uncomfortable Loudness Level (UCL) were assessed using a Grason Stadler GSI 61 audiometer for the first half of the study, followed by use of an AudioTraveller AA222 audiometer after a relocation of the study to another site. Prior to relocation a Grason Stadler GSI TympStar middle ear analyzer was used to assess tympanometry and acoustic reflexes, followed by use of the AudioTraveller AA222 following relocation. Distortion Product Otoacoustic Emissions (DPOAE) could only be assessed in the initial study location, where a Bio-Logic AuDX Pro was used. Finally, Auditory Brainstem Response (ABR) was assessed using the Teca Sapphire II 4Me system for the first half of the study and the EEG system that is part of the Neuromag VectorView MEG System following relocation.
Tympanometry can be measured without requiring a behavioral response and is therefore easily assessed in individuals who lack the language or cognitive abilities to participate in response-dependent assessment methods. Tympanometry reflects the changes in the physical properties of the middle ear (specifically the mobility of the tympanic membrane) in response to the application of systematically varied air pressure through the middle ear canal (Margolis & Hunter, 2000). Abnormal tympanometry can result from multiple causes (e.g., middle ear tumor, fluid in the middle ear, impacted ear wax, lack of contact between the conduction bones of the middle ear, perforated ear drum, scarring of the tympanic membrane, etc.), many of which are temporary (Seidman, Simpson & Khan, 2001), and thus, tympanometry cannot be used alone to diagnose hearing loss. Assessment of tympanometry included measures of ear canal volume, static compliance, and tympanic peak pressure. Ear canal volume and compliance was measured first by presenting a high negative air pressure to the outer ear canal, which results in a stiffening of the tympanic membrane, thereby creating an acoustic cavity consisting of the outer ear canal only, establishing the outer ear equivalent volume. A perforated tympanic membrane will also capture the volume of the middle ear in this measurement, resulting in an abnormally high ear canal volume (Fowler & Shanks 2002). Next, to assess compliance, the pressure was gradually varied from high positive to negative, resulting in progressively increasing compliance until air pressure was equal on both sides of the tympanic membrane and the highest compliance to sound waves was elicited. The amount of pressure at which this condition was achieved was the tympanic peak pressure. The cavity responding to sound under these conditions is then comprised of both the outer ear canal and middle ear, allowing for measurement of their combined equivalent volume. The equivalent volume of the middle ear, known as the compliance, was obtained by subtracting the outer ear canal equivalent volume from the combined equivalent volume (Interacoustics 2010). Compliance reflects the movement of the eardrum (in ml) in response to pressure and therefore the maximum amount of acoustic energy absorbed by the middle ear system (Onusko 2004). A final classification of normal versus abnormal was assigned based on these measures of ear canal volume, static compliance, and tympanic peak pressure as determined by a licensed audiologist.
The acoustic reflex threshold (ART) is the lowest decibel sound pressure level at which a stapedius muscle contraction reflex can be elicited. Higher thresholds can signify severe hearing loss, although this measure is less sensitive to mild and moderate hearing loss (Borg & Counter, 1989). ART was assessed ipsilaterally via an impedance probe and contralaterally via a contralateral headset. A 500Hz tone was presented at 65dB and increased at 5dB increments until a reflex contraction of the stapedius muscle was induced. This procedure was repeated for 1000Hz, 2000Hz, and 4000Hz tones ipsilaterally and contralaterally to determine the lowest decibel level for which a reflex was induced. Thresholds ≥ 95dB were classified as abnormal.
Measurement of otoacoustic emissions is another method of evaluating hearing in individuals who are difficult to assess through audibility threshold testing (Eiserman, Hartel, & Shisler et al., 2008). Otoacoustic emissions are the low intensity sounds produced by the cochlea. They reflect the cochlea's response to sound stimulation, although the emissions must be transmitted back to the recording microphone by the outer and middle ear as well. They can be transiently evoked using a click or tone-burst stimulus (TEOAE), or through presentation of a pair of primary tones (distortion product, or DPOAE). The responses to these stimuli occur at frequencies which are mathematically related to the primary stimulus frequencies in a healthy ear. OAEs cannot be used to fully describe an individual's auditory thresholds, but they may provide information which can be used to validate other threshold measures (Joint Committee on Infant Hearing, 2007). For example, emissions are frequently reduced in ears with minor hearing loss, and are generally absent when hearing loss exceeds 30 dB (Johnsen, Bagi, & Elberling, 1983); however, this test is most reliable for assessing the presence of moderate to severe hearing loss and was thus used as a pass/fail screener for abnormalities rather than a continuous measure of hearing threshold (Harlor & Bower, 2009). DPOAE was performed by positioning earplugs which house a measuring microphone and sound emitting speaker in the outer ear. Tone pairs are presented through the speakers over a range of frequencies at 65dB. The sounds enter the outer, middle, and inner ear and the recording microphone measures the echoed sound returning from the inner ear. OAEs less than 6dB are classified as atypical.
The ABR is an auditory evoked potential recorded via electrodes placed on the scalp. Although the main recording site is at the cranial cap, the ABR reflects activity in peripheral aspects of the auditory system including the cochlear nucleus, medial lemniscus, and brainstem nuclei. Because this is a neurophysiological response, it is possible to use this method to estimate pronounced hearing loss in very low functioning children, without the cooperation and cognitive task demands required by behavioral methods for estimating hearing thresholds. In considering the ABR as a method for evaluating the integrity of the peripheral auditory system, it should be recognized that the ABR is not a very sensitive measure of slight hearing loss, and it generally does not provide information on potential hearing loss at low frequencies. Thus, while the ABR is helpful for evaluating hearing in children who are difficult to assess with other methods, data are ideally interpreted within the context of additional testing. ABR data were acquired separately for each ear as recorded at Cz referenced to the mastoid contralateral to the side of presentation. Data were collected in response to clicks (80dB SPL) presented at a rate of 10Hz, with a total of 1200 stimuli presented to each ear. Data were analyzed with respect to the latencies of ABR waves I, III, and V, and also the I-V and III-V interpeak latencies. A response was considered abnormal if it was more than 3 standard deviations beyond literature reported means.
PTA was assessed by presenting puretone sounds ranging from 250-8000Hz unilaterally through headphones. Sounds of increasing intensity were presented at 5dB intervals until the participant indicated detection of the sound. The lowest dB HL detected for each sound was recorded. The process was repeated over 3 trials and the average dB HL was recorded for each frequency bilaterally. Hearing loss was defined according to the criteria outlined by Rosenhall et al. (1999). Specifically, a PTA value ≥ 20dB HL was classified as abnormal. In addition to classification as normal versus abnormal, PTA hearing threshold values also were examined as continuous measures to quantify degree of hearing loss.
UCL for speech sounds was assessed in a similar way. Speech sounds of increasing intensity were presented unilaterally through headphones until the participant indicated that a sound was too loud and uncomfortable. The dB level at which a sound was classified as too loud and uncomfortable was documented bilaterally at the participant's UCL. Sounds past the level of the participant's UCL or sounds over 115dB were not presented. Like PTA values, UCL thresholds could also be reliably examined as continuous variables to examine degree of loudness sensitivity in participants.
Statistical Analyses
First, preliminary χ2 analyses were performed to determine if rates of peripheral audiological abnormalities differed between data collection sites. Next, independent samples t-tests were performed to determine if there were difference in age and IQ for participants with missing versus collected data for each audiological measure. Then, to test the hypothesis that participants with ASD would show increased rates of abnormal peripheral audiology, χ2 analyses were performed between rates of abnormal audiological screening measures in the ASD and control groups. Because some screening measures are more sensitive than others to different types of audiological dysfunction or to participant compliance, concurrent examination of multiple measures of peripheral audiology within individual participants may provide the best estimate of intact versus impaired audiological functioning. Abnormal findings on more than one measure, however, may not necessarily indicate greater severity of hearing impairment. For example, a participant's high frequency hearing loss may be detected on measures of PTA and ABR; however, both of these results are indicative of the same hearing abnormality. Thus, dichotomization of the ASD group into those with (ASD+) and without (ASD-) at least one abnormal audiological screening result was the most appropriate method for testing the hypothesis that participants with audiological dysfunction would show weaker communication skills than those with intact peripheral audiology. To this end, a χ2 analysis was performed to determine if participants with ASD showed increased rates of audiological dysfunction when abnormality on at least one measure was considered dysfunctional. Oneway analyses of variance (ANOVAs) were then performed to examine the difference between ASD+, ASD-, and control groups on measures of communication. Following identification of significant group differences in ANOVAs for any given communication measure, post hoc paired comparisons were performed to determine which groups significantly differed from each other on that measure.
Finally, to examine group differences in qualities of hearing abnormalities, analyses of covariance (ANCOVAs) were performed, controlling for the effects of age and intellectual ability, between participants with ASD and controls across a range of PTA frequencies as well as UCL for speech sounds. Age was entered as a covariate in these analyses to control for variability in auditory maturation across the age range of the sample. FSIQ was entered as a covariate to control for the effects of cognitive functioning on task performance given the wide range of intellectual abilities in the ASD group. Finally, correlation analyses were performed between communication measures and PTA and UCL thresholds to assess whether variability in loudness sensitivity or hearing thresholds at specific frequencies were associated with general language skills, speech articulation, phonological processing, or affect recognition. PTA and UCL were selected for these analyses because they are the only two measures which can be reliably assessed on a continuum (e.g., other measures, which may be only sensitive to moderate or severe hearing loss were thus scored as normal versus abnormal and could not be compared in independent samples t-test or correlation analyses). Bonferroni corrections for multiple comparisons were applied for tests of all hypotheses.
Results
Preliminary analyses of site effects indicated that rates of audiological abnormalities did not significantly differ between study sites, with p>.05 for ART (χ2 = .14), UCL (χ2 = 3.57), PTA (χ2 = .49), Tympanometry (χ2 =.04), and ABR (χ2 = 1.07). Pie charts based on percentage values and corresponding chi square analyses examining group differences in rates of normal and abnormal findings for each audiological measure are presented in Figure 1. Missing data for communication measures, PTA, Tympanometry, ART, and ABR were due to limited comprehension of task demands or noncompliance with procedures. Additional missing data for UCL and DPOAE were due to unavailability of equipment following relocation of the study (temporary for UCL and permanently for DPOAE). Independent samples t-tests indicated that participants with missing data were significantly younger than those who were able to participate in PTA (t(74)=-2.25, p<.05) and ART assessments (t(74)=-2.39, p<.05). In contrast, participants with missing DPOAE data were significantly older than those for whom DPOAE data were collected (t(74)=2.27, p<.05). Participants with missing data for the remaining audiological tests did not significantly differ in age from those who participated in the assessments. Finally, FSIQ of participants with missing data did not significantly differ from those for whom data was collected for any of the audiological measures.
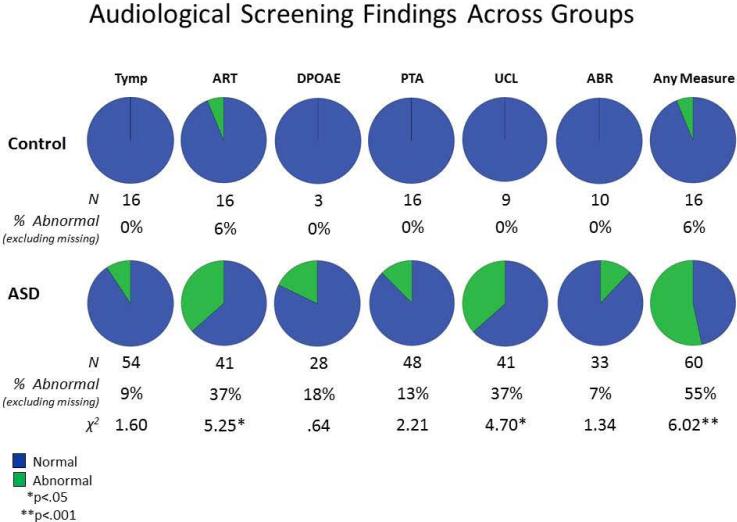
Figure 1.
Rates and χ2 values for normal versus abnormal findings on audiological screening measures in participants diagnosed with ASD and controls. The rightmost chart marked “Any Measure” indicates the rates of normal versus abnormal findings on at least one of the audiological screening measures.
To test the hypothesis that peripheral hearing abnormalities are more common in individuals on the autism spectrum compared to typically developing participants, differences in rates of audiological abnormalities were examined between groups. Results indicated that while abnormal audiological findings were generally more frequent in the ASD group on all measures, after Bonferroni correction for multiple comparisons χ2 analyses of these differences were only statistically significant when participants were dichotomized as having an abnormality on at least one measure versus having no abnormalities on any measure (p<.001).
To evaluate whether ASD+ (n=31), ASD- (n=19) and control (n=16) groups differed in communication abilities, a series of Oneway ANOVAs were performed to evaluate group differences on measures of speech articulation (GFTA), expressive, receptive, and overall language abilities (CELF), phonological awareness (CTOPP), and child (CVAR) and adult vocal affect recognition (AVAR). Preliminary independent samples t-tests indicated that the ASD+ group did not significantly differ from the ASD- group with respect to age (M=10.27, SD=3.31 for ASD- and M=11.20, SD=3.48 for ASD+; t(58)=-1.05, p = .30) or intellectual ability (FSIQ M=86.07, SD=21.04 for ASD- and M=79.41, SD=22.48 for ASD+; t(58)=.92, p = .36). Next, Oneway ANOVAs were performed on all communication measures with Bonferroni corrections for multiple comparisons resulting in a significance threshold of p<.007. Statistically significant differences were identified between groups on all measures of communication except for speech articulation and child vocal affect recognition, which did not survive correction for multiple comparisons. To determine which groups differed from each other on these measures, Bonferroni-corrected paired comparisons were performed. These analyses indicated that significant group differences were only detected between control and ASD groups for these comparisons and that ASD groups did not significantly differ from each other on any measures of communication. These results are summarized in Table 2.
Table 2.
Oneway ANOVA Results for Group Differences in Performance on Communication Measures
| Study Group |
|||||
|---|---|---|---|---|---|
| Measure | Control M±SD [Range] | ASD – M±SD [Range] | ASD + M±SD [Range] | F | η 2 |
| Speech Articulation | 103.38±1.93 [101-108] | 84.47±22.21 [40-105] | 93.16±18.33 [40-110] | 5.14 | .14 |
| Expressive Language | 110.25±10.46 [89-132] | 73.78±28.18a*** [45-118] | 75.46±26.63a*** [45-128] | 12.86*** | .30 |
| Receptive Language | 108.06±11.08 [92-134] | 75.06±28.50a*** [45-131] | 76.83±23.13a*** [45-128] | 11.98*** | .28 |
| Core Language | 113.38±8.01 [99-133] | 71.17±30.69a*** [40-120] | 73.14±26.70a*** [40-126] | 16.32*** | .35 |
| Phonological Awareness | 109.56±9.87 [91-124] | 87.13±20.05a*** [46-115] | 91.68±16.51a*** [61-124] | 8.96*** | .24 |
| Adult Vocal Affect Recognition | 105.96±9.00 [88.97-118.64] | 87.96±14.18a*** [50.45-106.96] | 87.89±15.43a*** [58.75-113.13] | 10.09*** | .27 |
| Child Vocal Affect Recognition | 98.45±11.18 [76.89-113.68] | 89.41±16.33 [52.57-114.09] | 82.82±19.69 [43.09-109.26] | 4.37 | .14 |
p≤.001
Significantly different from control group based on Bonferroni-corrected thresholds for paired comparisons
Note: ASD groups did not significantly differ from each other on any measures of communication.
Independent samples t-tests and correlational analyses were conducted to assess group differences in UCL and PTA values for the most impaired ear at each tested frequency as well as their relationships to communication measures. Bonferroni correction for multiple comparisons resulted in a significance threshold of p<.0045. Results indicated that group differences in hearing thresholds were significant only for UCL, with t(33.73)=-4.65, p<.001. Because of their limited variability in peripheral audiology, correlation analyses were not possible for the control group. In the ASD group, however, PTA at 2000Hz was significantly related to all language measures. Weaker associations were detected between affect recognition, phonological awareness and PTA values at 2000Hz and lower frequencies, although these associations were not statistically significant after correction for multiple comparisons. These correlations for the ASD group are presented in Table 3 and means, standard deviations, and ranges are reported for both groups in Tables 4 and 5. As would be expected, FSIQ was significantly associated with performance on several of the communication measures for these participants (GFTA r=.414, p=.007; CELF r=.893, p<.001; CTOPP r=.639, p<.001; CVAR r=.699, p<.001; AVAR r=.617, p<.001), and as such, correlation analyses were performed between FSIQ and PTA/UCL values for the ASD group. Like the communication measures, FSIQ was associated with PTA values at 2000Hz (r=-.44, p=.007), although this relationship was not statistically significant after correction for multiple comparisons.
Table 3.
Correlation Coefficients (r[N]) for FSIQ/Communication Measures and PTA/UCL in ASD Sample
| PTA (Hz) |
IQ | Articulation | Expressive Language |
Receptive Language |
Core Language |
Phonological Awareness |
Adult Affect |
Child Affect |
|---|---|---|---|---|---|---|---|---|
| 250 | −.15 [36] | −.27 [38] | −.13 [37] | −.06 [38] | −.13 [37] | −.13 [37] | .04 [35] | −.04 [35] |
| 500 | −.27 [37] | −.28 [39] | −.26 [38] | −.18 [39] | −.25 [38] | −.36* [38] | −.03 [36] | −.07 [36] |
| 750 | −.37* [35] | −.28 [37] | −.36* [36] | −.32 [37] | −.36* [36] | −.43** [36] | −.09 [34] | −.20 [34] |
| 1000 | −.18 [37] | −.18 [39] | −.19 [38] | −.18 [39] | −.20 [38] | −.32* [38] | −.06 [36] | −.10 [36] |
| 1500 | −.20 [34] | −.34* [36] | −.36* [35] | −.32 [36] | −.35* [35] | −.39* [35] | −.11 [33] | −.26 [33] |
| 2000 | −.44** [37] | −.62** [39] | −.49** [38] | −.51** [39] | −.53** [38] | −.42** [38] | −.35* [36] | −.36* [36] |
| 3000 | −.13 [34] | −.33 [36] | −.22 [35] | −.19 [36] | −.21 [35] | −.31 [35] | −.07 [33] | −.19 [33] |
| 4000 | −.16 [37] | −.21 [39] | −.16 [38] | −.11 [39] | −.16 [38] | −.33* [38] | −.08 [36] | −.17 [36] |
| 6000 | .05 [34] | −.07 [36] | −.03 [35] | .01 [36] | −.02 [35] | −.19 [35] | .12 [33] | .04 [33] |
| 8000 | −.25 [36] | −.13 [38] | −.21 [37] | −.18 [38] | −.22 [37] | −.25 [37] | −.07 [35] | −.22 [35] |
| UCL Speech | .09 [35] | −.17 [39] | .12 [37] | .16 [39] | .11 [38] | .05 [36] | .20 [35] | .14 [35] |
p<.05
p<.01
p<.0045 (statistically significant after correction for multiple comparisons)
Table 4.
Descriptive Statistics for ASD Group Hearing Thresholds and UCL in the Most Impaired Ear (dB HL)
| Hz | PTA 250 | PTA 500 | PTA 750 | PTA 1000 | PTA 1500 | PTA 2000 | PTA 3000 | PTA 4000 | PTA 6000 | PTA 8000 | UCL Speech |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 20.92 | 18.72 | 16.08 | 13.08 | 12.36 | 8.46 | 9.72 | 10.00 | 11.25 | 9.61 | 92.80 |
| SD | 11.38 | 11.79 | 11.00 | 10.43 | 8.24 | 8.12 | 6.96 | 9.67 | 8.73 | 9.03 | 15.85 |
| Min | 5.00 | .00 | .00 | .00 | .00 | −5.00 | .00 | .00 | −5.00 | −5.00 | 45.00 |
| Max | 50.00 | 60.00 | 45.00 | 45.00 | 40.00 | 40.00 | 30.00 | 50.00 | 40.00 | 25.00 | 115.00 |
Table 5.
Descriptive Statistics for Control Group Hearing Thresholds and UCL in the Most Impaired Ear (dB HL)
| Hz | PTA 250 | PTA 500 | PTA 750 | PTA 1000 | PTA 1500 | PTA 2000 | PTA 3000 | PTA 4000 | PTA 6000 | PTA 8000 | UCL Speech |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 23.84 | 20.00 | 16.92 | 10.94 | 9.62 | 2.81 | 6.92 | 5.00 | 7.69 | 6.15 | 107.78 |
| SD | 10.64 | 7.53 | 7.23 | 4.91 | 5.94 | 5.15 | 5.60 | 4.83 | 8.32 | 8.45 | 6.18 |
| Min | 10.00 | 5.00 | 5.00 | 5.00 | .00 | −5.00 | −5.00 | −5.00 | −10.00 | −5.00 | 100.00 |
| Max | 50.00 | 60.00 | 45.00 | 45.00 | 40.00 | 40.00 | 30.00 | 50.00 | 40.00 | 25.00 | 115.00 |
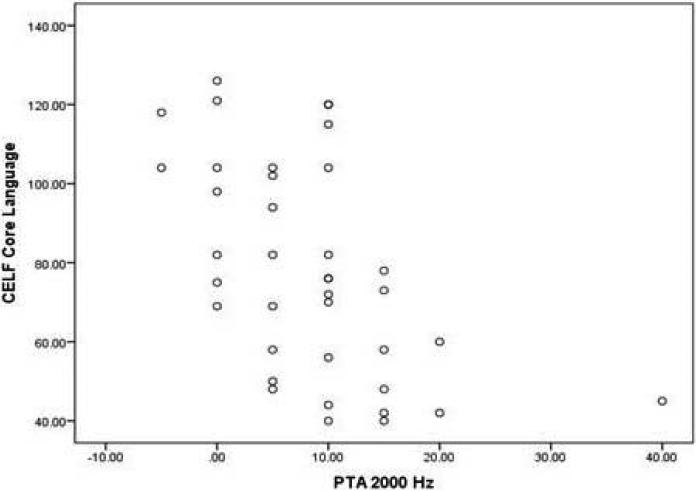
It is noteworthy that subclinical hearing loss substantially contributed to these significant correlations. For example, the maximum value in the 2000Hz condition was 40dB HL, which falls within the range of mild hearing loss. The central tendency of scores for hearing threshold at 2000Hz was substantially lower (M=8.46, SD=8.12), indicating that the relationships between PTA at 2000Hz and language abilities were substantially driven by hearing thresholds within the normal to subclinical range. Figure 2 illustrates this relationship with CELF Core Language scores.
Figure 2.
Scatterplot of hearing threshold values for 2000Hz PTA and scores on CELF Core Language Index
Discussion
The incidence of an abnormal finding on at least one measure of audiological functioning was higher in children with an ASD (55%) than the control group (6%) or estimates for the general population (14.9% of children aged 6-19 years according to CDC's Third National Health and Nutrition Examination Survey, 1988–1994). The presence of sound sensitivity in at least one ear was also considerably higher for the ASD population (37% of ASD participants versus 0% for the control group and general population estimates of 8-15%; Baguley, 2003). These findings are consistent with previous studies indicating increased rates of peripheral audiological dysfunction in individuals with ASD when assessed on more than one audiological measure (Jure et al., 1991; Rosenhall et al., 1999; Tas et al., 2007) and increased rates of hyperacusis (Rosenhall et al., 1999). These results are inconsistent with one study, however, in which increased rates of audiological abnormalities in ASD was not identified compared to a control group when PTA, ART, TEOAE, and DEOAE were examined (Gravel et al., 2006). These discrepant results may be attributable to several factors, such as the higher level of functioning for the participants in the Gravel et al. study compared to the present study in which participants with a diverse range of functioning were included. In addition, ABR was not assessed by Gravel et al., and abnormalities in ABR are one of the most replicated findings with regard to audiological dysfunction in ASD. Finally, Gravel et al. characterized hyperacusis as hearing thresholds ≤ 0 dB HL without reference to the participants’ levels of perceived discomfort with sounds of different intensities. This is an important distinction, as Stiegler and Davis (2010) have reported that while there is evidence of hyperacusis in some individuals with ASD based on behavioral response despite the lack of evidence of physiological contributions to sound sensitivity. They attribute this failure to identify an association between this perceived discomfort and audiological abnormalities to the fact that sound sensitivity or discomfort may be related to more complex cortical phenomena rather than peripheral auditory dysfunction.
Taken together, the results of the present study are consistent with the majority of previous literature identifying increased rates of audiological dysfunction in individuals with ASD. While some prior evidence suggests that this dysfunction may be limited to participants with lower levels of functioning (Gravel et al.), this suggests that peripheral audiological dysfunction may be associated with functional impairment in ASD. When participants with ASD were dichotomized into groups with and without evidence of audiological abnormality, however, significant differences were not identified on communication measures in the present study. Because only a small percentage of hearing-impaired children are diagnosed with an ASD, this has been regarded as evidence that peripheral auditory dysfunction is unrelated to ASD symptom presentation (Jure, Rapin, & Tuchman, 1991). The failure to identify group differences in communication measures in the current study would seemingly support this hypothesis. However, examination of these data in a dichotomized format (i.e., intact versus impaired audiology) fails to address the possibility that distorted perception may have more pervasive impact on functioning than a failure to hear at all, as has been suggested by Klin (1993).
Therefore, to examine the extent to which subclinical variability in hearing thresholds and sound sensitivity were related to ASD symptomatology, correlational analyses were performed between measures of PTA, UCL, and communication. Because this sample included participants who were lower functioning, and because of the foreseeable strong association between these communication measures and FSIQ, these correlations were also performed between PTA, UCL, and FSIQ. UCL thresholds were not significantly associated with any measure of communication. This is not surprising given that UCL levels for all but one participant were greater than 60dB, which is the volume of conversational speech. Thus, UCL values would need to be much lower if discomfort with the volume of conversational speech were expected to negatively impact language development. Results of these analyses did, however, indicate that variability in hearing thresholds were related to speech articulation and receptive and expressive language skills. Sound frequencies for speech production range from 250-1000Hz for vowel sounds and 1500-6000Hz for consonant sounds (Center for Disease Control and Prevention, 2012). In the present study there were robust findings for relationships between measures of PTA for middle range frequencies (2000Hz in particular) and all measures of audible communication and FSIQ, although only relationships between 2000Hz hearing thresholds and expressive, receptive, and core language abilities and articulation were significant after correction for multiple comparisons. Thus, these results may suggest that an association exists between subclinical hearing loss at 2000Hz and language abilities in individuals with ASD. The fact that the association between 2000Hz PTA and IQ was relatively weaker than the association between 2000Hz PTA and several of the communication measures (i.e., articulation, expressive, receptive, and core language abilities) suggests that the relationship between mild/subclinical hearing loss and language may be primary, having a subsequent effect on intellectual development rather than the other direction. This stands to reason, as the ability to effectively communicate with others can undoubtedly affect learning and development in other cognitive domains.
Notably, this 2000Hz frequency is within the range of consonant sounds on the human speech spectrum, which make up the greater part of phonemes in audible communication. Detailed examination of these results of correlation analyses suggests some additional specific trends in the relationship between hearing threshold and audible communication. Specifically, phonological awareness was more modestly related to middle and low frequency hearing thresholds. The fact that phonological awareness was associated, albeit not statistically significantly, with thresholds at the greatest range of frequencies on the human speech spectrum is consistent with expectation for this measure, as it specifically targets awareness of a range of consonant and vowel sounds. The relationships identified in the current study therefore suggest that subclinical hearing loss at frequencies on the human speech spectrum may be related to functional communication levels in ASD. Taken together, these findings provide evidence that dichotomized classification of clinical audiology may not provide a complete picture in understanding the role of subclinical hearing loss in ASD symptomatology and that dimensional examination of relationships between hearing level and communication abilities may be more informative in understanding the relationship between peripheral auditory dysfunction and communication in ASD. It is unclear if these relationships are causal in nature, or rather, if they are related to a common underlying etiology; however these findings may have implications for treatment response to mild or subclinical hearing loss in children with ASDs.
Limitations and Future Directions
There are several limitations to the present study. First, relocation of the study resulted in use of different equipment for many of the study measures, and although analysis of site effect suggests that rates of peripheral audiological abnormalities did not differ between sites, these results should be replicated with use of consistent equipment between subjects. A second limitation of the present study was the rate of missing data, which varied between subjects and tasks. While some of this missing data can be considered random (e.g., delay in availability of equipment immediately following relocation), other forms of missing data are more systematic (e.g., some lower functioning participants may not have bene able to participate in tasks requiring behavioral response, such as PTA or UCL). The latter of these effects may have resulted in an underestimation of rates of abnormalities or underestimation of correlations between PTA/UCL and performance on communication measures if these lower functioning participants also present with greater audiological dysfunction as prior research suggests (Hitoglou et at., 2010; Konstantareas & Homatidis, 1987). Notably, participants with missing data were older for DPOAE and younger for both PTA and ABR. Because DPOAE and ABR were scored normal versus abnormal, controlling for these differences in age was not possible; however, age was covaried in ANCOVAs between participant groups across all frequencies of PTA. Third, only PTA and UCL values were appropriate for correlational analyses with communication measures, and these measures were the most dependent on participant comprehension and behavioral compliance. This resulted in a group with less representation of lower functioning participants, and so the effects of subclinical hearing loss on communication may not generalize to those participants. Further, while considerable efforts were made to discontinue testing for any participants suspected of poor task comprehension or noncompliance, replication of these findings for specific speech frequencies will provide further evidence of the association between subclinical hearing loss at relevant human speech frequencies with communication skills as opposed to more random effects of measurement error. Finally, groups were not matched for age or general intellectual ability, and thus it is unclear to what extent these findings of audiological dysfunction are specific to ASD versus general intellectual impairment affecting those with ASD. This finding retains value, however, regardless of whether it applies to ASD per se versus representing a marker of low levels of functioning in ASD. For example, should these finding be replicated, the implications for audiological intervention would still apply irrespective of the cognitive domains that might benefit from this remediation. Further research is necessary to understand the relationship between subclinical hearing loss and development of communication and general intellectual abilities.
Replication of these findings and longitudinal research are necessary to further understand the developmental processes involved in the acquisition of these deficits. Future studies examining auditory processes may benefit from examination of auditory response to different sound frequencies, including 2000Hz and surrounding frequencies. Finally, the relationship between peripheral auditory dysfunction and atypical cortical auditory processing in ASD has not been established. Studies with comprehensive audiological screening and cortical measures of auditory processing are necessary to explore these relationships. These studies should incorporate longitudinal designs to elucidate the developmental role of sensory dysfunction in ASD.
Conclusions
This study was the first to provide a comprehensive examination of peripheral audiological functioning in individuals with ASD compared to a control group in relation to communication abilities. Results indicated increased rates of peripheral audiological abnormality compared to both controls and the general population. While group differences were not found on communication measures when participants were dichotomized by traditional classification of normal limits, subclinical hearing loss was related to communication abilities in correlational analyses for the ASD group. These findings warrant further study and may have implications for treatment of mild and subclinical hearing loss in ASD.
Acknowledgments
Grant Sponsor: NIH R01 awarded to primary investigator Jeffrey Lewine, Ph.D.
Grant Number: HD051747-01A1
Literature Cited
- American Speech-Language-Hearing Association . Auditory Integration Training (Technical Report) Author; Rockville, MD: 2004. [Google Scholar]
- Bachara GH, Raphael J, Phelan WJ. Empathy development in deaf preadolescents. American Annals of the Deaf. 1980;125:38–41. doi: 10.1353/aad.2012.1175. [DOI] [PubMed] [Google Scholar]
- Baguley DM. Hyperacusis. Journal of the Royal Society of Medicine. 2003;96:582–585. doi: 10.1258/jrsm.96.12.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich a. a., Choudhury N. a., Realpe-Bonilla T, Roesler CP. Plasticity in Developing Brain: Active Auditory Exposure Impacts Prelinguistic Acoustic Mapping. Journal of Neuroscience. 2014;34(40):13349–13363. doi: 10.1523/JNEUROSCI.0972-14.2014. doi:10.1523/JNEUROSCI.0972-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg E, Counter SA. The Middle-Ear Muscles. Scientific American. 1989;261(2):74–78. doi: 10.1038/scientificamerican0889-74. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention [March 9, 2014];Hearing loss in children: About sound. 2012 from http://www.cdc.gov/ncbddd/hearingloss/sound.html.
- Center for Disease Control and Prevention [March 14, 2012];Summary of 2009 National CDC EHDI Data. 2009 from http://www.cdc.gov/ncbddd/hearingloss/2009-Data/2009_EHDI_HSFS_Summary_508_OK.pdf.
- Dyck MJ, Farrugia C, Shochet IM, Holmes-Brown M. Emotion recognition/understanding ability in hearing or vision-impaired children: Do sounds, sights, or words make the difference? Journal of Child Psychology and Psychiatry. 2004;45:789–800. doi: 10.1111/j.1469-7610.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- Edgar JC, Khan SY, Blaskey L, Chow VY, Rey M, Gaetz W, Roberts TPL. Neuromagnetic Oscillations Predict Evoked-Response Latency Delays and Core Language Deficits in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1904-x. doi:10.1007/s10803-013-1904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserman W, Hartel D, Shisler L, et al. Using otoacoustic emissions to screen for hearing loss in early childhood care settings. International Journal of Pediatric Otorhinolaryngology. 2008;72:475–482. doi: 10.1016/j.ijporl.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Flanagan DP, Kaufman AS. Essentials of WISC-IV Assessment. John Wiley & Sons, Inc.; Hoboken, NJ: 2009. [Google Scholar]
- Fowler CG, Shanks JE. Tympanometry. In: Katz J, editor. Handbook of Clinical Audiology: 5th Edition. Lippincott, Williams & Wilkins; Baltimore: 2002. [Google Scholar]
- Gillberg C, Rosenhall U, Johansson E. Auditory brainstem responses in childhood psychosis. Journal of Autism and Developmental Disorders. 1983;13:181–195. doi: 10.1007/BF01531818. [DOI] [PubMed] [Google Scholar]
- Goldman R, Fristoe M. Goldman Fristoe-2: Test of Articulation. American Guidance Service, Inc.; Circle Pines, MN: 2000. [Google Scholar]
- Gravel JS, Dunn M, Lee WW, Ellis MA. Peripheral audition of children on the autistic spectrum. Ear and Hearing. 2006;27(3):299–312. doi: 10.1097/01.aud.0000215979.65645.22. doi:10.1097/01.aud.0000215979.65645.22. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Akshoomoff N. Brainstem and middle latency auditory evoked response postential in autism and developmental language disorder. Journal of Autism and Developmental Disorders. 1989;19:255–269. doi: 10.1007/BF02211845. [DOI] [PubMed] [Google Scholar]
- Harlor ADB, Bower C. Hearing assessment in infants and children: recommendations beyond neonatal screening. Pediatrics. 2009;124(4):1252–63. doi: 10.1542/peds.2009-1997. doi:10.1542/peds.2009-1997. [DOI] [PubMed] [Google Scholar]
- Hayes RW, Gordon AG. Auditory abnormalities in autistic children. Lancet. 1977;2:767. doi: 10.1016/s0140-6736(77)90278-1. [DOI] [PubMed] [Google Scholar]
- Hitoglou M, Ververi A, Antoniadis A, Zafeiriou DI. Childhood autism and auditory system abnormalities. Pediatric Neurology. 2010;42(5):309–14. doi: 10.1016/j.pediatrneurol.2009.10.009. doi:10.1016/j.pediatrneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Interacoustics . Audio Traveler AA222: Operation Manual. Interacoustics; Assens, Denmark: 2010. [Google Scholar]
- Johnsen NJ, Bagi P, Elberling C. Evoked acoustic emissions from the human ear III: Findings in neonates. Scandinavian Audiology. 1983;12:17–24. doi: 10.3109/01050398309076220. [DOI] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- Jure R, Rapin I, Tuchman RF. Hearing-impaired autistic children. Developmental Medicine and Child Neurology. 1991;33:1062–1072. doi: 10.1111/j.1469-8749.1991.tb14828.x. [DOI] [PubMed] [Google Scholar]
- Kasai K, Hashimoto O, Kawakubo Y, Yumoto M, Kamio S, Itoh K, Kato N. Delayed automatic detection of change in speech sounds in adults with autism: a magnetoencephalographic study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2005;116(7):1655–64. doi: 10.1016/j.clinph.2005.03.007. doi:10.1016/j.clinph.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Bruneau N, Roge B, Georgieff N, Veuillet E, Adrien J, Collet L. Peripheral auditory asymmetry in infantile autism. European Journal of Neuroscience. 2001;13:628–632. doi: 10.1046/j.1460-9568.2001.01423.x. [DOI] [PubMed] [Google Scholar]
- Kielinen M, Rantala H, Timonen E, Linna S-L, Moilanen I. Associated medical disorders and disabilities in children with Autistic Disorder: A population-based study. Autism. 2004;8:49–60. doi: 10.1177/1362361304040638. [DOI] [PubMed] [Google Scholar]
- Klin A. Auditory brainstem response in Autism: Brainstem dysfunction or peripheral hearing loss? Journal of Autism and Developmental Disorders. 1993;23(1):15–35. doi: 10.1007/BF01066416. [DOI] [PubMed] [Google Scholar]
- Konstantareas MM, Homatidis S. Ear infections in autistic and normal children. Journal of Autism and Developmental Disorders. 1987;17:585–594. doi: 10.1007/BF01486973. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental Science. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. doi:10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of Autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS): Manual. Western Psychological Services; Los Angeles, CA: 2001. [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of Autism & Developmental Disorders. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Storoschuk S, Rutter M, Pickles A. Using the ADI-R to diagnose autism in preschool children. Infant Mental Health. 1993;14:234–252. [Google Scholar]
- Margolis RH, Hunter LL. Acoustic immittance measurements. In: Roeser RJ, Valente M, Hosford-Dunn H, editors. Diagnosis.; Thieme Medical Publishers, Inc.; Audiology: New York: 2000. [Google Scholar]
- Most T, Aviner C. Auditory, visual and auditory–visual perception of emotions by individuals with cochlear implants, hearing aids, and normal hearing. Journal of Deaf Studies and Deaf Education. 2009;14:449–464. doi: 10.1093/deafed/enp007. [DOI] [PubMed] [Google Scholar]
- Most T, Michaelis H. Auditory, visual, and auditory–visual perceptions of emotions by young children with hearing loss versus children with normal hearing. Journal of Speech, Language, and Hearing Research. 2012;55:1148–1162. doi: 10.1044/1092-4388(2011/11-0060). [DOI] [PubMed] [Google Scholar]
- Nowicki S. Emory University; Atlanta, GA: 2010. Manual for the receptive tests of the diagnostic analysis of nonverbal accuracy 2. Unpublished manuscript. [Google Scholar]
- Nowicki S, Jr., Carton J. The measurement of emotional intensity from facial Expressions: the DANVA FACES 2. Journal of Social Psychology. 1993;133:749–751. doi: 10.1080/00224545.1993.9713934. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Duke MP. Individual differences in the nonverbal communication of affect: The diagnostic analysis of nonverbal accuracy scale. Journal of Nonverbal Behavior. 1994;18:9–35. [Google Scholar]
- Ornitz EM. Neurophysiology of infantile autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1985;24:251–262. doi: 10.1016/s0002-7138(09)61084-0. [DOI] [PubMed] [Google Scholar]
- Onusko E. Tympanometry. American Family Physician. 2004;70(9):1713–1720. [PubMed] [Google Scholar]
- Rimland B, Edelson S. Brief report: A pilot study of auditory integration training in autism. Journal of Autism and Developmental Disorders. 1995;25:61–70. doi: 10.1007/BF02178168. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Teale PD, Maharajh K, Kronberg E, Youngpeter K, Wilson LB, Hepburn S. Transient and steady-state auditory gamma-band responses in first-degree relatives of people with autism spectrum disorder. Molecular Autism. 2011;2(1):11. doi: 10.1186/2040-2392-2-11. doi:10.1186/2040-2392-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum SM, Arick JR, Krug DA, Stubbs EG, Young NB, Pelson RO. Auditory brainstem evoked responses in autistic children. Journal of Autism and Developmental Disorders. 1980;10:215–225. doi: 10.1007/BF02408472. [DOI] [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Sandstrom M, Ahlsen G, Gillberg C. Autism and hearing loss. Journal of Autism and Developmental Disorders. 1999;29(5):349–357. doi: 10.1023/a:1023022709710. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised: Manual. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Seidman MD, Simpson GT, II, Khan MJ. Common problems of the ear. In: Noble J, editor. Textbook of Primary Care Medicine, 3rd Edition. Mosby Elsevier; Philadelphia: 2001. [Google Scholar]
- Semel E, Wiig E, Secord WA. Clinical Evaluation of Language Fundamentals 4 (CELF-4) The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Smith DEP, McConnell JV, Walter TL, Miller SD. Effects of using an auditory trainer on the attentional, language, and social behavior of autistic children. Journal of Autism & Developmental Disorders. 1985;15:285–302. doi: 10.1007/BF01531499. [DOI] [PubMed] [Google Scholar]
- Smith DEP, Miller SD, Steward M, Walter TL, McConnell JV. Conductive hearing loss in autistic, learning-disabled, and normal children. Journal of Autism & Developmental Disorders. 1988;18:53–65. doi: 10.1007/BF02211818. [DOI] [PubMed] [Google Scholar]
- Tas A, Yagiz R, Tas M, et al. Evaluation of hearing in children with autism by using TEOAE and ABR. Autism. 2007;11(1):73–79. doi: 10.1177/1362361307070908. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Rosenblatt B, Linschoten L. Auditory brainstem response abnormalities in autistic children. Canadian Journal of Neurological Sciences. 1892;9:429–433. doi: 10.1017/s0317167100044346. [DOI] [PubMed] [Google Scholar]
- Thivierge J, Bedard C, Cote R, Maziade M. Brainstem auditory evoked response and subcortical abnormalities in autism. American Journal of Psychiatry. 1990;147:1609–1613. doi: 10.1176/ajp.147.12.1609. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgeson JK, Rashotte CA. Comprehensive Test of Phonological Processing (CTOPP) Pro-Ed, Inc.; Austin, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third Edition (WAIS-III) Pearson Assessments; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence—Third Edition (WPPSI-III) Pearson Assessments; San Antonio, TX: 2002. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Fourth Edition (WISC-IV) Pearson Assessments; San Antonio, TX: 2003. [Google Scholar]
- Williams PE, Weiss LG, Rolfhus EL. WISC-IV Technical Report 2: Psychometric Properties. The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biological Psychiatry. 2007;62(3):192–7. doi: 10.1016/j.biopsych.2006.07.002. doi:10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V, Wong SN. Brainstem auditory evoked potential study in children with Autistic Disorder. Journal of Autism and Developmental Disorders. 1991;21:329–340. doi: 10.1007/BF02207329. [DOI] [PubMed] [Google Scholar]