Abstract
Severe bloodstream-borne infection--i.e., sepsis--and the resulting multiorgan failure are now the most common cause of death in many intensive care units. One of the most fundamentally important and controversial issues concerning the pathophysiology of sepsis is the role of intracellular free calcium concentration ([Ca2+]i) in this disorder. Because of the critical role of calcium as an intracellular second messenger and as a potential cellular toxin, resolution of this issue is crucial. Using 19F NMR spectroscopy and the calcium indicator 5,5'-difluoro-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetate we demonstrate in the intact perfused organ, the rat thoracic aorta, that [Ca2+]i in aortic smooth muscle is increased > 2-fold during sepsis. Furthermore, we determined that sodium dantrolene, a drug that decreases release of calcium from the sarcoplasmic reticulum and that is lifesaving in malignant hyperthermia (a disorder due to increased [Ca2+]i), is able to reduce the elevated [Ca2+]i in sepsis to control values when added in vitro or when given in vivo to the animal. These results suggest that an increase in [Ca2+]i is an early event in sepsis and that increased [Ca2+]i may be responsible for, or contribute to, cellular injury. Dantrolene may offer a therapeutic strategy in the treatment of sepsis.
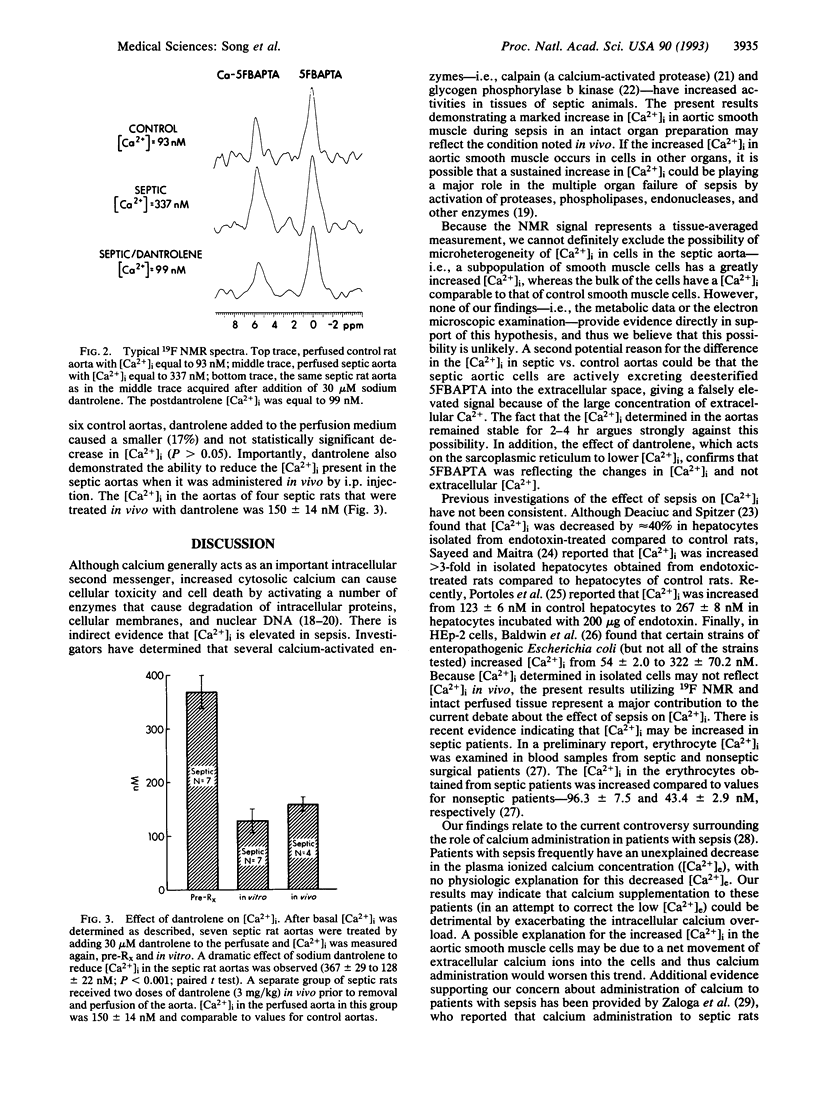
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. J., Ward W., Aitken A., Knutton S., Williams P. H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991 May;59(5):1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D., Cohen R. A., Levinsky N. G. Endotoxin inhibits contraction of vascular smooth muscle in vitro. Am J Physiol. 1990 Apr;258(4 Pt 2):H1187–H1192. doi: 10.1152/ajpheart.1990.258.4.H1187. [DOI] [PubMed] [Google Scholar]
- Beebe D. S., Belani K. G., Tuohy S. E., Sweeney M. F., Gillingham K., Komanduri V., Palahniuk R. J. Is dantrolene safe to administer in sepsis? The effect of dantrolene after endotoxin administration in dogs and rats. Anesth Analg. 1991 Sep;73(3):289–294. doi: 10.1213/00000539-199109000-00011. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J., Thompson K., Sayeed M. M. Calcium-dependent and calcium-independent protease activities in skeletal muscle during sepsis. Circ Shock. 1991 Oct;35(2):117–122. [PubMed] [Google Scholar]
- Carcillo J. A., Litten R. Z., Suba E. A., Roth B. L. Alterations in rat aortic alpha 1-adrenoceptors and alpha 1-adrenergic stimulated phosphoinositide hydrolysis in intraperitoneal sepsis. Circ Shock. 1988 Nov;26(3):331–339. [PubMed] [Google Scholar]
- Chernow B. Calcium: does it have a therapeutic role in sepsis? Crit Care Med. 1990 Aug;18(8):895–896. [PubMed] [Google Scholar]
- Crass M. F., 3rd, Hulsey S. M., Bulkley T. J. Use of a new pulsatile perfused rat aorta preparation to study the characteristics of the vasodilator effect of parathyroid hormone. J Pharmacol Exp Ther. 1988 May;245(2):723–734. [PubMed] [Google Scholar]
- Deaciuc I. V., Spitzer J. A. Rat liver free cytosolic Ca2+ and glycogen phosphorylase in endotoxicosis and sepsis. Am J Physiol. 1986 Nov;251(5 Pt 2):R984–R995. doi: 10.1152/ajpregu.1986.251.5.R984. [DOI] [PubMed] [Google Scholar]
- Frandsen A., Schousboe A. Mobilization of dantrolene-sensitive intracellular calcium pools is involved in the cytotoxicity induced by quisqualate and N-methyl-D-aspartate but not by 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionate and kainate in cultured cerebral cortical neurons. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2590–2594. doi: 10.1073/pnas.89.7.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut K., Desmedt J. E. Effect of dantrolene sodium on calcium movements in single muscle fibres. Nature. 1974 Dec 20;252(5485):728–730. doi: 10.1038/252728a0. [DOI] [PubMed] [Google Scholar]
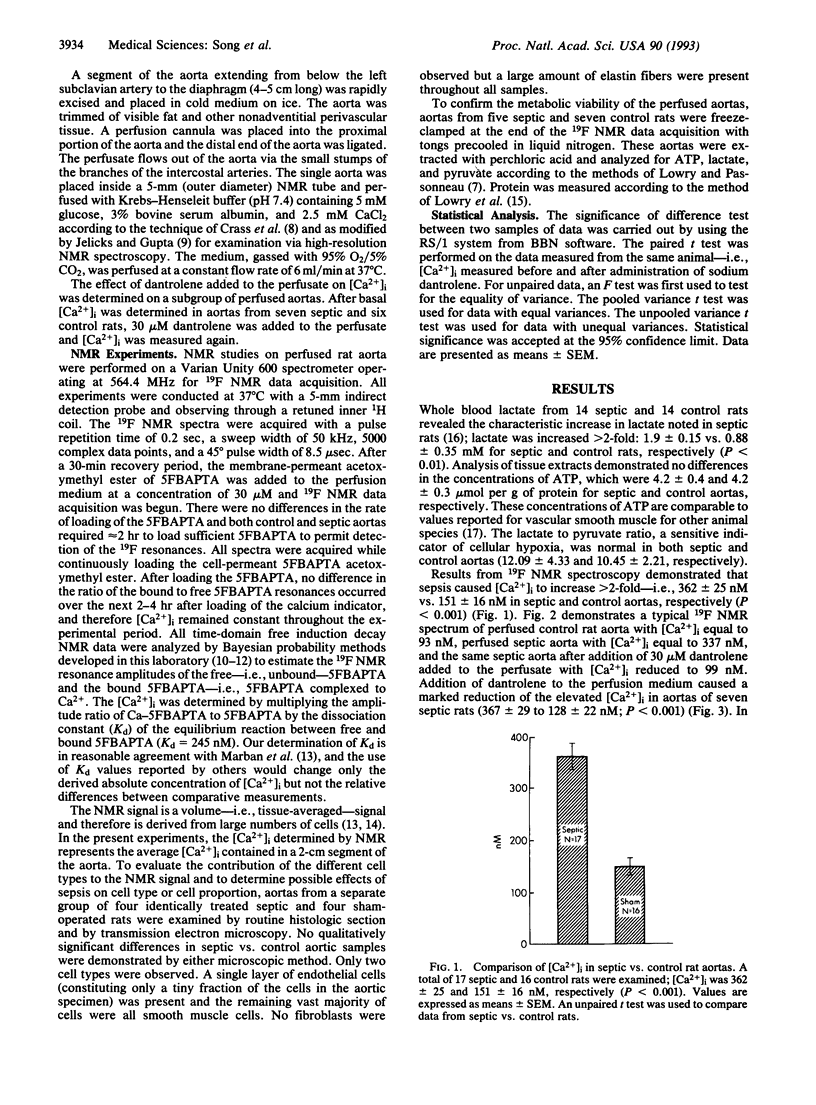
- Hotchkiss R. S., Karl I. E. Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA. 1992 Mar 18;267(11):1503–1510. [PubMed] [Google Scholar]
- Jelicks L. A., Gupta R. K. NMR measurement of cytosolic free calcium, free magnesium, and intracellular sodium in the aorta of the normal and spontaneously hypertensive rat. J Biol Chem. 1990 Jan 25;265(3):1394–1400. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu M. S., Kang G. F. Liver glycogen metabolism in endotoxin shock. II. Endotoxin administration increases glycogen phosphorylase activities in dog livers. Biochem Med Metab Biol. 1987 Feb;37(1):73–80. doi: 10.1016/0885-4505(87)90011-9. [DOI] [PubMed] [Google Scholar]
- Lopez J. R., Gerardi A., Lopez M. J., Allen P. D. Effects of dantrolene on myoplasmic free [Ca2+] measured in vivo in patients susceptible to malignant hyperthermia. Anesthesiology. 1992 May;76(5):711–719. doi: 10.1097/00000542-199205000-00008. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Koretsune Y., Yue D. T., Chacko V. P., Pike M. M. Quantification of [Ca2+]i in perfused hearts. Critical evaluation of the 5F-BAPTA and nuclear magnetic resonance method as applied to the study of ischemia and reperfusion. Circ Res. 1990 May;66(5):1255–1267. doi: 10.1161/01.res.66.5.1255. [DOI] [PubMed] [Google Scholar]
- Mine T., Kojima I., Kimura S., Ogata E. Assessment of the role of Ca2+ mobilization from intracellular pool(s), using dantrolene, in the glycogenolytic action of alpha-adrenergic stimulation in perfused rat liver. Biochim Biophys Acta. 1987 Feb 18;927(2):229–234. doi: 10.1016/0167-4889(87)90139-x. [DOI] [PubMed] [Google Scholar]
- Murphy E., Levy L., Raju B., Steenbergen C., Gerig J. T., Singh P., London R. E. Measurement of cytosolic calcium using 19F NMR. Environ Health Perspect. 1990 Mar;84:95–98. doi: 10.1289/ehp.908495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol. 1992;32:449–470. doi: 10.1146/annurev.pa.32.040192.002313. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Parker M. M., Natanson C., Suffredini A. F., Danner R. L., Cunnion R. E., Ognibene F. P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990 Aug 1;113(3):227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Portolés M. T., Ainaga M. J., Municio A. M., Pagani R. Intracellular calcium and pH alterations induced by Escherichia coli endotoxin in rat hepatocytes. Biochim Biophys Acta. 1991 Mar 19;1092(1):1–6. doi: 10.1016/0167-4889(91)90170-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. The calcium messenger system (1). N Engl J Med. 1986 Apr 24;314(17):1094–1101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- Reid I. R., Civitelli R., Halstead L. R., Avioli L. V., Hruska K. A. Parathyroid hormone acutely elevates intracellular calcium in osteoblastlike cells. Am J Physiol. 1987 Jul;253(1 Pt 1):E45–E51. doi: 10.1152/ajpendo.1987.253.1.E45. [DOI] [PubMed] [Google Scholar]
- Sayeed M. M., Maitra S. R. Effect of diltiazem on altered cellular calcium regulation during endotoxic shock. Am J Physiol. 1987 Oct;253(4 Pt 2):R549–R554. doi: 10.1152/ajpregu.1987.253.4.R549. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Soulsby M. E., Bennett C. L., Hess M. L. Canine arterial calcium transport during endotoxin shock. Circ Shock. 1980;7(2):139–148. [PubMed] [Google Scholar]
- Wakabayashi I., Hatake K., Kakishita E., Nagai K. Diminution of contractile response of the aorta from endotoxin-injected rats. Eur J Pharmacol. 1987 Sep 2;141(1):117–122. doi: 10.1016/0014-2999(87)90417-1. [DOI] [PubMed] [Google Scholar]
- Wichterman K. A., Baue A. E., Chaudry I. H. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980 Aug;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Zaloga G. P., Sager A., Black K. W., Prielipp R. Low dose calcium administration increases mortality during septic peritonitis in rats. Circ Shock. 1992 Jul;37(3):226–229. [PubMed] [Google Scholar]



